Abstract
Tolerance to submergence-induced hypoxia is an important agronomic trait especially for crops in lowland and flooding-affected areas. Although rice (Oryza sativa) is considered a flood-tolerant crop, only limited cultivars display strong tolerance to prolonged submergence and/or hypoxic stress. Therefore, characterization of hypoxic resistant genes and/or germplasms have important theoretical and practical significance for rice breeding and sustained improvements. Previous investigations have demonstrated that loss-of-function of OsPIN2, a gene encoding an auxin efflux transporter, results in the loss of root gravitropism due to disrupted auxin transport in the root tip. In this study, we revealed a novel connection between OsPIN2 and reactive oxygen species (ROS) in modulating root gravitropism and hypoxia tolerance in rice. It is shown that the OsPIN2 mutant had decreased accumulation of ROS in root tip, due to the downregulation of glycolate oxidase encoding gene OsGOX6, one of the main H2O2 sources. The morphological defects of root including waved rooting and agravitropism in OsPIN2 mutant may be rescued partly by exogenous application of H2O2. The OsPIN2 mutant exhibited increased resistance to ROS toxicity in roots due to treatment with H2O2. Furthermore, it is shown that the OsPIN2 mutant had increased tolerance to hypoxic stress accompanied by lower ROS accumulation in roots, because the hypoxia stress led to over production of ROS in the roots of the wild type but not in that of OsPIN2 mutant. Accordingly, the anoxic resistance-related gene SUB1B showed differential expression in the root of the WT and OsPIN2 mutant in response to hypoxic conditions. Notably, compared with the wild type, the OsPIN2 mutant displayed a different pattern of auxin distribution in the root under hypoxia stress. It was shown that hypoxia stress caused a significant increase in auxin distribution in the root tip of the WT but not in that of the war1 mutant. In summary, these results suggested that OsPIN2 may play a role in regulating ROS accumulation probably via mediating auxin transport and distribution in the root tip, affecting root gravitropism and hypoxic tolerance in rice seedlings. These findings may contribute to the genetic improvement and identification of potential hypoxic tolerant lines in rice.
1. Introduction
In recent years, as climate change leads to more severe weather extremes, submergence and waterlogging events are likely to become even more frequent and severe. Submergence or waterlogging resulting in root-zone hypoxia stress has become one of the major abiotic stresses for plant survival [,]. Hypoxia in plants could depress cellular respiration, substance metabolism, and plant growth and development [,]. In the lowland and flooding-affected area, hypoxia stress has become the most important adverse environmental factor that causes large loss of crop production [,]. Even for rice that adapts to grow in water-logged paddy fields, long time hypoxia stress may cause severe harm to rice production [,]. Therefore, exploration of genes or germplasm resistant to hypoxia stress is of great importance for genetic improvement in rice.
Extensive studies have shown that reactive oxygen species (ROS), such as superoxide (O2•−), hydrogen peroxide (H2O2) and hydroxyl radicals, play important roles in plant growth, development, and responses to various biotic and abiotic stresses [,]. For instance, plants suffering submergence or waterlogging will generally induce root-zone hypoxia and lead to increased ROS accumulation in root cells. Over accumulation of ROS can disturb cellular homeostasis between antioxidants and oxidants that will lead to oxidative stress, trigger membrane lipid peroxidation, and adversely affect membrane functions, and cause leakage of electrolytes, and serious damage of DNA and proteins [,]. In this respect, lower ROS accumulation during and following submergence may be associated with increased hypoxic tolerance [,]. However, on the other hand, ROS act as signaling molecules to regulate cellular signal transduction and various cellular processes at the physiological level []. This is particularly important regarding cell proliferation and differentiation in root growth and development []. In Arabidopsis, UPBEAT1 (UPB1) may directly regulate the expression of a set of peroxidases that modulate the balance of ROS between the zones of cell proliferation and the zones of cell elongation where differentiation begins in roots []. It is shown that higher levels of ROS can repress PHB3 expression and consequently activate the expression of AP2/ERF transcriptional factors ERF109 and ERF114, which promote root elongation and differentiation in the elongation and differentiation zones, while reduced ROS levels activate PHB3 which inhibits ERF115 to maintain the pluripotency of the quiescent center (QC) []. Moreover, in Arabidopsis thaliana, H2O2 treatment promotes root elongation by accelerating cell expansion in the myb30 mutant []. These investigations demonstrated that ROS signals may play extensive roles in regulating root growth and development as well as responses to various environmental stresses.
Phytohormones are the most important signaling molecules in plant cells that regulate a large range of developmental and physiological processes. Increasing evidence has shown a close connection between ROS and phytohormones in regulating many aspects of plant growth and development [,,]. The roles for ROS–hormone crosstalk in regulating root cell proliferation and differentiation and related processes have been demonstrated in many previous investigations [,,,]. For instance, accumulated ethylene enhances ROS production and subsequently promotes rice root elongation under flooding conditions []. It is revealed that H2O2 generation is involved in auxin-mediated lateral root formation [], and exogenous ROS such as H2O2 treatment may mimic lateral root induction mediated by auxin []. Moreover, it is proposed that auxin may induce ROS production through the modulation of the NAD(P)H oxidase RBOHD activity []. These investigations suggest that ROS–auxin interactions may play an important role in root cell proliferation and differentiation and root formation.
For higher plants, how the root senses gravity and directs its orientation, the so-called gravitropism, is a fundamental question in plant biology. Although extensive studies have shown the crucial role for polar auxin transport in regulating root gravitropism, ROS signals are also involved in gravitropic signaling [,,]. It is revealed that gravistimulation or unilateral application of auxin to vertical roots leads to a transient increase in intracellular ROS concentration in the root endodermis, while unilateral application of ROS to vertical roots pretreated with auxin transport inhibitor may also lead to gravitropic bending []. Furthermore, scavenging of ROS by antioxidants may inhibit root gravitropism, indicating the role of ROS in regulating root gravitropism []. These investigations suggest that the crosstalk between ROS and auxin may modulate root gravitropism and related physiological processes. Several previous studies have shown that the loss-of-function of rice OsPIN2, a gene encoding an auxin efflux transporter, led to the loss of root gravitropism due to disrupted auxin transport and distribution in the root cap [,,]. However, it remains unknown if additional signals were involved in OsPIN2-mediated auxin transport in regulating root gravitropism. Here, we report a novel connection between OsPIN2 and ROS signals in modulating not only root gravitropism but also hypoxic tolerance in rice seedlings. The findings will contribute to a novel understanding of the molecular mechanism for root gravitropism and genetic improvement of hypoxic tolerance in rice.
2. Results
2.1. OsPIN2 Mutant Showed Lower ROS Accumulation in Root Tip
The OsPIN2 mutants, war1-1 and war1-2, were derived from Oryza sativa L. indica cv. 9311 and Oryza sativa L. japonica cv. Nipponbare (Nip), respectively. The war1-1 and war1-2 mutant were also indicated as war1 and war1-cr#3 in the previous study [,,]. As is shown in Figure 1A, both war1-1 and war1-2 contain single base-pair deletions in the coding region of OsPIN2, representing the loss-of-function alleles in the OsPIN2 locus. Histochemical analysis was performed on the WT (cv. 9311 and cv. Nip) and OsPIN2 mutants using 3,3′-diaminobenzidine (DAB) and nitrotetrazolium blue chloride (NBT) staining to determine ROS (hydrogen peroxide and superoxide anion) production in the root tip. The results from DAB histochemical staining showed that the root tips of two WTs (cv. 9311 and cv. Nip) have darker staining, but the OsPIN2 mutants war1-1 and war1-2 have shallower staining (Figure 1B), indicating that the OsPIN2 mutants have less hydrogen peroxide (H2O2) accumulation in the root tip. The results from NBT histochemical staining also showed that war1-1 and war1-2 were less stained in the root tip (Figure 1C), indicating that less accumulation of superoxide anion (O2•−) in the root tip of OsPIN2 mutants. 2′,7′-dichlorodihydrofluorescein diacetate (H2DAF-DA) as a fluorescence probe may detect intracellular ROS (H2O2 and O2•−) and has been routinely used to quantify ROS in plant cells. We therefore used a H2DAF-DA fluorescence probe to measure the ROS levels in the WT and the mutants. The results showed that both war1-1 and war1-2 have weaker intensity of fluorescence signals in the root tip as compared with WT (Figure 1D,E), further indicating less accumulation of ROS in the OsPIN2 mutants.
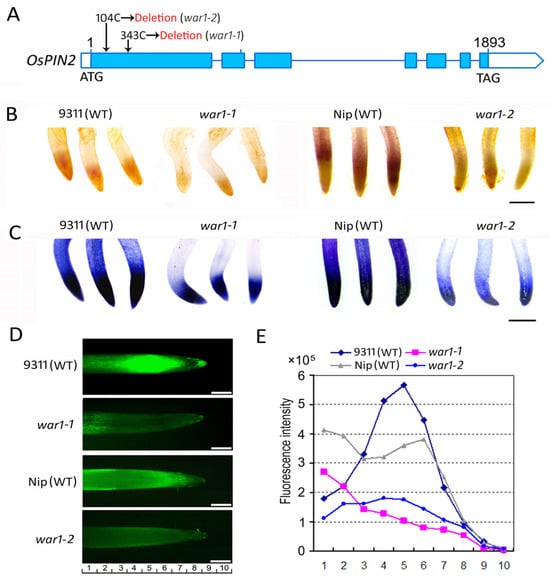
Figure 1.
The OsPIN2 mutants war1-1 and war1-2 showed lower ROS levels in the toot tips. (A) Schematic diagram of war1-1 and war1-2 mutation site in the OsPIN2 locus. Single base-pair deletions (104C deletion in war1-2 and 343C deletion in war1-1) were shown. (B,C) 3,3′-diaminobenzidine (DAB) and nitrotetrazolium blue chloride (NBT) histochemical staining were used to detect the levels of ROS hydrogen peroxide (B) and superoxide anion (C) in the root tips of WT (cv. 9311 and cv. Nip) and OsPIN2 mutants. (D) Detection of ROS (H2O2 and O2•−) accumulation by fluorescent staining with H2DAF-DA in the root tip of WT (cv. 9311 and cv. Nip) and OsPIN2 mutants. (E) ROS fluorescence intensity at the corresponding position of the root tip. The numbers underneath indicate the position from the root tips corresponding to that in (D). Scale bars = 1 mm (B,C), and 500 μm (D).
2.2. Expressions of ROS-Generating Genes in the Root of OsPIN2 Mutant
Previous study by Wang et al. [] had reported that the rice genome encodes nine NADPH oxidase genes (OsNOX1-9), also known as respiratory burst oxidase homologues (RBOH) genes, which are responsible for ROS generation in the plasma membrane and extracellular space. To investigate if the NADPH oxidase genes are responsible for the decrease in ROS in the OsPIN2 mutants, we analyzed the expressions of OsNOX1~9 genes in the root tips of the WT (cv. 9311) and war1-1 by RT-qPCR. It is revealed that the expressions of OsNOX1, OsNOX2, OsNOX3, OsNOX7 and OsNOX9 were slightly upregulated while OsNOX4 was slightly downregulated in the root of the war1-1 mutant as compared with that of the WT (Figure 2A). The expressions of OsNOX6 and OsNOX8 were more than 2-folds upregulation in the root tip of the war1 mutant, while OsNOX5 showed more than 6-folds downregulation in the war1-1 mutant (Figure 2A). In fact, ROS are not only produced in the cell membrane and extracellular space, but also are generated continuously in the mitochondria and chloroplasts. According to our previous RNA-seq data [], it showed that OsGOX6 (LOC_Os08g09860), a gene encoding glycolate oxidase or called hydroxyacid oxidase that catalyzes the oxidation in vivo of glycolic acid to glyoxylic acid and hydrogen peroxide, was significantly downregulated in the root of the war1-1 mutant. The RNA-seq data showed that the mRNA levels of OsGOX6 were 87.72, 100.03, and 90.57 in three independent biological replicates of WT (cv. 9311), but were 0, 0, and 0.06 in the three biological replicates of the war1-1 mutant. We further performed RT-qPCR to analyze the expression of OsGOX6 in the root of the WT (cv. 9311 and cv. Nip) and the two mutant lines. It was shown that the expression of OsGOX6 was down-regulated by 13-folds in the war1-1 mutant as compared with that in cv. 9311 (Figure 2B). Compared with that in cv. Nip, the expression of OsGOX6, was decreased by 4-folds in the root of the war1-2 mutant (Figure 2B). To explore which of these ROS-generating genes make more contribution to produce ROS in roots, we further compared their relative expression levels to reference gene OsActin1 in the root of the WT (Figure 2C). It was shown that the OsNOX1, OsNOX3 and OsGOX6 had relatively high expression levels in rice root (Figure 2B), indicating OsNOX1, OsNOX3 and OsGOX6 may have more contribution to produce ROS in rice root. Since OsNOX1 and OsNOX3 were slightly upregulated in the root of the war1-1 mutant and OsGOX6 was 13-folds down-regulated in the root of the war1-1 mutant, the results suggest that the downregulated expressions of OsGOX6 in war1-1 and war1-2 were possibly responsible for the decreased production of ROS in the root tip of OsPIN2 mutant lines.
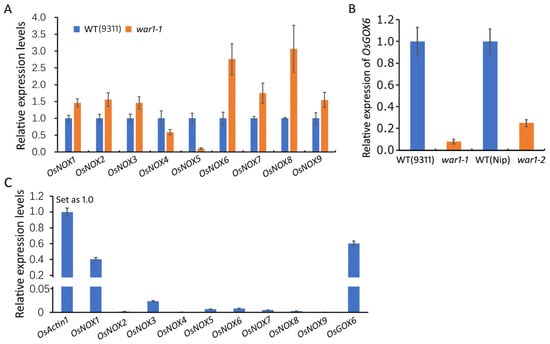
Figure 2.
Expression levels of ROS-generating genes OsNOX1~9 and OsGOX6 in the root of WT and OsPIN2 mutants. (A) Relative expression of OsNOX1~9 in the root of WT (cv. 9311) and the war1-1 mutant. (B) Relative expression of OsGOX6 in the WT (cv. 9311 and cv. Nip) and the OsPIN2 mutants (war1-1 and war1-2). The expression levels in (A,B) were analyzed by the 2−ΔΔCt method. (C) Relative expression levels of OsNOX1~9 and OsGOX6 in the root of WT (cv. 9311) as compared with internal reference gene OsActin1. The expression of OsActin1 was set as 1. The expression levels of OsNOX1~9 and OsGOX6 were analyzed according to the 2−ΔCt method.
2.3. Application of Hydrogen Peroxide Induces Root Gravitropic Curvature in OsPIN2 Mutant
Previous investigations have shown that the OsPIN2 mutants lack root gravitropism due to disrupted auxin transport in the root tip [,,]. In this study, the results that OsPIN2 mutants had less ROS accumulation in the root tip promoted us to investigate if exogenous application of hydrogen peroxide could rescue the gravitropic defects in war1. It is shown that the root tips of WT (cv. 9311 and cv. Nip) exhibited gravitropic curvature, while war1-1 and war1-2 had no gravitropic curvature at non-application with H2O2 (Figure 3A). However, applications of H2O2 induced gravitropic curvature of the root tip in war1-1 and war1-2 (Figure 3B–D). This result indicated that application of hydrogen peroxide may induce root gravitropic curvature in OsPIN2 mutants.
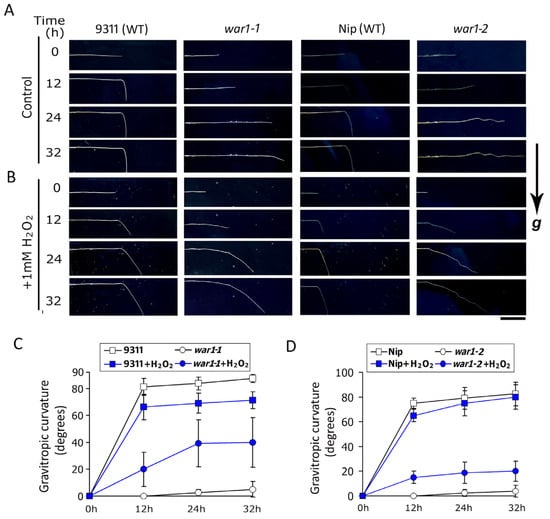
Figure 3.
The OsPIN2 mutants, war1-1 and war1-2, showed gravitropic curvature in root tip by application of hydrogen peroxide. (A,B) The roots of war1-1, war1-2 and their respective WT (cv. 9311 and cv. Nip) were embedded horizontally in vertical agar plates containing no H2O2 (A) or 1 mM H2O2 (B). Root images were captured at 0, 12, 24, and 32 h after embedding into the vertical plate. The direction of gravity is indicated by “g”. Scale bar = 1 cm. (C,D) Degrees of gravitropic curvature of root tip in cv. 9311 and war1-1 (C), and cv. Nip and war1-2 (D) at 0, 12, 24, and 32 h after embedding. Values are means ± SD (n = 6).
2.4. OsPIN2 Mutant Showed Increased Resistance to ROS Toxicity in Roots
The findings that OsPIN2 mutants have less ROS accumulation in the root tip and applications of H2O2 may induce gravitropic curvature in the root tip promoted us to further investigate if OsPIN2 mutants are more resistant to an exogenous application of a higher concentration of hydrogen peroxide. It showed that the application of 5 mM H2O2 in hydroponic medium may cause stronger repression of root elongation in WT than war1-1, as compared with that of the control (Figure 4A,B). The root length was significantly decreased in the WT but there was no significant change in war1-1 by application of H2O2 in the hydroponic medium (Figure 4C). The result indicated that OsPIN2 mutant had increased resistance to ROS toxicity. Moreover, we found that the application of H2O2 markedly represses the wavy rooting phenotype in war1-1, as compared with that of control (Figure 4A,B). The wavy frequency for the primary root was significantly decreased in war1-1 by application of H2O2 in a hydroponic medium (Figure 4D,E).
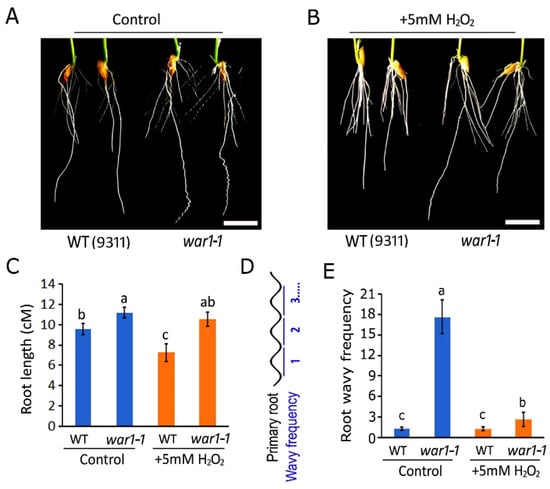
Figure 4.
The OsPIN2 mutant showed increased resistance to ROS hydrogen peroxide toxicity. (A,B) Germinated seeds of WT (cv. 9311) and war1-1 were cultured in hydroponic medium supplemented with no H2O2 (A) or 5 mM H2O2 (B) for 8 days. Scale bars = 1 cm (A,B). (C) Root length for WT (cv. 9311) and war1-1 cultured for 8 days in hydroponic medium supplemented with no H2O2 (control) or 5 mM H2O2. (D) Schematic diagram of wavy frequency for each primary root. (E) Wavy frequency for primary root in WT (cv. 9311) and war1-1 cultured in hydroponic medium supplemented with no H2O2 (control) or 5 mM H2O2. Values are means ± SD (n = 12). The letters above the bars indicate significant differences (p < 0.05) as determined by one-way ANVOA followed by Tukey’s test.
2.5. OsPIN2 Mutant Showed Lower ROS Accumulation and Stronger Tolerance under Hypoxic Stress
Because rice plants have higher background tolerance to hypoxia stress than upland crops, it is difficult to kill the plants by hypoxia stress under sufficient nutritional conditions. We therefore performed a hypoxia experiment to test the effects of hypoxia stress on root growth. It is shown that the root growth was significantly inhibited in the WT (cv. 9311 and cv. Nip) by 10 days of hypoxia stress with 0.1% agar solution (Figure 5A,B). It is interesting that hypoxia stress results in root tip curling in cv. Nip, but not in the war1-2 mutant (Figure 5B). Compared with wild type 9311 and Nip, hypoxia stress had a smaller inhibition on the root growth in war1-1 and war1-2 (Figure 5A,B). As is shown in Figure 5C, the hypoxia stress caused a 56.1% decrease in root length in 9311 but caused only a 22.3% decrease in root length in war1-1. It is surprising that the hypoxia stress did not cause a decrease in root length in war1-2 (Figure 5D). As is shown in Figure 5D, the hypoxia stress caused a 29.9% decrease in root length in Nip but caused a 13.3% increase in root length in war1-2. ROS detection by DAB staining was further performed in roots of Nip and war1-2. It is revealed that hypoxia stress led to increased accumulation of ROS in the roots of Nip but caused no significant increase in ROS accumulation in the roots of war1-2 (Figure 5E), indicating that the OsPIN2 mutation inhibited the production of ROS in roots and led to enhanced resistance to hypoxia stress. These results suggested that OsPIN2 mutants exhibiting stronger hypoxia tolerance were possibly associated with less ROS accumulation under hypoxic stress, because high levels of ROS accumulation are lethal to root cells.
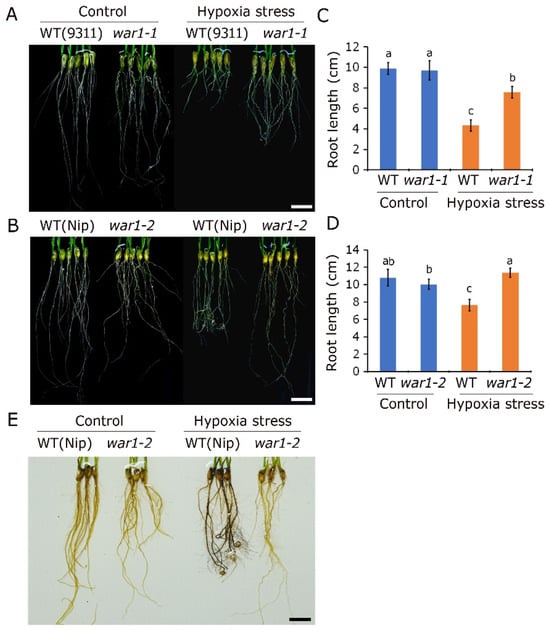
Figure 5.
The OsPIN2 mutant showed enhanced tolerance and decreased ROS accumulation under hypoxic stress. (A,B) The phenotypes of roots of WT (cv. 9311 and cv. Nip) and OsPIN2 mutants (war1-1 and war1-2) after non-stress (control) or hypoxic stress. (C,D) The root length of WT (cv. 9311 and cv. Nip) and OsPIN2 mutants (war1-1 and war1-2) after non-stress (control) or hypoxic stress. Values are means ± SD (n = 12). The letters above the bars indicate significant differences (p < 0.05) as determined by one-way ANVOA followed by Tukey’s test. (E) Detection of H2O2 accumulation by DAB staining in the root of Nip and OsPIN2 mutant war1-2 under non-stress (control) or hypoxic stress conditions. Scale bars = 2 cm (A,B,E).
2.6. Differential Expression of Anoxic Resistance-Related Gene SUB1B in the Root of WT and OsPIN2 Mutant under Hypoxic Stress
According to our previous RNA-seq data [], SUB1B (LOC_Os09g11460), an anoxic or submergence resistance-related gene, was significantly downregulated in the war1 mutant under non stressed conditions. It is shown that the mRNA levels of SUB1B were 12.02, 11.2, and 9.6 in the three independent biological replicates of WT, but were 5.31, 3.8, and 4.31 in the three biological replicates of the war1-1 mutant. In this study, we further investigate the gene expression of SUB1B in the root of the WT (cv.9311 and cv. Nip) and OsPIN2 mutants (war1-1 and war1-2) under non-stress or hypoxia stress conditions. The results from RT-qPCR showed that the SUB1B gene was downregulated in the root of war1-1 and war1-2 under non-stressed condition, as compared with that of the WT (Figure 6A,B). However, the hypoxia stress caused upregulations of SUB1B expressions in the WT (cv. 9311 and cv. Nip) but caused stronger upregulations of SUB1B in the root of war1-1 and war1-2 mutants (Figure 6A,B). The result suggested that loss-of-function of OsPIN2 may induce expression of SUB1B in response to hypoxia stress.
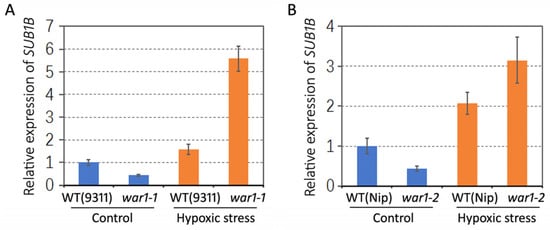
Figure 6.
Relative expressions of anoxic resistance-related gene SUB1B in the root of WT and OsPIN2 mutants under non-stress (control) or hypoxic stress conditions. (A) Expression levels of SUB1B in WT (cv. 9311) and war1-1 mutant. (B) Expression levels of SUB1B in WT (cv. Nip) and war1-2 mutant.
2.7. OsPIN2 Mutant Showed Reduced Auxin Distribution in the Root Tip under Hypoxia Stress
Since the roots of OsPIN2 mutant had stronger hypoxia tolerance and less ROS accumulation under hypoxic conditions, we speculated that these changes might be associated with disrupted auxin transport in the root tip. Therefore, we performed a DR5 promoter-GUS experiment to investigate auxin distribution in the root tip in response to hypoxia stress. It showed that WT and war1-1 mutant have different patterns of auxin distribution and accumulation in the root tip in response to hypoxic stress (Figure 7). Under non-stress conditions, auxin IAA as represented by GUS signals was mainly accumulated in the whole root cap, QC, and central vascular tissue in WT (Figure 7), but was confined to the stele, QC, columella and one side of the lateral root cap in war1-1 (Figure 7). Under hypoxic stress conditions, the distribution of auxin was altered in both the WT and war1-1 as compared with that in non-stress condition (control). It showed that hypoxia stress induced auxin distribution not only in the stele but also diffused distribution throughout the elongation zone in the root tip in the WT (Figure 7). However, auxin distribution was still located mainly in the root stele in the war1-1 mutant under hypoxia stress (Figure 7). Compared with one side of auxin distribution in the war1-1 root cap under non-stressed conditions (control), the hypoxic stress induced auxin distribution at two sides of the root cap in the war1-1 (Figure 7). Moreover, there has been a trend that auxin was diffused from the root cap to the meristem or division zone (Figure 7). Overall, it is noteworthy that hypoxic stress caused a significant increase in auxin distribution and accumulation in the root tip in the WT but caused a slight increase in auxin accumulation in the root tip of the war1-1 (Figure 7).
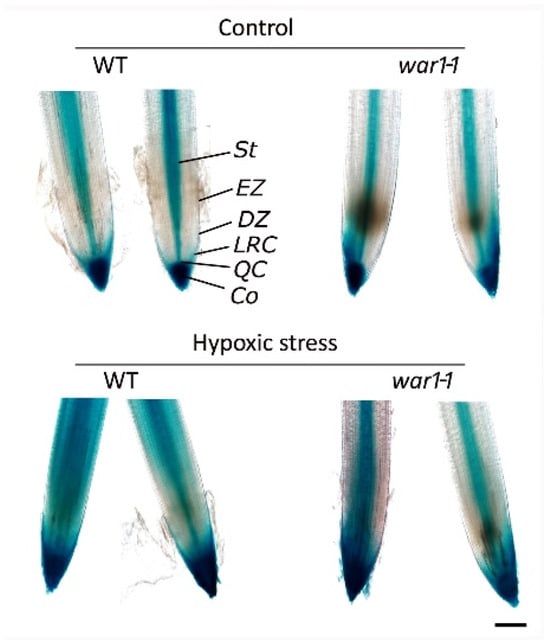
Figure 7.
Detection of auxin distribution in root tip by DR5 promoter-GUS experiment. GUS reporter gene under the control of auxin-inducible DR5 promoter was expressed in cv. 9311 (WT) and war1-1. GUS staining was performed with root tip from primary root of 10-day-old seedling. St, stele; EZ, elongation zone; DZ, division zone; Co, columella; LRC, lateral root cap; QC, quiescent center. Scale bars = 200 μm.
3. Discussion
Extensive studies have demonstrated that PIN-FORMED (PIN) auxin efflux carriers, especially PIN2, are required for root gravitropism in plants [,,,,], indicating the importance for auxin transport in regulating gravitropism []. Besides the hormone auxin, additional signal molecules such as Ca2+ [,], inositol 1,4,5-trisphosphate [] and nitric oxide [] are also necessary for gravitropism signal transduction and responsive processes. Furthermore, there is evidence that reactive oxygen species (ROS) are implicated in regulating root gravitropic perception []. ROS may accumulate on the lower side of roots in an auxin-dependent manner, where they have been shown to modulate the gravitropic curvature []. Since application of hydrogen peroxide may induce curvature even in roots treated with auxin transport inhibitor NPA, it is believed that ROS functions downstream of auxin signals []. Our previous study indicates that the OsPIN2 mutant, war1, produces a waved rooting phenotype due to the loss of root gravitropism []. In this study, application of hydrogen peroxide may rescue the waved rooting phenotype in the OsPIN2 mutant (Figure 4). The root tip of the OsPIN2 mutant showed no gravitropic curvature, but application of hydrogen peroxide may induce gravitropic curvature in the mutant (Figure 3), indicating that ROS may induce a root gravitropic response in OsPIN2 mutant. This result may suggest that ROS signals regulating root gravitropism are independent of OsPIN2 function. This conclusion was consistent with the previous hypothesis proposed by Joo et al. (2005) [] that ROS signals act as a downstream component of the auxin-mediated root geotropism signaling pathway.
Many investigations have shown that ROS are essential for numerous developmental and physiological processes throughout the plant life cycle [,]. the importance of ROS as growth regulators to modulate root developmental processes such as meristem maintenance, root elongation, lateral root and root hair formation, and endodermis and vascular differentiation has been indicated [,]. In fact, these processes generally involve a complex interplay between steady-state levels of ROS and phytohormone signals []. In this study, we focused on the connection between ROS and auxin actions since they have extensive interactions in regulating root growth and development. It is shown that localized auxin accumulation may increase ROS in most cell types []. In Arabidopsis, RBOH-mediated ROS production may facilitate lateral root emergence, suggesting the function of ROS as important signals during auxin-regulated lateral root formation []. Investigations on tomato (Solanum lycopersicum) further revealed that auxin regulates the level of H2O2 in the root tip and an increase in auxin level may trigger accumulation of H2O2 leading to inhibition of root cell elongation and root growth []. It has been revealed that the loss of OsPIN2 function altered auxin transport and distribution in the root, thus leading to abnormal root system architecture, root elongation growth and lateral root formation patterns [,,]. In this study, our data indicated that the loss of OsPIN2 function led to reduced H2O2 accumulation in the root tip (Figure 1), and the root defects in the OsPIN2 mutant may be rescued partly by application of H2O2, suggesting that OsPIN2-mediated auxin transport and distribution regulate root growth and development probably by modulating ROS homeostasis in the root tip. In plant cells, besides production by NADPH oxidase (or called RBOH), an alternative ROS-producing pathway can be mediated by glycolate oxidase (GOX) [], because GOX catalyzes glycolate or its derivatives into glyoxylate and H2O2 during photorespiration []. It is confirmed in Nicotiana benthamiana leaves that transgenic plants’ overexpression of GOX induces ROS accumulation []. In rice, it has been demonstrated that GOX isoforms are localized to the peroxisome []. The physical association–dissociation of GOX and catalase, in response to environmental stress, seems to serve as a specific mechanism to modulate H2O2 levels in rice []. In fact, the genes encoding GOX are not only present in plants and green algae, but are also widely found in animals and bacteria [,]. It is considered that plant and animal GOXs have a common eukaryotic ancestor and animal cells also possess GOX activities which produce glyoxylate used for the peroxisomal synthesis of glycine [,,], indicating that GOX may catalyze glycolate into glyoxylate and by-product H2O2 in non-photorespiratory pathways. In humans, glycolate oxidase is a potential drug target for treatment of primary hyperoxaluria, a genetic disorder where overproduction of oxalate results in the formation of kidney stones [,]. A previous investigation suggested that the Ricinus communis genome encodes a single GOX with different functions in photosynthetic and heterotrophic organs []. Schmitz et al. [] reporting a high level of glyoxylate in Ricinus communis roots have hypothesized that in heterotrophic tissues such as the root, the GOX is involved in the production of serine (from glyoxylate) to feed the folate pathway. In Arabidopsis, however, it was shown that GOX3, a glycolate oxidase homolog of yeast l-lactate cytochrome c oxidoreductase, is predominantly present in roots and may catalyze l-lactate oxidation []. In fact, more convincing evidence came from the study of rice that OsGLO4, a glycolate oxidase gene, is involved in the formation of iron plaque on the surface of roots by mediating GOX activity and H2O2 production under alternative wetting and drying condition []. In the present study, the GOX encoding gene OsGOX6 was greatly downregulated in the root of OsPIN2 mutants (Figure 2), suggesting that glycolate metabolism in roots was depressed due to the lack of OsPIN2 function. As indicated previously, GOX can also catalyze glycolate into glyoxylate and H2O2 during non-photorespiratory pathways; the downregulated expression of OsGOX6 could be responsible for the lower levels of H2O2 in the root of the OsPIN2 mutant. Since OsPIN2 encodes an auxin efflux transporter and its absence may disrupt the polar auxin transport in the root tip and lead to abnormal auxin levels in the root cap [,,]; our data may have indicated an interesting connection between OsPIN2-modulated auxin transport and OsGOX6-mediated H2O2 production in rice root.
It is widely believed that high levels of ROS can be lethal for plant cell integrity [], although cell death in response to biotic or abiotic stresses is often mediated by plant hormones. At lower concentrations, ROS may function in signaling pathways that regulate plant development in response to physiological and environmental stresses []. In this study, the OsPIN2 mutant showed less ROS accumulation in the root tip under non-stressed conditions. Hypoxia stress caused no significant increase in the ROS level in the OsPIN2 mutant root but overproduction of ROS in the WT (Figure 5). Since high levels of ROS may be lethal for the plant cell integrity, we draw a conclusion that the OsPIN2 mutant had stronger root activity under hypoxia stress due to less ROS accumulation in the root. Consistent with this conclusion, the hypoxia stress caused less inhibition of root growth in the OsPIN2 mutant but resulted in significant inhibition of root growth in the WT, suggesting that loss of OsPIN2 function led to enhanced resistance to hypoxia stress, owing to lower ROS accumulation in roots. We suggested that root growth inhibition in the WT was due to ROS-induced cell death in root cells. Previous investigations have suggested that hydrogen peroxide (H2O2) is required for ethylene-induced epidermal death and promotes the aerenchyma development in rice stems [,]. Furthermore, it is revealed that exposure of plant cells to different concentrations of H2O2 may induce different cell death pathways in a dose-dependent manner [,]. In the present study, the root elongation was significantly decreased in the WT but was not significantly decreased in the mutant by application of H2O2, suggesting that the OsPIN2 mutant was more resistant to ROS toxicity. This was consistent with the fact that the OsPIN2 mutant accumulated lower levels of ROS in the roots. These results indicated that loss-of-function of OsPIN2 led to a reduction in ROS accumulation and increase in root resistance to ROS toxicity.
Tolerance to flooding-induced hypoxia is an important agronomic trait especially for crops in lowland and flooding-affected areas. Although rice is considered a flood-tolerant crop, only limited cultivars display tolerance to prolonged submergence, which is largely attributed to the presence of the SUB1A, a gene encoding the AP2/ERF transcriptional factor [,]. However, for most rice cultivars, such as cv. Nipponbare (O. sativa L. ssp. japonica) and cv. 9311 (O. sativa L. ssp. indica), the SUB1A allele is absent in the genome. Alternatively, these cultivars possess two alleles of the SUB1 orthologue, SUB1B (LOC_Os09g11460, https://www.ricedata.cn/gene/list/365.htm, accessed on 1 November 2023) and SUB1C (LOC_Os09g11480, https://www.ricedata.cn/gene/list/362.htm, accessed on 1 November 2023), in the genome. In this study, it is shown that SUB1B expression was significantly decreased in the roots of the OsPIN2 mutant under non-stressed conditions, indicating that the loss of the OsPIN2 function led to repression of SUB1B expression. Considering that the SUB1-meidated resistant pathway is related to anaerobic respiration, plant hormone response, and the antioxidant system [], it seems that enhanced hypoxic tolerance in the OsPIN2 mutant was associated with strong induction of SUB1B expression under hypoxia stress. Previous investigations had shown that a set of AP2/ERF transcriptional factors are ROS responsive in Arabidopsis root. For instance, ERF109, ERF114 and ERF115 were highly upregulated in response to ROS accumulations, but the AP2/ERF transcriptional factors PLT1 and PLT2 were downregulated in their expression in response to ROS accumulation []. However, the AP2/ERF transcriptional factor SUB1B exhibited opposite patterns of expression between the WT and OsPIN2 mutant, indicating that additional factors instead of ROS might affect SUB1B expression under hypoxic stress.
It is shown that root tips are extremely sensitive to flooding stress and often die after a few hours of exposure to hypoxic conditions, compromising root survival following the re-establishment of normoxic oxygen levels []. For instance, maize root tips exposed to 4% oxygen for 24 h undergo massive cellular death, preceded by the accumulation of NO and ROS []. Some investigations suggested that root survival to hypoxic stress is dependent upon maintaining the function of the apical root meristem quiescent center (QC). This process is governed by PIN-mediated basipetal flow of auxin leading to the formation of an auxin maximum that is required for the establishment of a highly oxidized environment specifying the QC niche []. However, perturbations in auxin flow and/or distribution along root profile during hypoxic stress may shift the redox state of the QC towards a more reduced environment leading to the activation of the QC, degradation of the meristem, and root abortion []. The previous investigation indicated that the loss of OsPIN2 function resulted in abnormal auxin flow or distribution in root cap and QC under non-stressed conditions [,,]. In this study, it was shown that hypoxia stress resulted in higher accumulation of auxin in the root cap and elongation zone in WT but led to less accumulation of auxin in the tip (especially the elongation zone) in the OsPIN2 mutant. It seems that hypoxia-induced auxin accumulation was associated with ROS accumulation due to the lack of OsPIN2. According to our data, hypoxic stress induced higher accumulation for both ROS and auxin in the WT, while led to less accumulation for ROS and auxin in the OsPIN2 mutant. These results indicated a connection between auxin distribution and ROS accumulation in response to root hypoxia. We suggested that the loss-of-function of OsPIN2 resulted in decreased auxin transport and accumulation in the root tip, leading to less accumulation of ROS in the root tip, followed by enhanced hypoxia tolerance.
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
The OsPIN2 mutants, war1-1 derived from the rice cv. 9311 (Oryza sativa L. indica) by 60Co γ-ray mutagenesis and war1-2 (also indicated as war1-cr#3 previously) derived from the rice cv. Nipponbare (Nip, Oryza sativa L. japonica) by CRISPR/Cas9-mediated mutagenesis of OsPIN2, were characterized as root gravitropic mutants previously []. To observe the root phenotype, germinated seeds of wild type (cv. 9311 and cv. Nip) and OsPIN2 mutants (war1-1 and war1-2) were planted in Hoagland‘s hydroponic solution and supplemented with 0.5 mM MES monohydrate (pH 6.5) as described previously []. To investigate the effects of exogenous application of H2O2 on root growth, germinated seeds were transferred to hydroponic Hoagland’s medium supplemented with 5 mM H2O2. The hydroponic system was kept in a greenhouse with 12 h light (28 °C) and 12 h dark (25 °C) photoperiod at 180 µmol m−2 s−1 photon density and 70–80% humidity. The hydroponic culture was refreshed every three days. To examine the effects of exogenous application of H2O2 on root gravitropism, germinated seed of roots were placed horizontally in MS agar plates that were supplemented with or without 1 mM H2O2. The plates were then positioned vertically, and the downward bending angle of the root tip was measured at various time points.
4.2. Hypoxic Stress Treatment
The hypoxic stress treatment was performed as described by Li et al. [] with minor modification. For aerobic hydroponic culture (control), oxygen was supplied through an oxygen pump for 6 h daily, and the concentration of dissolved oxygen in the culture medium was maintained above 6 ppm. For hypoxic stress in hydroponic culture, 0.1% agar was added into the Hoagland’s culture medium to create a viscous deoxygenated environment, causing the dissolved oxygen content to decrease to about 0.5–1.0 ppm on the fourth day of culturing. Germinated seeds of wild type and OsPIN2 mutant were planted on aerobic hydroponic culture and hypoxic culture medium in a greenhouse with 12 h light (28 °C) and 12 h dark (25 °C) photoperiod at 180 µmol m−2 s−1 photon density and 70–80% humidity.
4.3. Detection of ROS Level in Roots
The detection of hydrogen peroxide (H2O2) and superoxide anion (O2•−) in roots was performed using 3,3′-diaminobenzidine (DAB) and nitrotetrazolium blue chloride (NBT) histochemical staining, respectively, as described by Xu et al. []. Briefly, root tips were immersed directly into 0.5 mg/mL DAB solution (prepared in distilled water, pH 3.8) for 3 h at 25 °C, and the production of hydrogen peroxide was monitored by observing the development of a brown stain in the root tip. For detection of superoxide anion, root tips were immersed directly in a reaction medium containing 0.25 Mm NBT in 50 Mm K-phosphate buffer (Ph 7.8) for 1 h at 25 °C. Superoxide anion was detected by the formation of blue formazan. The total level of hydrogen peroxide and superoxide anion was further measured by fluorescence microscopy after labeling with the cell-permeable fluorogenic probe, 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA), as described by Wang et al. (2016) [] with minor modifications. Briefly, root tips were immersed in 1 Ml of 10 Mm MES-KCl buffer (Ph 6.1), and then 1 µL of 10 Mm H2DCF-DA solution was added. After incubating for 8 min in the dark, the roots were washed 4 times with deionized water, and the fluorescence from the H2DCF-DA was recorded using a Leica DM5000B fluorescence microscope (Leica, Wetzlar, Germany) with a GFP filter.
4.4. RNA Extraction and RT-qPCR Analysis
Total RNA was extracted from 0.5 cm long root tips using the RNeasy Plant Mini Kit (Qiagen 74904, Qiagen Inc., Hilden, Germany) according to the manufacturer’s instructions. First-strand cDNA was synthesized using the M-MLV first strand kit (Invitrogen, Carlsbad, CA, USA) by reverse transcription. Real-time RT-qPCR was performed on the Bio-Rad CFX96 Real-Time PCR system (Bio-Rad Laboratories Inc., Hercules, CA, USA) in a 20 μL reaction volume containing 50 ng of cDNA template, 10 μmol of primers, 10 μL of 2× SYBR Green real-time PCR master mix, and deionized water. The amplification procedure comprised 40 cycles at 95 °C for 30 s, 95 °C for 5 s, and 60 °C for 30 s. The rice OsActin1 was used as the internal reference gene for RT-qPCR analysis of gene expression level. All primers for RT-qPCR in this study is listed in Table S1.
4.5. Detection of Auxin IAA Distribution by DR5 Promoter-GUS Experiment
The genetic materials of DR5 promoter-GUS plants used in this study were previously described by Li et al. []. Root tips from 10-day-old plants were examined for GUS-histochemical staining and microscopic observation under Leica DM5000 B microscope (Leica, Wetzlar, Germany) following the protocol by Zhou et al. [].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants13040476/s1, Table S1: The primers used for RT-qPCR analysis in this study.
Author Contributions
Conceptualization, B.H. and W.L.; methodology, B.H. and L.Q.; validation, B.H., R.Z. and C.Z.; investigation, B.H. and R.Z.; resources, N.W., Y.Z. and Y.X.; data curation, Q.L. and L.Q.; writing—original draft preparation, B.H.; writing—review and editing, W.L.; visualization, B.H. and W.L.; supervision, W.L.; project administration, W.L.; funding acquisition, W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (32070197) and Innovation and Entrepreneurship Training Program for College Students (S202210712726).
Data Availability Statement
The data presented in this study are available in the article and Supplementary Materials.
Acknowledgments
We thank Lan Cao and Zhongping Lei from the Teaching and Research Core Facility at the College of Life Sciences, Northwest A&F University, for technical assistance in microscopic observations.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bakshi, A.; Gilroy, S. Analysis of plant flooding response. Methods Enzymol. 2023, 680, 461–491. [Google Scholar] [PubMed]
- Jethva, J.; Schmidt, R.R.; Sauter, M.; Selinski, J. Try or Die: Dynamics of Plant Respiration and How to Survive Low Oxygen Conditions. Plants 2022, 11, 205. [Google Scholar] [CrossRef]
- Yuan, L.B.; Chen, M.X.; Wang, L.N.; Sasidharan, R.; Voesenek, L.; Xiao, S. Multi-stress resilience in plants recovering from submergence. Plant Biotechnol. J. 2023, 21, 466–481. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Ali, S.; Park, S.; Bae, H. Exploring the Potential of Multiomics and Other Integrative Approaches for Improving Waterlogging Tolerance in Plants. Plants 2023, 12, 1544. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Seo, Y.S.; Walia, H.; Cao, P.; Fukao, T.; Canlas, P.E.; Amonpant, F.; Bailey-Serres, J.; Ronald, P.C. The submergence tolerance regulator Sub1A mediates stress-responsive expression of AP2/ERF transcription factors. Plant Physiol. 2010, 152, 1674–1692. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Xu, X.; Fukao, T.; Canlas, P.; Maghirang-Rodriguez, R.; Heuer, S.; Ismail, A.M.; Bailey-Serres, J.; Ronald, P.C.; Mackill, D.J. Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature 2006, 442, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Waszczak, C.; Carmody, M.; Kangasjarvi, J. Reactive oxygen species in plant signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, A.; Van Breusegem, F. Reactive oxygen species in plant development. Development 2018, 145, dev164376. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef]
- Ateeq, M.; Khan, A.H.; Zhang, D.; Alam, S.M.; Shen, W.; Wei, M.; Meng, J.; Shen, X.; Pan, J.; Zhu, K.; et al. Comprehensive physio-biochemical and transcriptomic characterization to decipher the network of key genes under waterlogging stress and its recuperation in Prunus persica. Tree Physiol. 2023, 43, 1265–1283. [Google Scholar] [CrossRef] [PubMed]
- Pucciariello, C.; Perata, P. The Oxidative Paradox in Low Oxygen Stress in Plants. Antioxidants 2021, 10, 332. [Google Scholar] [CrossRef]
- Tsukagoshi, H. Control of root growth and development by reactive oxygen species. Curr. Opin. Plant Biol. 2016, 29, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Tsukagoshi, H.; Busch, W.; Benfey, P.N. Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 2010, 143, 606–616. [Google Scholar] [CrossRef]
- Kong, X.; Tian, H.; Yu, Q.; Zhang, F.; Wang, R.; Gao, S.; Xu, W.; Liu, J.; Shani, E.; Fu, C.; et al. PHB3 Maintains Root Stem Cell Niche Identity through ROS-Responsive AP2/ERF Transcription Factors in Arabidopsis. Cell Rep. 2018, 22, 1350–1363. [Google Scholar] [CrossRef] [PubMed]
- Mabuchi, K.; Maki, H.; Itaya, T.; Suzuki, T.; Nomoto, M.; Sakaoka, S.; Morikami, A.; Higashiyama, T.; Tada, Y.; Busch, W.; et al. MYB30 links ROS signaling, root cell elongation, and plant immune responses. Proc. Natl. Acad. Sci. USA 2018, 115, E4710–E4719. [Google Scholar] [CrossRef]
- Xia, X.J.; Zhou, Y.H.; Shi, K.; Zhou, J.; Foyer, C.H.; Yu, J.Q. Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J. Exp. Bot. 2015, 66, 2839–2856. [Google Scholar] [CrossRef]
- Qi, J.S.; Wang, J.L.; Gong, Z.Z.; Zhou, J.M. Apoplastic ROS signaling in plant immunity. Curr. Opin. Plant Biol. 2017, 38, 92–100. [Google Scholar] [CrossRef]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef]
- Zafari, S.; Vanlerberghe, G.C.; Igamberdiev, A.U. The Role of Alternative Oxidase in the Interplay between Nitric Oxide, Reactive Oxygen Species, and Ethylene in Tobacco (Nicotiana tabacum L.) Plants Incubated under Normoxic and Hypoxic Conditions. Int. J. Mol. Sci. 2022, 23, 7153. [Google Scholar] [CrossRef]
- Liu, Z.; Hartman, S.; van Veen, H.; Zhang, H.; Leeggangers, H.; Martopawiro, S.; Bosman, F.; de Deugd, F.; Su, P.; Hummel, M.; et al. Ethylene augments root hypoxia tolerance via growth cessation and reactive oxygen species amelioration. Plant Physiol. 2022, 190, 1365–1383. [Google Scholar] [CrossRef]
- Gorb, S.N.; Sauter, M.; Kovalev, A.; Steffens, B. Emerging roots alter epidermal cell fate through mechanical and reactive oxygen species signaling. Plant Cell 2012, 24, 3296–3306. [Google Scholar]
- Ma, F.; Wang, L.; Li, J.; Samma, M.K.; Xie, Y.; Wang, R.; Wang, J.; Zhang, J.; Shen, W. Interaction between HY1 and H2O2 in auxin-induced lateral root formation in Arabidopsis. Plant Mol. Biol. 2014, 85, 49–61. [Google Scholar] [CrossRef]
- Wang, P.; Du, Y.; Li, Y.; Ren, D.; Song, C.P. Hydrogen peroxide-mediated activation of MAP kinase 6 modulates nitric oxide biosynthesis and signal transduction in Arabidopsis. Plant Cell 2010, 22, 2981–2998. [Google Scholar] [CrossRef]
- Peer, W.A.; Cheng, Y.; Murphy, A.S. Evidence of oxidative attenuation of auxin signalling. J. Exp. Bot. 2013, 64, 2629–2639. [Google Scholar] [CrossRef]
- Su, S.H.; Gibbs, N.M.; Jancewicz, A.L.; Masson, P.H. Molecular Mechanisms of Root Gravitropism. Curr. Biol. 2017, 27, R964–R972. [Google Scholar] [CrossRef]
- Sato, E.M.; Hijazi, H.; Bennett, M.J.; Vissenberg, K.; Swarup, R. New insights into root gravitropic signalling. J. Exp. Bot. 2015, 66, 2155–2165. [Google Scholar] [CrossRef] [PubMed]
- Krieger, G.; Shkolnik, D.; Miller, G.; Fromm, H. Reactive Oxygen Species Tune Root Tropic Responses. Plant Physiol. 2016, 172, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.H.; Bae, Y.S.; Lee, J.S. Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol. 2001, 126, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guo, M.; Li, Y.; Ruan, W.; Mo, X.; Wu, Z.; Sturrock, C.J.; Yu, H.; Lu, C.; Peng, J.; et al. LARGE ROOT ANGLE1, encoding OsPIN2, is involved in root system architecture in rice. J. Exp. Bot. 2018, 69, 385–397. [Google Scholar] [CrossRef]
- Inahashi, H.; Shelley, I.J.; Yamauchi, T.; Nishiuchi, S.; Takahashi-Nosaka, M.; Matsunami, M.; Ogawa, A.; Noda, Y.; Inukai, Y. OsPIN2, which encodes a member of the auxin efflux carrier proteins, is involved in root elongation growth and lateral root formation patterns via the regulation of auxin distribution in rice. Physiol. Plant. 2018, 164, 216–225. [Google Scholar] [CrossRef]
- Li, W.; Zhang, M.; Qiao, L.; Chen, Y.; Zhang, D.; Jing, X.; Gan, P.; Huang, Y.; Gao, J.; Liu, W.; et al. Characterization of wavy root 1, an agravitropism allele, reveals the functions of OsPIN2 in fine regulation of auxin transport and distribution and in ABA biosynthesis and response in rice (Oryza sativa L.). Crop J. 2022, 10, 980–992. [Google Scholar] [CrossRef]
- Wang, G.-F.; Li, W.-Q.; Li, W.-Y.; Wu, G.-L.; Zhou, C.-Y.; Chen, K.-M. Characterization of rice NADPH oxidase genes and their expression under various environmental conditions. Int. J. Mol. Sci. 2013, 14, 9440–9458. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Challa, G.S.; Gupta, A.; Gu, L.; Wu, Y.; Li, W. Physiological and Transcriptomic Characterization of Sea-Wheatgrass-Derived Waterlogging Tolerance in Wheat. Plants 2022, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.; Guan, C.; Galweiler, L.; Tanzler, P.; Huijser, P.; Marchant, A.; Parry, G.; Bennett, M.; Wisman, E.; Palme, K. AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J. 1998, 17, 6903–6911. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, P.; Edwards, K.S.; Rahman, A.; DeLong, A.; Muday, G.K. PINOID Kinase Regulates Root Gravitropism through Modulation of PIN2-Dependent Basipetal Auxin Transport in Arabidopsis. Plant Physiol. 2009, 150, 722–735. [Google Scholar] [CrossRef]
- Sinclair, W.; Trewavas, A.J. Calcium in gravitropism. A re-examination. Planta 1997, 203, S85–S90. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.T.; Feldman, L.J. Light-regulated root gravitropism: A role for, and characterization of, a calcium/calmodulin-dependent protein kinase homolog. Planta 1997, 203, S91–S97. [Google Scholar] [CrossRef] [PubMed]
- Perera, I.Y.; Hung, C.Y.; Brady, S.; Muday, G.K.; Boss, W.F. A universal role for inositol 1,4,5-trisphosphate-mediated signaling in plant gravitropism. Plant Physiol. 2006, 140, 746–760. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Neill, S.J.; Tang, Z.; Cai, W. Nitric oxide mediates gravitropic bending in soybean roots. Plant Physiol. 2005, 137, 663–670. [Google Scholar] [CrossRef]
- Joo, J.H.; Yoo, H.J.; Hwang, I.; Lee, J.S.; Nam, K.H.; Bae, Y.S. Auxin-induced reactive oxygen species production requires the activation of phosphatidylinositol 3-kinase. FEBS Lett. 2005, 579, 1243–1248. [Google Scholar] [CrossRef]
- Eljebbawi, A.; Rondon, Y.; Dunand, C.; Estevez, J. Highlighting Reactive Oxygen Species (ROS) as multitaskers in root development. iScience 2020, 24, 101978. [Google Scholar] [CrossRef] [PubMed]
- Orman-Ligeza, B.; Parizot, B.; de Rycke, R.; Fernandez, A.; Himschoot, E.; Van Breusegem, F.; Bennett, M.J.; Perilleux, C.; Beeckman, T.; Draye, X. RBOH-mediated ROS production facilitates lateral root emergence in Arabidopsis. Development 2016, 143, 3328–3339. [Google Scholar] [CrossRef] [PubMed]
- Ivanchenko, M.G.; den Os, D.; Monshausen, G.B.; Dubrovsky, J.G.; Bednarova, A.; Krishnan, N. Auxin increases the hydrogen peroxide (H2O2) concentration in tomato (Solanum lycopersicum) root tips while inhibiting root growth. Ann. Bot. 2013, 112, 1107–1116. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, Y.; Xie, Z.; Li, X.; He, Z.H.; Peng, X.X. Association-Dissociation of Glycolate Oxidase with Catalase in Rice: A Potential Switch to Modulate Intracellular H2O2 Levels. Mol. Plant 2016, 9, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Rojas, C.; Mysore, K.S. Glycolate oxidase is an alternative source for H2O2 production during plant defense responses and functions independently from NADPH oxidase. Plant Signal. Behav. 2012, 7, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, Z.; Zhang, K.; Zhang, X.; Zhang, Y.; Wang, X.; Han, C.; Yu, J.; Xu, K.; Li, D. Barley Stripe Mosaic Virus gammab Interacts with Glycolate Oxidase and Inhibits Peroxisomal ROS Production to Facilitate Virus Infection. Mol. Plant 2018, 11, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.S.; Lu, Y.S.; Zhai, L.G.; Deng, R.S.; Jiang, J.; Li, Y.; He, Z.H.; Peng, X.X. Glycolate Oxidase Isozymes Are Coordinately Controlled by GLO1 and GLO4 in Rice. PLoS ONE 2012, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Esser, C.; Kuhn, A.; Groth, G.; Lercher, M.J.; Maurino, V.G. Plant and animal glycolate oxidases have a common eukaryotic ancestor and convergently duplicated to evolve long-chain 2-hydroxy acid oxidases. Mol. Biol. Evol. 2014, 31, 1089–1101. [Google Scholar] [CrossRef]
- Yamada, M.; Higashiyama, T.; Kishino, S.; Kataoka, M.; Ogawa, J.; Shimizu, S.; Isobe, K. Novel alcohol oxidase with glycolate oxidase activity from Ochrobactrum sp. AIU 033. J. Mol. Catal. B-Enzym. 2014, 105, 41–48. [Google Scholar] [CrossRef]
- Williams, E.; Cregeen, D.; Rumsby, G. Identification and expression of a cDNA for human glycolate oxidase. Biochim. Biophys. Acta. 2000, 1493, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Pennati, A.; Gadda, G. Involvement of ionizable groups in catalysis of human liver glycolate oxidase. J. Biol. Chem. 2009, 284, 31214–31222. [Google Scholar] [CrossRef]
- Vignaud, C.; Pietrancosta, N.; Williams, E.L.; Rumsby, G.; Lederer, F. Purification and characterization of recombinant human liver glycolate oxidase. Arch. Biochem. Biophys. 2007, 465, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.; Hudig, M.; Meier, D.; Linka, N.; Maurino, V.G. The genome of Ricinus communis encodes a single glycolate oxidase with different functions in photosynthetic and heterotrophic organs. Planta 2020, 252, 100. [Google Scholar] [CrossRef] [PubMed]
- Engqvist, M.K.; Schmitz, J.; Gertzmann, A.; Florian, A.; Jaspert, N.; Arif, M.; Balazadeh, S.; Mueller-Roeber, B.; Fernie, A.R.; Maurino, V.G. GLYCOLATE OXIDASE3, a Glycolate Oxidase Homolog of Yeast l-Lactate Cytochrome c Oxidoreductase, Supports l-Lactate Oxidation in Roots of Arabidopsis. Plant Physiol. 2015, 169, 1042–1061. [Google Scholar] [CrossRef]
- Yu, X.L.; Wu, D.M.; Fu, Y.Q.; Yang, X.J.; Baluska, F.; Shen, H. OsGLO4 is involved in the formation of iron plaques on surface of rice roots grown under alternative wetting and drying condition. Plant Soil 2018, 423, 111–123. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Steffens, B.; Geske, T.; Sauter, M. Aerenchyma formation in the rice stem and its promotion by H2O2. New Phytol. 2011, 190, 369–378. [Google Scholar] [CrossRef]
- Steffens, B.; Sauter, M. Epidermal cell death in rice is confined to cells with a distinct molecular identity and is mediated by ethylene and H2O2 through an autoamplified signal pathway. Plant Cell 2009, 21, 184–196. [Google Scholar] [CrossRef]
- Gechev, T.S.; Hille, J. Hydrogen peroxide as a signal controlling plant programmed cell death. J. Cell Biol. 2005, 168, 17–20. [Google Scholar] [CrossRef]
- Houot, V.; Etienne, P.; Petitot, A.S.; Barbier, S.; Blein, J.P.; Suty, L. Hydrogen peroxide induces programmed cell death features in cultured tobacco BY-2 cells, in a dose-dependent manner. J. Exp. Bot. 2001, 52, 1721–1730. [Google Scholar] [PubMed]
- Niroula, R.K.; Pucciariello, C.; Ho, V.T.; Novi, G.; Fukao, T.; Perata, P. SUB1A-dependent and -independent mechanisms are involved in the flooding tolerance of wild rice species. Plant J. 2012, 72, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.K.; Callis, J.; Wemmer, D.; Walbot, V.; Jardetzky, O. Mechanisms of cytoplasmic pH regulation in hypoxic maize root tips and its role in survival under hypoxia. Proc. Natl. Acad. Sci. USA 1984, 81, 3379–3383. [Google Scholar] [CrossRef]
- Mira, M.M.; Hill, R.D.; Stasolla, C. Phytoglobins Improve Hypoxic Root Growth by Alleviating Apical Meristem Cell Death. Plant Physiol. 2016, 172, 2044–2056. [Google Scholar] [CrossRef]
- Mira, M.M.; El-Khateeb, E.A.; Gaafar, R.M.; Igamberdiev, A.U.; Hill, R.D.; Stasolla, C. Stem cell fate in hypoxic root apical meristems is influenced by phytoglobin expression. J. Exp. Bot. 2020, 71, 1350–1362. [Google Scholar] [CrossRef]
- Xu, Q.T.; Yang, L.; Zhou, Z.Q.; Mei, F.Z.; Qu, L.H.; Zhou, G.S. Process of aerenchyma formation and reactive oxygen species induced by waterlogging in wheat seminal roots. Planta 2013, 238, 969–982. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.M.; Wang, Y.J.; Gao, Y.T.; Li, R.; Wang, G.F.; Li, W.Q.; Liu, W.T.; Chen, K.M. The plasma membrane NADPH oxidase OsRbohA plays a crucial role in developmental regulation and drought-stress response in rice. Physiol. Plant. 2016, 156, 421–443. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, F.; Wang, X.; Yang, Y.; Yu, C.; Liu, H.; Cheng, Y.; Yan, C.; Chen, J. Specific expression of DR5 promoter in rice roots using a tCUP derived promoter-reporter system. PLoS ONE 2014, 9, e87008. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).