The Antioxidant Activity of Wild-Growing Plants Containing Phenolic Compounds in Latvia
Abstract
:1. Introduction
2. Results
2.1. Phenolic Compound Characterization
2.1.1. Determination of Total Phenolic Content in Acetone and Ethanol Extracts
2.1.2. Determination of Total Flavonoid Content
2.1.3. Determination of Tannin Contents in Herbal Decoctions
2.1.4. Phenolic Compound Characterisation via High-Performance Liquid Chromatography (HPLC)
2.2. Determination of Antioxidant Activity Using a DPPH (2,2-Diphenyl-1-picrylhydrazyl) Radical Scavenging Assay
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Chemicals and Reagents
4.3. Extract Preparation
4.4. Determination of the Total Phenolic Content
4.5. Determination of the Total Flavonoid Content
4.6. Determination of Tannin Content in Herbal Decoctions
4.7. Phenolic Compound Characterisation Analysed Using High-Performance Liquid Chromatography (HPLC)
4.8. Determination of Antioxidant Activity Using a DPPH (2,2-Diphenyl-1-picrylhydrazyl) Radical Scavenging Assay
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tresserra-Rimbau, A.; Lamuela-Raventos, R.M.; Moreno, J.J. Polyphenols, Food and Pharma. Current Knowledge and Directions for Future Research. Biochem. Pharmacol. 2018, 156, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T. Systematics and Health Effects of Chemically Distinct Tannins in Medicinal Plants. Phytochemistry 2005, 66, 2012–2031. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Yoshida, T.; Hatano, T. Pharmacologically Active Tannins Isolated from Medicinal Plants. In Plant Polyphenols. Basic Life Sciences; Hemingway, R.W., Laks, P.E., Eds.; Springer: Boston, MA, USA, 1992; Volume 59, pp. 539–569. ISBN 978-1-4613-6540-2. [Google Scholar]
- Okuda, T.; Yoshida, T.; Hatano, T. Ellagitannins as Active Constituents of Medicinal Plants. Planta Med. 1989, 55, 117–122. [Google Scholar] [CrossRef]
- Hou, A.-J.; Liu, Y.-Z.; Yang, H.; Lin, Z.-W.; Sun, H.-D. Hydrolyzable Tannins and Related Polyphenols from Eucalyptus globulus. J. Asian Nat. Prod. Res. 2000, 2, 205–212. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information PubChem Compound Summary for CID 370, Gallic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Gallic-acid (accessed on 14 October 2022).
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic Acid: Pharmacological Activities and Molecular Mechanisms Involved in Inflammation-Related Diseases. Biomed. Pharmacother. 2021, 133, 110985. [Google Scholar] [CrossRef]
- Singla, E.; Dharwal, V.; Naura, A.S. Gallic Acid Protects against the COPD-Linked Lung Inflammation and Emphysema in Mice. Inflamm. Res. 2020, 69, 423–434. [Google Scholar] [CrossRef]
- BenSaad, L.A.; Kim, K.H.; Quah, C.C.; Kim, W.R.; Shahimi, M. Anti-Inflammatory Potential of Ellagic Acid, Gallic Acid and Punicalagin A&B Isolated from Punica Granatum. BMC Complement. Altern. Med. 2017, 17, 47. [Google Scholar] [CrossRef]
- Siddiqui, S.; Kamal, A.; Khan, F.; Jamali, K.S.; Saify, Z.S. Gallic and Vanillic Acid Suppress Inflammation and Promote Myelination in an in Vitro Mouse Model of Neurodegeneration. Mol. Biol. Rep. 2019, 46, 997–1011. [Google Scholar] [CrossRef] [PubMed]
- Shree, A.; Islam, J.; Vafa, A.; Mohammad Afzal, S.; Sultana, S. Gallic Acid Prevents 1, 2-Dimethylhydrazine Induced Colon Inflammation, Toxicity, Mucin Depletion, and Goblet Cell Disintegration. Environ. Toxicol. 2020, 35, 652–664. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information PubChem Compound Summary for CID 5281855, Ellagic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ellagic-acid (accessed on 14 October 2022).
- Sharifi-Rad, J.; Quispe, C.; Castillo, C.M.S.; Caroca, R.; Lazo-Vélez, M.A.; Antonyak, H.; Polishchuk, A.; Lysiuk, R.; Oliinyk, P.; De Masi, L.; et al. Ellagic Acid: A Review on Its Natural Sources, Chemical Stability, and Therapeutic Potential. Oxid. Med. Cell. Longev. 2022, 2022, 3848084. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information PubChem Compound Summary for CID 1794427, Chlorogenic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Chlorogenic-acid (accessed on 14 October 2022).
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic Acid: A Comprehensive Review of the Dietary Sources, Processing Effects, Bioavailability, Beneficial Properties, Mechanisms of Action, and Future Directions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3130–3158. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The Biological Activity Mechanism of Chlorogenic Acid and Its Applications in Food Industry: A Review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-Antioxidant Activity Relationships of Flavonoids and Phenolic Acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Sile, I.; Romane, E.; Reinsone, S.; Maurina, B.; Tirzite, D.; Dambrova, M. Data on Medicinal Plants in the Records of Latvian Folk Medicine from the 19th Century. Data Brief. 2020, 28, 105024. [Google Scholar] [CrossRef]
- Kļaviņa, A.; Keidāne, D.; Šukele, R.; Bandere, D.; Kovaļčuka, L. Traditional Latvian Herbal Medicinal Plants Used to Treat Parasite Infections of Small Ruminants: A Review. Vet. World 2021, 14, 1548. [Google Scholar] [CrossRef]
- Šukele, R.; Skadiņš, I.; Koka, R.; Bandere, D. Antibacterial Effects of Oak Bark (Quercus robur) and Heather Herb (Calluna vulgaris L.) Extracts against the Causative Bacteria of Bovine Mastitis. Vet. World 2022, 15, 2315–2322. [Google Scholar] [CrossRef]
- Šukele, R.; Bārzdiņa, A.; Koka, R.; Skadins, I.; Lauberte, L.; Brangule, A.; Kovalcuka, L.; Bandere, D. Antibacterial Activity of Tanacetum vulgare L. Extracts against Clinical Isolates of Bovine Mastitis. Appl. Sci. 2023, 13, 3369. [Google Scholar] [CrossRef]
- Puertollano, M.A.; Puertollano, E.; de Cienfuegos, G.A.; de Pablo, M.A. Dietary Antioxidants: Immunity and Host Defense. Curr. Top. Med. Chem. 2011, 11, 1752–1766. [Google Scholar] [CrossRef]
- Dróżdż, P.; Pyrzynska, K. Assessment of Polyphenol Content and Antioxidant Activity of Oak Bark Extracts. Eur. J. Wood Wood Prod. 2018, 76, 793–795. [Google Scholar] [CrossRef]
- Hathway, D.E. Oak-Bark Tannins. Biochem. J. 1958, 70, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Cai, J.; Duan, C.-Q.; Reeves, M.; He, F. A Review of Polyphenolics in Oak Woods. Int. J. Mol. Sci. 2015, 16, 6978–7014. [Google Scholar] [CrossRef] [PubMed]
- Council of Europe Oak Bark 01/2008:1887 Corrected 6.0 European Pharmacopeia; Directorate for the Quality of Medicines & HealthCare of the Council of Europe (EDQM): Strasbourg, France, 2013; Volume 2, p. 1335. ISBN 978-92-871-7525-0.
- Chepel, V.; Lisun, V.; Skrypnik, L. Changes in the Content of Some Groups of Phenolic Compounds and Biological Activity of Extracts of Various Parts of Heather (Calluna Vulgaris (L.) Hull) at Different Growth Stages. Plants 2020, 9, 926. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, F.; Chylinski, C.; Hutchings, M.R.; Lima, J.; Davidson, R.; Kelly, R.; Macrae, A.; Salminen, J.-P.; Engström, M.T.; Maurer, V.; et al. Comparative Analysis of the Anthelmintic Efficacy of European Heather Extracts on Teladorsagia Circumcincta and Trichostrongylus Colubriformis Egg Hatching and Larval Motility. Parasit. Vectors 2022, 15, 409. [Google Scholar] [CrossRef]
- Ekiert, H.; Pajor, J.; Klin, P.; Rzepiela, A.; Ślesak, H.; Szopa, A. Significance of Artemisia vulgaris L. (Common mugwort) in the History of Medicine and Its Possible Contemporary Applications Substantiated by Phytochemical and Pharmacological Studies. Molecules 2020, 25, 4415. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Thiruvengadam, M.; Chung, I.-M.; Nagella, P. Polyphenol Composition and Antioxidant Activity from the Vegetable Plant “Artemisia Absinthium” L. Aust. J. Crop Sci. 2013, 7, 1921–1926. [Google Scholar]
- Nurzyńska-Wierdak, R.; Sałata, A.; Kniaziewicz, M. Tansy (Tanacetum vulgare L.)—A Wild-Growing Aromatic Medicinal Plant with a Variable Essential Oil Composition. Agronomy 2022, 12, 277. [Google Scholar] [CrossRef]
- Rohloff, J.; Mordal, R.; Dragland, S. Chemotypical Variation of Tansy (Tanacetum vulgare L.) from 40 Different Locations in Norway. J. Agric. Food Chem. 2004, 52, 1742–1748. [Google Scholar] [CrossRef] [PubMed]
- Muresan, M.; Benedec, D.; Vlase, L.; Oprean, R.; Toiu, A.; Oniga, I. Screening of Polyphenolic Compounds, Antioxidant and Antimicrobial Properties of Tanacetum vulgare from Transylvania. Stud. Univ. Babes-Bolyai. Chem. 2015, 60, 12718. [Google Scholar]
- Aćimović, M.; Puvača, N. Tanacetum vulgare L.—A Systematic Review. Technol. Eng. Manag. J. Agron. Technol. Eng. Manag. 2020, 2020, 416–422. [Google Scholar]
- Baranauskienė, R.; Kazernavičiūtė, R.; Pukalskienė, M.; Maždžierienė, R.; Venskutonis, P.R. Agrorefinery of Tanacetum vulgare L. into Valuable Products and Evaluation of Their Antioxidant Properties and Phytochemical Composition. Ind. Crops Prod. 2014, 60, 113–122. [Google Scholar] [CrossRef]
- Sowa, P.; Marcinčáková, D.; Miłek, M.; Sidor, E.; Legáth, J.; Dżugan, M. Analysis of Cytotoxicity of Selected Asteraceae Plant Extracts in Real Time, Their Antioxidant Properties and Polyphenolic Profile. Molecules 2020, 25, 5517. [Google Scholar] [CrossRef]
- Juan-Badaturuge, M.; Habtemariam, S.; Jackson, C.; Thomas, M.J.K. Antioxidant Principles of Tanacetum vulgare L. Aerial Parts. Nat. Prod. Commun. 2009, 4, 1561–1564. [Google Scholar] [CrossRef]
- Ivănescu, B.; Tuchiluș, C.; Corciovă, A.; Lungu, C.; Teodor Mihai, C.; Gheldiu, A.; Vlase, L. Antioxidant, Antimicrobial and Cytotoxic Activity of Tanacetum vulgare, Tanacetum Corymbosum and Tanacetum Macrophyllum Extracts. Farmacia 2018, 66, 282–288. [Google Scholar]
- Martins, G.R.; Monteiro, A.F.; do Amaral, F.R.L.; da Silva, A.S. A Validated Folin-Ciocalteu Method for Total Phenolics Quantification of Condensed Tannin-Rich Açaí (Euterpe Oleracea Mart.) Seeds Extract. J. Food Sci. Technol. 2021, 58, 4693–4702. [Google Scholar] [CrossRef]
- Muflihah, Y.M.; Gollavelli, G.; Ling, Y.-C. Correlation Study of Antioxidant Activity with Phenolic and Flavonoid Compounds in 12 Indonesian Indigenous Herbs. Antioxidants 2021, 10, 1530. [Google Scholar] [CrossRef]
- Motta, S.; Guaita, M.; Cassino, C.; Bosso, A. Relationship between Polyphenolic Content, Antioxidant Properties and Oxygen Consumption Rate of Different Tannins in a Model Wine Solution. Food Chem. 2020, 313, 126045. [Google Scholar] [CrossRef]
- Antasionasti, I.; Datu, O.S.; Lestari, U.S.; Abdullah, S.S.; Jayanto, I. Correlation Analysis of Antioxidant Activities with Tannin, Total Flavonoid, and Total Phenolic Contents of Nutmeg (Myristica Fragrans Houtt) Fruit Precipitated by Egg White. Borneo J. Pharm. 2021, 4, 301–310. [Google Scholar] [CrossRef]
- Amarowicz, R. Tannins: The New Natural Antioxidants? Eur. J. Lipid Sci. Technol. 2007, 109, 549–551. [Google Scholar] [CrossRef]
- Taib, M.; Rezzak, Y.; Bouyazza, L.; Lyoussi, B. Medicinal Uses, Phytochemistry, and Pharmacological Activities of Quercus Species. Evid.-Based Complement. Altern. Med. 2020, 2020, 1920683. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Luís, Â.; Ferreira, S.; Duarte, A.P. Antioxidant and Antimicrobial Activity and Potential of Heather (Erica spp.) Extracts in the Control of Listeria monocytogenes. Int. J. Food Sci. Technol. 2019, 54, 862–870. [Google Scholar] [CrossRef]
- Rodrigues da Silva, L.; Campos Chisté, R.; Fernandes, E. Chemical and Antioxidant Characterization of the Portuguese Heather Honey from Calluna vulgaris. Separations 2021, 8, 177. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D. Chlorogenic Acid: Recent Advances on Its Dual Role as a Food Additive and a Nutraceutical against Metabolic Syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Xiang, L. Pharmacological Action and Potential Targets of Chlorogenic Acid. In Advances in Pharmacology; Du, G., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 87, pp. 71–88. ISBN 9780128201855. [Google Scholar]
- Gao, J.; Hu, J.; Hu, D.; Yang, X. A Role of Gallic Acid in Oxidative Damage Diseases: A Comprehensive Review. Nat. Prod. Commun. 2019, 14, 1934578X1987417. [Google Scholar] [CrossRef]
- Temraz, A.; El-Tantawy, W.H. Characterization of Antioxidant Activity of Extract from Artemisia Vulgaris. Pak. J. Pharm. Sci. 2008, 21, 321–326. [Google Scholar]
- Oyedemi, S.O.; Coopoosamy, R.M. Preliminary Studies on the Antibacterial and Antioxidative Potentials of Hydroalcoholic Extract from the Whole Parts of Artemisia vulgaris L. Int. J. Pharmacol. 2015, 11, 561–569. [Google Scholar] [CrossRef]
- Galiñanes, C.; Freire, M.S.; González-Álvarez, J. Antioxidant Activity of Phenolic Extracts from Chestnut Fruit and Forest Industries Residues. Eur. J. Wood Wood Prod. 2015, 73, 651–659. [Google Scholar] [CrossRef]
- Msaada, K.; Salem, N.; Bachrouch, O.; Bousselmi, S.; Tammar, S.; Alfaify, A.; Al Sane, K.; Ben Ammar, W.; Azeiz, S.; Haj Brahim, A.; et al. Chemical Composition and Antioxidant and Antimicrobial Activities of Wormwood (Artemisia absinthium L.) Essential Oils and Phenolics. J. Chem. 2015, 2015, 804658. [Google Scholar] [CrossRef]
- Canadanovic-Brunet, J.M.; Djilas, S.M.; Cetkovic, G.S.; Tumbas, V.T. Free-Radical Scavenging Activity of Wormwood (Artemisia absinthium L.) Extracts. J. Sci. Food Agric. 2005, 85, 265–272. [Google Scholar] [CrossRef]
- Monschein, M.; Iglesias Neira, J.; Kunert, O.; Bucar, F. Phytochemistry of Heather (Calluna vulgaris (L.) Hull) and Its Altitudinal Alteration. Phytochem. Rev. 2010, 9, 205–215. [Google Scholar] [CrossRef]
- Iason, G.R.; Hartley, S.E.; Duncan, A.J. Chemical Composition of Calluna vulgaris (Ericaceae): Do Responses to Fertilizer Vary with Phenological Stage? Biochem. Syst. Ecol. 1993, 21, 315–321. [Google Scholar] [CrossRef]
- Jalal, M.A.F.; Read, D.J.; Haslam, E. Phenolic Composition and Its Seasonal Variation in Calluna vulgaris. Phytochemistry 1982, 21, 1397–1401. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and Development of DPPH Method of Antioxidant Assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Besharati, M.; Maggiolino, A.; Palangi, V.; Kaya, A.; Jabbar, M.; Eseceli, H.; De Palo, P.; Lorenzo, J.M. Tannin in Ruminant Nutrition: Review. Molecules 2022, 27, 8273. [Google Scholar] [CrossRef]
- Hassan, Z.M.; Manyelo, T.G.; Selaledi, L.; Mabelebele, M. The Effects of Tannins in Monogastric Animals with Special Reference to Alternative Feed Ingredients. Molecules 2020, 25, 4680. [Google Scholar] [CrossRef]
- Molino, S.; Lerma-Aguilera, A.; Jiménez-Hernández, N.; Gosalbes, M.J.; Rufián-Henares, J.Á.; Francino, M.P. Enrichment of Food with Tannin Extracts Promotes Healthy Changes in the Human Gut Microbiota. Front. Microbiol. 2021, 12, 570. [Google Scholar] [CrossRef]
- Mattioli, L.B.; Corazza, I.; Micucci, M.; Pallavicini, M.; Budriesi, R. Tannins-Based Extracts: Effects on Gut Chicken Spontaneous Contractility. Molecules 2023, 28, 395. [Google Scholar] [CrossRef] [PubMed]
- Kļaviņa, A.; Keidāne, D.; Ganola, K.; Lūsis, I.; Šukele, R.; Bandere, D.; Kovalcuka, L. Anthelmintic Activity of Tanacetum vulgare L. (Leaf and Flower) Extracts against Trichostrongylidae Nematodes in Sheep In Vitro. Animals 2023, 13, 2176. [Google Scholar] [CrossRef] [PubMed]
- Sallam, I.E.; Abdelwareth, A.; Attia, H.; Aziz, R.K.; Homsi, M.N.; von Bergen, M.; Farag, M.A. Effect of Gut Microbiota Biotransformation on Dietary Tannins and Human Health Implications. Microorganisms 2021, 9, 965. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guidelines on Good Agricultural and Collection Practices (GACP) for Medicinal Plants; World Health Organization: Geneva, Switzerland, 2003; pp. 5–78.
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.-P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant Activity of Plant Extracts Containing Phenolic Compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Wang, W.; Li, J.; Zhang, H.; Wang, X.; Fan, J.; Zhang, X. Phenolic Compounds and Bioactivity Evaluation of Aqueous and Methanol Extracts of Allium mongolicum Regel. Food Sci. Nutr. 2019, 7, 779–787. [Google Scholar] [CrossRef]
- European Council. European Directorate for the Quality of Medicines & HealthCare (EDQM) European Pharmacopoeia-8th Edition 2.8.14. Tannins in Herbal Drugs, 8th ed.; European Council: Strasbourg, France, 2013; ISBN 978-92-871-7525-0.
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
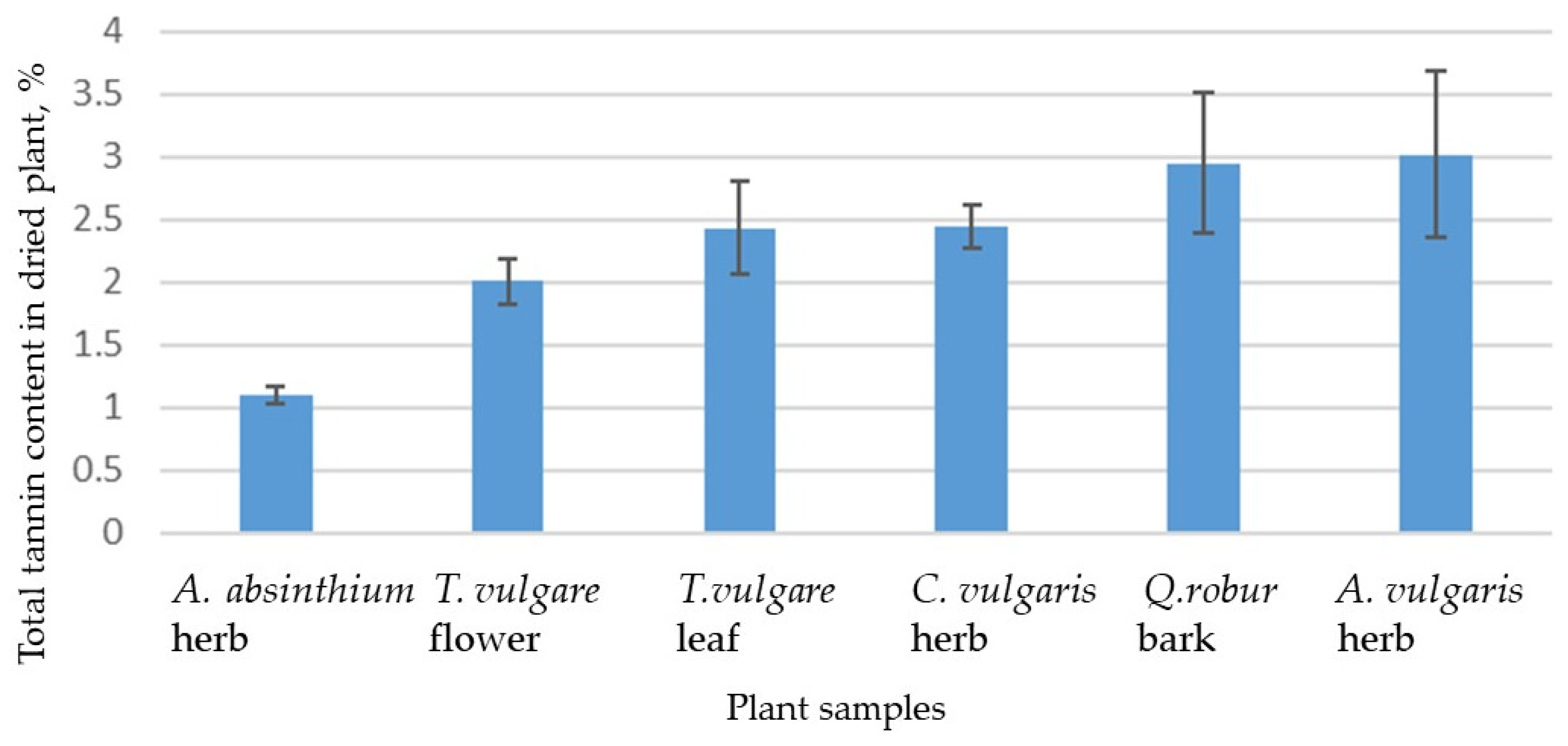
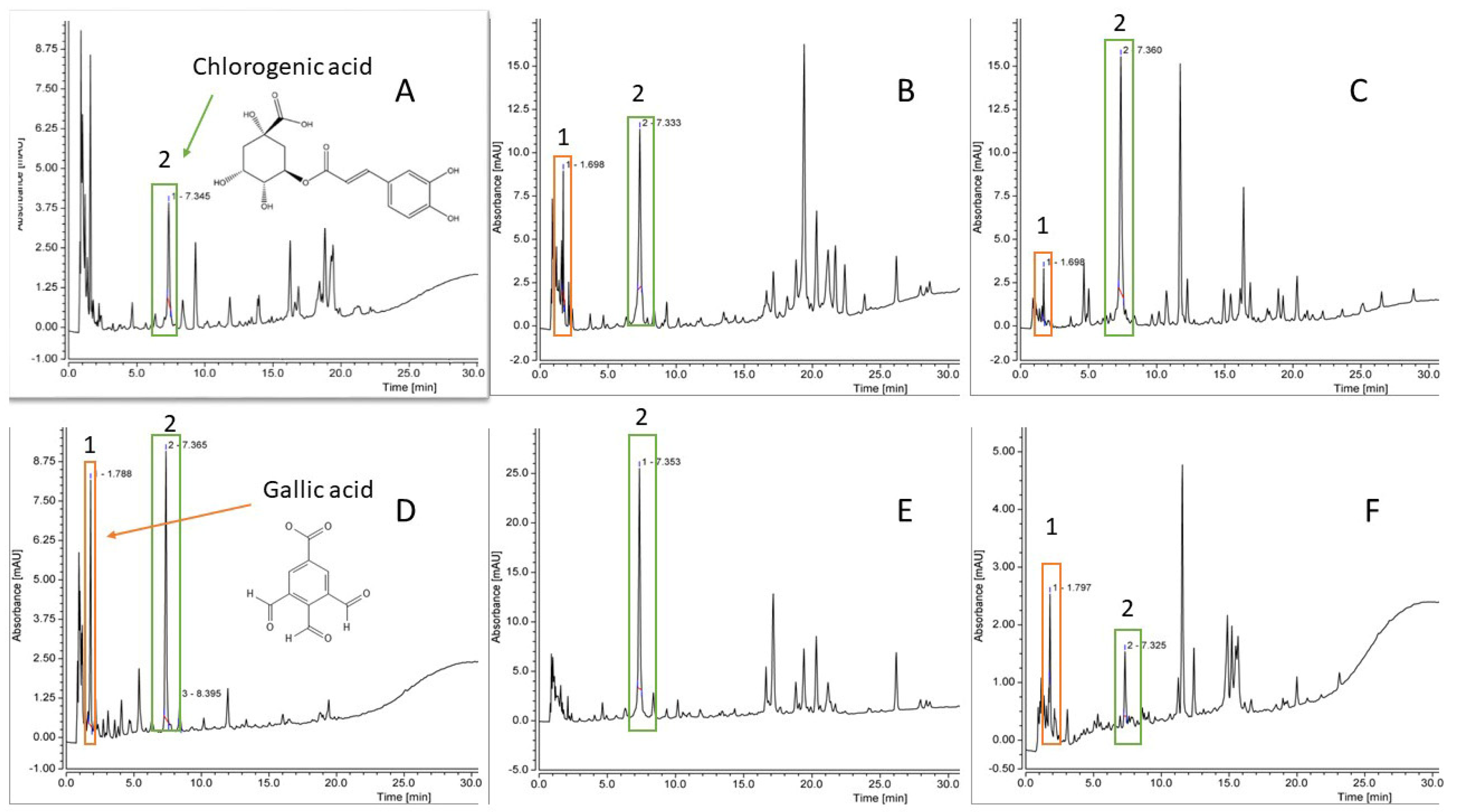
| Plant Sample | TPC (mg GAE/g of Lyophilized Extract wt), ±SD | TFC (mg QE/g of Lyophilized Extract wt), ±SD | IC50 Value of DPPH Radical Scavenging Activity (µg/mL), ±SD |
|---|---|---|---|
| Ascorbic acid | - | - | 43.92 ± 1.15 |
| Artemisia absinthium herb a | 68.22 ± 1.62 | 3.79 ± 0.43 | 509.33 ± 1.11 |
| Artemisia vulgaris herb a | 125.04 ± 6.06 | 15.25 ± 0.51 | 195.43 ± 1.12 |
| Calluna vulgaris herb a | 294.88 ± 14.20 | 51.13 ± 0.29 | 127.06 ± 1.07 |
| Quercus robur bark a | 301.39 ± 10.17 | 5.11 ± 0.32 | 96.16 ± 1.03 |
| Tanacetum vulgare flower a | 154.11 ± 7.95 | 25.12 ± 2.53 | 193.64 ± 1.10 |
| Tanacetum vulgare leaf a | 158.48 ± 15.57 | 46.15 ± 0.29 | 185.35 ± 1.12 |
| Artemisia absinthium herb b | 91.12 ± 1.09 | 5.26 ± 0.99 | 322.85 ± 1.13 |
| Artemisia vulgaris herb b | 179.21 ± 2.29 | 16.06 ± 0.53 | 164.44 ± 1.13 |
| Calluna vulgaris herb b | 285.61 ± 5.41 | 55.08 ± 2.23 | 104.71 ± 1.07 |
| Quercus robur bark b | 316.02 ± 21.54 | 6.20 ± 0.22 | 83.95 ± 1.04 |
| Tanacetum vulgare flower b | 155.38 ± 3.17 | 29.69 ±0.02 | 181.97 ± 1.07 |
| Tanacetum vulgare leaf b | 225.99 ± 3.69 | 52.75 ± 2.37 | 146.55 ± 1.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teterovska, R.; Sile, I.; Paulausks, A.; Kovalcuka, L.; Koka, R.; Mauriņa, B.; Bandere, D. The Antioxidant Activity of Wild-Growing Plants Containing Phenolic Compounds in Latvia. Plants 2023, 12, 4108. https://doi.org/10.3390/plants12244108
Teterovska R, Sile I, Paulausks A, Kovalcuka L, Koka R, Mauriņa B, Bandere D. The Antioxidant Activity of Wild-Growing Plants Containing Phenolic Compounds in Latvia. Plants. 2023; 12(24):4108. https://doi.org/10.3390/plants12244108
Chicago/Turabian StyleTeterovska, Renāte, Inga Sile, Artūrs Paulausks, Liga Kovalcuka, Rudīte Koka, Baiba Mauriņa, and Dace Bandere. 2023. "The Antioxidant Activity of Wild-Growing Plants Containing Phenolic Compounds in Latvia" Plants 12, no. 24: 4108. https://doi.org/10.3390/plants12244108
APA StyleTeterovska, R., Sile, I., Paulausks, A., Kovalcuka, L., Koka, R., Mauriņa, B., & Bandere, D. (2023). The Antioxidant Activity of Wild-Growing Plants Containing Phenolic Compounds in Latvia. Plants, 12(24), 4108. https://doi.org/10.3390/plants12244108







