A Multiplex PCR System of Novel Microsatellite Loci for Population Genetic Application in Walnuts
Abstract
1. Introduction
2. Results
2.1. Primer Screening, Development and Selection
2.2. Simplex PCR
2.3. Multiplex PCR System and Validation
2.4. Allelic Ladders
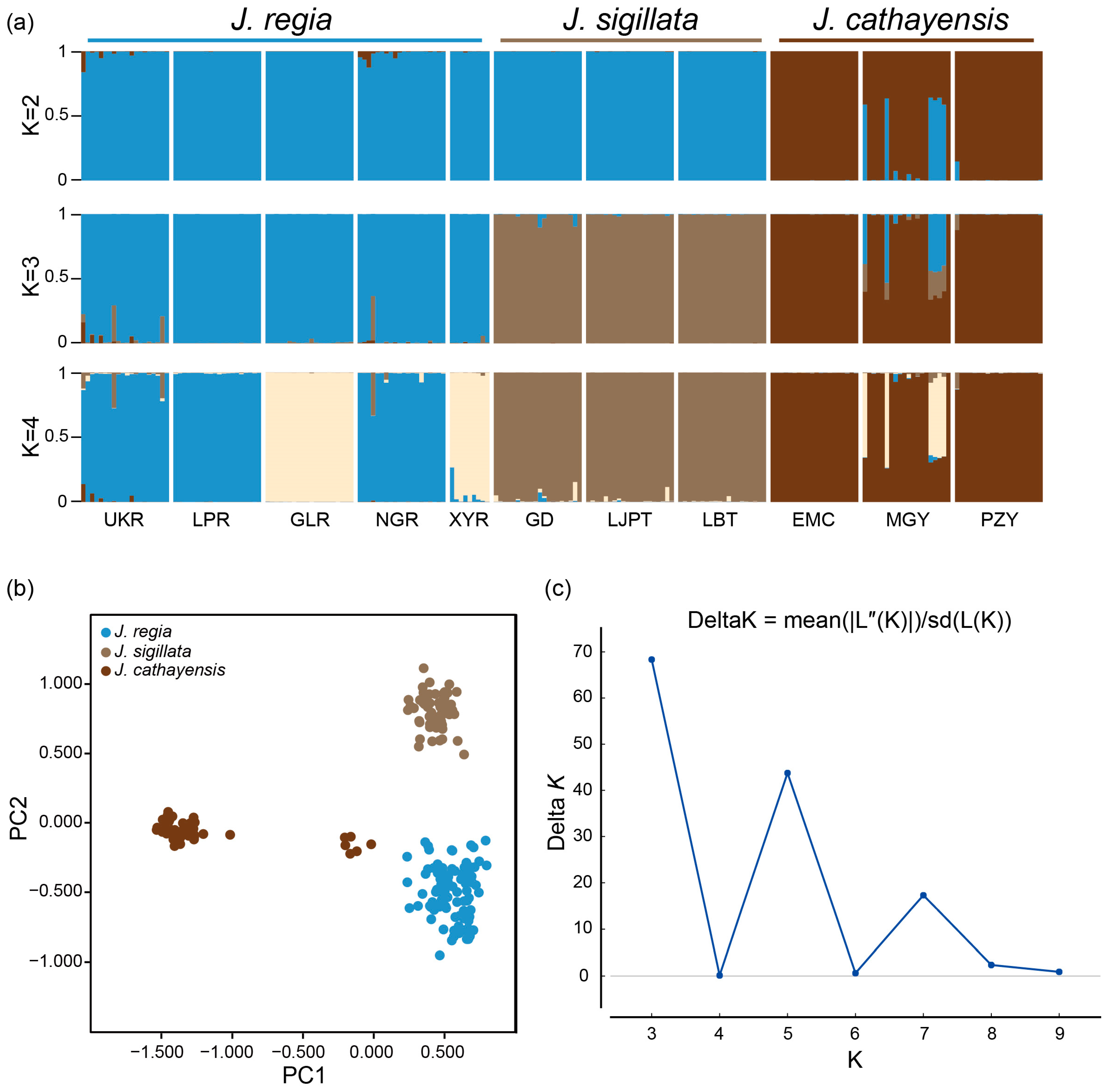
2.5. Genetic Diversity and Population Structure Analysis
3. Discussion
3.1. Genomic SSR Markers and Transferability
3.2. Polymorphism of the SSR Markers and Genetic Diversity
3.3. Multiplex PCR System
4. Materials and Methods
4.1. Sample Collection and DNA Extraction
4.2. Data Mining and Primer Design
4.3. Primer Screening
4.4. Simplex PCR Amplification
4.5. Development of m-PCR System and Validation
4.6. Development of Allelic Ladders
4.7. Data Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manning, W.E. The classification within the Juglandaceae. Ann. Mo. Bot. Gard. 1978, 65, 1058–1087. [Google Scholar] [CrossRef]
- Xu, H.L.; Yu, X.F.; Qu, S.C.; Zhang, R.; Qu, X.R.; Chen, Y.P.; Ma, X.Y.; Sui, D.Y. Anti-proliferative effect of Juglone from Juglans mandshurica Maxim on human leukemia cell HL-60 by inducing apoptosis through the mitochondria-dependent pathway. Eur. J. Pharmacol. 2010, 645, 14–22. [Google Scholar] [CrossRef]
- Taha, N.A.; Al-wadaan, M.A. Utility and importance of walnut, Juglans regia Linn: A. Afr. J. Microbiol. Res. 2011, 5, 5796–5805. [Google Scholar]
- Lu, A.M.; Stone, D.E.; Grauke, L.J. Juglandaceae. In Flora of China, 1st ed.; Wu, C.Y., Raven, P.H., Eds.; Science Press & Missouri Botanical Garden Press: St. Louis, MO, USA, 1999; Volume 4, pp. 277–285. [Google Scholar]
- Xi, R.T.; Zhang, Y.P. Walnut flora. In China Fruit-Plant Monograph; Zhao, H.C., Tian, B.F., Eds.; China Forestry Publishing House: Beijing, China, 1996; Volume 14, p. 51. [Google Scholar]
- Bernard, A.; Lheureux, F.; Dirlewanger, E. Walnut: Past and future of genetic improvement. Tree Genet. Genomes 2017, 14, 1. [Google Scholar] [CrossRef]
- Kalia, R.K.; Rai, M.K.; Kalia, S.; Singh, R.; Dhawan, A. Microsatellite markers: An overview of the recent progress in plants. Euphytica 2011, 177, 309–334. [Google Scholar] [CrossRef]
- White, G.; Powell, W. Isolation and characterization of microsatellite loci in Swietenia humilis (Meliaceae): An endangered tropical hardwood species. Mol. Ecol. 1997, 6, 851–860. [Google Scholar] [CrossRef]
- Zane, L.; Bargelloni, L.; Patarnello, T. Strategies for microsatellite isolation: A review. Mol. Ecol. 2002, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Thiel, T.; Michalek, W.; Varshney, R.; Graner, A. Exploiting EST databases for the development and characterization of gene-derived SSR-markers in barley (Hordeum vulgare L.). Theor. Appl. Genet. 2003, 106, 411–422. [Google Scholar] [CrossRef]
- Zalapa, J.E.; Cuevas, H.; Zhu, H.Y.; Steffan, S.; Senalik, D.; Zeldin, E.; McCown, B.; Harbut, R.; Simon, P. Using next-generation sequencing approaches to isolate simple sequence repeat (SSR) loci in the plant sciences. Am. J. Bot. 2012, 99, 193–208. [Google Scholar] [CrossRef]
- Markoulatos, P.; Siafakas, N.; Moncany, M. Multiplex polymerase chain reaction: A practical approach. J. Clin. Lab. Anal. 2002, 16, 47–51. [Google Scholar] [CrossRef]
- Sint, D.; Raso, L.; Traugott, M. Advances in multiplex PCR: Balancing primer efficiencies and improving detection success. Methods Ecol. Evol. 2012, 3, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.E.; Popowitch, E.B.; Miller, M.B. Evaluation of a novel multiplex PCR panel compared to quantitative bacterial culture for diagnosis of lower respiratory tract infections. J. Clin. Microbiol. 2020, 58, e02013–e02019. [Google Scholar] [CrossRef] [PubMed]
- Li, J.C.; Li, J.P.; Xu, S.G.; Xiong, S.Y.; Yang, J.N.; Chen, X.; Wang, S.W.; Qiao, X.L.; Zhou, T. A rapid and reliable multiplex PCR assay for simultaneous detection of fourteen animal species in two tubes. Food Chem. 2019, 295, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Sadredinamin, M.; Shabani, M.; Karimi, A.; Sohrabi, M.-R.; Karimi-Yazdi, M.; Ghalavand, Z.; Alebouyeh, M. Virulence genes expression profiling of different Shigella flexneri serotypes in response to sub-inhibitory concentrations of azithromycin and ciprofloxacin. Gut. Pathog. 2022, 14, 10. [Google Scholar] [CrossRef]
- Koh, J.C.O.; Barbulescu, D.M.; Norton, S.; Redden, B.; Salisbury, P.A.; Kaur, S.; Cogan, N.; Slater, A.T. A multiplex PCR for rapid identification of Brassica species in the triangle of U. Plant Methods 2017, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, J.J.; Zhang, L.K.; Luo, J.; Zhao, H.; Zhang, J.; Wen, C.L. A new SNP genotyping technology Target SNP-seq and its application in genetic analysis of cucumber varieties. Sci. Rep. 2020, 10, 5623. [Google Scholar] [CrossRef]
- Yao, C.X.; Zhang, C.M.; Bi, C.; Zhou, S.; Dong, F.S.; Liu, Y.W.; Yang, F.; Jiao, B.; Zhao, H.; Lyu, M.Y. Establishment and application of multiplex PCR systems based on molecular markers for HMW-GSs in wheat. Agriculture 2022, 12, 556. [Google Scholar] [CrossRef]
- Woeste, K.; Burns, R.; Rhodes, O.; Michler, C. Thirty polymorphic nuclear microsatellite loci from black walnut. J. Hered. 2002, 93, 58–60. [Google Scholar] [CrossRef]
- Hoban, S.; Anderson, R.; McCleary, T.; Schlarbaum, S.; Romero-Severson, J. Thirteen nuclear microsatellite loci for butternut (Juglans cinerea L.). Mol. Ecol. Resour. 2008, 8, 643–646. [Google Scholar] [CrossRef]
- Chen, C.M.; Han, S.J.; Yuan, S.S.; Wang, C.J.; Yu, J.H. Isolation and characterization of 20 polymorphic microsatellite markers for Juglans mandshurica (Juglandaceae). Appl. Plant Sci. 2013, 1, 1200009. [Google Scholar] [CrossRef]
- Xu, Z.C.; Jin, Y.C.; Milne, R.I.; Xiahou, Z.Y.; Qin, H.T.; Ye, L.J.; Gao, L.M.; Liu, J.; Li, D.Z. Development of 32 novel microsatellite loci in Juglans sigillata using genomic data. Appl. Plant Sci. 2020, 8, e11328. [Google Scholar] [CrossRef]
- Ćelepirović, N.; Karija Vlahović, M.; Dounavi, A.; Ivanković, M. Optimizations of high throughput multiplex polymerase chain reaction with simple sequence repeat markers for genotyping of common walnut populations (Juglans regia L.). Silva Fenn. 2016, 50, 1674. [Google Scholar] [CrossRef][Green Version]
- Martínez-García, P.J.; Crepeau, M.W.; Puiu, D.; Gonzalez-Ibeas, D.; Whalen, J.; Stevens, K.A.; Paul, R.; Butterfield, T.S.; Britton, M.T.; Reagan, R.L. The walnut (Juglans regia) genome sequence reveals diversity in genes coding for the biosynthesis of non-structural polyphenols. Plant J. 2016, 87, 507–532. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.N.; Yan, P.C.; Zhang, B.W.; Woeste, K.E.; Lin, K.; Zhang, D.Y. Demographically idiosyncratic responses to climate change and rapid Pleistocene diversification of the walnut genus Juglans (Juglandaceae) revealed by whole-genome sequences. New Phytol. 2018, 217, 1726–1736. [Google Scholar] [CrossRef] [PubMed]
- Vischi, M.; Chiabà, C.; Raranciuc, S.; Poggetti, L.; Messina, R.; Ermacora, P.; Cipriani, G.; Paffetti, D.; Vettori, C.; Testolin, R. Genetic diversity of walnut (Juglans regia L.) in the Eastern Italian Alps. Forests 2017, 8, 81. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, J.; Jin, Q.; Wang, Y.; Pang, X.; Li, Y. Development and characterization of new microsatellites for walnut (Juglans regia). Genet. Mol. Res. 2013, 12, 4723–4734. [Google Scholar] [CrossRef] [PubMed]
- Topçu, H.; Ikhsan, A.S.; Sütyemez, M.; Çoban, N.; Güney, M.; Kafkas, S. Development of 185 polymorphic simple sequence repeat (SSR) markers from walnut (Juglans regia L.). Sci. Hortic. 2015, 194, 160–167. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, T.; Gao, X.X.; Wang, Y.; Zhang, Q.; Zhou, H.J.; Zhao, G.F.; Wang, M.L.; Woeste, K.E.; Zhao, P. De novo assembly and characterization of the leaf, bud, and fruit transcriptome from the vulnerable tree Juglans mandshurica for the development of 20 new microsatellite markers using Illumina sequencing. Mol. Genet. Genom. 2016, 291, 849–862. [Google Scholar] [CrossRef] [PubMed]
- Huang, S. Analysis of EST-SSR Genetic Diversity and Primers’ Transferability on Part of Walnut (Juglans regia L.) Resources in Xinjiang. Master’s Thesis, Xinjiang Agricultural University, Urumqi, China, 2013. [Google Scholar]
- Tóth, G.; Gáspári, Z.; Jurka, J. Microsatellites in different eukaryotic genomes: Survey and analysis. Genome Res. 2000, 10, 967–981. [Google Scholar] [CrossRef]
- Liu, J.; Gao, L.M.; Li, D.Z.; Zhang, D.Q.; Möller, M. Cross-species amplification and development of new microsatellite loci for Taxus wallichiana (Taxaceae). Am. J. Bot. 2011, 98, e70–e73. [Google Scholar] [CrossRef]
- Reddy, M.; Rathour, R.; Kumar, N.; Katoch, P.; Sharma, T. Cross-genera legume SSR markers for analysis of genetic diversity in Lens species. Plant Breed. 2010, 129, 514–518. [Google Scholar] [CrossRef]
- Azevedo, A.L.S.; Costa, P.P.; Machado, J.C.; Machado, M.A.; Vander Pereira, A.; da Silva Lédo, F.J. Cross species amplification of Pennisetum glaucum microsatellite markers in Pennisetum purpureum and genetic diversity of Napier grass accessions. Crop Sci. 2012, 52, 1776. [Google Scholar] [CrossRef]
- Satya, P.; Paswan, P.K.; Ghosh, S.; Majumdar, S.; Ali, N. Confamiliar transferability of simple sequence repeat (SSR) markers from cotton (Gossypium hirsutum L.) and jute (Corchorus olitorius L.) to twenty two Malvaceous species. 3 Biotech 2016, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Serrote, C.M.L.; Reiniger, L.R.S.; Silva, K.B.; Dos Santos Rabaiolli, S.M.; Stefanel, C.M. Determining the Polymorphism Information Content of a molecular marker. Gene 2020, 726, 144175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhu, A.D.; Wang, X.J.; Yu, J.; Zhang, H.R.; Gao, J.S.; Cheng, Y.J.; Deng, X.X. Development of Juglans regia SSR markers by data mining of the EST database. Plant Mol. Biol. Rep. 2010, 28, 646–653. [Google Scholar] [CrossRef]
- Ikhsan, A.S.; Topçu, H.; Sütyemez, M.; Kafkas, S. Novel 307 polymorphic SSR markers from BAC-end sequences in walnut (Juglans regia L.): Effects of motif types and repeat lengths on polymorphism and genetic diversity. Sci. Hortic. 2016, 213, e11328. [Google Scholar] [CrossRef]
- Wambulwa, M.C.; Fan, P.Z.; Milne, R.; Wu, Z.Y.; Luo, Y.H.; Wang, Y.H.; Wang, H.; Gao, L.M.; Xiahou, Z.Y.; Jin, Y.C. Genetic analysis of walnut cultivars from southwest China: Implications for germplasm improvement. Plant Divers. 2022, 44, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Shahi Shavvon, R.; Qi, H.L.; Mafakheri, M.; Fan, P.Z.; Wu, H.Y.; Bazdid Vahdati, F.; Al-Shmgani, H.S.; Wang, Y.H.; Liu, J. Unravelling the genetic diversity and population structure of common walnut in the Iranian Plateau. BMC Plant Biol. 2023, 23, 201. [Google Scholar] [CrossRef]
- Lu, A.M. On the geographical distribution of the Juglandaceae. J. Syst. Evol. 1982, 20, 257–274. [Google Scholar]
- Ji, F.Y.; Ma, Q.G.; Zhang, W.T.; Liu, J.; Feng, Y.; Zhao, P.; Song, X.B.; Chen, J.X.; Zhang, J.P.; Wei, X. A genome variation map provides insights into the genetics of walnut adaptation and agronomic traits. Genome Biol. 2021, 22, 300. [Google Scholar] [CrossRef]
- Ding, Y.M.; Cao, Y.; Zhang, W.P.; Chen, J.; Liu, J.; Li, P.; Renner, S.S.; Zhang, D.Y.; Bai, W.N. Population-genomic analyses reveal bottlenecks and asymmetric introgression from Persian into iron walnut during domestication. Genome Biol. 2022, 23, 145. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Pan, G.; Ma, Q.G.; Zhang, J.P.; Pei, D. The genetic diversity and introgression of Juglans regia and Juglans sigillata in Tibet as revealed by SSR markers. Tree Genet. Genomes 2015, 11, 1. [Google Scholar] [CrossRef]
- Wang, H.; Pei, D.; Gu, R.S.; Wang, B.Q. Genetic diversity and structure of walnut populations in central and southwestern China revealed by microsatellite markers. J. Am. Soc. Hortic. Sci. 2008, 133, 197–203. [Google Scholar] [CrossRef]
- Zhang, W.P.; Cao, L.; Lin, X.R.; Ding, Y.M.; Liang, Y.; Zhang, D.Y.; Pang, E.L.; Renner, S.S.; Bai, W.N. Dead-end hybridization in walnut trees revealed by large-scale genomic sequence data. Mol. Biol. Evol. 2022, 39, msab308. [Google Scholar] [CrossRef]
- Liu, J.; Magige, E.A.; Fan, P.Z.; Wambulwa, M.C.; Luo, Y.H.; Qi, H.L.; Gao, L.M.; Milne, R.I. Genetic imprints of grafting in wild iron walnut populations in southwestern China. BMC Plant Biol. 2023, 23, 423. [Google Scholar] [CrossRef]
- Jin, X.Y.; Wei, Y.Y.; Cui, W.; Chen, C.; Guo, Y.X.; Zhang, W.Q.; Zhu, B.F. Development of a novel multiplex polymerase chain reaction system for forensic individual identification using insertion/deletion polymorphisms. Electrophoresis 2019, 40, 1691–1698. [Google Scholar] [CrossRef]
- Hill, C.R.; Butler, J.M.; Vallone, P.M. A 26plex autosomal STR assay to aid human identity testing. J. Forensic Sci. 2009, 54, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.M. Advanced Topics in Forensic DNA Typing: Methodology; Elsevier Science: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Meglécz, E.; Pech, N.; Gilles, A.; Dubut, V.; Hingamp, P.; Trilles, A.; Grenier, R.; Martin, J.F. QDD version 3.1: A user-friendly computer program for microsatellite selection and primer design revisited: Experimental validation of variables determining genotyping success rate. Mol. Ecol. Resour. 2014, 14, 1302–1313. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Rychlik, W. OLIGO 7 Primer Analysis Software. In PCR Primer Design; Yuryev, A., Ed.; Humana Press: Totowa, NJ, USA, 2007; pp. 35–59. [Google Scholar]
- Kalinowski, S.T.; Taper, M.L.; Marshall, T.C. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 2007, 16, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Smouse, R.P.P.; Peakall, R. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar]
- Takezaki, N.; Nei, M.; Tamura, K. POPTREE2: Software for constructing population trees from allele frequency data and computing other population statistics with Windows interface. Mol. Biol. Evol. 2010, 27, 747–752. [Google Scholar] [CrossRef]
- Rousset, F. GENEPOP’007: A complete re-implementation of the genepop software for Windows and Linux. Mol. Ecol. Resour. 2008, 8, 103–106. [Google Scholar] [CrossRef]
- Earl, D.A.; VonHoldt, B.M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Rosenberg, N.A. DISTRUCT: A program for the graphical display of population structure. Mol. Ecol. Notes 2004, 4, 137–138. [Google Scholar] [CrossRef]



| Locus | J. sigillata | J. regia | J. cathayensis | J. mandshurica | Total (n = 95) | PCR Amplification Rate | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GD (n = 20) | LJPT (n = 20) | LBT (n = 20) | GLR (n = 20) | XYR (n = 9) | MGY (n = 3) | DTL (n = 3) | |||||||||||||||||
| HO | HE | NA | HO | HE | NA | HO | HE | NA | HO | HE | NA | HO | HE | NA | HO | HE | NA | HO | HE | NA | NA | ||
| JR01 | 0.000 | 0.095 | 2 * | 0.200 | 0.180 | 2 | 0.050 | 0.049 | 2 | 0.450 | 0.546 | 4 | 0.000 | 0.000 | 1 | 0.333 | 0.500 | 3 | 0.000 | 0.000 | 1 | 7 | 100% |
| JR02 | 0.588 | 0.689 | 5 | 0.700 | 0.586 | 5 | 0.500 | 0.685 | 4 * | 0.050 | 0.386 | 3 * | 0.444 | 0.568 | 3 | 0.667 | 0.667 | 4 | 0.667 | 0.667 | 4 | 9 | 97% |
| JR03 | 0.706 | 0.685 | 5 | 0.300 | 0.429 | 3 | 0.550 | 0.516 | 4 | 0.400 | 0.584 | 4 * | 0.444 | 0.494 | 3 | 0.333 | 0.500 | 3 | 0.000 | 0.000 | 1 | 7 | 97% |
| JR04 | 0.700 | 0.754 | 6 | 0.550 | 0.544 | 4 | 0.500 | 0.488 | 7 | 0.500 | 0.738 | 4 * | 0.444 | 0.642 | 4 | 0.000 | 0.667 | 3 | 0.000 | 0.444 | 2 | 12 | 100% |
| JR05 | 0.600 | 0.530 | 5 | 0.650 | 0.580 | 5 | 0.550 | 0.584 | 4 | 0.250 | 0.219 | 2 | 0.111 | 0.204 | 3 | 0.333 | 0.500 | 3 | 0.000 | 0.444 | 2 | 9 | 100% |
| JR06 | 0.400 | 0.656 | 4 * | 0.400 | 0.566 | 4 | 0.700 | 0.559 | 3 | 0.647 | 0.554 | 3 | 0.556 | 0.611 | 3 | 0.000 | 0.444 | 2 | 0.333 | 0.278 | 2 | 6 | 97% |
| JR07 | 0.450 | 0.566 | 4 | 0.400 | 0.569 | 4 * | 0.579 | 0.661 | 6 | 0.125 | 0.443 | 4 * | 0.500 | 0.602 | 3 | 0.000 | 0.444 | 2 | 0.000 | 0.000 | 1 | 7 | 94% |
| JR08 | 0.500 | 0.526 | 3 | 0.400 | 0.446 | 4 | 0.450 | 0.581 | 4 * | 0.100 | 0.395 | 3 * | 0.556 | 0.512 | 3 | 1.000 | 0.500 | 2 | 0.000 | 0.000 | 1 | 5 | 100% |
| JR09 | 0.200 | 0.228 | 4 | 0.400 | 0.448 | 5 | 0.313 | 0.361 | 3 * | 0.125 | 0.576 | 4 * | 0.556 | 0.611 | 3 | 0.333 | 0.722 | 4 | 0.000 | 0.444 | 2 | 8 | 92% |
| JR10 | 0.200 | 0.440 | 5 | 0.000 | 0.455 | 2 * | 0.150 | 0.410 | 4 * | 0.000 | 0.480 | 2 * | 0.778 | 0.549 | 3 | 0.500 | 0.625 | 3 | 0.333 | 0.611 | 3 | 5 | 99% |
| JR11 | 0.650 | 0.576 | 3 | 0.550 | 0.696 | 4 | 0.650 | 0.708 | 5 * | 0.400 | 0.485 | 3 * | 0.667 | 0.623 | 4 | 0.333 | 0.611 | 3 | 0.333 | 0.278 | 2 | 5 | 100% |
| JR12 | 0.100 | 0.095 | 2 | 0.550 | 0.504 | 4 | 0.300 | 0.329 | 3 | 0.650 | 0.489 | 2 | 0.111 | 0.401 | 2 | 0.667 | 0.667 | 4 | 0.667 | 0.667 | 3 | 6 | 100% |
| JS02 | 0.650 | 0.545 | 4 | 0.632 | 0.536 | 3 | 0.500 | 0.480 | 2 | 0.000 | 0.000 | 1 | 0.000 | 0.000 | 1 | 0.000 | 0.000 | 1 | 0.000 | 0.444 | 2 | 7 | 99% |
| JS03 | 0.650 | 0.731 | 5 | 0.400 | 0.445 | 5 | 0.750 | 0.560 | 5 | 0.474 | 0.411 | 2 | 0.111 | 0.105 | 2 | 1.000 | 0.500 | 2 | 0.667 | 0.611 | 3 | 6 | 98% |
| JS04 | 0.550 | 0.696 | 5 | 0.950 | 0.775 | 6 | 0.400 | 0.700 | 5 * | 0.400 | 0.471 | 3 | 0.444 | 0.660 | 3 | 0.333 | 0.500 | 3 | 0.000 | 0.000 | 1 | 7 | 100% |
| JS05 | 0.650 | 0.565 | 3 | 0.750 | 0.518 | 5 | 0.550 | 0.526 | 3 | 0.000 | 0.000 | 1 | 0.333 | 0.500 | 2 | 0.000 | 0.000 | 1 | 0.000 | 0.000 | 1 | 8 | 96% |
| JS06 | 0.700 | 0.599 | 3 * | 0.600 | 0.595 | 3 | 0.550 | 0.511 | 3 | 0.000 | 0.000 | 1 | 0.667 | 0.494 | 2 | 0.000 | 0.000 | 1 | 0.000 | 0.000 | 1 | 4 | 98% |
| JS07 | 0.350 | 0.499 | 2 | 0.700 | 0.495 | 2 | 0.550 | 0.545 | 3 | 0.000 | 0.320 | 2 * | 0.556 | 0.475 | 2 | 0.000 | 0.444 | 2 | 0.000 | 0.000 | 1 | 6 | 100% |
| JS09 | 0.550 | 0.443 | 4 | 0.400 | 0.341 | 4 | 0.500 | 0.415 | 5 | 0.000 | 0.000 | 1 | 0.444 | 0.426 | 3 | 0.333 | 0.500 | 3 | 0.000 | 0.000 | 1 | 6 | 100% |
| JS12 | 0.450 | 0.421 | 3 * | 0.700 | 0.579 | 3 | 0.550 | 0.629 | 3 | 0.350 | 0.469 | 2 | 0.667 | 0.648 | 4 | 0.000 | 0.444 | 2 | 0.000 | 0.444 | 2 | 8 | 100% |
| JS13 | 0.600 | 0.585 | 3 | 0.500 | 0.619 | 5 | 0.500 | 0.686 | 6 | 0.000 | 0.000 | 1 | 0.667 | 0.648 | 3 | 0.333 | 0.611 | 3 | 1.000 | 0.611 | 3 | 6 | 99% |
| JS14 | 0.650 | 0.546 | 4 | 0.800 | 0.636 | 4 | 0.550 | 0.659 | 3 | 0.000 | 0.000 | 1 | 0.375 | 0.430 | 2 | 0.000 | 0.000 | 1 | 1.000 | 0.500 | 2 | 5 | 98% |
| JS15 | 0.100 | 0.095 | 2 | 0.600 | 0.670 | 4 | 0.600 | 0.574 | 5 | 0.300 | 0.255 | 2 | 0.556 | 0.512 | 3 | 0.000 | 0.444 | 2 | 0.000 | 0.000 | 1 | 6 | 100% |
| JS19 | 0.368 | 0.499 | 4 * | 0.368 | 0.716 | 6 * | 0.400 | 0.713 | 6 * | 0.000 | 0.000 | 1 | 0.000 | 0.000 | 1 | 0.000 | 0.444 | 2 | 0.333 | 0.278 | 2 | 10 | 97% |
| JS22 | 0.650 | 0.785 | 6 | 0.600 | 0.606 | 4 | 0.600 | 0.718 | 7 | 0.000 | 0.000 | 1 | 0.444 | 0.346 | 2 | 0.000 | 0.444 | 2 | 0.333 | 0.278 | 2 | 10 | 100% |
| JS28 | 0.050 | 0.049 | 2 | 0.316 | 0.359 | 4 | 0.200 | 0.261 | 3 | 0.400 | 0.420 | 2 | 0.111 | 0.105 | 2 | 0.333 | 0.500 | 3 | 0.000 | 0.000 | 1 | 5 | 99% |
| BFU-Jr277 | 0.600 | 0.486 | 3 | 0.421 | 0.486 | 4 | 0.400 | 0.429 | 3 | 0.500 | 0.455 | 2 | 0.222 | 0.346 | 2 | 0.000 | 0.000 | 1 | 0.000 | 0.000 | 1 | 5 | 96% |
| BFU-Jr38 | 0.400 | 0.515 | 3 * | 0.700 | 0.696 | 5 | 0.600 | 0.581 | 7 | 0.316 | 0.432 | 2 | 0.667 | 0.599 | 4 | 0.000 | 0.000 | 1 | 0.000 | 0.000 | 1 | 12 | 98% |
| CUJRD102 | 0.200 | 0.255 | 2 | 0.333 | 0.292 | 3 | 0.316 | 0.332 | 2 | 0.421 | 0.499 | 2 | 0.222 | 0.198 | 2 | 0.333 | 0.500 | 3 | 0.333 | 0.611 | 3 | 6 | 96% |
| CUJRD462 | 0.100 | 0.185 | 3 | 0.550 | 0.661 | 5 | 0.300 | 0.404 | 4 | 0.650 | 0.489 | 2 | 0.222 | 0.346 | 2 | 0.333 | 0.611 | 3 | 0.000 | 0.444 | 2 | 6 | 100% |
| JM5446 | 0.350 | 0.374 | 3 | 0.250 | 0.301 | 3 | 0.350 | 0.289 | 2 | 0.000 | 0.000 | 1 | 0.111 | 0.105 | 2 | 0.333 | 0.611 | 3 | 0.333 | 0.611 | 3 | 8 | 100% |
| SSR18 | 0.850 | 0.674 | 8 | 0.700 | 0.596 | 3 | 0.650 | 0.569 | 4 | 0.400 | 0.434 | 3 | 0.778 | 0.660 | 3 | 0.667 | 0.500 | 3 | 0.333 | 0.278 | 2 | 9 | 100% |
| ZMZ7 | 0.100 | 0.095 | 2 | 0.350 | 0.503 | 4 | 0.750 | 0.630 | 6 | 0.450 | 0.399 | 2 | 0.222 | 0.346 | 2 | 0.333 | 0.278 | 2 | 0.333 | 0.500 | 3 | 8 | 100% |
| Primer Pair Sequence (5′–3′) | Repeat Motif | Size Range (bp) | Ta (°C) | Fluorescence | |
|---|---|---|---|---|---|
| M1 | 57 | ||||
| JS09 | F: TTCGACCGCGTTTCCAGTTA | (TTC)7 | 116–131 | 56 | FAM |
| R: CCAGACTCACGGTCAGTTCC | |||||
| JS12 | F: TCAACATTGGCGAGGTGACA | (TTA)7 | 128–155 | 55 | TAMRA |
| R: AGGCAAGTCTACTTCTTTCCCT | |||||
| ZMZ7 | F: GAACAAATAGACCAGGCACG | (TCC)7 | 215–236 | 56 | TAMRA |
| R: TAACGACAACCGATGAAACC | |||||
| JR03 | F: ATACGGATCTGATGGCATGG | (GAC)6 | 235–268 | 57 | HEX |
| R: AGACAGCAATATCCACCCTT | |||||
| M2 | 58 | ||||
| CUJRD462 | F: TGCTCATTTTCATCCACTATC | (GAA)8 | 250–268 | 55 | HEX |
| R: ACTTCCTCTCCTTCCTCTTTC | |||||
| JS03 | F: TGACGAGGTTTACCAGATGGG | (GAA)5 | 90–105 | 58 | TAMRA |
| R: CGTTCTTCTTTCAGAGTGCTGTT | |||||
| JS04 | F: CATACATATGTGGGTGGCCT | (GAA)6 | 349–367 | 57 | TAMRA |
| R: TCCTCCTCTCTCTTCCCTTT | |||||
| JS07 | F: ACCAGCAGTTCCATGTACGG | (GAG)9 | 111–132 | 57 | HEX |
| R: GCTCATGCCATTATCTGCTTCG | |||||
| JS19 | F: AGATGATTTATGGCAGCCAATGA | (AAG)7 | 216–252 | 56 | FAM |
| R: TGCTGGGTAAACGCATGAGT | |||||
| JR05 | F: GTCGCAAGCTCAGCAAATAA | (AAAG)8 | 194–214 | 57 | HEX |
| R: TGTATGTATGGGAGGGGGAT | |||||
| JR07 | F: TCTTAAGAAGAGCCAATCGC | (ACCA)5 | 303–330 | 56 | FAM |
| R: GCTGTGTACCTCTTAGGGTT | |||||
| JR11 | F: AGCTAGCTCTCAAACAACAAGC | (GCAGTA)8 | 140–164 | 53 | FAM |
| R: ACAAACATGGCAACCTTCGTG | |||||
| M3 | 56 | ||||
| BFU-Jr38 | F: AGCTCCTCAAGCAAGGCTTA | (GAT)13 | 127–145 | 60 | FAM |
| R: GTGCATGGAACCACACTCAG | |||||
| BFU-Jr277 | F: TATTCACCCGGAGGTTTCAG | (GAT)10 | 235–250 | 61 | FAM |
| R: CCGAAGCCAGTCGAGTTATC | |||||
| JM5446 | F: ATGCATGCAGCTCCTACCTC | (CTAG)5 | 221–249 | 56 | HEX |
| R: GGACGTGTCCTGGGTTTTCA | |||||
| JS05 | F: CGGCATTACAGTCGGCAGTA | (GAA)10 | 93–120 | 57 | TAMRA |
| R: ACAATTCCCGTGCTGCATCT | |||||
| JS15 | F: ATCTCCGTGACTCCGCTCCT | (TTG)5 | 352–377 | 60 | TAMRA |
| R: ACCCGCCACCATCTTCATCTACCAA | |||||
| JR02 | F: GTTGGGCTGCCAGAGATTCT | (TTC)7 | 141–168 | 56 | HEX |
| R: ACGCTTCATTGGTAAACGAACG | |||||
| JR06 | F: TTGGAGCCCAATCAAGGATT | (ACAG)5 | 299–319 | 57 | HEX |
| R: CACACAGAAAAGACCAGCAG | |||||
| JR08 | F: ACTCCTGTCACTTGTATGCC | (CACG)5 | 329–359 | 57 | FAM |
| R: CCCGAGACATCAGAACCTTT | |||||
| JR12 | F: GCCTCTCCTCGTGCTCATTT | (GAA)18 | 212–230 | 56 | TAMRA |
| R: ACTCGCTACTTTTCAGGCCC | |||||
| M4 | 58 | ||||
| CUJRD102 | F: GACAGCAGCCTTATTTTGTAAC | (GAG)8 | 169–184 | 53 | HEX |
| R: TTCGTCCTCTTCTTCTTCAAC | |||||
| JS06 | F: CCCTGCATGCAATCAATCACA | (AGT)5 | 96–111 | 55 | TAMRA |
| R: ATGGGACGAGTGATGGACTC | |||||
| JS14 | F: CACATCGAGTGTTTCAAGTGACA | (TGC)6 | 134–149 | 57 | FAM |
| R: TGCACATGAGGAATTAACTGCTT | |||||
| JS22 | F: AAAGTTGCTCCTCAGCTTGG | (ATC)7 | 266–293 | 56 | FAM |
| R: TAATTAGCAATGAACAGATGGTGG | |||||
| JR04 | F: TGTTCTACCATTGCTCCGAA | (TCA)6 | 348–381 | 57 | FAM |
| R: ACACCTAGTTAGGAGCTGGA | |||||
| JR10 | F: TGGGAAGGGATTTCGTGTTGT | (TCTGA)5 | 195–215 | 56 | TAMRA |
| R: TAAGGACGCCCATTGCCATT | |||||
| M5 | 55 | ||||
| JS02 | F: CAACTCTGTGATTGCATGGG | (AAAG)8 | 383–411 | 57 | HEX |
| R: GGTAACTCTCATCGCTAGGG | |||||
| JS13 | F: TCTTGTCAGCATACTAAGCTTGTT | (TTCT)5 | 129–158 | 56 | HEX |
| R: ACTAACTGCATATAGGATCAACCA | |||||
| JS28 | F: AAAGGGTGAAGGAAGAAATTAGGAT | (AAGAG)5 | 316–332 | 57 | HEX |
| R: CCAAATTAAGCCAAACATGGTTGC | |||||
| JR01 | F: GAGCAGCTATGAAGAGGATGA | (AGA)6 | 236–263 | 57 | HEX |
| R: CTGAAATTTGTGGGGGTTCC | |||||
| JR09 | F: ATCACCTGATGTGGAAGCAA | (GAGGA)5 | 359–394 | 57 | TAMRA |
| R: CCATAGGACCCATAACGTGA | |||||
| SSR18 | F: GGAAAGGGATTTGAGGAGAGAT | (TTC)8 | 297–303 | 60 | TAMRA |
| R: GAAGAGGAGGAAGAAGAGGAGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiahou, Z.-Y.; Wambulwa, M.C.; Xu, Z.-C.; Ye, L.-J.; Fan, P.-Z.; Magige, E.A.; Luo, Y.-H.; Liu, J. A Multiplex PCR System of Novel Microsatellite Loci for Population Genetic Application in Walnuts. Plants 2023, 12, 4101. https://doi.org/10.3390/plants12244101
Xiahou Z-Y, Wambulwa MC, Xu Z-C, Ye L-J, Fan P-Z, Magige EA, Luo Y-H, Liu J. A Multiplex PCR System of Novel Microsatellite Loci for Population Genetic Application in Walnuts. Plants. 2023; 12(24):4101. https://doi.org/10.3390/plants12244101
Chicago/Turabian StyleXiahou, Zuo-Ying, Moses C. Wambulwa, Zu-Chang Xu, Lin-Jiang Ye, Peng-Zhen Fan, Ephie A. Magige, Ya-Huang Luo, and Jie Liu. 2023. "A Multiplex PCR System of Novel Microsatellite Loci for Population Genetic Application in Walnuts" Plants 12, no. 24: 4101. https://doi.org/10.3390/plants12244101
APA StyleXiahou, Z.-Y., Wambulwa, M. C., Xu, Z.-C., Ye, L.-J., Fan, P.-Z., Magige, E. A., Luo, Y.-H., & Liu, J. (2023). A Multiplex PCR System of Novel Microsatellite Loci for Population Genetic Application in Walnuts. Plants, 12(24), 4101. https://doi.org/10.3390/plants12244101







