Postharvest Storage Differentially Modulates the Enzymatic and Non-Enzymatic Antioxidant System of the Exocarp and Mesocarp of Hass Avocado: Implications for Disorders
Abstract
:1. Introduction
2. Results
2.1. Physiological Disorders at the Exocarp and Mesocarp Level in Hass Avocados Stored in Refrigeration and Controlled Atmosphere and at Their Respective Consumption Maturity
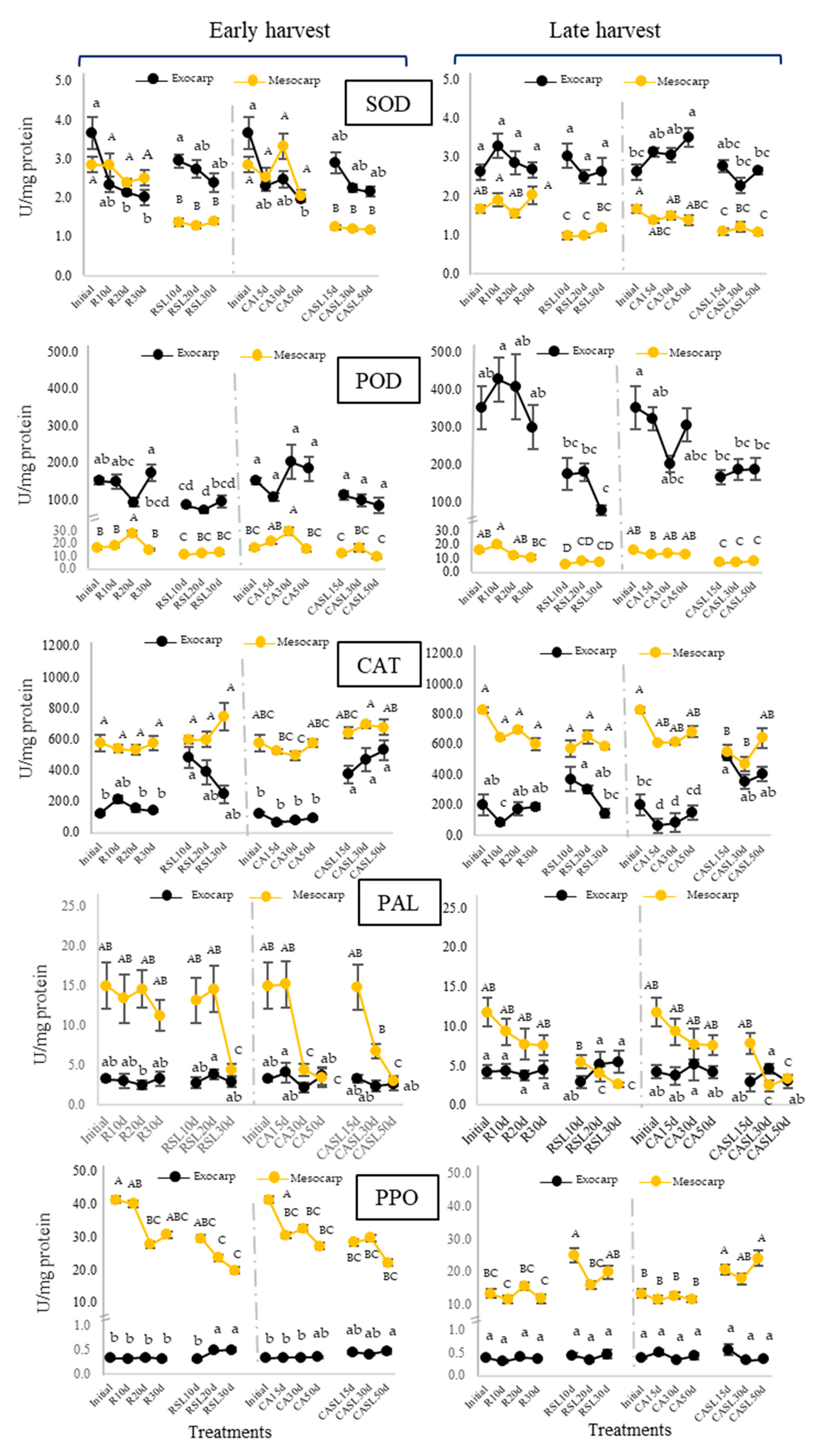
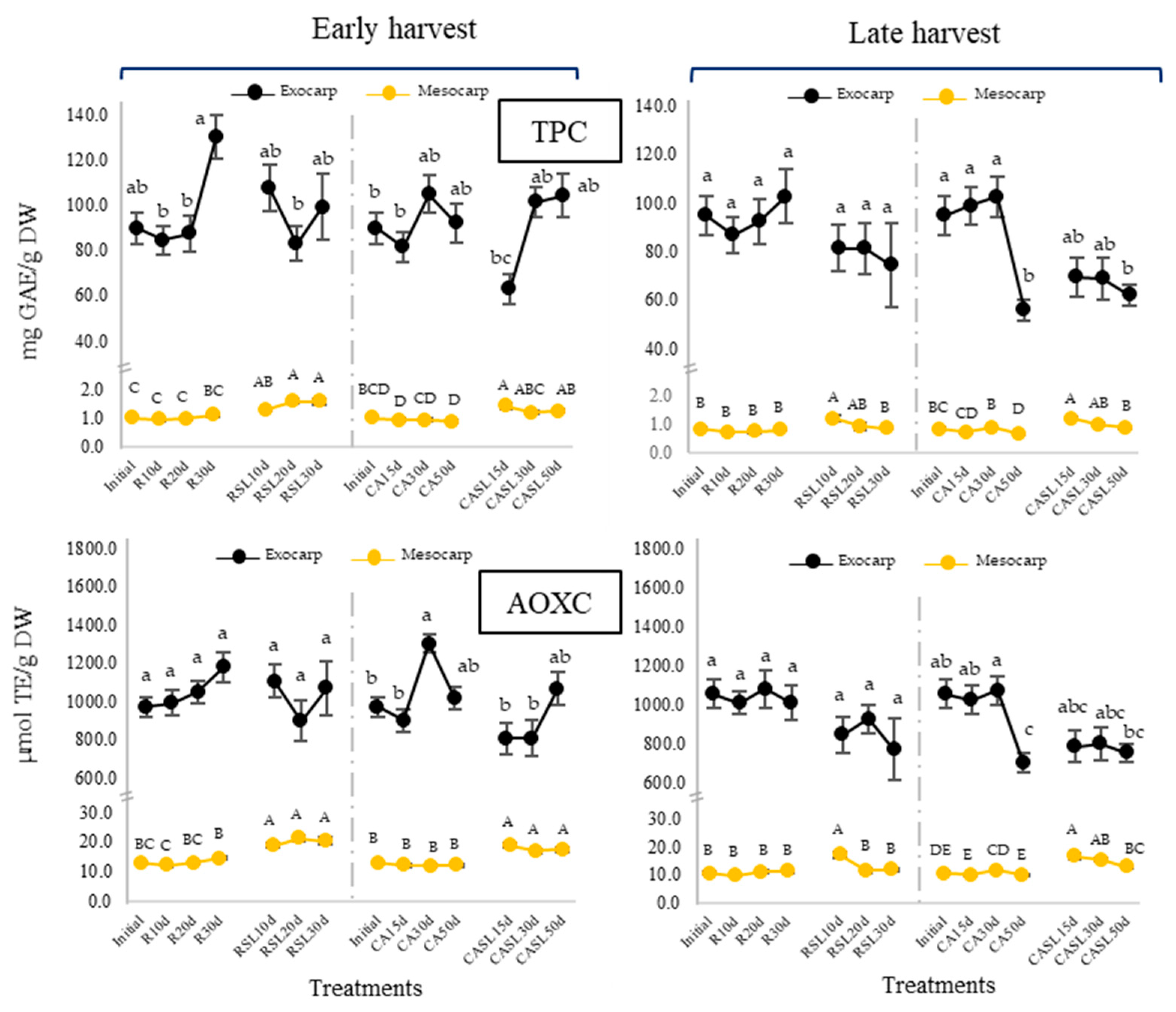
2.2. Enzymatic and Non-Enzymatic Antioxidant System in Hass Avocados at Harvest, during Refrigerated and Controlled Atmosphere Storage and at Consumption Maturity
2.3. Profile of Phenolic Compounds Determined by UPLC-PAD in Hass Avocados at Harvest and at Consumption Maturity Subjected to Different Storage Treatments
2.4. Multivariate Analysis of the Variables Evaluated in Hass Avocado at Harvest and Consumption Maturity
3. Discussion
3.1. Physiological Disorders at the Level of Exocarp and Mesocarp
3.2. Enzymatic and Non-Enzymatic Antioxidant System in Hass Avocados at Harvest, during Storage under Refrigeration and Controlled Atmosphere for Prolonged Periods and at Their Consumption Maturity
3.3. Total Phenolic Compounds (UPLC-DA) in Hass Avocados at Harvest and at Consumption Maturity of Samples from Refrigeration and Controlled Atmosphere Treatments
4. Materials and Methods
4.1. Sampling Material
4.2. Postharvest Storage: Refrigeration and Controlled Atmosphere and Shelf-Life Conditions
4.3. Dry Matter Content
4.4. Assessment of Exocarp and Mesocarp Physiological Disorders
4.5. Specific Enzymatic Activities
4.6. Determination of Total Phenolic Compounds and Antioxidant Capacity
4.7. Profile and Content of Phenolic Compounds Determined by UPLC-PAD
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dreher, M.L.; Davenport, A.J. Hass Avocado Composition and Potential Health Effects. Crit. Rev. Food Sci. Nutr. 2013, 53, 738–750. [Google Scholar] [CrossRef] [PubMed]
- Yahia, E.M. Avocado. In Crop Post-Harvest: Science and Technology-Perishables, 1st ed.; Rees, D., Farrell, G., Orchard, J., Eds.; Wiley-Blackwell: Chichester, UK, 2012; Volume 3, pp. 159–186. [Google Scholar]
- Villa-Rodríguez, J.; Molina-Corral, F.J.; Ayala-Zavala, J.F.; Olivas, G.; Gonzáles-Aguilar, G. Effect of maturity stage on the content of fatty acids and antioxidant activity of ‘Hass’ avocado. Food Res. Int. 2011, 44, 1231–1237. [Google Scholar] [CrossRef]
- Campos, D.; Terán-Hilares, F.; Chirinos, R.; Aguilar, A.; García, D.; Pacheco-Avalos, A.; Pedreschi, R. Bioactive compounds and antioxidant activity from harvest to edible ripeness of avocado cv. Hass (Persea americana) throughout the harvest seasons. Int. J. Food Sci. Technol. 2020, 55, 2208–2218. [Google Scholar] [CrossRef]
- Doberti, M.J. Radiografía a la Exportación de Palta en América Latina: ¿Qué Países Llevan la Delantera? Grupo DF. 2003. Available online: https://dfsud.com/america/radiografia-a-la-exportacion-de-palta-en-america-latina-que-paises (accessed on 12 October 2023). (In Spanish).
- Hernández, I.; Uarrota, V.; Paredes, D.; Fuentealba, C.; Defilippi, B.G.; Campos-Vargas, R.; Meneses, C.; Hertog, M.; Pedreschi, R. Can Metabolites at Harvest Be Used as Physiological Markers for Modelling the Softening Behaviour of Chilean “Hass” AvocadosDestined to Local and Distant Markets? Postharvest Biol. Technol. 2021, 174, 111457. [Google Scholar] [CrossRef]
- Defilippi, B.G.; Robledo, P.; Ferreyra, R.; Soto, S.; Saavedra, J. Preharvest Factors Influencing “Hass” Avocado (Persea americana Mill.) Quality during Long Term Storage. Acta Hortic. 2015, 1071, 137–142. [Google Scholar] [CrossRef]
- Blakey, R.J.; Bower, J.P.; Bertling, I. Influence of water and ABA supply on the ripening pattern of avocado (Persea americana Mill.) fruit and the prediction of water content using Near Infrared Spectroscopy. Postharvest Biol. Technol. 2009, 53, 72–76. [Google Scholar] [CrossRef]
- Woolf, A.B.; Cox, K.A.; White, A.; Ferguson, I.B. Low temperature conditioning treatments reduce external chilling injury of “Hass” avocados. Postharvest Biol. Technol. 2003, 28, 113–122. [Google Scholar] [CrossRef]
- Artés, F. El envasado en atmósfera modificada mejora la calidad de consumo de los productos hortofrutícolas intactos y mínimamente procesados en fresco. Rev. Iberoam. Tecnol. Postcosecha 2006, 7, 61–85. (In Spanish) [Google Scholar]
- Burdon, J.; Lallu, N.; Haynes, G.; McDermott, K.; Billing, D. The Effect of Delays in Establishment of a Static or Dynamic Controlled Atmosphere on the Quality of “Hass” Avocado Fruit. Postharvest Biol. Technol. 2008, 49, 61–68. [Google Scholar] [CrossRef]
- Uarrota, V.; Hernández, I.; Ponce, E.; Vidal, J.; Fuentealba, C.; Defilippi, B.; Lindh, V.; Zulueta, C.; Chirinos, R.; Campos, D.; et al. Unravelling factors associated with ‘blackspot’ disorder in stored Hass avocado (Persea americana Mill) fruit. J. Hortic. Sci. 2020, 95, 804–815. [Google Scholar] [CrossRef]
- Arpaia, M.L.; Collin, S.; Sievert, J.; Obenland, D. Influence of cold storage prior to and after ripening on quality factors and sensory attributes of “Hass” avocados. Postharvest Biol. Technol. 2015, 110, 149–157. [Google Scholar] [CrossRef]
- Everett, K.; Hallett, I.; Rees-George, J.; Chynoweth, R.; Pak, H. Avocado lenticel damage: The cause and the effect on fruit quality. Postharvest Biol. Technol. 2008, 48, 383–390. [Google Scholar] [CrossRef]
- Lindh, V.; Uarrota, V.; Zulueta, C.; Alvaro, J.E.; Valdenegro, M.; Cuneo, I.F.; Mery, D.; Pedreschi, R. Image análisis reveals that lenticel damage does not result in black spot development but enhances dehydration in Persea americana mill. cv. Hass during prolonged storage. Agronomy 2021, 11, 1699. [Google Scholar] [CrossRef]
- Chirinos, R.; Ramon, K.; Mendoza, M.; Figueroa-Merma, A.; Pacheco-Ávalos, A.; Campos, D.; Pedreschi, P. Effect of Prolonged Cold Storage on the Dynamics of the Enzymatic and Non-Enzymatic Antioxidant System in the Mesocarp of Avocado (Persea americana) cv. Hass: Relationship with Oxidative Processes. Horticulturae 2022, 8, 880. [Google Scholar] [CrossRef]
- Fuentealba, C.; Vidal, J.; Zulueta, C.; Ponce, E.; Uarrota, V.; Defilippi, B.G.; Pedreschi, R. Controlled Atmosphere Storage Alleviates Hass Avocado Black Spot Disorder. Horticulturae 2022, 8, 369. [Google Scholar] [CrossRef]
- Ramírez-Gil, J.; Henao-Rojas, J.C.; Morales-Osorio, J.G. Postharvest diseases and disorders in avocado cv. Hass and their relationship to preharvest management practices. Heliyon 2021, 7, e05905. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, J.L.; Manzano, S.; Palma, F.; Carvajal, F.; Garrido, D.; Jamilena, M. Oxidative Stress Associated with Chilling Injury in Immature Fruit: Postharvest Technological and Biotechnological Solutions. Int. J. Mol. Sci. 2017, 18, 1467. [Google Scholar] [CrossRef] [PubMed]
- Turkan, I. Emerging roles for ROS and RNS—Versatile molecules in plants. J. Exp. Bot. 2017, 68, 4413–4416. [Google Scholar] [CrossRef]
- Rhodes, D.; Nadolska-Orczyk, A. Plant Stress Physiology. In Encyclopedia of Life Sciences; John Wiley & Sons, Ltd.: Chichester, UK, 2001; pp. 1–7. [Google Scholar]
- Hodges, D.M.; Lester, G.E.; Munro, K.D.; Toivonen, P.M. Oxidative stress: Importance for postharvest quality. Hort. Sci. 2004, 39, 924–929. [Google Scholar] [CrossRef]
- Lado, J.; Rodrigo, M.J.; López-Climent, M.; Gómez-Cadenas, A.; Zacarías, L. Implication of the antioxidant system in chilling injury tolerance in the red peel of grapefruit. Postharvest Biol. Technol. 2016, 111, 214–223. [Google Scholar] [CrossRef]
- Oliveira, L.S.; Rodrigues, D.C.; Lopes, M.M.A.; Moura, C.F.H.; Oliveira, A.B.; Miranda, M.R.A. Changes in postharvest quality and antioxidant metabolism during development and ripening of sapodilla (Manilkara zapota L.). Int. Food Res. J. 2017, 24, 2427–2434. [Google Scholar]
- Haminiuk, C.; Maciel, G.; Plata-Oviedo, M.; Peralta, R. Phenolic compounds in fruits—An overview. Int. J. Food Sci. Technol. 2012, 47, 2023–2044. [Google Scholar] [CrossRef]
- Glowacz, M.; Bill, M.; Tinyane, P.P.; Sivakumar, D. Maintaining postharvest quality of cold stored “Hass” avocados by altering the fatty acids content and composition with the use of natural volatile compounds—Methyl jasmonate and methyl salicylate. J. Food Sci. Technol. 2017, 97, 5186–5193. [Google Scholar] [CrossRef]
- Uarrota, V.G.; Hernández, I.; Ponce, E.; Bauer, C.M.; Maraschin, M.; Pedreschi, R. Metabolic profiling and biochemical analysis of stored Hass avocado fruit by GC-MS and UHPLC-UV-VIS revealed oxidative stress as the main driver of ‘blackspot’ physiological disorder. Int. J. Food Sci. Technol. 2022, 57, 7896–7916. [Google Scholar] [CrossRef]
- Hofman, P.; Jobin-Décor, M.; Giles, J. Percentage of dry matter and oil content are not reliable indicators of fruit maturity or quality in late- harvested “Hass” Avocado. HortScience 2000, 35, 694–695. [Google Scholar] [CrossRef]
- Yahia, E.; Woolf, A. Avocado (Persea americana Mill). In Postharvest Biology & Technology of Tropical and Sub-Tropical Fruits; Yahia, E.M., Ed.; Woodhead Publishing: Cambridge, UK, 2011; Volume 2, pp. 125–185. [Google Scholar]
- Mendieta, B.; Olaeta, J.A.; Pedreschi, R.; Undurraga, P. Reduction of cold damage during cold storage of Hass avocado by a combined use of pre-conditioning and waxing. Sci. Hort. 2016, 200, 119–124. [Google Scholar] [CrossRef]
- Sharma, S.; Barman, K.; Prasad, R.N.; Singh, J. Chilling Stress During Postharvest Storage of Fruits and Vegetables. In New Frontiers in Stress Management for Durable Agriculture; Rakshit, A., Singh, H., Singh, A., Singh, U., Fraceto, L., Eds.; Springer: Singapore, 2020; pp. 75–100. [Google Scholar]
- Woolf, A.B.; Requejo-tapia, C.; Cox, K.A.; Jackman, R.C.; Gunson, A.; Lu, M.; White, A. 1–MCP Reduces Physiological Storage Disorders of ‘Hass’ Avocados. Postharvest Biol. Technol. 2005, 35, 43–60. [Google Scholar] [CrossRef]
- Thorp, T.; Hutching, D.; Lowe, T.; Marsh, K. Survey of fruit mineral concentrations and postharvest quality of New Zealand-grown ‘Hass’ avocado (Hylocereus undatus Persea americana Mill.). N. Z. J. Crop Hortic. Sci. 2010, 25, 251–260. [Google Scholar] [CrossRef]
- Imahori, Y.; Takemura, M.; Bai, J. Chilling-induced oxidative stress and antioxidant responses in mume (Prunus mume) fruit during low temperature storage. Postharvest Biol. Technol. 2008, 49, 54–60. [Google Scholar] [CrossRef]
- Balois-Morales, R.; Colinas-León, M.T.; Peña-Valdivia, C.B.; Chávez-Franco, S.H.; Alia-Tejacal, I. Sistema enzimático antisenescencia, catalasa-superóxido dismutasa, de frutos de pitahaya (Hylocereus undatus) almacenados con frío. Rev. Chapingo. Ser. Hortic. 2008, 14, 295–299. (In Spanish) [Google Scholar] [CrossRef]
- Baquero, D.L.E.; Castro, R.J.A.; Narváez, C.C.E. Catalasa, peroxidasa y polifenoloxidasa en pitaya amarilla (Acanthocereus pitajaya): Maduración y senescencia. Acta Biol. Colomb. 2005, 10, 49–59. (In Spanish) [Google Scholar]
- Bukowska, B.; Michałowicz, J.; Pieniążek, D.; Sicińska, P.; Duda, W. Superoxide Dismutases and Their Inhibitors—The Role in Some Diseases. Curr. Enzym. Inhib. 2006, 2, 379–397. [Google Scholar] [CrossRef]
- Chirinos, R.; Campos, D.; Martínez, S.; Llanos, S.; Betalleluz-Pallardel, I.; García-Ríos, D.; Pedreschi, R. The effect of hydrothermal treatment on metabolite composition of Hass avocados stored in a controlled atmosphere. Plants 2021, 10, 2427. [Google Scholar] [CrossRef]
- Pieczul, K.; Dobrzycka, A.; Wolko, J.; Perek, A.; Zielezinska, M.; Bocianowski, J.; Rybus-Zajax, M. The activity of β-glucosidase and guaiacol peroxidase in different genotypes of winter oilseed rape (Brassica napus L.) infected by Alternaria black spot fungi. Acta Physiol. Plant. 2020, 42, 142. [Google Scholar] [CrossRef]
- Kolodyaznaya, V.S.; Evgenievna, V.T.; Kiprushkina, E.I.; Shestopalova, I.A.; Broyko, Y.V.; Filippov, V.I.; Klementiev, D.A.; Kostyuk, V.A. Dynamics of physiological and biochemical processes in avocado fruit treated with preparations during storage. Prog. Chem. Appl. Chitin Deriv. 2020, 25, 111–123. [Google Scholar] [CrossRef]
- Al-Amrani, M.; Al-Alawi, A.; Al-Marhobi, I. Assessment of Enzymatic Browning and Evaluation of Antibrowning Methods on Dates. Int. J. Food Sci. 2020, 8380461. [Google Scholar] [CrossRef]
- Switala, J.; Loewen, P.C. Diversity of Properties Among Catalases. Arch. Biochem. Biophys. 2002, 401, 145–154. [Google Scholar] [CrossRef]
- Wang, B.; Wu, C.; Wang, G.; He, J.; Zhu, S. Transcriptomic analysis reveals a role of phenylpropanoid pathway in the enhancement of chilling tolerance by pre-storage cold acclimation in cucumber fruit. Sci. Hortic. 2021, 288, 110282. [Google Scholar] [CrossRef]
- Habibi, F.; Ramezanian, A.; Rahemi, M.; Eshghi, S.; Guillén, F.; Serrano, M.; Valero, D. Postharvest treatments with γ-aminobutyric acid, methyl jasmonate or methyl salicylate enhance chilling tolerance of blood orange fruit at prolonged cold storage. J. Sci. Food Agric. 2019, 99, 6408–6417. [Google Scholar] [CrossRef]
- Villa-Rodriguez, J.A.; Yahia, E.M.; González-León, A.; Ifie, I.; Robles-Zepeda, R.E.; Domínguez-Avila, J.A.; González-Aguilar, G.A. Ripening of “Hass” avocado mesocarp alters its phytochemical profile and the in vitro cytotoxic activity of its methanolic extracts. S. Afr. J. Bot. 2020, 128, 1–8. [Google Scholar] [CrossRef]
- Colombo, R.; Papetti, A. Avocado (Persea americana Mill.) by-products and their impact: From bioactive compounds to biomass energy and sorbent material for removing contaminants. A review. Int. J. Food Sci. Technol. 2019, 54, 943–951. [Google Scholar] [CrossRef]
- Jiménez-Velázquez, P.; Valle-Guadarrama, S.; Alia-Tejacal, I.; Salinas-Moreno, Y.; García-Cruz, L.; Pérez-López, A.; Guerra-Ramírez, D. Separation of bioactive compounds from epicarp of ‘Hass’ avocado fruit through aqueous two-phase systems. Food Bioprod. Process. 2020, 123, 238–250. [Google Scholar] [CrossRef]
- Serrano-García, I.; Domínguez-García, J.; Hurtado-Fernández, E.; González-Fernández, J.J.; Hormaza, J.I.; Beiro-Valenzuela, M.G.; Monasterio, R.; Pedreschi, R.; Olmo-García, L.; Carrasco-Pancorbo, A. Assessing the RP-LC-MS-Based Metabolic Profile of Hass Avocados Marketed in Europe from Different Geographical Origins (Peru, Chile, and Spain) over the Whole Season. Plants 2023, 12, 3004. [Google Scholar] [CrossRef]
- Martin, L.; Rose, J. There’s more than one way to skin a fruit: Formation and functions of fruit cuticles. J. Exp. Bot. 2013, 65, 4639–4651. [Google Scholar] [CrossRef]
- Lye, H.S.; Ong, M.K.; Teh, L.K.; Chang, C.C.; Wei, L.K. Avocado. In Valorization of Fruit Processing By-Products; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 67–93. [Google Scholar]
- Wang, D.; Yeats, T.; Uluisik, S.; Rose, J.; Seymour, G. Fruit Softening: Revisiting the Role of Pectin. Trends Plant Sci. 2018, 23, 302–310. [Google Scholar] [CrossRef]
- Tesfay, S.Z.; Bertling, I.; Bower, J.P. Effects of postharvest potassium silicate application on phenolics and other anti-oxidant systems aligned to avocado fruit quality. Postharvest Biol. Technol. 2011, 60, 92–99. [Google Scholar] [CrossRef]
- Hernández, I.; Fuentealba, C.; Olaeta, J.; Poblete-Echeverríab, C.; Defilippi, B.; González-Agüeroc, M.; Campos-Vargas, R.; Lurie, S.; Pedreschi, R. Effects of heat shock and nitrogen shock pre-treatments on ripening heterogeneity of Hass avocados stored in controlled atmosphere. Sci. Hortic. 2017, 225, 408–415. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis of AOAC International, 18th ed.; Horwitz, W., Ed.; EE.UU.: Washington, DC, USA, 2005. [Google Scholar]
- AgroFórum. Postcosecha de la Palta Hass: Guía Fotográfica de los Atributos de Calidad. 2018. Available online: https://www.agroforum.pe/agro-noticias/postcosecha-de-palta-hass-guia-fotografica-de-atributos-de-calidad-13374/?langid=1 (accessed on 10 September 2023). (In Spanish).
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Rodríguez-Carpena, J.G.; Morcuende, D.; Andrade, M.J.; Kylli, P.; Estévez, M. Avocado (Persea americana Mill.) Phenolics, In Vitro Antioxidant and Antimicrobial Activities, and Inhibition of Lipid and Protein Oxidation in Porcine Patties. J. Agric. Food Chem. 2011, 59, 5625–5635. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant capacity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]




| Peak N° | Retention Time (min) | Phenolic Compound Assignment (mg/100 g DM)/ Harvest Stage | Refrigeration | Controlled Atmosphere | |||||
|---|---|---|---|---|---|---|---|---|---|
| Initial (0 d) | Shelf Life—Consumption Maturity (RSL) * | Shelf Life—Consumption Maturity (CASL) * | |||||||
| 10 d | 20 d | 30 d | 15 d | 30 d | 50 d | ||||
| 1 | 11.06 | Chlorogenic Acid | |||||||
| Early (I) | 312.9 ± 54.9 abA | 393.3 ± 94.0 abA | 442.6 ± 109.5 aA | 117.5 ± 18.3 cB | 239.1 ± 15.6 bcA | 107.1 ± 6.8 cA | 217.7 ± 69.3 bcA | ||
| Late (II) | 288.3 ± 0.4 aA | 53.5 ± 6.1 eB | 61.4 ± 11.8 eB | 139.2 ± 19.6 cA | 104.9 ± 11.8 cdB | 118.5 ± 63.9 bcA | 218.0 ± 57.8 bA | ||
| 2 | 12.87 | Epicatechin derivative 1 (EpiD1) | |||||||
| Early (I) | 666.2 ± 100.1 cA | 1515.4 ± 483.5 abA | 2023.4 ± 352.6 aA | 139.8 ± 6.2 deB | 743.0 ± 218.1 cA | 1356.6 ± 555.6 abA | 324.0 ± 109.1 dA | ||
| Late (II) | 119.3 ± 9.4 dB | 54.2 ± 27.6 eB | 167.2 ± 1.7 cB | 345.1 ± 74.3 abA | 250.9 ± 53.2 bB | 356.4 ± 29.6 aB | 249.7 ± 4.8 bA | ||
| 3 | 14.08 | Epicatechin | |||||||
| Early (I) | 428.9 ± 115.6 cdA | 1086.6 ± 229.1 abA | 1502.7 ± 213.6 aA | 298.7 ± 33.3 eA | 571.9 ± 134.6 cA | 693.8 ± 147.4 cA | 238.4 ± 53.6 eA | ||
| Late (II) | 132.9 ± 13.2 bB | 72.8 ± 14.8 cB | 184.1 ± 25.0 abB | 229.0 ± 4.8 aB | 228.6 ± 21.9 aB | 205.2 ± 32.8 aB | 136.4 ± 13.3 bB | ||
| 4 | 16.03 | Epicatechin derivative 2 (EpiD2) | |||||||
| Early (I) | 429.2 ± 134.0 abA | 684.5 ± 121.1 abA | 171.5 ± 85.4 cA | 124.4 ± 4.5 cdB | 522.6 ± 147.2.6 abA | 792.1 ± 229.5 aA | 208.1 ± 42.6 cA | ||
| Late (II) | 127.7 ± 20.6 cB | 39.8 ± 0.4 dB | 108.0 ± 18.8 cA | 197.6 ± 10.0 aA | 171.7 ± 19.4 abB | 206.7 ± 17.4 aB | 183.2 ± 17.7 aA | ||
| 5 | 17.16 | Epicatechin derivative 3 (EpiD3) | |||||||
| Early (I) | 402.5 ± 3.97 bc | 459.9 ± 119.0 b | 846.85 ± 85.6 a | 133.1 ± 8.5 d | 367.8 ± 158.4 bc | 484.6 ± 200.3 b | 141.5 ± 25.0 d | ||
| Late (II) | ND | ND | ND | ND | ND | ND | ND | ||
| 6 | 18.20 | Epicatechin derivative 4 (EpiD4) | |||||||
| Early (I) | 45.3 ± 16.7 d | 310.7 ± 124.5 bc | 546.5 ± 37.5 a | 198.3 ± 28.8 c | 258.3 ± 12.4 bc | 420.8 ± 10.9 b | 153.1 ± 32.8 c | ||
| Late (II) | ND | ND | ND | ND | ND | ND | ND | ||
| 7 | 20.07 | Epicatechin derivative 5 (EpiD5) | |||||||
| Early (I) | 135.9 ± 45.6 b | 492.3 ± 129.9 a | 513.6 ± 96.4 a | 107.8 ± 24.6 bc | 78.1 ± 5.1 c | 393.8 ± 42.9 ab | 183.5 ± 13.0 b | ||
| Late (II) | ND | ND | ND | ND | ND | ND | ND | ||
| Total phenolic compounds (TPC-UPLC) | |||||||||
| Early (I) | 2421.1 ± 429.8 bA | 4942.9 ± 1301.4 abA | 6047.3 ± 541.5 aA | 1163.1 ± 85.8 cA | 2781.2 ± 691.7 bA | 4239.0 ± 1193.7 abA | 1466.5 ± 280.1 cA | ||
| Late (II) | 668.2 ± 42.7 bB | 220.3 ± 35.9 dB | 520.6 ± 30.3 cB | 911.0 ± 108.7 aAB | 756.2 ± 62.5 abB | 886.8 ± 143.7 abB | 787.2 ± 48.7 abB | ||
| Peak N° | Retention Time (min) | Phenolic Compound Assignment (mg/100 DM)/ Harvest Stage | Refrigeration | Controlled Atmosphere | |||||
|---|---|---|---|---|---|---|---|---|---|
| Initial (0 d) | Shelf Life—Consumption Maturity (RSL) * | Shelf Life—Consumption Maturity (CASL) * | |||||||
| 10 d | 20 d | 30 d | 15 d | 30 d | 50 d | ||||
| 1 | 7.9 | Syringique acid derivative (SAD) | |||||||
| Early (I) | Tr | 2.92 ± 1.31 abA | 3.23 ± 1.34 abA | 3.01 ± 1.29 abA | ND | 2.02 ± 0.53 bA | 4.07 ± 1.32 aA | ||
| Late (II) | Tr | 1.61 ± 0.32 bA | 0.31 ± 0.09 cB | 0.15 ± 0.01 cB | ND | 1.67 ± 0.39 bA | 3.19 ± 0.68 aA | ||
| 2 | 11.52 | p-coumaric acid derivative 1 (PCAD1) | |||||||
| Early (I) | Tr | 0.33 ± 0.16 dB | 0.80 ± 0.27 cB | 2.85 ± 1.00 aA | 0.65 ± 0.10 cB | 0.78 ± 0.31 cB | 1.40 ± 0.19 abAB | ||
| Late (II) | Tr | 7.47 ± 2.40 aA | 5.34 ± 0.25 bA | 3.36 ± 0.48 cA | 6.85 ± 0.43 aA | 3.87 ± 0.56 cA | 2.14 ± 0.72 dA | ||
| 3 | 12.52 | p-coumaric acid derivative 2 (PCAD2) | |||||||
| Early (I) | ND | ND | ND | ND | 0.36 ± 0.14 aB | 0.27 ± 0.05 aB | 0.28 ± 0.02 aB | ||
| Late (II) | ND | 1.40 ± 0.46 ab | 0.52 ± 0.12 b | 0.11 ± 0.02 c | 1.60 ± 0.22 aA | 0.92 ± 0.39 bA | 0.89 ± 0.25 bA | ||
| 4 | 13.12 | Caffeic acid derivative 1 (CAD1) | |||||||
| Early (I) | ND | ND | ND | ND | ND | ND | ND | ||
| Late (II) | ND | 1.59 ± 0.85 ab | 2.60 ± 0.70 a | ND | 0.10 ± 0.03 c | 0.11 ± 0.03 c | ND | ||
| 5 | 13.91 | p-coumaric acid derivative 3 (PCAD3) | |||||||
| Early (I) | ND | ND | ND | ND | ND | ND | ND | ||
| Late (II) | ND | 0.69 ± 0.03 a | 0.61 ± 0.10 a | 0.08 ± 0.02 | 0.07 ± 0.02 c | 0.15 ± 0.01 b | 0.06 ± 0.01 c | ||
| 6 | 16.35 | p-coumaric acid | |||||||
| Early (I) | Tr | 0.26 ± 0.01 bB | ND | ND | 0.50 ± 0.20 aB | 0.42 ± 0.09 aB | 0.28 ± 0.09 bA | ||
| Late (II) | Tr | 1.76 ± 0.17 abA | 1.10 ± 0.04 c | 0.22 ± 0.07 d | 2.10 ± 0.32 aA | 1.80 ± 0.13 abA | 0.17 ± 0.01 dB | ||
| 7 | 17.18 | p-coumaric acid derivative 4 (PCAD4) | |||||||
| Early (I) | ND | ND | ND | ND | ND | ND | ND | ||
| Late (II) | ND | 2.11 ± 0.01 a | 0.99 ± 0.09 b | 0.11 ± 0.01 d | 2.24 ± 0.24 a | 1.21 ± 0.52 b | 0.52 ± 0.18 c | ||
| 8 | 18.8 | p-coumaric acid derivative 5 (PCAD5) | |||||||
| Early (I) | Tr | 0.63 ± 0.06 cB | 1.67 ± 0.31 bB | 1.15 ± 0.30 bB | 3.93 ± 0.16 aB | 3.22 ± 0.86 aB | 3.22 ± 1.13 aAB | ||
| Late (II) | Tr | 30.29 ± 7.00 aA | 11.44 ± 2.63 cA | 5.17 ± 0.94 dA | 23.56 ± 1.16 abA | 13.06 ± 5.32 cA | 5.18 ± 1.53 dA | ||
| 9 | 19.79 | p-coumaric acid derivative 6 (PCAD6) | |||||||
| Early (I) | Tr | 2.01 ± 0.68 aB | 1.96 ± 0.55 abA | 0.76 ± 0.28 cA | 2.43 ± 1.10 aB | 1.63 ± 0.36 abB | 2.43 ± 0.27 aA | ||
| Late (II) | Tr | 6.89 ± 1.46 aA | 3.21 ± 2.16 bcAB | 0.32 ± 0.02 dB | 6.42 ± 0.27 aA | 4.07 ± 1.61 bA | 2.73 ± 0.15 bA | ||
| 10 | 19.88 | Caffeic acid derivative 2 (CAD2) | |||||||
| Early (I) | ND | ND | ND | ND | ND | ND | ND | ||
| Late (II) | ND | 2.33 ± 0.23 a | 1.70 ± 0.21 b | 0.17 ± 0.04 c | 0.70 ± 0.02 c | 0.85 ± 0.49 c | 0.17 ± 0.01 c | ||
| 11 | 20.02 | p-coumaric acid derivative 7 (PCAD7) | |||||||
| Early (I) | ND | ND | ND | ND | ND | ND | ND | ||
| Late (II) | ND | 4.79 ± 1.47 a | 6.28 ± 4.69 a | 0.10 ± 0.01 d | 2.30 ± 0.11 ab | 0.73 ± 0.29 c | 0.21 ± 0.02 d | ||
| 12 | 20.54 | p-coumaric acid derivative 8 (PCAD8) | |||||||
| Early (I) | ND | ND | ND | ND | 0.17 ± 0.11 aB | 0.15 ± 0.04 aA | 0.12 ± 0.03 aA | ||
| Late (II) | ND | 0.86 ± 0.20 a | 0.81 ± 0.63 a | 0.10 ± 0.03 b | 0.51 ± 0.10 aA | 0.15 ± 0.05 bA | 0.04 ± 0.00 cB | ||
| 13 | 20.44 | p-coumaric acid derivative 9 (PCAD9) | |||||||
| Early (I) | ND | ND | ND | ND | 0.70 ± 0.15 aB | 0.23 ± 0.02 bB | 0.24 ± 0.02 bB | ||
| Late (II) | ND | 2.44 ± 0.15 b | 1.90 ± 1.36 c | 0.29 ± 0.31 d | 5.51 ± 0.53 aA | 3.10 ± 1.5 bA | 2.20 ± 0.75 bcA | ||
| Total phenolic compounds (TPC-UPLC) | |||||||||
| Early (I) | ND | 6.15 ± 2.21 bB | 7.66 ± 2.46 bB | 7.76 ± 0.29 bB | 8.74 ± 1.96 abB | 8.73 ± 2.27 abB | 10.18 ± 0.33 aB | ||
| Late (II) | ND | 64.22 ± 14.21 aA | 36.83 ± 11.48 cA | 10.17 ± 0.05 eA | 51.96 ± 1.06 abA | 31.71 ± 12.29 cA | 17.50 ± 4.20 dA | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chirinos, R.; Delgado-Pariona, J.; Aguilar-Galvez, A.; Figueroa-Merma, A.; Pacheco-Ávalos, A.; Campos, D.; Pedreschi, R. Postharvest Storage Differentially Modulates the Enzymatic and Non-Enzymatic Antioxidant System of the Exocarp and Mesocarp of Hass Avocado: Implications for Disorders. Plants 2023, 12, 4008. https://doi.org/10.3390/plants12234008
Chirinos R, Delgado-Pariona J, Aguilar-Galvez A, Figueroa-Merma A, Pacheco-Ávalos A, Campos D, Pedreschi R. Postharvest Storage Differentially Modulates the Enzymatic and Non-Enzymatic Antioxidant System of the Exocarp and Mesocarp of Hass Avocado: Implications for Disorders. Plants. 2023; 12(23):4008. https://doi.org/10.3390/plants12234008
Chicago/Turabian StyleChirinos, Rosana, Jahaira Delgado-Pariona, Ana Aguilar-Galvez, Andrés Figueroa-Merma, Alejandro Pacheco-Ávalos, David Campos, and Romina Pedreschi. 2023. "Postharvest Storage Differentially Modulates the Enzymatic and Non-Enzymatic Antioxidant System of the Exocarp and Mesocarp of Hass Avocado: Implications for Disorders" Plants 12, no. 23: 4008. https://doi.org/10.3390/plants12234008
APA StyleChirinos, R., Delgado-Pariona, J., Aguilar-Galvez, A., Figueroa-Merma, A., Pacheco-Ávalos, A., Campos, D., & Pedreschi, R. (2023). Postharvest Storage Differentially Modulates the Enzymatic and Non-Enzymatic Antioxidant System of the Exocarp and Mesocarp of Hass Avocado: Implications for Disorders. Plants, 12(23), 4008. https://doi.org/10.3390/plants12234008






