Understanding Burkholderia glumae BGR1 Virulence through the Application of Toxoflavin-Degrading Enzyme, TxeA
Abstract
:1. Introduction
2. Results
2.1. Construction of Toxoflavin-Deficient Mutant Strains to Assess Whether Metagenome-Derived TxeA Is Functional in B. glumae BGR1 In Vivo
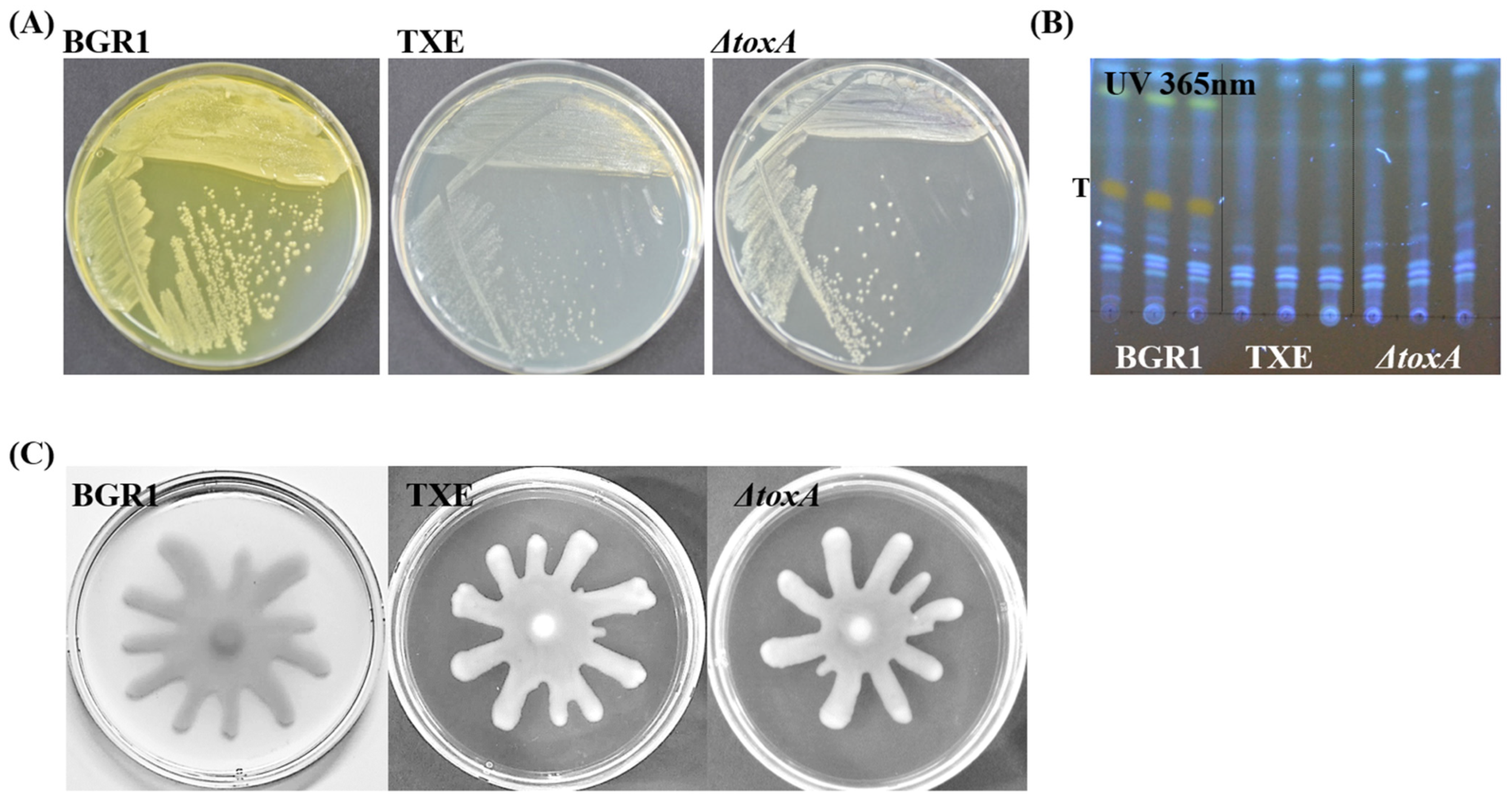
2.2. Phenotypic Analysis of Toxoflavin Production and Bacterial Motility in B. glumae BGR1 and Mutant Strains
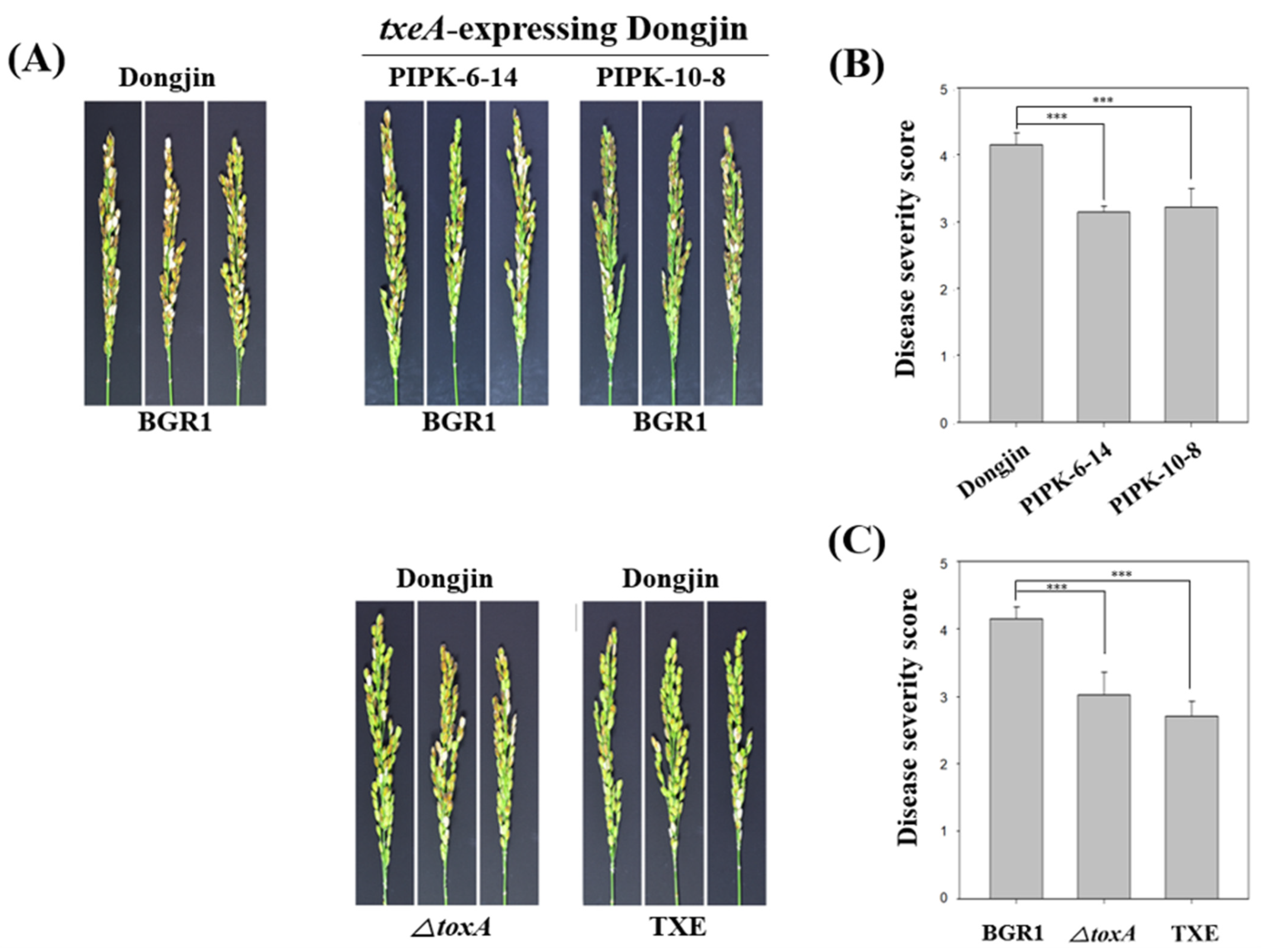
2.3. Association between Toxoflavin and Virulence in a Mutant Strain That Does Not Produce Toxoflavin through an In Vivo Virulence Assay
2.4. txeA-Induced Resistance against B. glumae BGR1 in Rice Plants
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains Plasmids and Growth Conditions
4.2. Construction of B. glumae BGR1 Expressing the txeA Gene Encoding Toxoflavin-Degrading Enzyme (TxeA)
4.3. Generation of Marker-Less Deletion Mutant Strains in B. glumae BGR1
4.4. Observation and Detection of the Virulence Factors in B. glumae BGR1
4.5. In Vivo Virulence Assays at Various Developmental Stages of Rice Plants
4.6. Construction of txeA-Expressing Rice Plants
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeong, Y.; Kim, J.; Kim, S.; Kang, Y. Toxoflavin Produced by Burkholderia glumae Causing Rice Grain Rot Is Responsible for Inducing Bacterial Wilt in Many Field Crops. Plant Dis. 2003, 87, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Kim, J.J.; Kim, I.; Mannaa, M.; Park, J.; Kim, J.; Lee, H.; Lee, S.; Park, D.; Sul, W.J.; et al. Type VI secretion systems of plant-pathogenic Burkholderia glumae BGR1 play a functionally distinct role in interspecies interactions and virulence. Mol. Plant Pathol. 2020, 21, 1055–1069. [Google Scholar] [CrossRef]
- Kim, J.; Kang, Y.; Choi, O.; Jeong, Y.; Jeong, J.-E.; Lim, J.Y.; Kim, M.; Moon, J.S.; Suga, H.; Hwang, I. Regulation of polar flagellum genes is mediated by quorum sensing and FlhDC in Burkholderia glumae. Mol. Microbiol. 2007, 64, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Kim, J.; Kim, S.; Kim, H.; Lim, J.Y.; Kim, M.; Kwak, J.; Moon, J.S.; Hwang, I. Proteomic analysis of the proteins regulated by HrpB from the plant pathogenic bacterium Burkholderia glumae. Proteomics 2008, 8, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, J.; Kim, S.; Park, I.; Seo, Y.-S. Differential regulation of toxoflavin production and its role in the enhanced virulence of Burkholderia gladioli. Mol. Plant Pathol. 2016, 17, 65–76. [Google Scholar] [CrossRef]
- Jung, B.; Park, J.; Kim, N.; Li, T.; Kim, S.; Bartley, L.E.; Kim, J.; Kim, I.; Kang, Y.; Yun, K.; et al. Cooperative interactions between seed-borne bacterial and air-borne fungal pathogens on rice. Nat. Commun. 2018, 9, 31. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Kang, Y.; Jang, J.Y.; Jog, G.J.; Lim, J.Y.; Kim, S.; Suga, H.; Nagamatsu, T.; Hwang, I. Quorum sensing and the LysR-type transcriptional activator ToxR regulate toxoflavin biosynthesis and transport in Burkholderia glumae. Mol. Microbiol. 2004, 54, 921–934. [Google Scholar] [CrossRef]
- Stern, K.G. Oxidation–reduction potentials of toxoflavin. Biochem. J. 1934, 29, 500–508. [Google Scholar] [CrossRef]
- Latuasan, H.E.; Berends, W. On the origin of the toxicity of toxoflavin. Biochim. Biophys. Acta 1996, 30, 502–508. [Google Scholar] [CrossRef]
- Suzuki, F.; Sawada, H.; Azegami, K.; Tsuchiya, K. Molecular characterization of the tox operon involved in toxoflavin biosynthesis of Burkholderia glumae. J. Gen. Plant Pathol. 2004, 70, 97–107. [Google Scholar] [CrossRef]
- Lelis, T.; Peng, J.; Barphagha, I.; Chen, R.; Ham, J.H. The virulence function and regulation of the metalloprotease gene prtA in the plant-pathogenic bacterium Burkholderia glumae. Mol. Plant-Microbe Interact. 2019, 31, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, M.K.; Philmus, B.; Begley, T.P.; Ealick, S.E. Burkholderia glumae ToxA Is a Dual-Specificity Methyltransferase That Catalyzes the Last Two Steps of Toxoflavin Biosynthesis. Biochemistry 2016, 55, 2748–2759. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.; Choi, O.; Goo, E.; Kim, N.; Kim, H.; Kang, Y.; Kim, J.; Moon, J.S.; Hwang, I. The quorum sensing-dependent gene katG of Burkholderia glumae is important for protection from visible light. J. Bacteriol. 2009, 191, 4152–4157. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-E.; Nguyen, C.M.; Lee, B.; Park, J.H.; Oh, J.Y.; Choi, J.S.; Kim, J.-C.; Song, J.K. Isolation and characterization of a novel metagenomic enzyme capable of degrading bacterial phytotoxin toxoflavin. PLoS ONE 2018, 13, e0183893. [Google Scholar] [CrossRef]
- Fenwick, M.K.; Philmus, B.; Begley, T.P.; Ealick, S.E. Toxoflavin lyase requires a novel 1-His-2-carboxylate facial triad. Biochemistry 2011, 50, 1091–1100. [Google Scholar] [CrossRef]
- Dong, Y.H.; Wang, L.H.; Xu, J.L.; Zhang, H.B.; Zhang, X.F.; Zhang, L.H. Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature 2001, 411, 813–817. [Google Scholar] [CrossRef]
- Devescovi, G.; Bigirimana, J.; Degrassi, G.; Cabrio, L.; LiPuma, J.J.; Kim, J.; Hwang, I.; Venturi, V. Involvement of a quorum-sensing-regulated lipase secreted by a clinical isolate of Burkholderia glumae in severe disease symptoms in rice. Appl. Environ. Microbiol. 2007, 73, 4950–4958. [Google Scholar] [CrossRef]
- Chen, R.; Barphagha, I.K.; Ham, J.H. Identification of potential genetic components involved in the deviant quorum-sensing signaling pathways of Burkholderia glumae through a functional genomics approach. Front. Cell. Infect. Microbiol. 2015, 5, 22. [Google Scholar] [CrossRef]
- Karki, H.S.; Shrestha, B.K.; Han, J.W.; Groth, D.E.; Barphagha, I.K.; Rush, M.C.; Melanson, R.A.; Kim, B.S.; Ham, J.H. Diversities in Virulence, Antifungal Activity, Pigmentation and DNA Fingerprint among Strains of Burkholderia glumae. PLoS ONE 2012, 7, e045376. [Google Scholar] [CrossRef]
- Chen, R.; Barphagha, I.K.; Karki, H.S.; Ham, J.H. Dissection of Quorum-Sensing Genes in Burkholderia glumae Reveals Non-Canonical Regulation and the New Regulatory Gene tofM for Toxoflavin Production. PLoS ONE 2012, 7, e052150. [Google Scholar] [CrossRef]
- Jang, M.S.; Goo, E.; An, J.H.; Kim, J.; Hwang, I. Quorum sensing controls flagellar morphogenesis in Burkholderia glumae. PLoS ONE 2014, 9, e084831. [Google Scholar] [CrossRef] [PubMed]
- Kalogeraki, V.S.; Winans, S.C. Suicide plasmids containing promoterless reporter genes can simultaneously disrupt and create fusions to target genes of diverse bacteria. Gene 1997, 188, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.; Tauch, A.; Jäger, W.; Kalinowski, J.; Thierbach, G.; Pühler, A. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19 selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 1994, 145, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Kovach, M.E.; Elzer, P.H.; Hill, D.S.; Robertson, G.T.; Farris, M.A.; Roop, R.M., II; Peterson, K.M. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 1995, 166, 175–176. [Google Scholar] [CrossRef]
- Bang, B.; Lee, J.; Kim, S.; Park, J.; Nguyen, T.T.; Seo, Y.-S. A rapid and efficient method for construction of an infectious clone of tomato yellow leaf curl virus. Plant Pathol. J. 2014, 30, 310–315. [Google Scholar] [CrossRef]


| Name | Characteristics | Source |
|---|---|---|
| Bacterial strains | ||
| B. glumae | ||
| BGR1 | Burkholderia glumae isolate from rice, wild type, Rifr | [1] |
| ΔtoxA | BGR1 derivative, deletion of 384 bp within bglu_2g06400 (toxA) | This study |
| TXE | BGR1 containing pBBR1MCS2::txeA | This study |
| A.tumefaciens | ||
| GV3101 | Agrobacterium tumefaciens, wild type, Rif | This study |
| AgroTXE | GV3101 containing pBBR1MCS2::txeA | This study |
| E. coli | ||
| E. coli DH5α λpir | F− 80dlacZΔM15 (lacZYA-argF) U169 recA1 endA1hsdR17 (rk-, mk+) phoAsupE44 -thi-1 gyrA96 relA1 | Lab collection |
| E. coli S17-1 λpir | hsdR recA pro RP4-2 (Tc::Mu; Km::Tn7) (λ pir) | [22] |
| Plasmids | ||
| pK18mobsacB | Allelic exchange suicide vector, sacB Kmr | [23] |
| pK18toxA | For constructing toxA KO mutant, pK18mobsacB:: 448bp upstream-downstream of toxA region restricted by EcoRI-HindIII, respectively. | This study |
| pBBR1MCS2 | Broad-host-range plasmid, Kmr, used to construct txeA-expressing strains. | [24] |
| pBBR1MCS2::txeA | For expressing the gene of toxoflavin degrading enzyme (TxeA) mutant strain, pBBR1MCS2::CDS of toxA | This study |
| pCAMBIA3301 | Binary vector, Kmr, PPTr, used to construct txeA-expressing transgenic plant | [25] |
| pCAMBIA3301::txeA | For expressing the gene of toxoflavin degrading enzyme (TxeA) transgenic plants, pCAMBIA3301::CDS of txeA, txeA gene driven by CaMV 35S promoter. | This study |
| Name | Sequence (5′→3′) | Use |
|---|---|---|
| toxA_LF | *TTTGGATCCTTGGCCCATCGATAGTGATT | To amplify the L fragment of toxA (bglu_2g06400) |
| toxA_LR | CGCGCCGATAGATTTCAC | To amplify the L fragment of toxA (bglu_2g06400) |
| toxA_RF | TTTTCGGGCGTGAAAAGCCAGTTCAGCTTCTAC | To amplify the R fragment of toxA (bglu_2g06400) |
| toxA_RR | *TTTAAGCTTGATTTGCGCAGAGTCTGGA | To amplify the R fragment of toxA (bglu_2g06400) |
| toxA_UP_F | CGAGGGAACATAGTGGCATT | To confirm the disruption of toxA (bglu_2g06400) |
| toxA_DOWN_R | TCTCAGGTTCGGGAGATACG | To confirm the disruption of toxA (bglu_2g06400) |
| pk18_DOWN_R | GTG AAG CTA GCT TAT CGC CAT | To confirm the first crossover in the process of constructing deletion mutant. |
| pBBR_TxeA_cF | *AAGGTACCAATGAATCAGCCTCCTCCT | Amplifying the txeA gene to be cloned in pBBR1MCS2. |
| pBBR_TxeA_cR | *TTTAAGCTTTCAATAGATCTTCCAAAT | Amplifying the txeA gene to be cloned in pBBR1MCS2. |
| pBBR1MCS2_dR | GACTCACTATAGGGCGAATTG | To confirm transformation in the process of constructing inserted txeA strains |
| pCAM_TxeA_cF | *AAAGGATCCATGAATCAGCCTCCTCCTCC | Amplifying the txeA gene to be cloned in pCAMBIA3301. |
| pCAM_TxeA_cR | *AAAAAGAATTCATAGATCTTCCAAATCCCAG | Amplifying the txeA gene to be cloned in pCAMBIA3301. |
| txeA_RT_sp_dF | GCGCATTTTAGAAACTTGTC | RT-PCR analysis to determine the txeA expression level |
| txeA_RT_sp_dR | TGAGGTGGGGATAGTTCCAAAC | RT-PCR analysis to determine the txeA expression level |
| txeA_cDNA_R | AGATCTTCCAAATCCCAGG | To construct the cDNA of txeA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, N.; Lee, D.; Lee, S.-B.; Lim, G.-H.; Kim, S.-W.; Kim, T.-J.; Park, D.-S.; Seo, Y.-S. Understanding Burkholderia glumae BGR1 Virulence through the Application of Toxoflavin-Degrading Enzyme, TxeA. Plants 2023, 12, 3934. https://doi.org/10.3390/plants12233934
Kim N, Lee D, Lee S-B, Lim G-H, Kim S-W, Kim T-J, Park D-S, Seo Y-S. Understanding Burkholderia glumae BGR1 Virulence through the Application of Toxoflavin-Degrading Enzyme, TxeA. Plants. 2023; 12(23):3934. https://doi.org/10.3390/plants12233934
Chicago/Turabian StyleKim, Namgyu, Duyoung Lee, Sais-Beul Lee, Gah-Hyun Lim, Sang-Woo Kim, Tae-Jin Kim, Dong-Soo Park, and Young-Su Seo. 2023. "Understanding Burkholderia glumae BGR1 Virulence through the Application of Toxoflavin-Degrading Enzyme, TxeA" Plants 12, no. 23: 3934. https://doi.org/10.3390/plants12233934
APA StyleKim, N., Lee, D., Lee, S.-B., Lim, G.-H., Kim, S.-W., Kim, T.-J., Park, D.-S., & Seo, Y.-S. (2023). Understanding Burkholderia glumae BGR1 Virulence through the Application of Toxoflavin-Degrading Enzyme, TxeA. Plants, 12(23), 3934. https://doi.org/10.3390/plants12233934








