Micropropagation and Acclimatization of Gymnocalycium cv. Fancy (Cactaceae): Developmental Responses to Different Explant Types and Hormone Conditions
Abstract
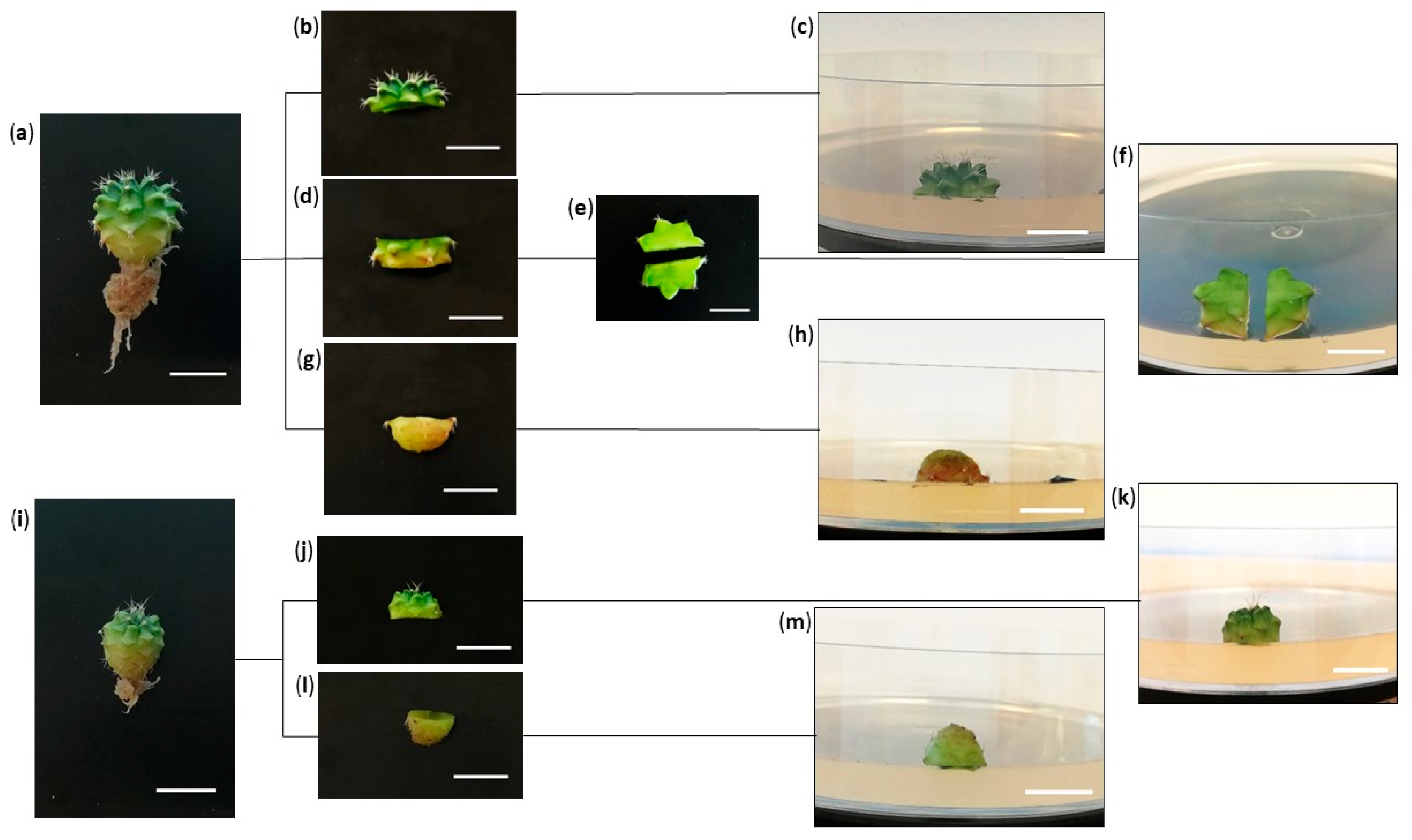
:1. Introduction
2. Results and Discussion
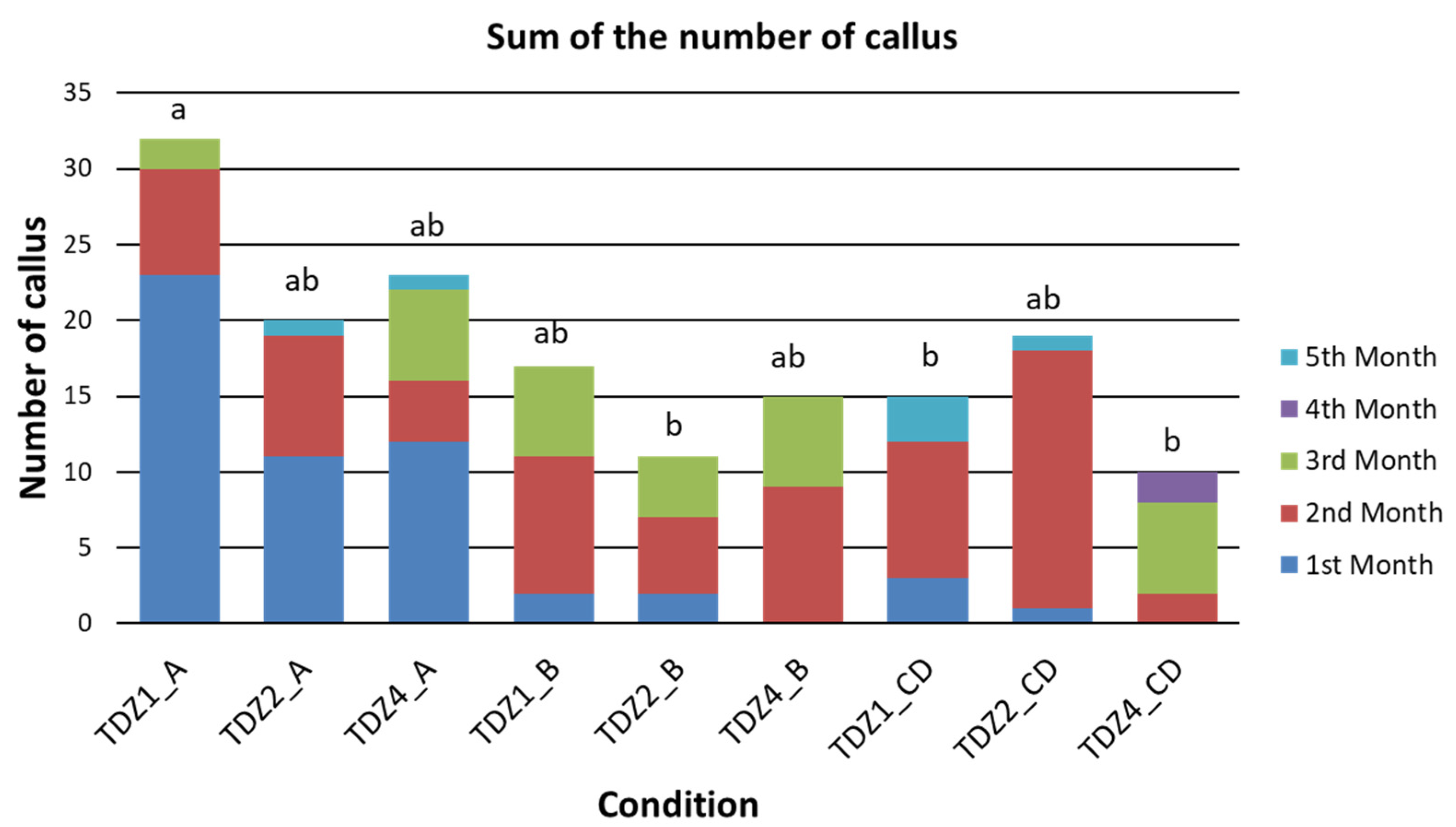
2.1. Callus
2.1.1. Productivity of TDZ Treatments Related to Callus Production
2.1.2. Efficiency of TDZ Treatments in Relation to Callus/Areole Production
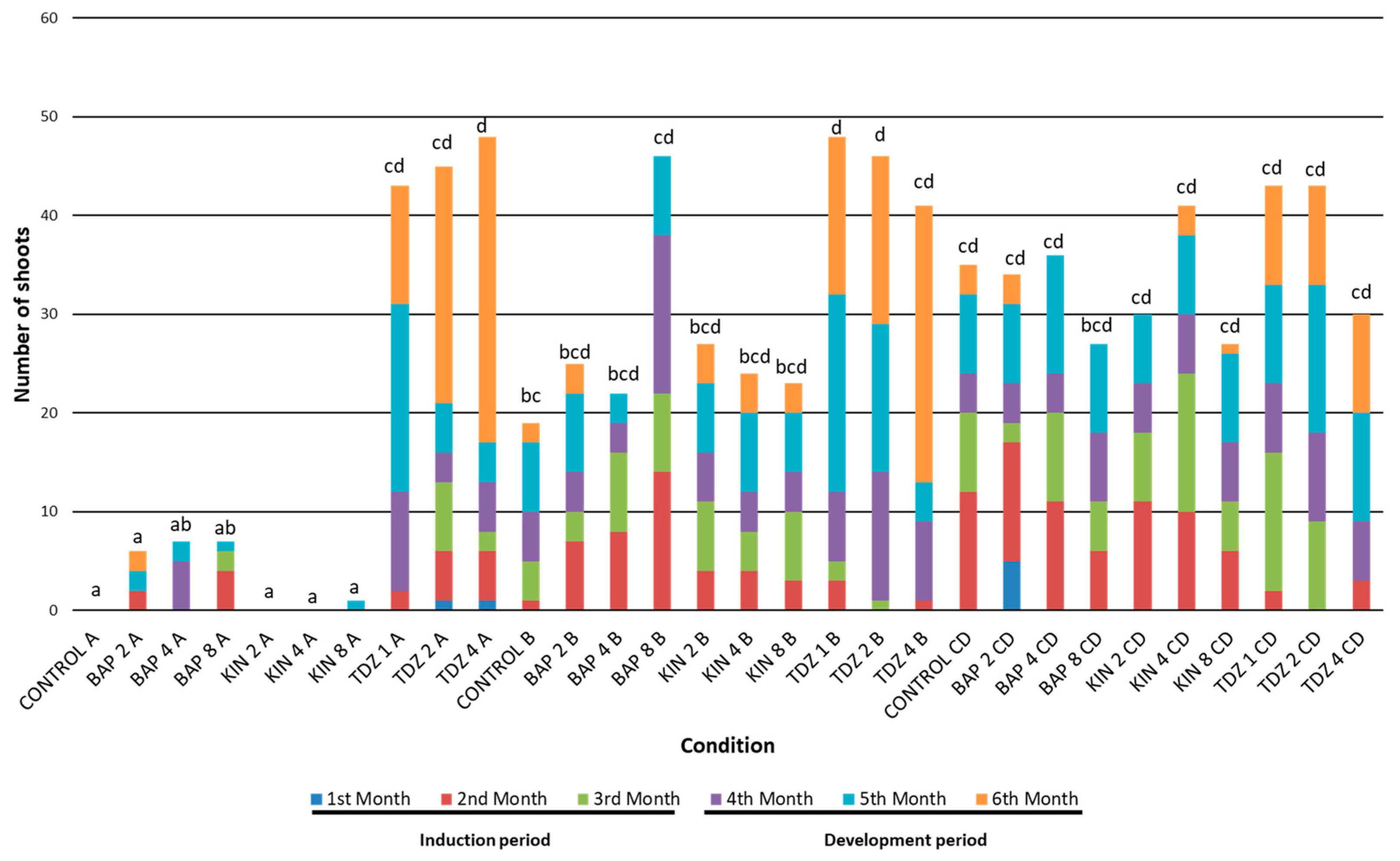
2.2. Shoots
2.2.1. Productivity Related to Shoot Production
2.2.2. Efficiency Related to Shoot Production
2.2.3. Shoot Production Based on Treatment Factor Combination
2.3. General Trends Related to the Induction Period
2.4. General Trends Related to the Growth Period
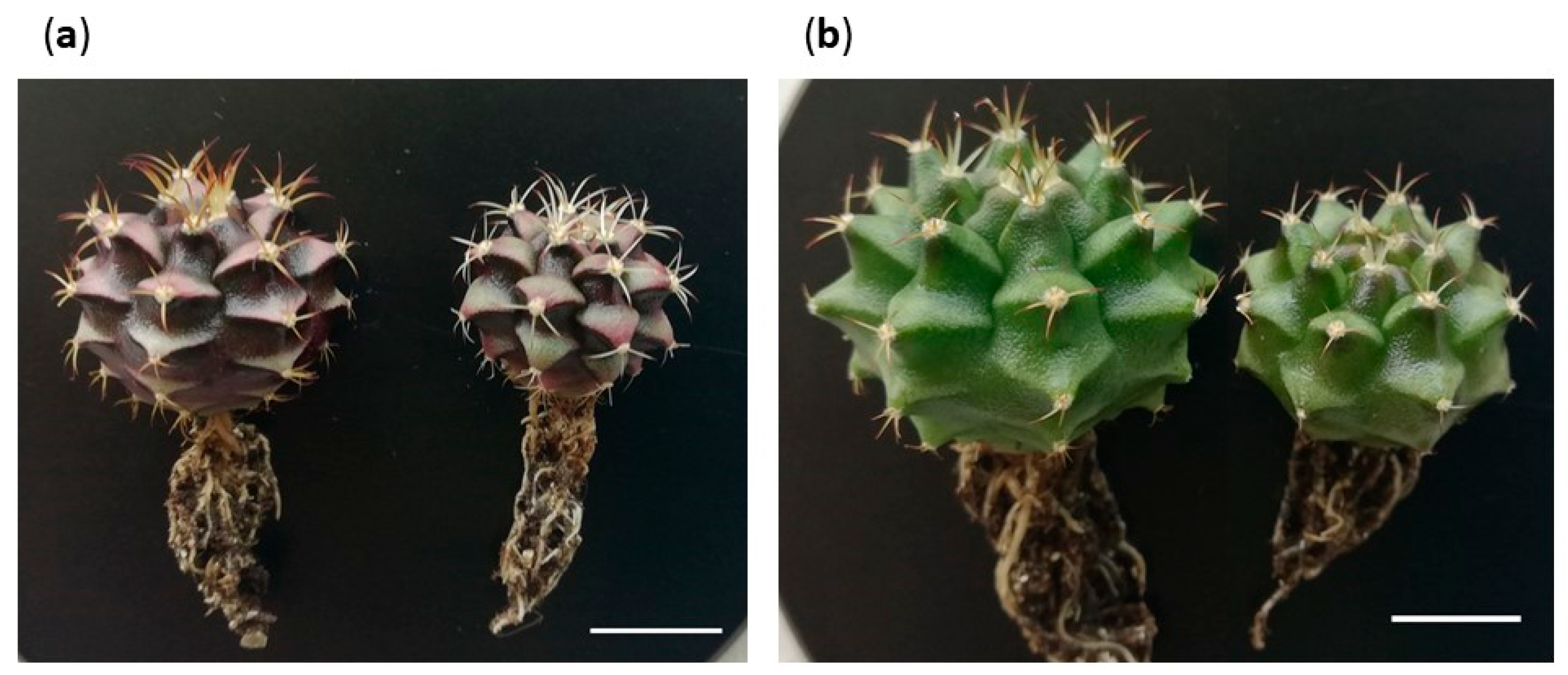
2.5. Descriptions of Morphotypes
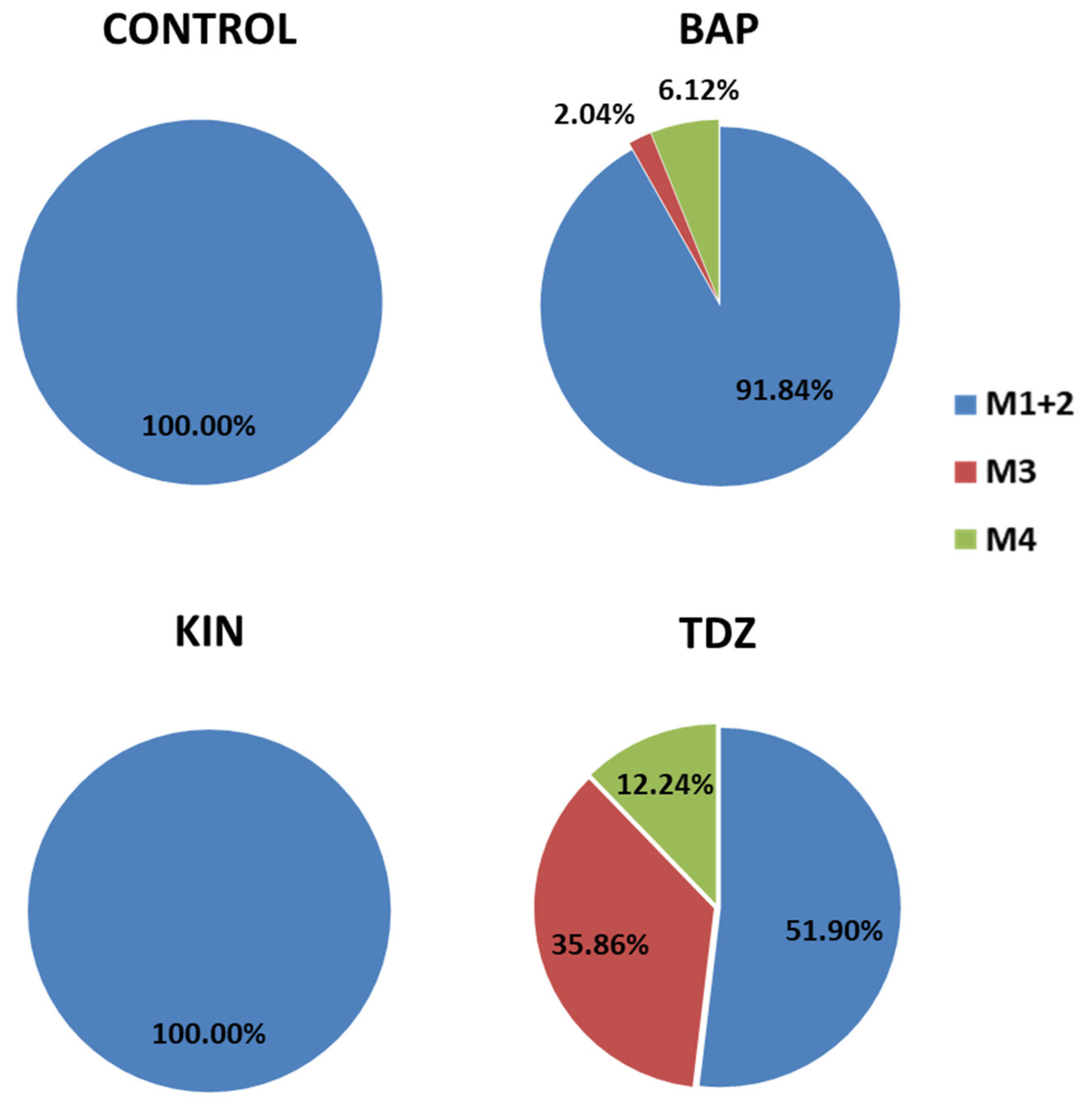
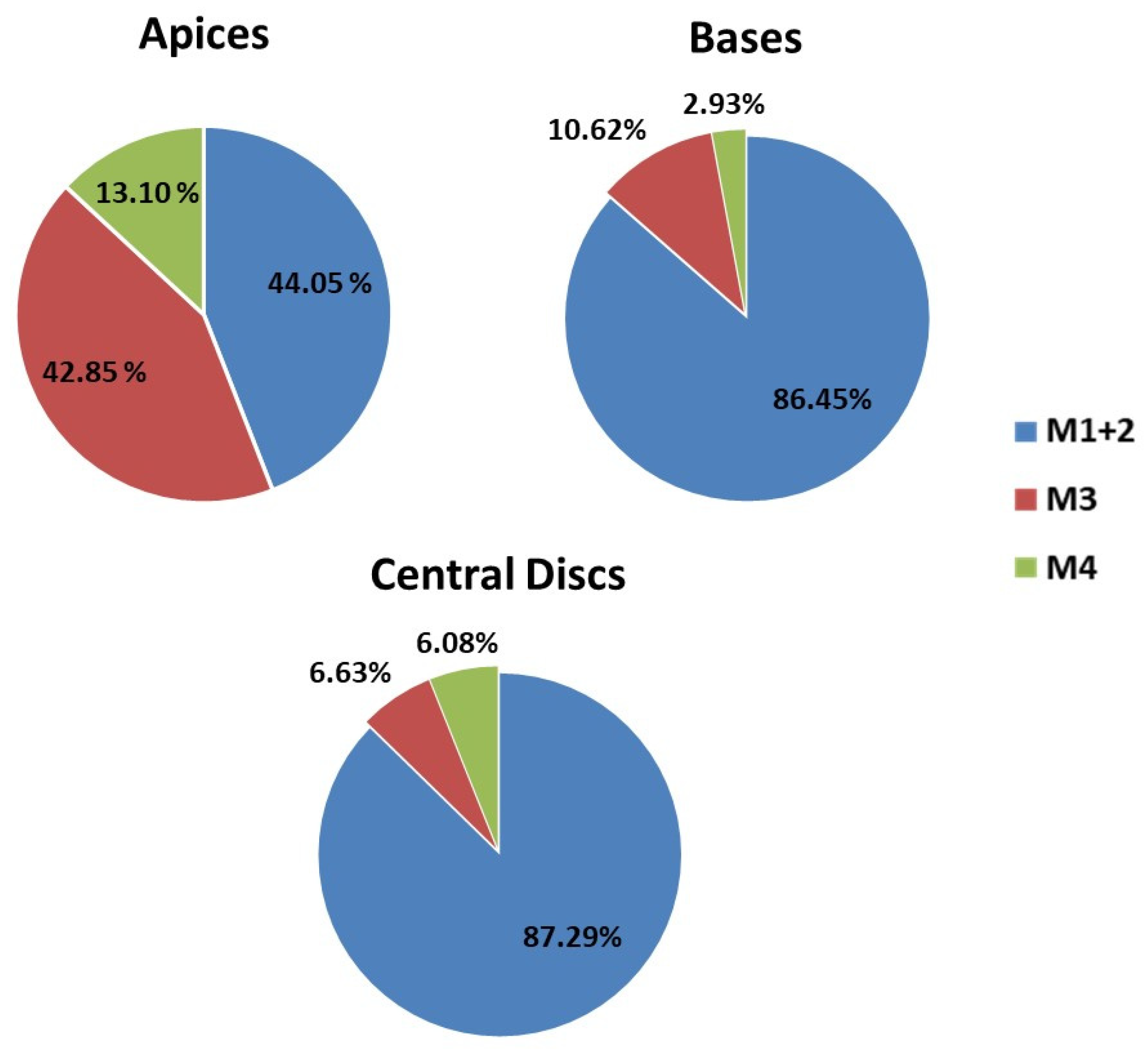
2.5.1. Occurrence of Expected Morphotypes
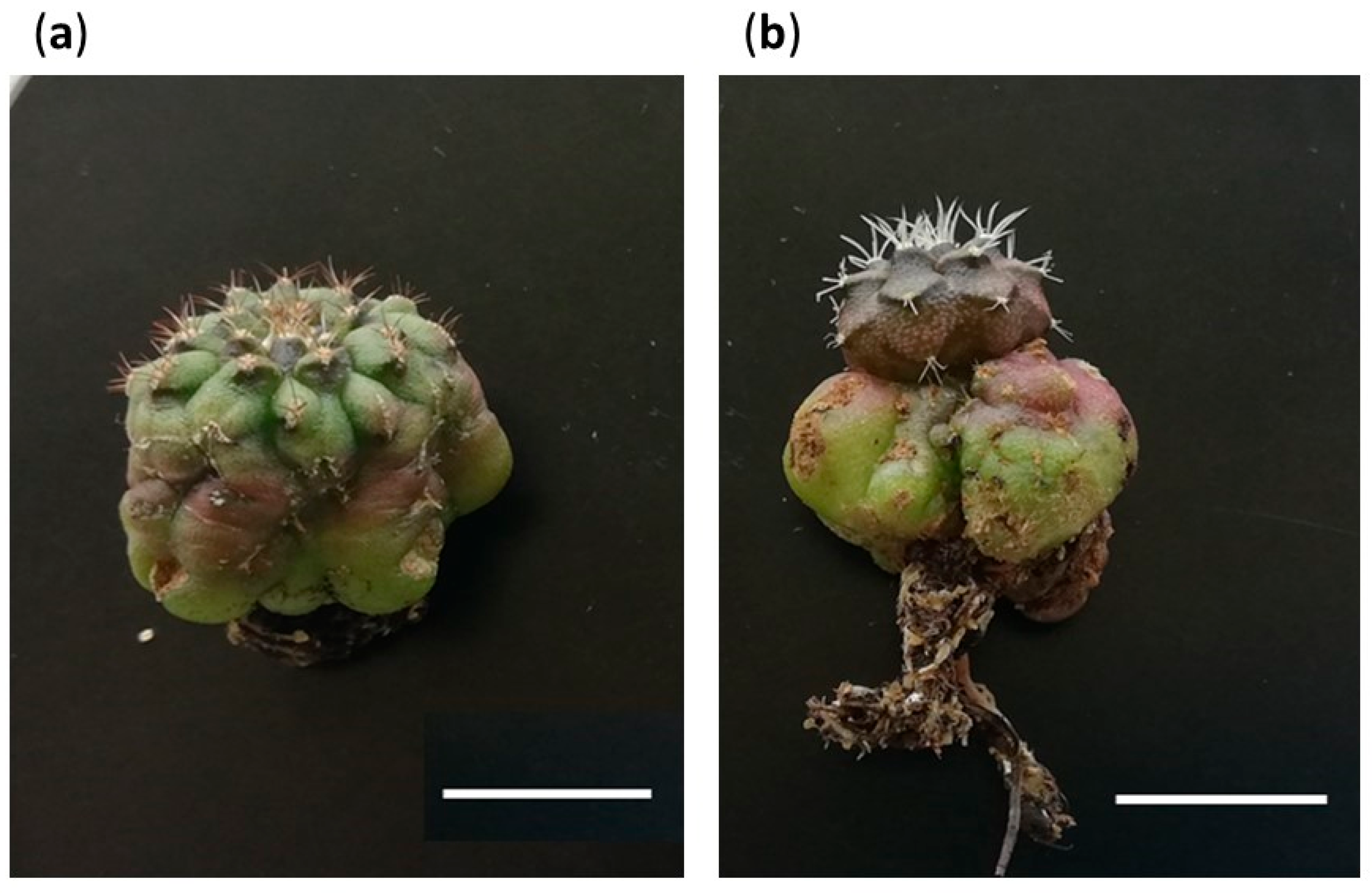
2.5.2. Occurrence of Unexpected Morphotypes
2.6. Root Emergence
2.7. Acclimatization
3. Materials and Methods
3.1. Plant Material and Disinfection
3.2. In Vitro Establishment and Culture Conditions
3.3. Induction and Tissue Culture Conditions
3.4. Experimental Design
3.5. Statistical Analysis
3.6. Acclimatization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Britton, N.L.; Rose, J.N. The Cactaceae: Descriptions and Illustrations of Plants of the Cactus Family; Courier Corporation: Chelmsford, MA, USA, 1963. [Google Scholar]
- Demaio, P.H.; Barfuss, M.H.J.; Kiesling, R.; Till, W.; Chiapella, J.O. Molecular phylogeny of Gymnocalycium (Cactaceae): Assessment of alternative infrageneric systems, a new subgenus, and trends in the evolution of the genus. Am. J. Bot. 2011, 98, 1841–1854. [Google Scholar] [CrossRef]
- Martino, P.A.; Bauk, K.; Ferrero, M.C.; Gurvich, D.E.; Las Peñas, M.L. Ecological significance of determinate primary root growth: Inter- and intra-specific differences in two species of Gymnocalycium (Cactaceae) along elevation gradients. Flora 2018, 248, 70–75. [Google Scholar] [CrossRef]
- Prisa, D. Gigaspora Margarita use to improve flower life in Notocactus and Gymnocalycium plants and roots protection against Fusarium sp. World J. Biol. Pharm. Health Sci. 2020, 2020, 2582–5542. [Google Scholar] [CrossRef]
- Repka, R.; Koutecký, P.; Mendel, P.; Frélich, R. Gymnocalycium × applanatum (Cactaceae, Cactoideae)—The first reported nothospecies between the subgenera Gymnocalycium and Trichomosemineum. Folia Geobot. 2021, 56, 255–269. [Google Scholar] [CrossRef]
- Perotti, S.B.; Aliscioni, N.L.; Delbón, N.E.; Perea, M.; Hammann, A.; Gurvich, D.E.; Biomass Partitioning, M.; Bao, Y.; Perotti, S.B.; Aliscioni, N.L.; et al. Biomass Partitioning and Morphoanatomical Traits of Six Gymnocalycium (Cactaceae) Species Occurring along a Precipitation Gradient. Diversity 2022, 14, 749. [Google Scholar] [CrossRef]
- Putnam, E.W. Gymnocalycium; The National Cactus and Succulent Society: Manchester, UK, 1978. [Google Scholar]
- Ortiz, D.G. Four taxa of the genus Gymnocalycium Pfeiffer ex Mittler, marketed in the Iberian Peninsula. Bouteloua 2009, 6, 3–6. [Google Scholar]
- Pérez-Molphe-Balch, E.; Santos-Díaz, M.D.S.; Ramírez-Malagón, R.; Ochoa-Alejo, N. Tissue culture of ornamental cacti. Sci. Agric. 2015, 72, 540–561. [Google Scholar] [CrossRef]
- Boonyawiwat, N.; Siritrakulsak, T.; Senakun, C.; Boontiang, K. Effect of Colchicine on Morphological and Anatomical Traits of Gymnocalycium mihanovichii (Frič & Gürke) Britton & Rose. Trends Sci. 2023, 20, 6597. [Google Scholar] [CrossRef]
- Torres-Silva, G.; Correia, L.N.F.; Koehler, A.D.; Batista, D.S.; Faria, D.V.; Resende, S.V.; Strickler, S.R.; Fouracre, J.; Romanel, E.; Specht, C.D.; et al. Expression of Melocactus glaucescens SERK1 sheds new light on the mechanism of areolar activation in cacti. Plant Cell Tissue Organ Cult. 2021, 147, 437–451. [Google Scholar] [CrossRef]
- CITES Appendices Disponible en. Available online: https://cites.org/eng/app/appendices.php (accessed on 13 February 2023).
- Giusti, P.; Vitti, D.; Fiocchetti, F.; Colla, G.; Saccardo, F.; Tucci, M. In vitro propagation of three endangered cactus species. Sci. Hortic. 2002, 95, 319–332. [Google Scholar] [CrossRef]
- Thorpe, T.A. History of plant tissue culture. Mol. Biotechnol. 2007, 37, 169–180. [Google Scholar] [CrossRef]
- Bhatia, S. Plant Tissue Culture. In Modern Applications of Plant Biotechnology in Pharmaceutical Sciences; Bhatia, S., Sharma, K., Dahiya, R., Bera, T., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 31–107. [Google Scholar] [CrossRef]
- Bouzroud, S.; El Maaiden, E.; Sobeh, M.; Devkota, K.P.; Boukcim, H.; Kouisni, L.; El Kharrassi, Y. Micropropagation of Opuntia and other cacti species through axillary shoot proliferation: A Comprehensive Review. Front. Plant Sci. 2022, 13, 926653. [Google Scholar] [CrossRef]
- Ballester-Olmos, J.F. Cactus y Plantas Suculentas; Guillén, R., Ed.; Floraprint; Verdecora: Madrid, Spain, 1997. [Google Scholar]
- Mulas, M.; D’Hallewin, G.; Pellizaro, G.; Spano, D. Rooting of Opuntia ficus-indica Mill. young cladodes. Adv. Hortic. Sci. 1992, 6, 44–46. [Google Scholar] [CrossRef]
- Estrada-Luna, A.A.; de Jesús Martínez-Hernández, J.; Torres-Torres, M.E.; Chablé-Moreno, F. In vitro micropropagation of the ornamental prickly pear cactus Opuntia lanigera Salm–Dyck and effects of sprayed GA3 after transplantation to ex vitro conditions. Sci. Hortic. 2008, 117, 378–385. [Google Scholar] [CrossRef]
- Ghaffari, A.; Hasanloo, T.; Nekouei, M. Micropropagation of tuna (Opuntia ficus—indica) and effect of medium composition on proliferation and rooting. Int. J. Biosci. 2013, 3, 129–139. [Google Scholar] [CrossRef]
- Radi, H.; Bouchiha, F.; El Maataoui, S.; Oubassou, E.Z.; Rham, I.; Alfeddy, M.N.; Aissam, S.; Mazri, M.A. Morphological and physio-biochemical responses of cactus pear (Opuntia ficus indica (L.) Mill.) organogenic cultures to salt and drought stresses induced in vitro. Plant Cell Tissue Organ Cult. 2023, 154, 337–350. [Google Scholar] [CrossRef]
- Marhri, A.; Tikent, A.; Garros, L.; Merah, O.; Elamrani, A.; Hano, C.; Abid, M.; Addi, M. Rapid and Efficient In vitro propagation protocol of endangered wild prickly pear growing in Eastern Morocco. Horticulturae 2023, 9, 491. [Google Scholar] [CrossRef]
- Fan, Q.J.; Zheng, S.C.; Yan, F.X.; Zhang, B.X.; Qiao, G.; Wen, X.P. Efficient regeneration of dragon fruit (Hylocereus undatus) and an assessment of the genetic fidelity of in vitro-derived plants using ISSR markers. J. Hortic. Sci. Biotechnol. 2015, 88, 631–637. [Google Scholar] [CrossRef]
- Martínez-Arroyo, M.C.; Mancilla-Álvarez, E.; Spinoso-Castillo, J.L.; Bello-Bello, J.J. Evaluation of the effect of different culture systems on photomixotrophic capacity during in vitro multiplication of pitahaya (Hylocereus undatus). S. Afr. J. Bot. 2023, 159, 396–404. [Google Scholar] [CrossRef]
- Cisneros, A.; Garcia, R.B.; Tel-Zur, N. Creation of novel interspecific-interploid Hylocereus hybrids (Cactaceae) via embryo rescue. Euphytica 2013, 189, 433–443. [Google Scholar] [CrossRef]
- Qin, J.; Wang, Y.; He, G.; Chen, L.; Cheng, X.; Xu, K.; Zhang, D. High-efficiency micropropagation of dormant buds in spine base of red pitaya (Hylocereus polyrhizus) for industrial breeding. Int. J. Agric. Biol. 2017, 19, 193–198. [Google Scholar] [CrossRef]
- Bello-Bello, J.J.; Schettino-Salomón, S.; Ortega-Espinoza, J.; Spinoso-Castillo, J.L. A temporary immersion system for mass micropropagation of pitahaya (Hylocereus undatus). 3 Biotech 2021, 11, 437. [Google Scholar] [CrossRef]
- Mállap-Detquizán, G.; Vilca-Valqui, N.C.; Meléndez-Mori, J.B.; Huaman-Huaman, E.; Oliva, M. In vitro multiplication of yellow dragon fruit (Hylocereus megalanthus) from seedlings obtained in vitro. Agron. Mesoam. 2022, 33, 45472. [Google Scholar] [CrossRef]
- Mohamed-Yasseen, Y. Micropropagation of pitaya (Hylocereus undatus Britton et Rose). Vitr. Cell. Dev. Biol.—Plant 2002, 38, 427–429. [Google Scholar] [CrossRef]
- Silos-Espino, H.; Valdez-Ortiz, A.; Rascón-Cruz, Q.; Rodríguez-Salazar, E.; Paredes-López, O. Genetic transformation of prickly-pear cactus (Opuntia ficus-indica) by Agrobacterium tumefaciens. Plant Cell Tissue Organ Cult. 2006, 86, 397–403. [Google Scholar] [CrossRef]
- De Andrade, R.A.; Martins, A.B.G.; Silva, M.T.H. Influence of the material source and the cicatrize time in vegetative propagation of red dragon fruit (Hylocereus undatus Haw). Rev. Bras. Frutic. 2007, 29, 183–186. [Google Scholar] [CrossRef]
- Benega-Garcia, R.; Cisneros, A.; Schneider, B.; Tel-Zur, N. Gynogenesis in the vine cacti Hylocereus and Selenicereus (Cactaceae). Plant Cell Rep. 2009, 28, 719–726. [Google Scholar] [CrossRef]
- Benega-Garcia, R.; Schneider, B.; Tel-Zur, N. Androgenesis in the vine cacti Selenicereus and Hylocereus (Cactaceae). Plant Cell Tissue Organ Cult. 2009, 96, 191–199. [Google Scholar] [CrossRef]
- Viñas, M.; Fernández-Brenes, M.; Azofeifa, A.; Jiménez, V.M. In vitro propagation of purple pitahaya (Hylocereus costaricensis [F.A.C. Weber] Britton & Rose) cv. Cebra. Vitr. Cell. Dev. Biol.-Plant 2012, 48, 469–477. [Google Scholar] [CrossRef]
- Barrell, P.J.; Meiyalaghan, S.; Jacobs, J.M.E.; Conner, A.J. Applications of biotechnology and genomics in potato improvement. Plant Biotechnol. J. 2013, 11, 907–920. [Google Scholar] [CrossRef]
- Rocha, D.I.; Monte-Bello, C.C.; Dornelas, M.C. Alternative induction of de novo shoot organogenesis or somatic embryogenesis from in vitro cultures of mature zygotic embryos of passion fruit (Passiflora edulis Sims) is modulated by the ratio between auxin and cytokinin in the medium. Plant Cell Tissue Organ Cult. 2015, 120, 1087–1098. [Google Scholar] [CrossRef]
- Kulus Dariusz Selected aspects of ornamental plants micropropagation in Poland and worldwide. Life Sci. 2015, 4, 10–25. [CrossRef]
- Krishna, H.; Alizadeh, M.; Singh, D.; Singh, U.; Chauhan, N.; Eftekhari, M.; Sadh, R.K. Somaclonal variations and their applications in horticultural crops improvement. 3 Biotech 2016, 6, 1–18. [Google Scholar] [CrossRef]
- Balch, E.P.M.; Reyes, M.E.P.; Amador, E.V.; Rangel, E.M.; Ruiz, L.D.R.M.; Viramontes, H.J.L. Micropropagation of 21 species of Mexican cacti by axillary proliferation. Vitr. Cell. Dev. Biol.-Plant 1998, 34, 131–135. [Google Scholar] [CrossRef]
- Kevers, C.; Franck, T.; Strasser, R.J.; Dommes, J.; Gaspar, T. Hyperhydricity of micropropagated shoots: A typically stress-induced change of physiological state. Plant Cell Tissue Organ Cult. 2004, 77, 181–191. [Google Scholar] [CrossRef]
- Olmos, E.; Piqueras, A.; Ramón Martínez-Solano, J.; Hellín, E. The subcellular localization of peroxidase and the implication of oxidative stress in hyperhydrated leaves of regenerated carnation plants. Plant Sci. 1997, 130, 97–105. [Google Scholar] [CrossRef]
- Lin, R.-S. Studies on tissue culture of cactus (Gymnocalycium minansvichii Var.). Agric. Lab. 1982, 31, 220–224. [Google Scholar]
- Park, H.-C.; Yoon, Y.-J.; Hahn, E.-J.; Paek, K.-Y. Effect of medium and culture temperature on in vitro tubercle formation of grafted-cacti «Bimoran» (Gymnocalycium mihanovichii Br. & R.). In Proceedings of the Korean Society of Plant Biotechnology Conference, Daejeon, Republic of Korea, 6–11 August 2003; pp. 361–763. [Google Scholar]
- Vidican, I.T. Investigation on the of 2,4-dichlorophenoxyacetic acid (2,4-D) on the process of callus from in vitro cultures Echinocactus (Pfiff.) mihanovichii. Analele Univ. Oradea Fasc. Protecția Mediu. 2012, 19, 305–311. [Google Scholar]
- Vidican, I.T. Study on regeneration capacity plant Echinocactus (Pfiff.) mihanovichii grown supplemented culture media with a mixture of equal parts of 3-indolilbutiric (AIB), and benzyladenine (BA), added in various concentrations. Analele Univ. Oradea Fasc. Protecția Mediu. 2014, 23, 277–284. [Google Scholar]
- Vidican, I.T. Research on the regenerative capacity and organogenous vitro cultures of Echinocactus (Pfiff.) mihanovichii the presence in the culture medium cytokinins benzyladenine (BA). Analele Univ. Oradea Fasc. Protecția Mediu. 2016, 26, 97–104. [Google Scholar]
- Vidican, I.T.; Lazăr, A.N.; Stanciu, A.Ș. Study on the in vitro culture increase and development by Echinocactus mihanovichii at the composition of the additional cultural environment with different contains of 3-indolyl butyric acid (IBA). Analele Univ. Oradea Fasc. Protecția Mediu. 2018, 31, 83–90. [Google Scholar]
- Rittirong, C.; Kiriphet, S.; Rodduang, P.; Rammasuit, S. Propagation of Gymnocalycium damsii by tissue culture techniques. J. Phys. Gen. Sci. 2019, 3, 9–17. [Google Scholar]
- Phakarat, R.; Chattarika, R.; Sujira, K.; Supawadee, R. Effect of plant growth regulators on the production of anthocyanins from the cultivation of Gymnocalycium damsii shoots from the cultivation of the upper parts of the maternal gymnocactus species. Wichcha J. 2020, 39, 116–128. [Google Scholar]
- Vidican, I.T.; Lazar, A.; Iancu, C.; Carbunar, M. Comparative study on regenerative and organogenic capacity of Echinocactus mihanovichii and Echinopsis (Zucc.) chamaecereus f. lutea cultivation in vitro in the presence in the cultural medium of 3-indutyl butyric acid (AIB) added in different concentrati. Analele Univ. Oradea Fasc. Protecția Mediu. 2020, 34, 143–152. [Google Scholar]
- Rodríguez-Garay, B.; Rubluo, A. In vitro morphogenetic responses of the endangered cactus Aztekium ritteri (Boedeker). Cactus Succul. J. 1992, 64, 116–119. [Google Scholar]
- Kaviani, B. Some Useful Information about Micropropagation. J. Ornam. Plants 2015, 5, 29–40. [Google Scholar]
- Astello-García, M.G.; Robles-Martínez, M.; Barba-de la Rosa, A.P.; Santos-Díaz, M.d.S. Establishment of callus from Opuntia robusta Wendl., a wild and medicinal cactus, for phenolic compounds production. Afr. J. Biotechnol. 2013, 12, 3204–3207. [Google Scholar]
- Cabañas-García, E.; Areche, C.; Gómez-Aguirre, Y.A.; Borquez, J.; Muñoz, R.; Cruz-Sosa, F.; Balch, E.P.M. Biomass production and secondary metabolite identification in callus cultures of Coryphantha macromeris (Engelm.) Britton & Rose (Cactaceae), a traditional medicinal plant. S. Afr. J. Bot. 2021, 137, 1–9. [Google Scholar] [CrossRef]
- Iliev, I.; Gajdosova, A.; Libiakova, G.; Jain, S.M. Techniques of micropropagation. In En Plant Cell Culture: Essential Methods; Davey, M.R., Anthony, P., Eds.; John Wiley & Sons, Ltd., Publication: London, UK, 2010; pp. 7–19. ISBN 0470686510. [Google Scholar]
- Jain, S.M. Protocols for In Vitro Propagation of Ornamental Plants; Jain, S.M., Ochatt, S.J., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; Volume 589, ISBN 978-1-60327-390-9. [Google Scholar]
- Mauseth, J.D. Structure function relationships in highly modified shoots of Cactaceae. Ann. Bot. 2006, 98, 901. [Google Scholar] [CrossRef]
- Lázaro-Castellanos, J.O.; Mata-Rosas, M.; González, D.; Arias, S.; Reverchon, F. In vitro propagation of endangered Mammillaria genus (Cactaceae) species and genetic stability assessment using SSR markers. Vitr. Cell. Dev. Biol.-Plant 2018, 54, 518–529. [Google Scholar] [CrossRef]
- Alatar, A.A. Thidiazuron induced efficient in vitro multiplication and ex vitro conservation of Rauvolfia serpentina—A potent antihypertensive drug producing plant. Biotechnol. Biotechnol. Equip. 2015, 29, 489–497. [Google Scholar] [CrossRef]
- Ajithkumar, D.; Seeni, S. Rapid clonal multiplication through in vitro axillary shoot proliferation of Aegle marmelos (L.) Corr., a medicinal tree. Plant Cell Rep. 1998, 17, 422–426. [Google Scholar] [CrossRef]
- Li, H.; Murch, S.J.; Saxena, P.K. Thidiazuron induced de novo shoot organogenesis on seedlings, etiolated hypocotyls and stem segments of Huang-qin. Plant Cell Tissue Organ Cult. 2000, 62, 169–173. [Google Scholar] [CrossRef]
- Ahmad, N.; Siddique, I.; Anis, M. Improved plant regeneration in Capsicum annuum L. from nodal segments. Biol. Plant. 2006, 50, 701–704. [Google Scholar] [CrossRef]
- Guo, B.; Abbasi, B.H.; Zeb, A.; Xu, L.L.; Wei, Y.H. Thidiazuron: A multi-dimensional plant growth regulator. Afr. J. Biotechnol. 2011, 10, 8984–9000. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, H.; Guo, F.; Luo, X. Effect of thidiazuron on somatic embryogenesis of Cayratia japonica. Plant Cell Tissue Organ Cult. 1994, 36, 73–79. [Google Scholar] [CrossRef]
- Sankhla, D.; Davis, T.D.; Sankhla, N. Thidiazuron-induced in vitro shoot formation from roots of intact seedlings of Albizzia julibrissin. Plant Growth Regul. 1994, 14, 267–272. [Google Scholar] [CrossRef]
- Vyskot, B.; JáRa, Z. Clonal propagation of cacti through axillary buds in vitro. J. Hortic. Sci. 1984, 59, 449–452. [Google Scholar] [CrossRef]
- Martínez-Vázquez, O.; Rubluo, A. In vitro mass propagation of the near-extinct Mammillaria san-angelensis Sánchez-Mejorada. J. Hortic. Sci. 1989, 64, 99–105. [Google Scholar] [CrossRef]
- Clayton, P.W.; Hubstenberger, J.F.; Phillips, G.C.; Butler-Nance, S.A. Micropropagation of Members of the Cactaceae Subtribe Cactinae. J. Am. Soc. Hortic. Sci. 1990, 115, 337–343. [Google Scholar] [CrossRef]
- Monostori, T.; Tanács, L.; Mile, L. Studies on in vitro propagation methods in cactus species of the genera Melocactus, Cereus and Lobivia. Acta Hortic. 2012, 937, 255–261. [Google Scholar] [CrossRef]
- Torres-Silva, G.; Resende, S.V.; Lima-Brito, A.; Bezerra, H.B.; de Santana, J.R.F.; Schnadelbach, A.S. In vitro shoot production, morphological alterations and genetic instability of Melocactus glaucescens (Cactaceae), an endangered species endemic to eastern Brazil. S. Afr. J. Bot. 2018, 115, 100–107. [Google Scholar] [CrossRef]
- Radhakrishnan, D.; Kareem, A.; Durgaprasad, K.; Sreeraj, E.; Sugimoto, K.; Prasad, K. Shoot regeneration: A journey from acquisition of competence to completion. Curr. Opin. Plant Biol. 2018, 41, 23–31. [Google Scholar] [CrossRef]
- Pilbeam, J. Gymnocalycium a Collector’s Guide; A.A. Balkema Publishers: Rotterdam, The Netherlands, 1995. [Google Scholar]
- Demaio, P.H.; Chiapella, J.O. New species in Gymnocalycium: A call for common sense. Cactaceae Syst. Initiat. 2014, 32, 4–6. [Google Scholar]
- Weiskopf, S.R.; Rubenstein, M.A.; Crozier, L.G.; Gaichas, S.; Griffis, R.; Halofsky, J.E.; Hyde, K.J.W.; Morelli, T.L.; Morisette, J.T.; Muñoz, R.C.; et al. Climate change effects on biodiversity, ecosystems, ecosystem services, and natural resource management in the United States. Sci. Total Environ. 2020, 733, 137782. [Google Scholar] [CrossRef]
- Taylor, N. Rhipsalis Pilocarpa: Cactáceas. Curtis’s Bot. Mag. 1997, 14, 125–129. [Google Scholar] [CrossRef]
- Sanchez-Martinez, E.; Hernandez-Martinez, M.M.; Hernandez-Oria, J.G.; Campos, G.R.; Vara, P.M.; Torres-Galeana, L.E. Mammillaria herrerae Werderm., a Mexican species on the brink of extinction. Mitteilungsblatt Arbeitskreises Mammillarienfreunde 2009, 33, 26–37. [Google Scholar]
- Perrino, E.V.; Calabrese, G. Endangered segetal species in southern Italy: Distribution, conservation status, trends, actions and ethnobotanical notes. Genet. Resour. Crop. Evol. 2018, 65, 2107–2134. [Google Scholar] [CrossRef]
- Anderson, E.F.; Arias Montes, S.; Taylor, N.P.; Cattabriga, A. Threatened Cacti of Mexico; Botanical Gardens Kew: Kew, UK, 1994. [Google Scholar]
- Malda, G.; Suzán, H.; Backhaus, R. In vitro culture as a potential method for the conservation of endangered plants possessing crassulacean acid metabolism. Sci. Hortic. 1999, 81, 71–87. [Google Scholar] [CrossRef]
- Sajeva, M.; Augugliaro, C.; Smith, M.J.; Oddo, E. Regulating Internet Trade in CITES Species. Conserv. Biol. 2013, 27, 429. [Google Scholar] [CrossRef]
- Pisani, D.; Pazienza, P.; Perrino, E.V.; Caporale, D.; De Lucia, C. The economic valuation of ecosystem services of biodiversity components in protected areas: A Review for a Framework of Analysis for the Gargano National Park. Sustainability 2021, 13, 11726. [Google Scholar] [CrossRef]
- Wagensommer, R.P.; Perrino, E.V.; Albano, A.; Medagli, P.; Passalacqua, N.G. Article Lectotypification of four Lacaita’s names in the genus Centaurea (Asteraceae). Phytotaxa 2016, 269, 54–058. [Google Scholar] [CrossRef]
- Lema-Rumińska, J.; Kulus, D. Micropropagation of Cacti—A Review. Haseltonia 2014, 2014, 46–63. [Google Scholar] [CrossRef]
- De Oliveira, S.A.; da Silva-Machado, M.J.P.; Prioli, A.J.; Aparecida-Mangolin, C. In vitro propagation of Cereus peruvianus mill. (cactaceae). Vitr. Cell. Dev. Biol.-Plant 1995, 31, 47–50. [Google Scholar] [CrossRef]
- Polivanova, O.B.; Bedarev, V.A. Hyperhydricity in plant tissue culture. Plants 2022, 11, 3313. [Google Scholar] [CrossRef]
- Al-Mayahi, A.M.W. In vitro propagation and assessment of genetic stability in date palm as affected by chitosan and thidiazuron combinations. J. Genet. Eng. Biotechnol. 2022, 20, 165. [Google Scholar] [CrossRef]
- Hesami, M.; Pepe, M.; Baiton, A.; Salami, S.A.; Jones, A.M.P. New Insight into Ornamental Applications of Cannabis: Perspectives and Challenges. Plants 2022, 11, 2383. [Google Scholar] [CrossRef]
- Datta, S.K. Conclusion of Mutation Work on Ornamentals in a Nutshell. In Role of Mutation Breeding In Floriculture Industry; Springer: Singapore, 2023; pp. 355–371. [Google Scholar] [CrossRef]
- Corneanu, M.M.; Corneanu, G.C.; Copacescu, S.N. Plant regeneration with somaclonal variability from Mammillaria sp. callus. In Proceedings of the Abstracts of the VIIth International Congress on Plant Tissue and Cell Culture, Amsterdam, The Netherlands, 24–29 June 1990; p. 99. [Google Scholar]
- Mangolin, C.A.; Prioli, A.J.; Machado, M.F.P.S. Isozyme patterns in callus cultures and in plants regenerated from calli of Cereus peruvianus (Cactaceae). Biochem. Genet. 1994, 32, 237–247. [Google Scholar] [CrossRef]
- McClelland, M.T.; Smith, M.A.L.; Carothers, Z.B. The effects of in vitro and ex vitro root initiation on subsequent microcutting root quality in three woody plants. Plant Cell Tissue Organ Cult. 1990, 23, 115–123. [Google Scholar] [CrossRef]
- Amghar, I.; Ibriz, M.; Ibrahimi, M.; Boudra, A.; Gaboun, F.; Meziani, R.; Iraqi, D.; Mazri, M.A.; Diria, G.; Abdelwahd, R. In vitro root induction from Argan (Argania spinosa (L.) Skeels) adventitious shoots: Influence of ammonium nitrate, auxins, silver nitrate and putrescine, and evaluation of plantlet acclimatization. Plants 2021, 10, 1062. [Google Scholar] [CrossRef]
- Fenning, T.; O’Donnell, M.; Preedy, K.; Bézanger, A.; Kenyon, D.; Lopez, G. The rooting ability of in vitro shoot cultures established from a UK collection of the common ash (Fraxinus excelsior L.) and their ex vitro survival. Ann. For. Sci. 2022, 79, 1–16. [Google Scholar] [CrossRef]
- Retes-Pruneda, J.L.; Valadez-Aguilar, M.d.L.; Pérez-Reyes, M.E.; Pérez-Molphe-Balch, E. In vitro propagation of Echinocereus, Escontria, Mammillaria, Melocactus and Polaskia species (Cactaceae). Bot. Sci. 2007, 81, 9–16. [Google Scholar] [CrossRef]
- Pai, S.R.; Desai, N.S. Effect of TDZ on various plant cultures. In Thidiazuron: From Urea Derivative to Plant Growth Regulator; Springer: Berlin/Heidelberg, Germany, 2018; pp. 439–454. [Google Scholar] [CrossRef]
- Rubluo, A.; Marín-Hernández, T.; Duval, K.; Vargas, A.; Márquez-Guzmán, J. Auxin induced morphogenetic responses in long-term in vitro subcultured Mammillaria san-angelensis Sánchez-Mejorada (Cactaceae). Sci. Hortic. 2002, 95, 341–349. [Google Scholar] [CrossRef]
- Su, Y.H.; Liu, Y.B.; Zhang, X.S. Auxin cytokinin interaction regulates meristem development. Mol. Plant 2011, 4, 616–625. [Google Scholar] [CrossRef]
- Otiende, M.A.; Fricke, K.; Nyabundi, J.O.; Ngamau, K.; Hajirezaei, M.R.; Druege, U. Involvement of the auxin-cytokinin homeostasis in adventitious root formation of rose cuttings as affected by their nodal position in the stock plant. Planta 2021, 254, 1–17. [Google Scholar] [CrossRef]
- Huetteman, C.A.; Preece, J.E. Thidiazuron: A potent cytokinin for woody plant tissue culture. Plant Cell Tissue Organ Cult. 1993, 33, 105–119. [Google Scholar] [CrossRef]
- Vinocur, B.; Carmi, T.; Altman, A.; Ziv, M. Enhanced bud regeneration in aspen (Populus tremula L.) roots cultured in liquid media. Plant Cell Rep. 2000, 19, 1146–1154. [Google Scholar] [CrossRef]
- Cortés-Olmos, C.; Gurrea-Ysasi, G.; Prohens, J.; Rodríguez-Burruezo, A.; Fita, A. In vitro germination and growth protocols of the ornamental Lophophora williamsii (Lem.) Coult. as a tool for protecting endangered wild populations. Sci. Hortic. 2018, 237, 120–127. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Teixeira, S.L.; Ribeiro, J.M.; Teixeira, M.T. Influence of NaClO on nutrient medium sterilization and on pineapple (Ananas comosus cv Smooth cayenne) behavior. Plant Cell Tissue Organ Cult. 2006, 86, 375–378. [Google Scholar] [CrossRef]

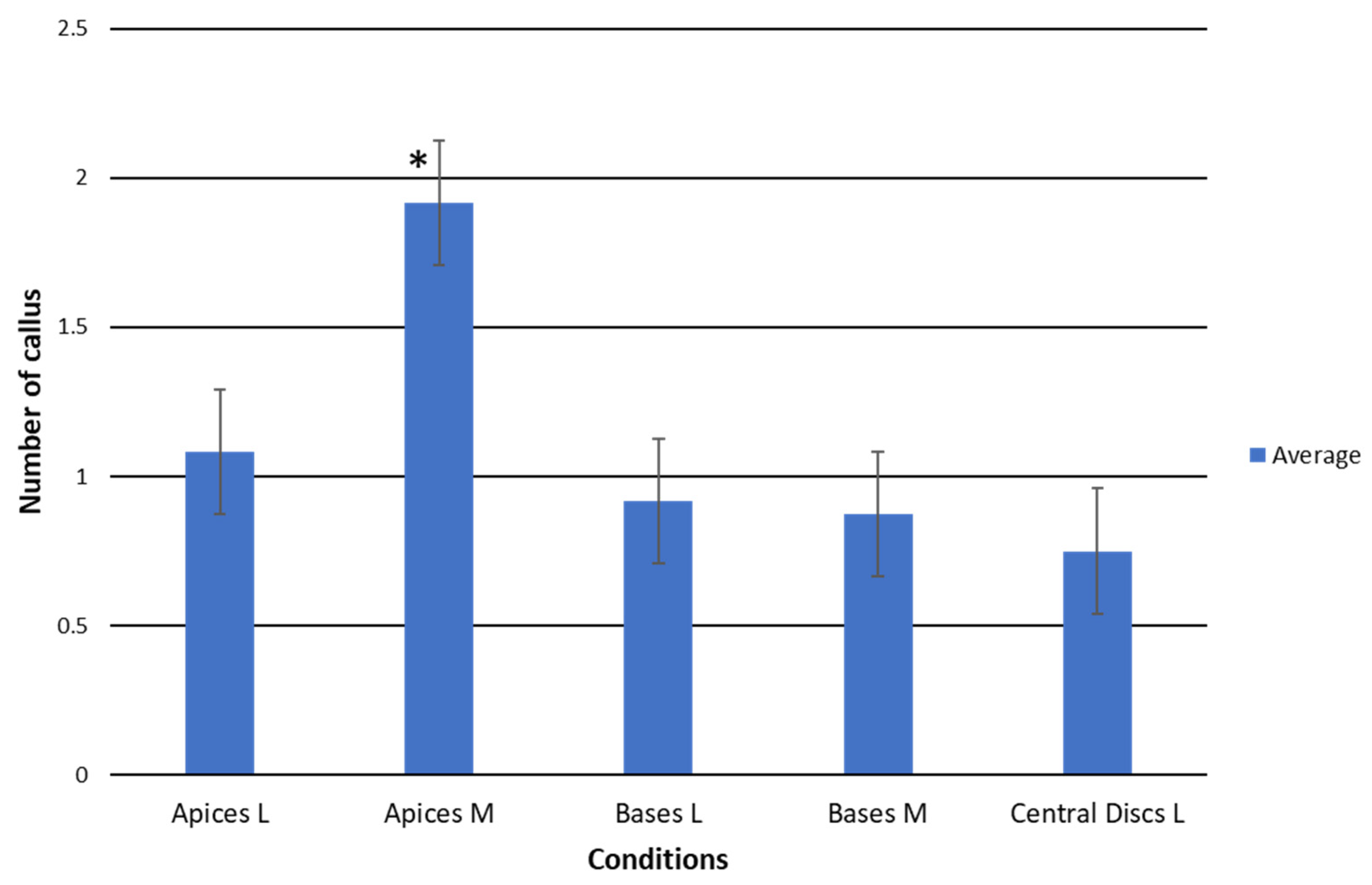
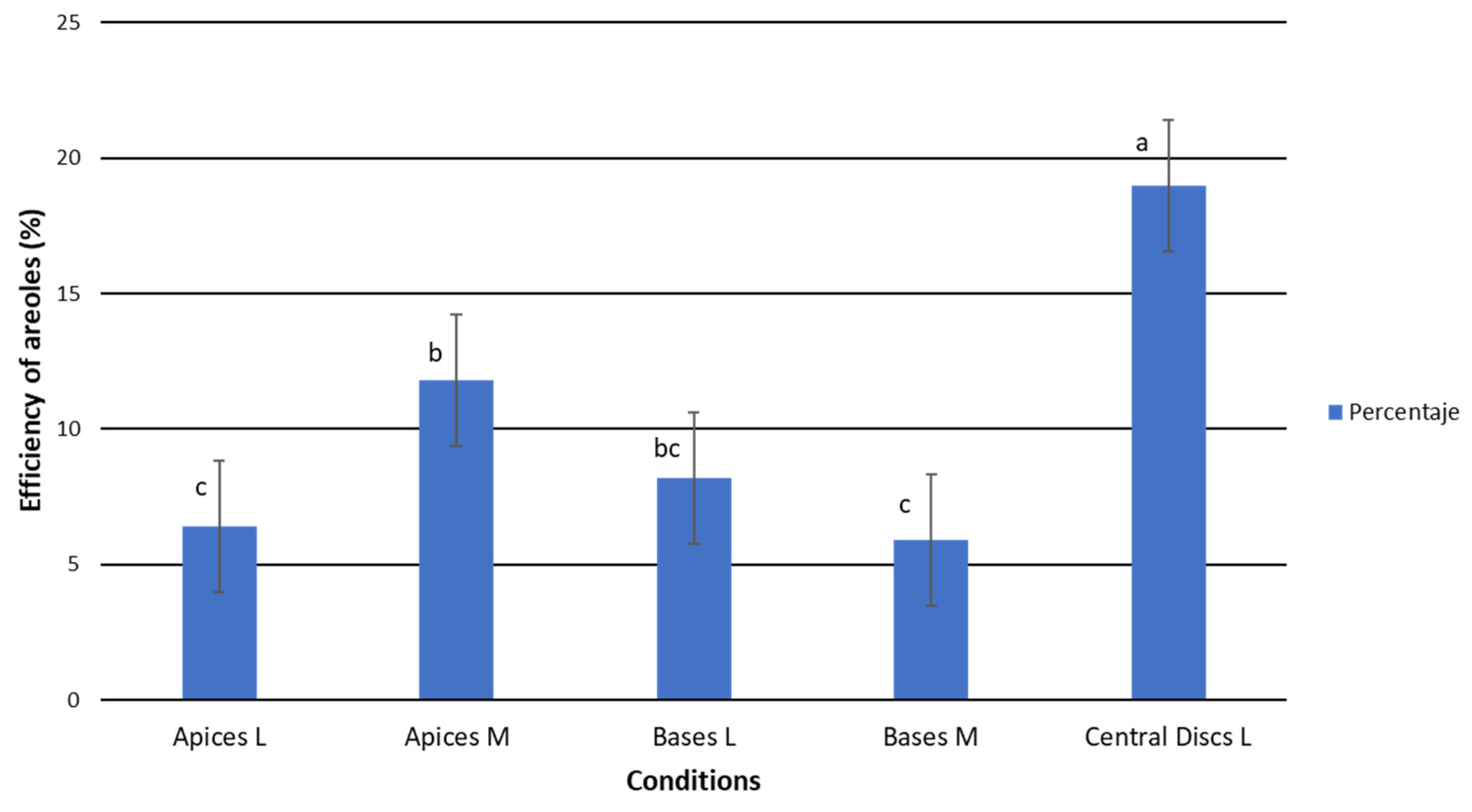




| Explant | Total Areoles Average | Initial Size (1) | Areoles Average (2) |
|---|---|---|---|
| Apices | 15.32 ± 0.14 | L | 15.58 ± 0.20 |
| Apices | M | 15.07 ± 0.20 | |
| Bases | 12.70 ± 0.23 | L | 12.24 ± 0.32 |
| Bases | M | 13.17 ± 0.31 | |
| Central Discs | 4.12 ± 0.09 | L | 4.12 ± 0.09 |
| Total Number of Explants | Activated Explants | % of Activated Explants | % of Activated Areoles (1) | ||
|---|---|---|---|---|---|
| APICES | TDZ1 (2) | 16 | 13 | 81.25 | 14.58 c |
| TDZ2 | 16 | 11 | 68.75 | 10.12 c | |
| TDZ4 | 16 | 12 | 75.00 | 11.30 c | |
| BASES | TDZ1 | 16 | 11 | 68.75 | 13.50 c |
| TDZ2 | 16 | 8 | 50.00 | 9.66 c | |
| TDZ4 | 16 | 9 | 56.25 | 12.54 c | |
| CENTRAL DISCS | TDZ1 | 16 | 7 | 43.75 | 47.55 b |
| TDZ2 | 16 | 10 | 62.50 | 32.98 a | |
| TDZ4 | 16 | 7 | 43.75 | 35.48 a |
| Principal Factors | Gl (1) | Induction Period | Development Period | ||||
|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | 6th | ||
| Hormones (H) | 2 | 0.311 | 0.001 * | 0.007 * | 0.816 | 0.102 | 0.000 * |
| Type of Explant (TE) | 2 | 0.228 | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * |
| Hormone Concentration (HC) | 3 | 0.319 | 0.891 | 0.461 | 0.008 * | 0.006 * | 0.000 * |
| Seedling Size (SS) | 1 | 0.436 | 0.238 | 0.497 | 0.907 | 0.317 | 0.855 |
| Total Cases | 576 | ||||||
| Condition (2) | Induction Period in Presence of PGRs (1) | Development Period in Absence of PGRs | ||||
|---|---|---|---|---|---|---|
| 1st Month | 2nd Month | 3rd Month | 4th Month | 5th Month | 6th Month | |
| Control_A | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Control_B | 0.00 ± 0.00 | 0.09 ± 0.05 | 0.34 ± 0.12 | 0.64 ± 0.31 | 1.06 ± 0.25 | 1.22 ± 0.27 |
| Control_CD | 0.00 ± 0.00 | 0.75 ± 0.20 | 1.25 ± 0.22 | 1.49 ± 0.32 | 2.03 ± 0.29 | 2.19 ± 0.30 |
| BAP2_A | 0.00 ± 0.00 | 0.13 ± 0.13 | 0.13 ± 0.13 | 0.13 ± 0.13 | 0.19 ± 0.14 | 0.25 ± 0.19 |
| BAP4_A | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.32± 0.16 | 0.44 ± 0.22 | 0.44 ± 0.22 |
| BAP8_A | 0.00 ± 0.00 | 0.25 ± 0.14 | 0.38 ± 0.18 | 0.38 ± 0.18 | 0.44 ± 0.20 | 0.44 ± 0.20 |
| BAP2_B | 0.00 ± 0.00 | 0.44 ± 0.27 | 0.63 ± 0.34 | 0.86 ± 0.35 | 1.38 ± 0.58 | 1.56 ± 0.56 |
| BAP4_B | 0.00 ± 0.00 | 0.50 ± 0.22 | 1.00 ± 0.26 | 1.18 ± 0.36 | 1.38 ± 0.33 | 1.38 ± 0.33 |
| BAP8_B | 0.00 ± 0.00 | 0.88 ± 0.31 | 1.38 ± 0.41 | 2.36 ± 0.28 | 2.88 ± 0.82 | 2.88 ± 0.82 |
| BAP2_CD | 0.31 ± 0.22 | 1.06 ± 0.32 | 1.19 ± 0.34 | 1.44 ± 0.29 | 1.94 ± 0.35 | 2.13 ± 0.40 |
| BAP4_CD | 0.00 ± 0.00 | 0.69 ± 0.18 | 1.25 ± 0.23 | 1.49 ± 0.30 | 2.25 ± 0.27 | 2.25 ± 0.27 |
| BAP8_CD | 0.00 ± 0.00 | 0.38 ± 0.15 | 0.69 ± 0.27 | 1.12 ± 0.34 | 1.69 ± 0.44 | 1.69 ± 0.44 |
| KIN2_A | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| KIN4_A | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| KIN8_A | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.06 ± 0.06 | 0.06 ± 0.06 |
| KIN2_B | 0.00 ± 0.00 | 0.25 ± 0.14 | 0.69 ± 0.20 | 1.00 ± 0.35 | 1.44 ± 0.34 | 1.69 ± 0.43 |
| KIN4_B | 0.00 ± 0.00 | 0.25 ± 0.14 | 0.50 ± 0.18 | 0.74 ± 0.22 | 1.25 ± 0.19 | 1.50 ± 0.26 |
| KIN8_B | 0.00 ± 0.00 | 0.19 ± 0.10 | 0.63 ± 0.24 | 0.86 ± 0.28 | 1.25 ± 0.25 | 1.44 ± 0.27 |
| KIN2_CD | 0.00 ± 0.00 | 0.69 ± 0.25 | 1.13 ± 0.27 | 1.44 ± 0.29 | 1.88 ± 0.29 | 1.88 ± 0.29 |
| KIN4_CD | 0.00 ± 0.00 | 0.63 ± 0.18 | 1.50 ± 0.27 | 1.87 ± 0.25 | 2.38 ± 0.18 | 2.56 ± 0.26 |
| KIN8_CD | 0.00 ± 0.00 | 0.40 ± 0.16 | 0.73 ± 0.21 | 1.06 ± 0.24 | 1.73 ± 0.27 | 1.80 ± 0.30 |
| TDZ1_A | 0.00 ± 0.00 | 0.13 ± 0.13 | 0.13 ± 0.13 | 0.74 ± 0.23 | 1.94 ± 0.35 | 2.69 ± 0.33 |
| TDZ2_A | 0.06 ± 0.06 | 0.38 ± 0.26 | 0.81 ± 0.39 | 1.01 ± 0.38 | 1.31 ± 0.48 | 2.81 ± 0.54 |
| TDZ4_A | 0.06 ± 0.06 | 0.38 ± 0.26 | 0.50 ± 0.26 | 0.80 ± 0.41 | 1.06 ± 0.35 | 3.00 ± 0.43 |
| TDZ1_B | 0.00 ± 0.00 | 0.19 ± 0.14 | 0.31 ± 0.20 | 0.75 ± 0.34 | 2.00 ± 0.45 | 3.00 ± 0.50 |
| TDZ2_B | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.06 ± 0.06 | 0.86 ± 0.34 | 1.81 ± 0.31 | 2.88 ± 0.36 |
| TDZ4_B | 0.00 ± 0.00 | 0.06 ± 0.06 | 0.06 ± 0.06 | 0.57 ± 0.27 | 0.81 ± 0.26 | 2.56 ± 0.62 |
| TDZ1_CD | 0.00 ± 0.00 | 0.13 ± 0.09 | 1.00 ± 0.32 | 1.44 ± 0.34 | 2.06 ± 0.44 | 2.69 ± 0.39 |
| TDZ2_CD | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.56 ± 0.22 | 1.12 ± 0.28 | 2.06 ± 0.35 | 2.69 ± 0.41 |
| TDZ4_CD | 0.00 ± 0.00 | 0.19 ± 0.19 | 0.19 ± 0.19 | 0.57 ± 0.25 | 1.25 ± 0.32 | 1.88 ± 0.29 |
| Condition (1) | N° of Explants | M1 | M2 | M3 | M4 |
|---|---|---|---|---|---|
| Control_A | 48 | 0 | 0 | 0 | 0 |
| Control_B | 48 | 45 | 0 | 0 | 0 |
| Control_CD | 48 | 84 | 0 | 0 | 0 |
| BAP2_A | 16 | 1 | 0 | 0 | 2 |
| BAP4_A | 16 | 4 | 0 | 0 | 0 |
| BAP8_A | 16 | 2 | 0 | 3 | 0 |
| BAP2_B | 16 | 13 | 9 | 0 | 0 |
| BAP4_B | 16 | 20 | 1 | 0 | 0 |
| BAP8_B | 16 | 31 | 14 | 0 | 1 |
| BAP2_CD | 16 | 23 | 1 | 1 | 7 |
| BAP4_CD | 16 | 34 | 2 | 0 | 0 |
| BAP8_CD | 16 | 17 | 8 | 0 | 2 |
| KIN2_A | 16 | 0 | 0 | 0 | 0 |
| KIN4_A | 16 | 0 | 0 | 0 | 0 |
| KIN8_A | 16 | 1 | 0 | 0 | 0 |
| KIN2_B | 16 | 20 | 3 | 0 | 0 |
| KIN4_B | 16 | 20 | 0 | 0 | 0 |
| KIN8_B | 16 | 10 | 9 | 0 | 0 |
| KIN2_CD | 16 | 30 | 0 | 0 | 0 |
| KIN4_CD | 16 | 36 | 2 | 0 | 0 |
| KIN8_CD | 16 | 22 | 4 | 0 | 0 |
| TDZ1_A | 16 | 7 | 0 | 18 | 8 |
| TDZ2_A | 16 | 11 | 0 | 9 | 1 |
| TDZ4_A | 16 | 11 | 0 | 6 | 0 |
| TDZ1_B | 16 | 20 | 0 | 8 | 4 |
| TDZ2_B | 16 | 18 | 0 | 7 | 3 |
| TDZ4_B | 16 | 3 | 0 | 14 | 0 |
| TDZ1_CD | 16 | 25 | 1 | 2 | 5 |
| TDZ2_CD | 16 | 16 | 0 | 16 | 4 |
| TDZ4_CD | 16 | 11 | 0 | 5 | 4 |
| Total | 576 | 535 | 54 | 89 | 41 |
| Total % | 74.41 | 7.51 | 12.38 | 5.7 |
| Expected Morphotypes (1 + 2) | Morphotype 3 | Morphotype 4 | |||||
|---|---|---|---|---|---|---|---|
| Cases | p-Value (1) | Mean (2) | p-Value | Mean | p-Value | Mean | |
| H (3) | 0.01 | 0.00 | 0.00 | ||||
| BAP | 192 | 1.20 ± 0.09 b | 0.02 ± 0.03 a | 0.06 ± 0.03 a | |||
| KIN | 192 | 1.04 ± 0.09 ab | 0.00 ± 0.03 a | 0.00 ± 0.03 a | |||
| TDZ | 192 | 0.82 ± 0.09 a | 0.44 ± 0.03 b | 0.15 ± 0.03 b | |||
| TE | 0.00 | 0.45 | 0.14 | ||||
| Apices | 192 | 0.19 ± 0.09 a | 0.19 ± 0.03 a | 0.06 ± 0.03 a | |||
| Bases | 192 | 1.21 ± 0.09 b | 0.15 ± 0.03 a | 0.04 ± 0.03 a | |||
| Central Discs | 192 | 1.66 ± 0.09 c | 0.12 ± 0.03 a | 0.11 ± 0.03 a | |||
| HC | 0.06 | 0.00 | 0.00 | ||||
| Control | 144 | 0.89 ± 0.11 a | 0.00 ± 0.04 a | 0.00 ± 0.03 a | |||
| Low | 144 | 1.06 ± 0.11 a | 0.20 ± 0.04 b | 0.18 ± 0.03 b | |||
| Medium | 144 | 1.14 ± 0.11 a | 0.22 ± 0.04 b | 0.06 ± 0.03 a | |||
| High | 144 | 0.99 ± 0.11 a | 0.19 ± 0.04 b | 0.05 ± 0.03 a | |||
| H × TE | 0.00 | 0.73 | 0.70 | ||||
| H × HC | 0.12 | 0.00 | 0.05 | ||||
| TE × HC | 0.12 | 0.01 | 0.58 | ||||
| H × TE × HC | 0.01 | 0.00 | 0.48 | ||||
| Total cases | 576 | ||||||
| Total mean | 1.021 ± 0.061 | 0.155 ± 0.023 | 0.071 ± 0.016 | ||||
| PGR (1) | Concentration | Type of Explant | Rooting after 4 Weeks (2) | Rooting after 8 Weeks |
|---|---|---|---|---|
| CONTROL | 0 | A | 84.38 ab | 90.63 a |
| 0 | CD | - | - | |
| 0 | B | - | - | |
| BAP | 2 | A | 93.75 a | 93.75 a |
| 2 | CD | 12.50 d | 37.50 b | |
| 2 | B | - | - | |
| 4 | A | 56.25 ab | 75.00 a | |
| 4 | CD | - | - | |
| 4 | B | - | - | |
| 8 | A | 50.00 bc | 93.75 a | |
| 8 | CD | - | - | |
| 8 | B | - | - | |
| KIN | 2 | A | 75.00 ab | 93.75 a |
| 2 | CD | - | - | |
| 2 | B | - | - | |
| 4 | A | 87.50 ab | 93.75 a | |
| 4 | CD | 18.75 cd | 25.00 b | |
| 4 | B | - | - | |
| 8 | A | 81.25 ab | 87.50 a | |
| 8 | CD | 18.75 cd | 31.25 b | |
| 8 | B | - | - | |
| TDZ | 1 | A | - | - |
| 1 | CD | - | - | |
| 1 | B | - | - | |
| 2 | A | - | - | |
| 2 | CD | - | - | |
| 2 | B | - | - | |
| 4 | A | - | - | |
| 4 | CD | - | - | |
| 4 | B | - | - |
| PGRs (1) | Concentration (µM) | Type of Explant | Initial Size (2) | N° of Explants |
|---|---|---|---|---|
| BAP | 2 | Apex | M | 8 |
| Apex | L | 8 | ||
| Base | M | 8 | ||
| Base | L | 8 | ||
| Central Disc | L | 16 | ||
| BAP | 4 | Apex | M | 8 |
| Apex | L | 8 | ||
| Base | M | 8 | ||
| Base | L | 8 | ||
| Central Disc | L | 16 | ||
| BAP | 8 | Apex | M | 8 |
| Apex | L | 8 | ||
| Base | M | 8 | ||
| Base | L | 8 | ||
| Central Disc | L | 16 | ||
| KIN | 2 | Apex | M | 8 |
| Apex | L | 8 | ||
| Base | M | 8 | ||
| Base | L | 8 | ||
| Central Disc | L | 16 | ||
| KIN | 4 | Apex | M | 8 |
| Apex | L | 8 | ||
| Base | M | 8 | ||
| Base | L | 8 | ||
| Central Disc | L | 16 | ||
| KIN | 8 | Apex | M | 8 |
| Apex | L | 8 | ||
| Base | M | 8 | ||
| Base | L | 8 | ||
| Central Disc | L | 16 | ||
| TDZ | 1 | Apex | M | 8 |
| Apex | L | 8 | ||
| Base | M | 8 | ||
| Base | L | 8 | ||
| Central Disc | L | 16 | ||
| TDZ | 2 | Apex | M | 8 |
| Apex | L | 8 | ||
| Base | M | 8 | ||
| Base | L | 8 | ||
| Central Disc | L | 16 | ||
| TDZ | 4 | Apex | M | 8 |
| Apex | L | 8 | ||
| Base | M | 8 | ||
| Base | L | 8 | ||
| Central Disc | L | 16 | ||
| CONTROL | Apex | M | 16 | |
| Apex | L | 16 | ||
| Base | M | 16 | ||
| Base | L | 16 | ||
| Central Disc | L | 32 | ||
| Total | 528 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortés-Olmos, C.; Guerra-Sandoval, V.M.; Blanca-Giménez, V.; Rodríguez-Burruezo, A. Micropropagation and Acclimatization of Gymnocalycium cv. Fancy (Cactaceae): Developmental Responses to Different Explant Types and Hormone Conditions. Plants 2023, 12, 3932. https://doi.org/10.3390/plants12233932
Cortés-Olmos C, Guerra-Sandoval VM, Blanca-Giménez V, Rodríguez-Burruezo A. Micropropagation and Acclimatization of Gymnocalycium cv. Fancy (Cactaceae): Developmental Responses to Different Explant Types and Hormone Conditions. Plants. 2023; 12(23):3932. https://doi.org/10.3390/plants12233932
Chicago/Turabian StyleCortés-Olmos, Carles, Vladimir Marín Guerra-Sandoval, Vicente Blanca-Giménez, and Adrián Rodríguez-Burruezo. 2023. "Micropropagation and Acclimatization of Gymnocalycium cv. Fancy (Cactaceae): Developmental Responses to Different Explant Types and Hormone Conditions" Plants 12, no. 23: 3932. https://doi.org/10.3390/plants12233932
APA StyleCortés-Olmos, C., Guerra-Sandoval, V. M., Blanca-Giménez, V., & Rodríguez-Burruezo, A. (2023). Micropropagation and Acclimatization of Gymnocalycium cv. Fancy (Cactaceae): Developmental Responses to Different Explant Types and Hormone Conditions. Plants, 12(23), 3932. https://doi.org/10.3390/plants12233932







