Cannabis Virome Reconstruction and Antiviral RNAi Characterization through Small RNA Sequencing
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification and Genome Reconstruction of Cannabis Virome Components
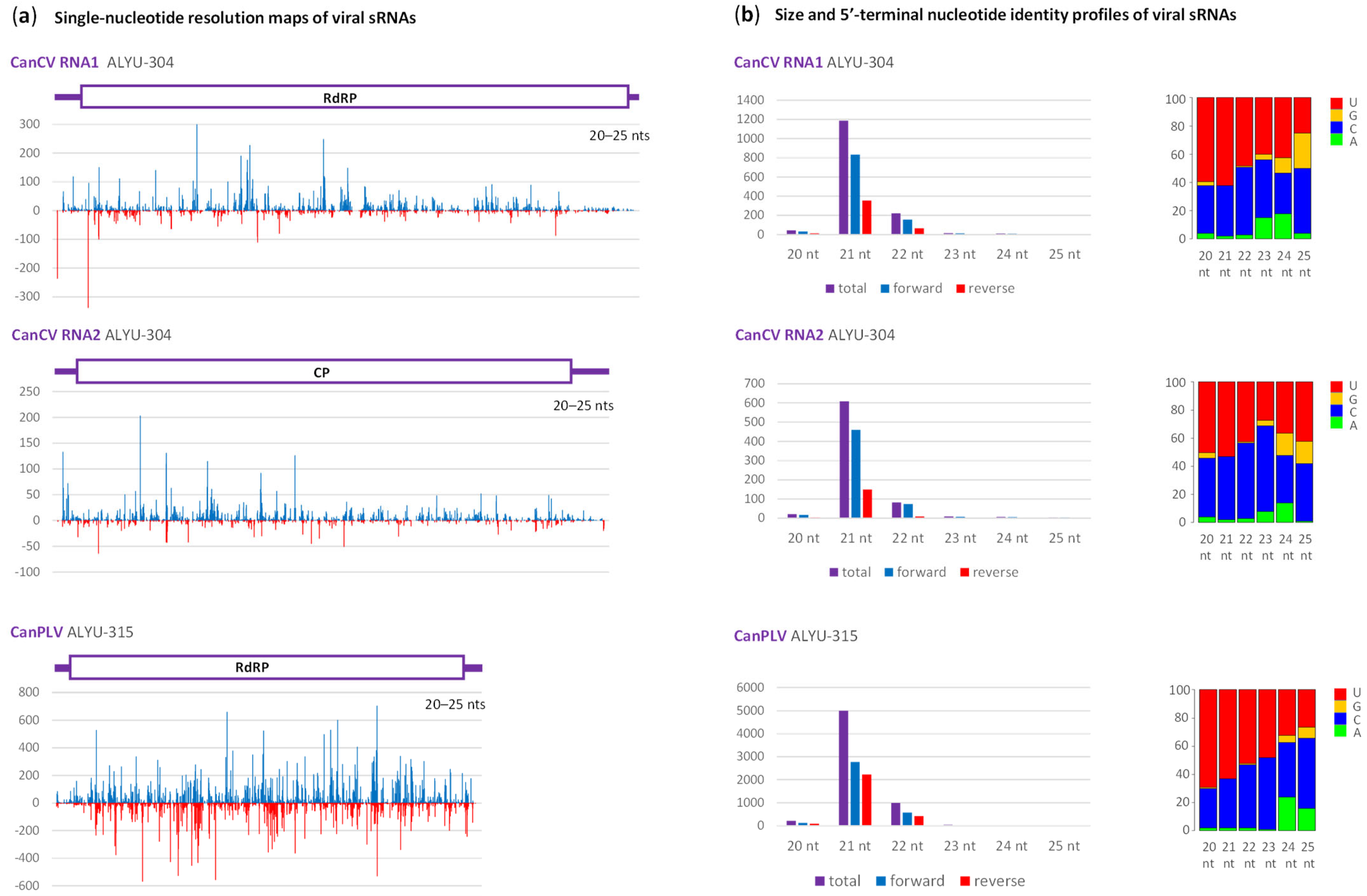
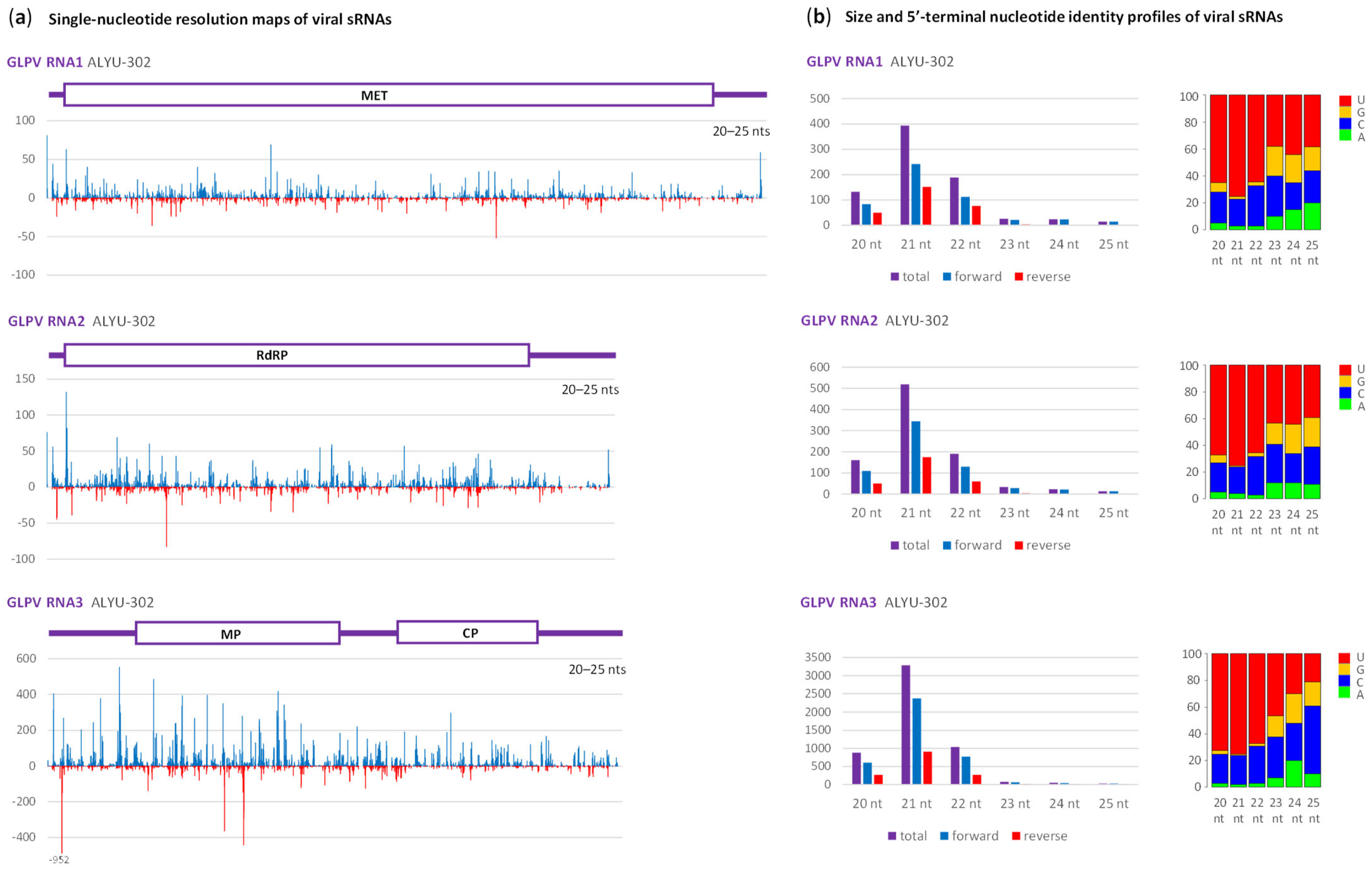
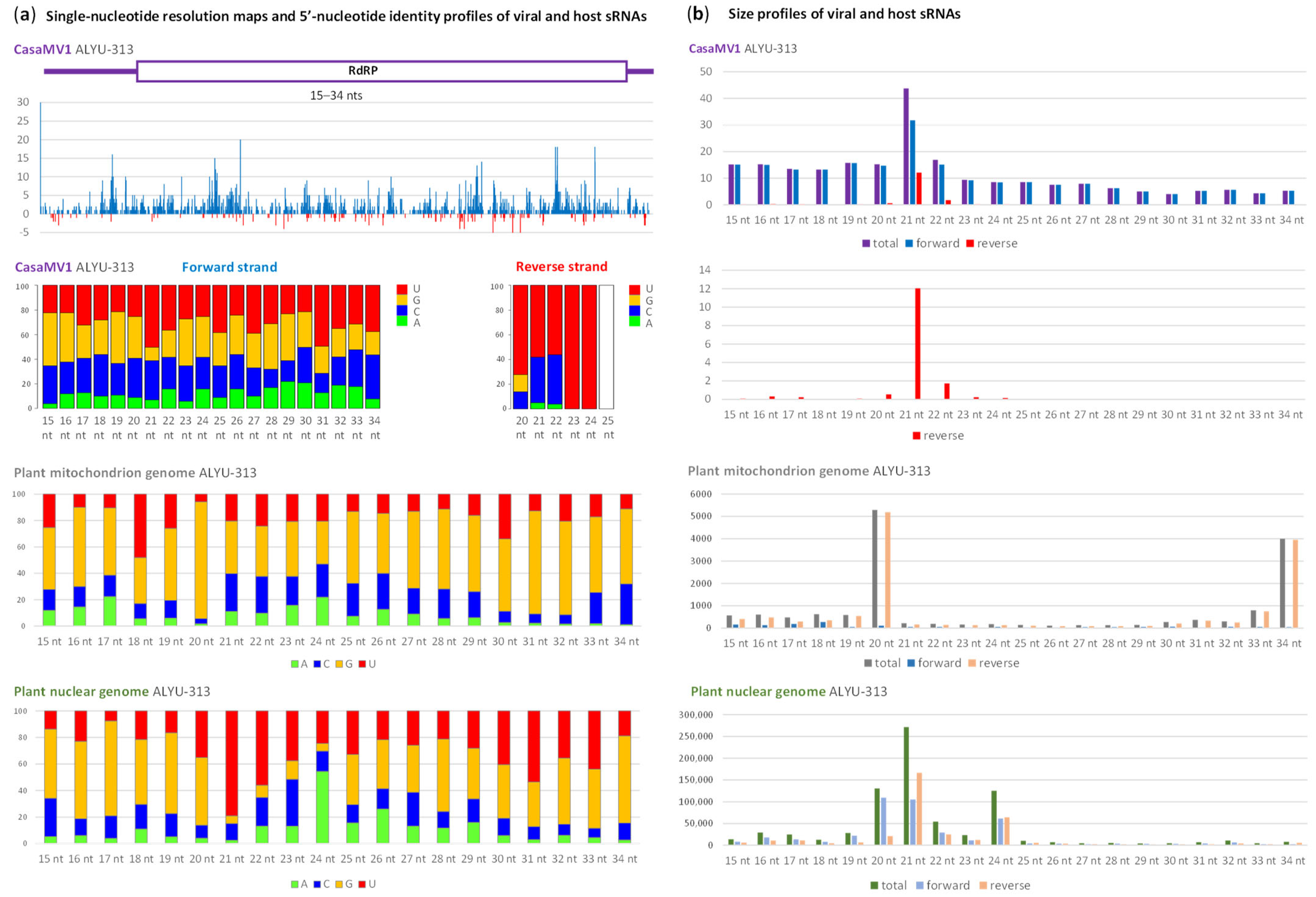
2.2. Interactions of Cannabis Virome Components with the Plant RNAi Machinery
3. Materials and Methods
3.1. Plant Samples
3.2. RNA Extraction and Validation for Illumina Sequencing
3.3. Illumina Sequencing and Bioinformatic Analysis of sRNAs
3.4. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Andre, C.M.; Hausman, J.F.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Schilling, S.; Dowling, C.A.; Shi, J.; Ryan, L.; Hunt, D.; OReilly, E.; Perry, A.S.; Kinnane, O.; McCabe, P.F.; Melzer, R. The Cream of the Crop: Biology, Breeding and Applications of Cannabis sativa. Authorea 2020. preprint. [Google Scholar] [CrossRef]
- Punja, Z.K. Emerging Diseases of Cannabis sativa and Sustainable Management. Pest. Man. Sci. 2021, 77, 3857–3870. [Google Scholar] [CrossRef] [PubMed]
- Miotti, N.; Passera, A.; Ratti, C.; Dall’Ara, M.; Casati, P. A Guide to Cannabis Virology: From the Virome Investigation to the Development of Viral Biotechnological Tools. Viruses 2023, 15, 1532. [Google Scholar] [CrossRef] [PubMed]
- Bektaş, A.; Hardwick, K.M.; Waterman, K.; Kristof, J. Occurrence of Hop Latent Viroid in Cannabis sativa with Symptoms of Cannabis Stunting Disease in California. Plant Dis. 2019, 103, 2699. [Google Scholar] [CrossRef]
- Warren, J.G.; Mercado, J.; Grace, D. Occurrence of Hop Latent Viroid Causing Disease in Cannabis sativa in California. Plant Dis. 2019, 103, 2699. [Google Scholar] [CrossRef]
- Hadad, L.; Luria, N.; Smith, E.; Sela, N.; Lachman, O.; Dombrovsky, A. Lettuce Chlorosis Virus Disease: A New Threat to Cannabis Production. Viruses 2019, 11, 802. [Google Scholar] [CrossRef]
- Giladi, Y.; Hadad, L.; Luria, N.; Cranshaw, W.; Lachman, O.; Dombrovsky, A. First Report of Beet Curly Top Virus Infecting Cannabis sativa in Western Colorado. Plant Dis. 2020, 104, 999. [Google Scholar] [CrossRef]
- Hu, J.; Masson, R.; Dickey, L. First Report of Beet Curly Top Virus Infecting Industrial Hemp (Cannabis sativa) in Arizona. Plant Dis. 2021, 105, 1233. [Google Scholar] [CrossRef]
- Chiginsky, J.; Langemeier, K.; MacWilliams, J.; Albrecht, T.; Cranshaw, W.; Fulladolsa, A.C.; Kapuscinski, M.; Stenglein, M.; Nachappa, P. First Insights Into the Virus and Viroid Communities in Hemp (Cannabis sativa). Front. Agron. 2021, 3, 778433. [Google Scholar] [CrossRef]
- Borges, F.; Martienssen, R.A. The expanding world of small RNAs in plants. Nat. Rev. Mol. Cell Biol. 2015, 16, 727–741. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Qi, Y. RNAi in Plants: An Argonaute-Centered View. Plant Cell 2016, 28, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Blevins, T.; Rajeswaran, R.; Shivaprasad, P.V.; Beknazariants, D.; Si-Ammour, A.; Park, H.-S.; Vazquez, F.; Robertson, D.; Meins, F.; Hohn, T.; et al. Four Plant Dicers Mediate Viral Small RNA Biogenesis and DNA Virus Induced Silencing. Nucleic Acids Res. 2006, 34, 6233–6246. [Google Scholar] [CrossRef] [PubMed]
- Seguin, J.; Rajeswaran, R.; Malpica-López, N.; Martin, R.R.; Kasschau, K.; Dolja, V.V.; Otten, P.; Farinelli, L.; Pooggin, M.M. De novo reconstruction of consensus master genomes of plant RNA and DNA viruses from siRNAs. PLoS ONE 2014, 9, e88513. [Google Scholar] [CrossRef]
- Pooggin, M.M. Small RNA-Omics for Plant Virus Identification, Virome Reconstruction, and Antiviral Defense Characterization. Front. Microbiol. 2018, 9, 2779. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Li, Y.; Li, J.; Xie, Z.; Luan, M.; Gao, C.; Shi, Y.; Chen, S. Genome-Wide Analysis of Alternative Splicing and Non-Coding RNAs Reveal Complicated Transcriptional Regulation in Cannabis sativa L. Int. J. Mol. Sci. 2021, 22, 11989. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, A.; Matoušek, J.; Steger, G.; Schubert, J. Complete Sequence of a Cryptic Virus from Hemp (Cannabis sativa). Arch. Virol. 2012, 157, 383–385. [Google Scholar] [CrossRef]
- Righetti, L.; Paris, R.; Ratti, C.; Calassanzio, M.; Onofri, C.; Calzolari, D.; Menzel, W.; Knierim, D.; Magagnini, G.; Pacifico, D.; et al. Not the One, but the Only One: About Cannabis Cryptic Virus in Plants Showing ‘Hemp Streak’ Disease Symptoms. Eur. J. Plant Pathol. 2018, 150, 575–588. [Google Scholar] [CrossRef]
- Vainio, E.J.; Chiba, S.; Ghabrial, S.A.; Maiss, E.; Roossinck, M.; Sabanadzovic, S.; Suzuki, N.; Xie, J.; Nibert, M. ICTV Virus Taxonomy Profile: Partitiviridae. J. Gen. Virol. 2018, 99, 17–18. [Google Scholar] [CrossRef] [PubMed]
- Elbeaino, T.; Kontra, L.; Demian, E.; Jaksa-Czotter, N.; Slimen, A.B.; Fabian, R.; Lazar, J.; Tamisier, L.; Digiaro, M.; Massart, S.; et al. Complete Sequence, Genome Organization and Molecular Detection of Grapevine Line Pattern Virus, a New Putative Anulavirus Infecting Grapevine. Viruses 2020, 12, 602. [Google Scholar] [CrossRef] [PubMed]
- Nibert, M.L.; Vong, M.; Fugate, K.K.; Debat, H.J. Evidence for contemporary plant mitoviruses. Virology 2018, 518, 14–24. [Google Scholar] [CrossRef] [PubMed]
- van Bakel, H.; Stout, J.M.; Cote, A.G.; Tallon, C.M.; Sharpe, A.G.; Hughes, T.R.; Page, J.E. The Draft Genome and Transcriptome of Cannabis sativa. Genome Biol. 2011, 12, R102. [Google Scholar] [CrossRef] [PubMed]
- Shahi, S.; Eusebio-Cope, A.; Kondo, H.; Hillman, B.I.; Suzuki, N. Investigation of Host Range of and Host Defense against a Mitochondrially Replicating Mitovirus. J. Virol. 2019, 93, e01503-18. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Lin, X.D.; Tian, J.H.; Chen, L.J.; Chen, X.; Li, C.X.; Qin, X.C.; Li, J.; Cao, J.P.; Eden, J.S.; et al. Redefining the invertebrate RNA virosphere. Nature 2016, 540, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, P.; Ferreira, F.; Da Silva, F.; Oliveira, L.S.; Marques, J.T.; Goes-Neto, A.; Aguiar, E.; Gruber, A. Characterization of a Novel Mitovirus of the Sand Fly Lutzomyia Longipalpis Using Genomic and Virus–Host Interaction Signatures. Viruses 2020, 13, 9. [Google Scholar] [CrossRef]
- Xin, M.; Cao, M.; Liu, W.; Ren, Y.; Lu, C.; Wang, X. The Genomic and Biological Characterization of Citrullus Lanatus Cryptic Virus Infecting Watermelon in China. Virus Res. 2017, 232, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Golyaev, V.; Candresse, T.; Rabenstein, F.; Pooggin, M.M. Plant Virome Reconstruction and Antiviral RNAi Characterization by Deep Sequencing of Small RNAs from Dried Leaves. Sci. Rep. 2019, 9, 19268. [Google Scholar] [CrossRef]
- Malpica-López, N.; Rajeswaran, R.; Beknazariants, D.; Seguin, J.; Golyaev, V.; Farinelli, L.; Pooggin, M.M. Revisiting the Roles of Tobamovirus Replicase Complex Proteins in Viral Replication and Silencing Suppression. Mol. Plant Microbe Interact. 2018, 31, 125–144. [Google Scholar] [CrossRef]
- Turco, S.; Golyaev, V.; Seguin, J.; Gilli, C.; Farinelli, L.; Boller, T.; Schumpp, O.; Pooggin, M.M. Small RNA-Omics for Virome Reconstruction and Antiviral Defense Characterization in Mixed Infections of Cultivated Solanum Plants. Mol. Plant Microbe Interact. 2018, 31, 707–723. [Google Scholar] [CrossRef]
- Zerbino, D.R.; Birney, E. Velvet: Algorithms for de Novo Short Read Assembly Using de Bruijn Graphs. Genome Res. 2008, 18, 821–829. [Google Scholar] [CrossRef]
- Schulz, M.H.; Zerbino, D.R.; Vingron, M.; Birney, E. Oases: Robust de Novo RNA-Seq Assembly across the Dynamic Range of Expression Levels. Bioinformatics 2012, 28, 1086–1092. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and Accurate Long-Read Alignment with Burrows–Wheeler Transform. Bioinformatics 2010, 26, 589–595. [Google Scholar] [CrossRef]
- Seguin, J.; Otten, P.; Baerlocher, L.; Farinelli, L.; Pooggin, M.M. MISIS-2: A Bioinformatics Tool for in-Depth Analysis of Small RNAs and Representation of Consensus Master Genome in Viral Quasispecies. J. Virol. Methods 2016, 233, 37–40. [Google Scholar] [CrossRef]
- Strausbaugh, C.A.; Eujayl, I.A.; Wintermantel, W.M. Beet Curly Top Virus Strains Associated with Sugar Beet in Idaho, Oregon, and a Western U.S. Collection. Plant Dis. 2017, 101, 1373–1382. [Google Scholar] [CrossRef]
- Matoušek, J.; Patzak, J. A Low Transmissibility of Hop Latent Viroid through a Generative Phase of Humulus lupulus L. Biologia Plant 2000, 43, 145–148. [Google Scholar] [CrossRef]
| Sample/ Isolate | Cultivar/ Variety | Dio- or Mono-Ecious | CanCV (RPM *) | GLPV (RPM *) | CasaMV1 (RPM *) | CanPLV (RPM *) | Symptoms | Sampled in | Stored at | Signs of RNA Degradation |
|---|---|---|---|---|---|---|---|---|---|---|
| ALYU-297 | Gerola | Dio | + (58) | No | September 2021 | −30 °C | Yes | |||
| ALYU-298 | Gerola | Dio | + (73) | Dwarfism and deformed leaves | September 2021 | −30 °C | Yes | |||
| ALYU-299 | Gerola | Dio | Dwarfism and deformed leaves | September 2021 | −30 °C | Yes | ||||
| ALYU-300 | Gerola | Dio | + (60) | Elongated internodes thin/narrow leaves | September 2021 | −30 °C | Yes | |||
| ALYU-301 | Perugina | Dio | +++ (1389) | No | September 2021 | −30 °C | Yes | |||
| ALYU-302 | Perugina | Dio | ++++ (8734) | Yellow/orange spots on leaves | September 2021 | −30 °C | Yes | |||
| ALYU-303 | Perugina | Dio | Desiccated parts, dense new sprouts | September 2021 | −30 °C | Yes | ||||
| ALYU-304 | Felina 32 | Mono | +++ (2366) | No | June 2022 | −80 °C | No | |||
| ALYU-305 | USO 31 | Mono | Leaves wrinkling and deformation | April 2021 | −30 °C | Yes | ||||
| ALYU-306 | Felina 32 | Mono | ++ (588) | + (39) | Leaves wrinkling and deformation | April 2021 | −30 °C | Yes | ||
| ALYU-307 | Futura 75 | Mono | Leaves wrinkling and deformation | April 2021 | −30 °C | Yes | ||||
| ALYU-308 | Feno A1 | Dio | + (22) | Variegation | June 2022 | −80 °C | No | |||
| ALYU-309 | Feno A2 | Dio | ++ (308) | Leaves wrinkling and deformation | June 2022 | −80 °C | No | |||
| ALYU-310 | Feno BB | Dio | Leaves wrinkling | June 2022 | −80 °C | No | ||||
| ALYU-311 | K 1 | Dio | ++ (608) | Leaves deformation | June 2022 | −80 °C | No | |||
| ALYU-312 | K 3 | Dio | ++ (156) | Leaves deformation | June 2022 | −80 °C | No | |||
| ALYU-313 | K 4 | Dio | ++ (226) | Leaves deformation | June 2022 | −80 °C | No | |||
| ALYU-314 | Sweet 6 | Dio | ++ (190) | + (26) | Leaves deformation | June 2022 | −80 °C | No | ||
| ALYU-315 | Queen sangria | Dio | ++++ (6327) | Leaves wrinkling and deformation | June 2022 | −80 °C | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miotti, N.; Sukhikh, N.; Laboureau, N.; Casati, P.; Pooggin, M.M. Cannabis Virome Reconstruction and Antiviral RNAi Characterization through Small RNA Sequencing. Plants 2023, 12, 3925. https://doi.org/10.3390/plants12233925
Miotti N, Sukhikh N, Laboureau N, Casati P, Pooggin MM. Cannabis Virome Reconstruction and Antiviral RNAi Characterization through Small RNA Sequencing. Plants. 2023; 12(23):3925. https://doi.org/10.3390/plants12233925
Chicago/Turabian StyleMiotti, Niccolo’, Natalia Sukhikh, Nathalie Laboureau, Paola Casati, and Mikhail M. Pooggin. 2023. "Cannabis Virome Reconstruction and Antiviral RNAi Characterization through Small RNA Sequencing" Plants 12, no. 23: 3925. https://doi.org/10.3390/plants12233925
APA StyleMiotti, N., Sukhikh, N., Laboureau, N., Casati, P., & Pooggin, M. M. (2023). Cannabis Virome Reconstruction and Antiviral RNAi Characterization through Small RNA Sequencing. Plants, 12(23), 3925. https://doi.org/10.3390/plants12233925






