A Novel Non-Specific Lipid Transfer Protein Gene, CmnsLTP6.9, Enhanced Osmotic and Drought Tolerance by Regulating ROS Scavenging and Remodeling Lipid Profiles in Chinese Chestnut (Castanea mollissima Blume)
Abstract
:1. Introduction
2. Results
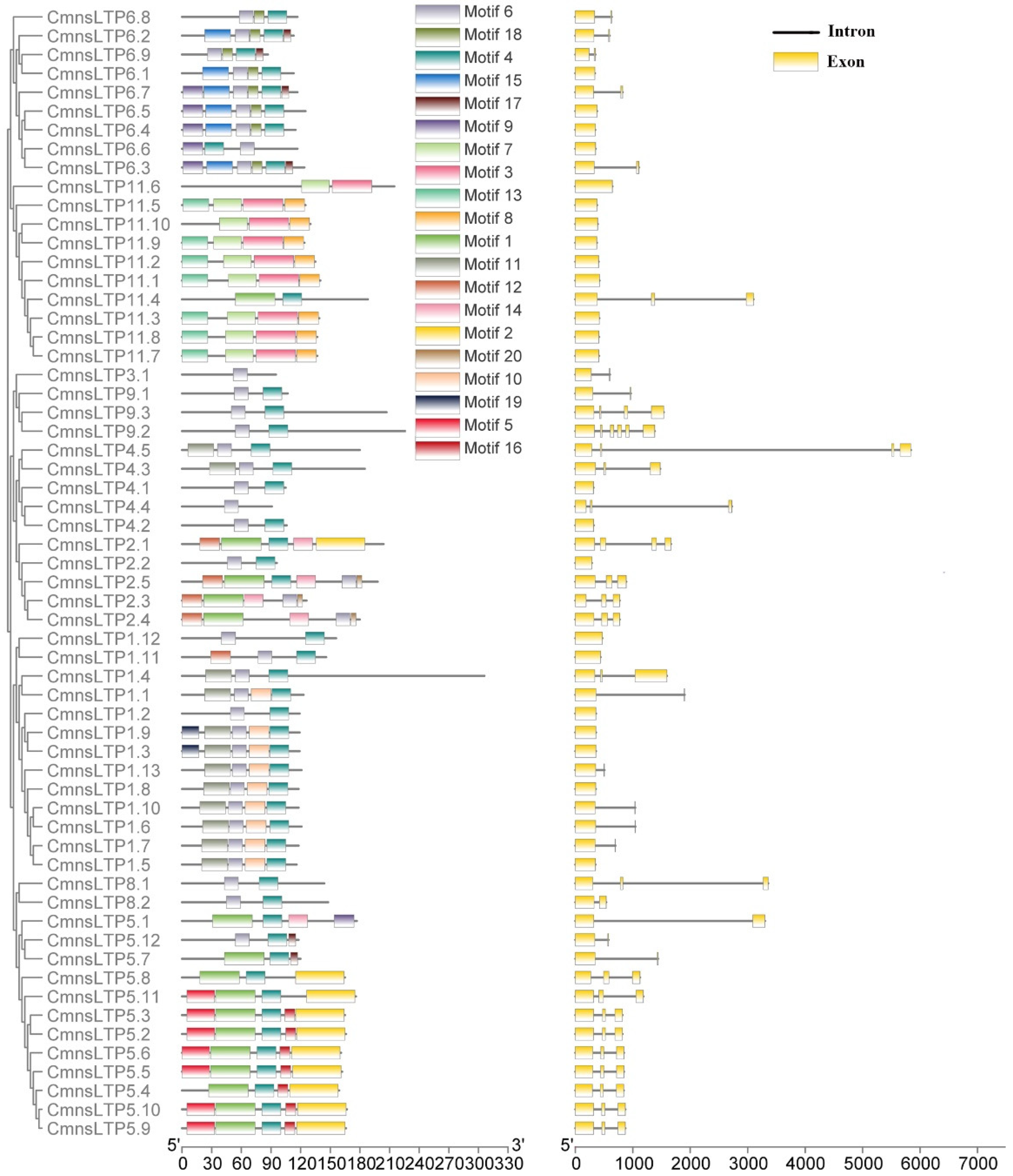
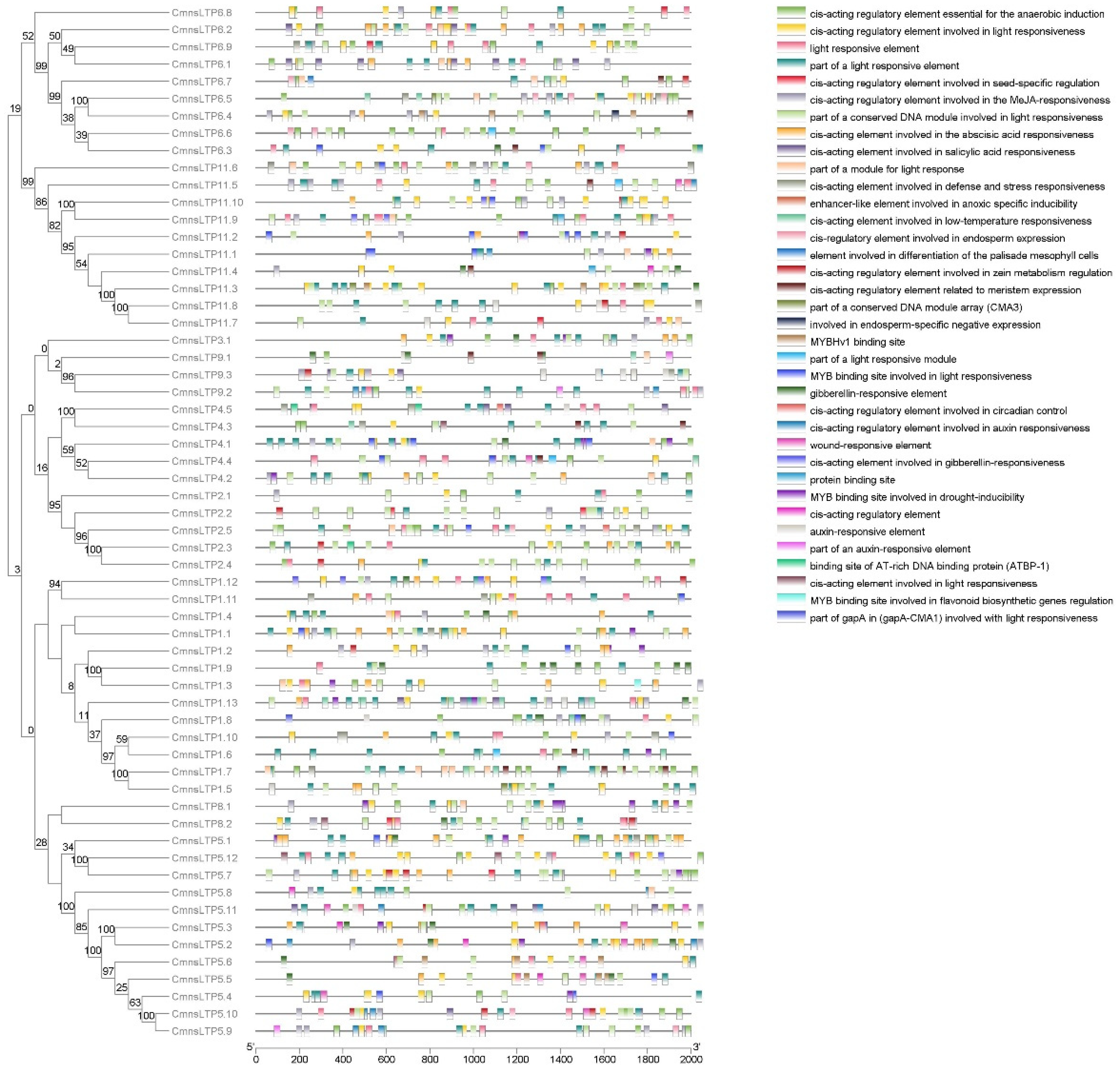
2.1. Phylogenetic Analysis of Chestnut nsLTPs (CmnsLTPs)
2.2. Gene Structures and Conserved Domains of CmnsLTPs
2.3. Regulatory Elements of CmnsLTPs
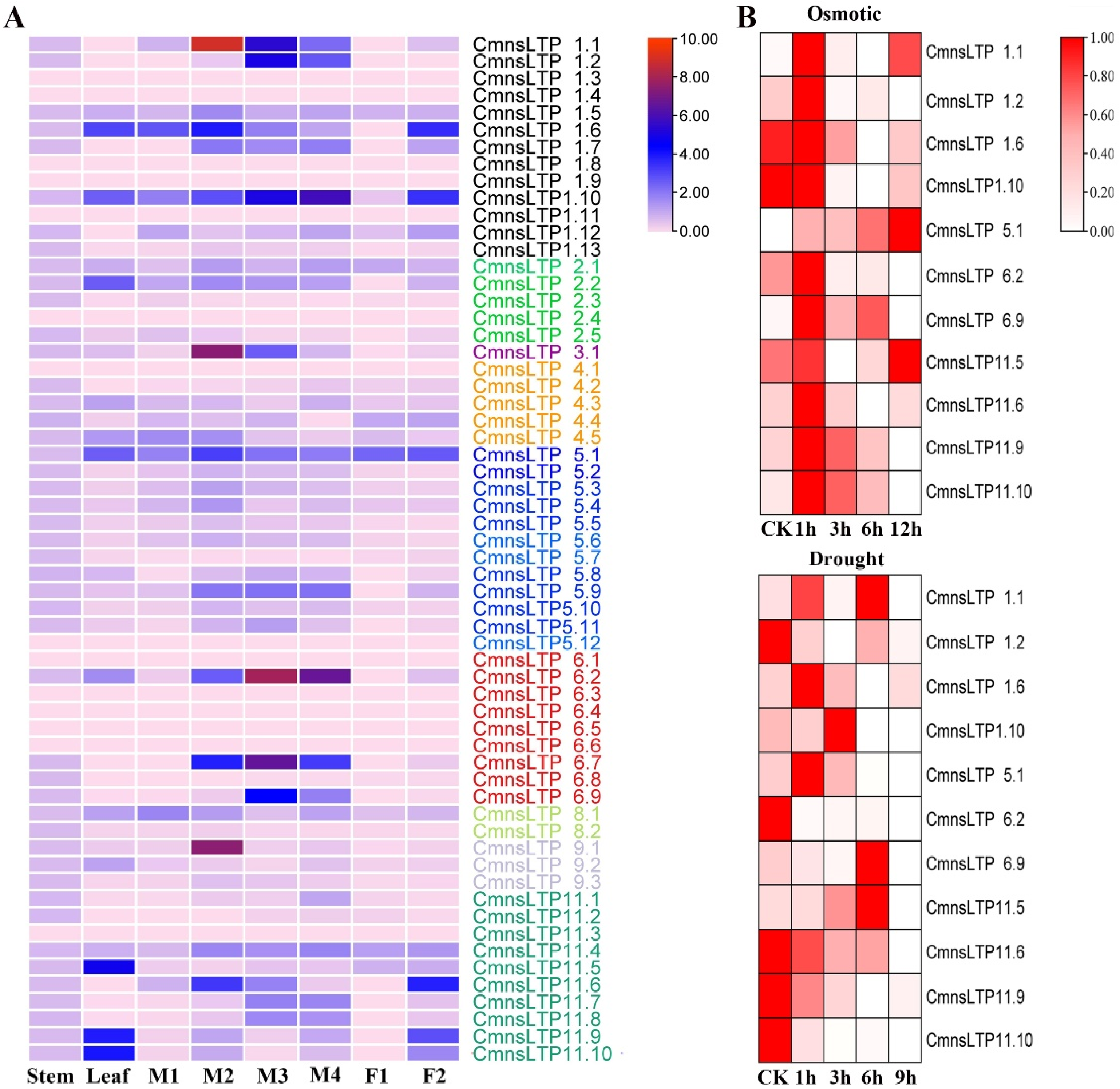
2.4. Expression Profiling of CmnsLTPs
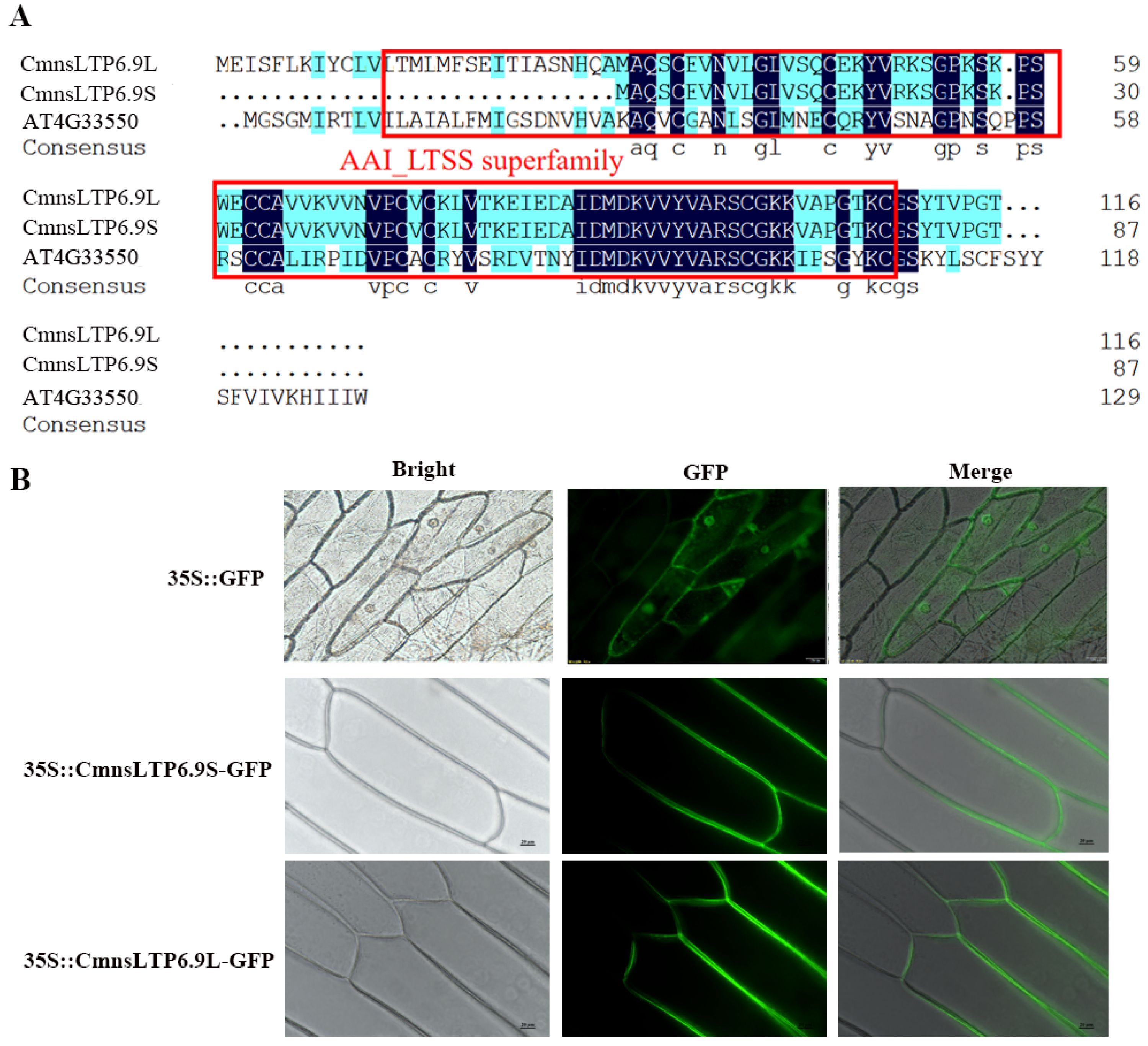
2.5. Sequence Characterization and Subcellular Localization of CmnsLTP6.9
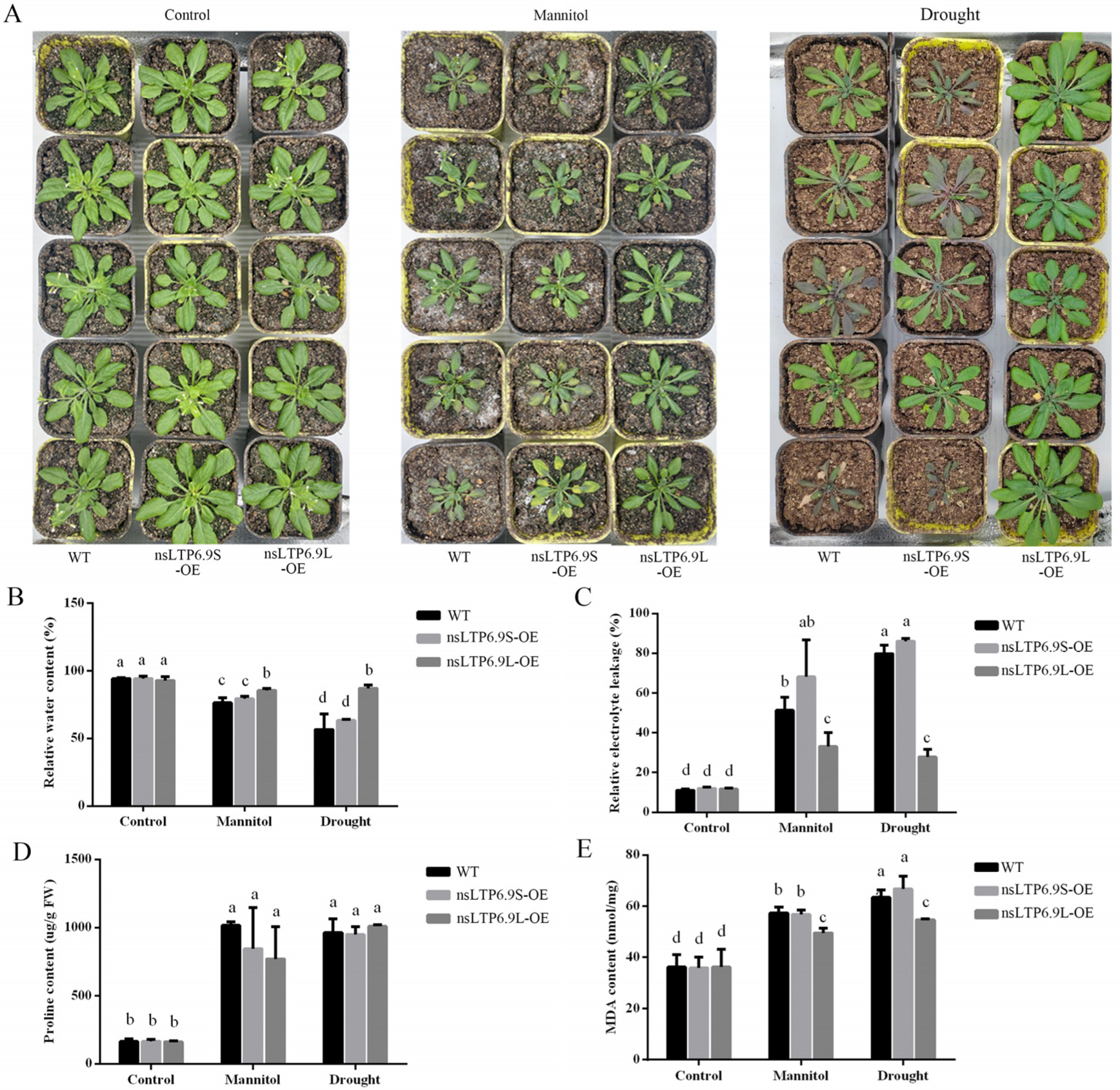
2.6. Overexpression of CmnsLTP6.9-Enhanced Tolerance to Osmotic and Drought Stress in A. thaliana
2.7. Overexpression of CmnsLTP6.9 Stimulated ROS Scavenging in A. thaliana under Osmotic and Drought Stress
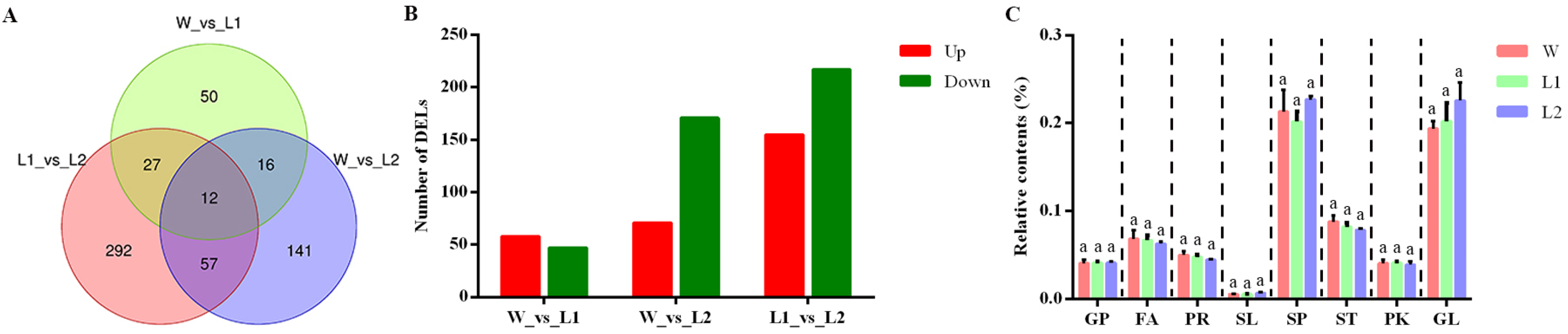
2.8. Lipidomic Analysis of Transgenic Arabidopsis
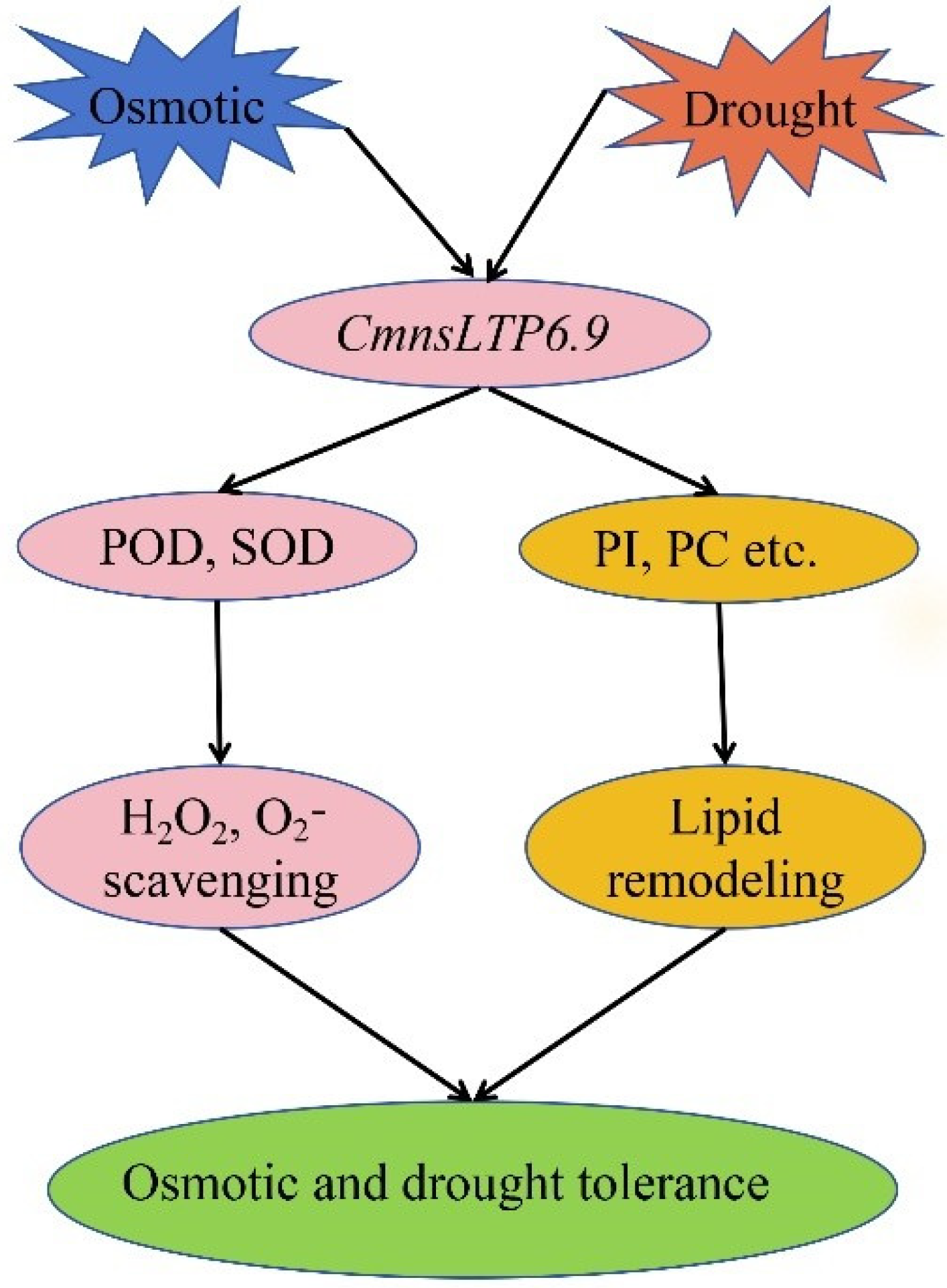
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Phylogenetic Analysis of the nsLTP Genes in Chestnut
4.3. Conserved Motifs, Gene Structure, and Promoter Analysis
4.4. RNA Extraction and qRT-PCR Assay
4.5. Subcellular Localization of CmnsLTP6.9S and CmnsLTP6.9L
4.6. Genetic Transformation and Fluorescence Analysis of A. thaliana
4.7. Osmotic and Drought Treatment
4.8. Oxidative Stress Analysis
4.9. Lipidomics Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fen, T.; Xie, C.Y.; Li, H.N.; Lei, S.C.; Li, J.; Huang, X.W.; Yang, F. Effect of in vitro digestion on chestnut outer-skin and inner-skin bioaccessibility: The relationship between biotransformation and antioxidant activity of polyphenols by metabolomics. Food Chem. 2021, 363, 130277. [Google Scholar]
- Zha, J.J.; Fan, B.; He, J.R.; He, C.Y.; Dong, X.D.; Feng, Y.Y.; Liu, Y.N.; Ji, H.Y.; Yu, S.S.; Liu, A.J.; et al. A novel polysaccharide from Castanea mollissima Blume: Preparation, characteristics and antitumor activities in vitro and in vivo. Carbohydr. Polym. 2020, 240, 116323. [Google Scholar]
- Ma, C.L. Valorization of Biomass to Furfural by Chestnut Shell-based Solid Acid in Methyl Isobutyl Ketone-Water-Sodium Chloride System. Appl. Biochem. Biotech. 2022, 194, 2021–2035. [Google Scholar]
- Zhao, J.B.; Du, C.J.; Ma, C.M.; Sun, J.C.; Han, Z.T.; Yan, D.H.; Jiang, Z.P.; Shi, S.Q. Response of photosynthesis and carbon/nitrogen metabolism to drought stress in Chinese chestnut ‘Yanshanzaofeng’ seedlings. J. Appl. Ecol. 2020, 31, 3674–3680. [Google Scholar]
- Wen, X.L.; Gu, C.Q.; Zhu, D.X.; Liu, P.; Lai, Y.P.; Zeng, Q.Z. Water stress affects on cell membrane lipid oxidation and calcification of chestnut (Castanea mollissima Bl.). Postharvest Biol. Tec. 2017, 126, 34–39. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, L.; Fu, Y.; Jiang, J.C. Bioactive constituents, nutritional benefits and woody food applications of Castanea mollissima: A comprehensive review. Food Chem. 2022, 393, 133380. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Wang, W.; Chen, Y.; Zhao, Y.; Zhang, S.; Shi, F.; Khalil-Ur-Rehman, M.; Nieuwenhuizen, N.J. Transcriptomics and antioxidant analysis of two chinese chestnut (Castanea mollissima BL.) varieties provides new insights into the mechanisms of resistance to gall wasp dryocosmus kuriphilus infestation. Front. Plant Sci. 2022, 13, 874434. [Google Scholar] [CrossRef]
- Carlson, E.; Stewart, K.; Baier, K.; McGuigan, L.; Culpepper, M.T.; Powell, W. Pathogen-induced expression of a blight tolerance transgene in American chestnut. Mol. Plant Pathol. 2022, 23, 370–382. [Google Scholar] [CrossRef]
- Jiang, L.; Shen, W.; Liu, C.; Tahir, M.M.; Li, X.; Zhou, S.; Ma, F.; Guan, Q. Engineering drought-tolerant apple by knocking down six GH3 genes and potential application of transgenic apple as a rootstock. Hortic. Res. 2022, 9, uhac122. [Google Scholar] [CrossRef]
- Nadarajah, K.K. ROS homeostasis in abiotic stress tolerance in plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef]
- Nam, J.W.; Lee, H.G.; Do, H.; Kim, H.U.; Seo, P.J. Transcriptional regulation of triacylglycerol accumulation in plants under environmental stress conditions. J. Exp. Bot. 2022, 73, 2905–2917. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Saito, K. Integrated metabolomics for abiotic stress responses in plants. Curr. Opin. Plant Biol. 2015, 24, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; You, J.; Shi, L.; Sheng, C.; Zhou, W.; Dossou, S.S.K.; Dossa, K.; Wang, L.; Zhang, X. Genome-wide analysis of nsLTP gene family and identification of SiLTPs contributing to high oil accumulation in Sesame (Sesamum indicum L.). Int. J. Mol. Sci. 2021, 22, 5291. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Okazaki, Y.; Myouga, F.; Shinozaki, K.; Saito, K. Landscape of the lipidome and transcriptome under heat stress in Arabidopsis thaliana. Sci. Rep. 2015, 5, 10533. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.J.; Wang, N.; Bao, J.J.; Zhu, H.X.; Wang, L.J.; Chen, X.Y. Lipidomic Analysis reveals the importance of GIPCs in Arabidopsis leaf extracellular vesicles. Mol. Plant. 2020, 13, 1523–1532. [Google Scholar] [CrossRef]
- Jacq, A.; Pernot, C.; Martinez, Y.; Domergue, F.; Payré, B.; Jamet, E.; Burlat, V.; Pacquit, V.B. The Arabidopsis Lipid Transfer Protein 2 (AtLTP2) is involved in cuticle-cell wall interface integrity and in etiolated hypocotyl permeability. Front. Plant Sci. 2017, 8, 263. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ji, C.; Zhou, D.; Gou, X.; Tang, J.; Jiang, Y.; Han, J.; Liu, Y.G.; Chen, L.; Xie, Y. OsLTP47 may function in a lipid transfer relay essential for pollen wall development in rice. J. Genet. Genom. 2022, 49, 481–491. [Google Scholar] [CrossRef]
- Tao, Y.; Zou, T.; Zhang, X.; Liu, R.; Chen, H.; Yuan, G.; Zhou, D.; Xiong, P.; He, Z.; Li, G.; et al. Secretory lipid transfer protein OsLTPL94 acts as a target of EAT1 and is required for rice pollen wall development. Plant J. 2021, 108, 358–377. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S.B.; Kim, H.J.; Min, M.K.; Hwang, I.; Suh, M.C. Characterization of glycosylphosphatidylinositol-anchored lipid transfer protein 2 (LTPG2) and overlapping function between LTPG/LTPG1 and LTPG2 in cuticular wax export or accumulation in Arabidopsis thaliana. Plant Cell Physiol. 2012, 53, 1391–1403. [Google Scholar] [CrossRef]
- Li, F.; Fan, K.; Guo, X.; Liu, J.; Zhang, K.; Lu, P. Genome-wide identification, molecular evolution and expression analysis of the non-specific lipid transfer protein (nsLTP) family in Setaria italica. BMC Plant Biol. 2022, 22, 547. [Google Scholar] [CrossRef]
- Yang, Y.; Li, P.; Liu, C.; Wang, P.; Cao, P.; Ye, X.; Li, Q. Systematic analysis of the non-specific lipid transfer protein gene family in Nicotiana tabacum reveal its potential roles in stress responses. Plant Physiol. Biochem. 2022, 172, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhang, C.; Shan, R.; Li, X.; Tseke Inkabanga, A.; Li, L.; Jiang, H.; Chai, Y. Genome-wide identification and expression analysis of nsLTP gene family in rapeseed (Brassica napus) reveals their critical roles in biotic and abiotic stress responses. Int. J. Mol. Sci. 2022, 23, 8372. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, J.Y.; Shi, Y.; Fu, H.Y.; Huang, M.T.; Meng, J.Y.; Gao, S.J. Systematic and functional analysis of non-specific lipid transfer protein family genes in sugarcane under Xanthomonas albilineans infection and salicylic acid treatment. Front. Plant Sci. 2022, 13, 1014266. [Google Scholar] [CrossRef]
- Wang, D.; Song, J.; Lin, T.; Yin, Y.; Mu, J.; Liu, S.; Wang, Y.; Kong, D.; Zhang, Z. Identification of potato Lipid transfer protein gene family and expression verification of drought genes StLTP1 and StLTP7. Plant Direct. 2023, 7, e491. [Google Scholar] [CrossRef] [PubMed]
- Duo, J.; Xiong, H.; Wu, X.; Li, Y.; Si, J.; Zhang, C.; Duan, R. Genome-wide identification and expression profile under abiotic stress of the barley non-specific lipid transfer protein gene family and its Qingke Orthologues. BMC Genom. 2021, 22, 674. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, S.; Qin, J.; Sun, C.; Liu, F. The lipid transfer protein OsLTPL159 is involved in cold tolerance at the early seedling stage in rice. Plant Biotechnol. J. 2020, 18, 756–769. [Google Scholar] [CrossRef]
- Yang, Y.; Song, H.; Yao, P.; Zhang, S.; Jia, H.; Ye, X. NtLTPI.38, a plasma membrane-localized protein, mediates lipid metabolism and salt tolerance in Nicotiana tabacum. Int. J. Biol. Macromol. 2023, 242, 125007. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Li, J.N.; Li, M.M.; Cheng, Z.M.; Xu, Q.A.; Song, X.H.; Shang, X.G.; Guo, W.Z. Overexpression of a cotton nonspecific lipid transfer protein gene, GhLTP4, enhances drought tolerance by remodeling lipid profiles, regulating abscisic acid homeostasis and improving tricarboxylic acid cycle in cotton. Environ. Exp. Bot. 2022, 201, 104991. [Google Scholar] [CrossRef]
- Hairat, S.; Baranwal, V.K.; Khurana, P. Identification of triticum aestivum nsLTPs and functional validation of two members in development and stress mitigation roles. Plant Physiol. Biochem. 2018, 130, 418–430. [Google Scholar] [CrossRef]
- Zou, H.W.; Tian, X.H.; Ma, G.H.; Li, Z.X. Isolation and functional analysis of ZmLTP3, a homologue to Arabidopsis LTP3. Int. J. Mol. Sci. 2013, 14, 5025–5035. [Google Scholar] [CrossRef]
- Guo, L.; Yang, H.; Zhang, X.; Yang, S. Lipid transfer protein 3 as a target of MYB96 mediates freezing and drought stress in Arabidopsis. J. Exp. Bot. 2013, 64, 1755–1767. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Cao, M.Y.; Zhu, P.X.; Zhang, Q.P.; Lam, H.M. Nonspecific lipid transfer protein 1 enhances immunity against tobacco mosaic virus in tobacco. J. Exp. Bot. 2023, 29, erad202. [Google Scholar]
- Wang, C.; Gao, H.; Chu, Z.; Ji, C.; Xu, Y.; Cao, W.; Zhou, S.; Song, Y.; Liu, H.; Zhu, C. A nonspecific lipid transfer protein, StLTP10, mediates resistance to Phytophthora infestans in potato. Mol. Plant Pathol. 2021, 22, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Bi, W.; Zhao, S.; Su, J.; Li, M.; Ma, L.; Yu, X.; Wang, X. Wheat apoplast-localized lipid transfer protein TaLTP3 enhances defense responses against Puccinia triticina. Front. Plant Sci. 2021, 12, 771806. [Google Scholar] [CrossRef]
- McLaughlin, J.E.; Bin-Umer, M.A.; Widiez, T.; Finn, D.; McCormick, S.; Tumer, N.E. A lipid transfer protein increases the glutathione content and enhances Arabidopsis resistance to a Trichothecene mycotoxin. PLoS ONE 2015, 10, e0130204. [Google Scholar] [CrossRef]
- Wei, H.; Movahedi, A.; Liu, G.; Zhu, S.; Chen, Y.; Yu, C.; Zhong, F.; Zhang, J. Characteristics, expression profile, and function of non-specific lipid transfer proteins of Populus trichocarpa. Int. J. Biol. Macromol. 2022, 202, 468–481. [Google Scholar] [CrossRef]
- Wei, H.; Liu, G.; Qin, J.; Zhang, Y.; Chen, J.; Zhang, X.; Yu, C.; Chen, Y.; Lian, B.; Zhong, F.; et al. Genome-wide characterization, chromosome localization, and expression profile analysis of poplar non-specific lipid transfer proteins. Int. J. Biol. Macromol. 2023, 231, 123226. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Wang, Y.; Wu, X.; Liu, Y.; Wan, M.; Wang, L.; Wang, X.; Zhang, C.; Wang, X.; et al. Identification of nsLTP family in Chinese white pear (Pyrus bretschneideri) reveals its potential roles in russet skin formation. Planta 2023, 257, 113. [Google Scholar] [CrossRef]
- Ghelli, R.; Brunetti, P.; Marzi, D.; Cecchetti, V.; Costantini, M.; Lanzoni-Rossi, M.; Scaglia Linhares, F.; Costantino, P.; Cardarelli, M. The full-length auxin response factor 8 isoform ARF8.1 controls pollen cell wall formation and directly regulates TDF1, AMS and MS188 expression. Plant J. 2023, 113, 851–865. [Google Scholar] [CrossRef]
- Li, X.; He, G.; Jiang, S.; Yang, C.; Yang, B.; Ming, F. Function of two splicing variants of RcCPR5 in the resistance of Rosa chinensis to powdery mildew. Plant Sci. 2023, 335, 111678. [Google Scholar] [CrossRef]
- Li, Q.; Lin, Y.C.; Sun, Y.H.; Song, J.; Chen, H.; Zhang, X.H.; Sederoff, R.R.; Chiang, V.L. Splice variant of the SND1 transcription factor is a dominant negative of SND1 members and their regulation in Populus trichocarpa. Proc. Natl. Acad. Sci. USA 2012, 109, 14699–14704. [Google Scholar] [CrossRef]
- Ellur, V.; Wei, W.; Ghogare, R.; Solanki, S.; Vandemark, G.; Brueggeman, R.; Chen, W. Unraveling the genomic reorganization of polygalacturonase-inhibiting proteins in chickpea. Front. Genet. 2023, 14, 1189329. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Ufer, G.; Bartels, D. Lipid signalling in plant responses to abiotic stress. Plant Cell Environ. 2016, 39, 1029–1048. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.C.; Stevens, D.M.; Little, H.; Coaker, G.L.; Bostock, R.M. Overlapping local and systemic defense induced by an oomycete fatty acid MAMP and brown seaweed extract in tomato. Mol. Plant Microbe Interact. 2023, 36, 359–371. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, R.; Wu, Y.; Wu, Q.; Jiang, Q.; Ma, J.; Zhang, Y.; Qi, P.; Chen, G.; Jiang, Y.; et al. TaRBP1 stabilizes TaGLTP and negatively regulates stripe rust resistance in wheat. Mol. Plant Pathol. 2023, 24, 1205–1219. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zhang, B.; Wang, Y.; Su, R. Characterization of SEC14 family in wheat and the function of TaSEC14-7B in salt stress tolerance. Plant Physiol. Biochem. 2023, 202, 107926. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.; Prasad, P.V.; Welti, R. Alterations in wheat pollen lipidome during high day and night temperature stress. Plant Cell Environ. 2018, 41, 1749–1761. [Google Scholar] [CrossRef]
- Sun, M.; Liu, X.; Gao, H.; Zhang, B.; Peng, F.; Xiao, Y. Phosphatidylcholine enhances homeostasis in peach seedling cell membrane and increases its salt stress tolerance by phosphatidic acid. Int. J. Mol. Sci. 2022, 23, 2585. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, S.; Luo, J.; Lü, L.; Zhang, L.; Cui, J. Lipidomics and RNA-Seq study of lipid regulation in Aphis gossypii parasitized by Lysiphlebia japonica. Sci. Rep. 2017, 7, 1364. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Y.; Xiao, C.; He, X.; Yang, X.; Tong, Z.; Wang, Z.; Sun, Z.; Qiu, W. A Novel Non-Specific Lipid Transfer Protein Gene, CmnsLTP6.9, Enhanced Osmotic and Drought Tolerance by Regulating ROS Scavenging and Remodeling Lipid Profiles in Chinese Chestnut (Castanea mollissima Blume). Plants 2023, 12, 3916. https://doi.org/10.3390/plants12223916
Xiao Y, Xiao C, He X, Yang X, Tong Z, Wang Z, Sun Z, Qiu W. A Novel Non-Specific Lipid Transfer Protein Gene, CmnsLTP6.9, Enhanced Osmotic and Drought Tolerance by Regulating ROS Scavenging and Remodeling Lipid Profiles in Chinese Chestnut (Castanea mollissima Blume). Plants. 2023; 12(22):3916. https://doi.org/10.3390/plants12223916
Chicago/Turabian StyleXiao, Yuxiong, Cui Xiao, Xiujuan He, Xin Yang, Zhu Tong, Zeqiong Wang, Zhonghai Sun, and Wenming Qiu. 2023. "A Novel Non-Specific Lipid Transfer Protein Gene, CmnsLTP6.9, Enhanced Osmotic and Drought Tolerance by Regulating ROS Scavenging and Remodeling Lipid Profiles in Chinese Chestnut (Castanea mollissima Blume)" Plants 12, no. 22: 3916. https://doi.org/10.3390/plants12223916
APA StyleXiao, Y., Xiao, C., He, X., Yang, X., Tong, Z., Wang, Z., Sun, Z., & Qiu, W. (2023). A Novel Non-Specific Lipid Transfer Protein Gene, CmnsLTP6.9, Enhanced Osmotic and Drought Tolerance by Regulating ROS Scavenging and Remodeling Lipid Profiles in Chinese Chestnut (Castanea mollissima Blume). Plants, 12(22), 3916. https://doi.org/10.3390/plants12223916





