Clarithromycin Suppresses Apple Proliferation Phytoplasma in Explant Cultures
Abstract
:1. Introduction
2. Results
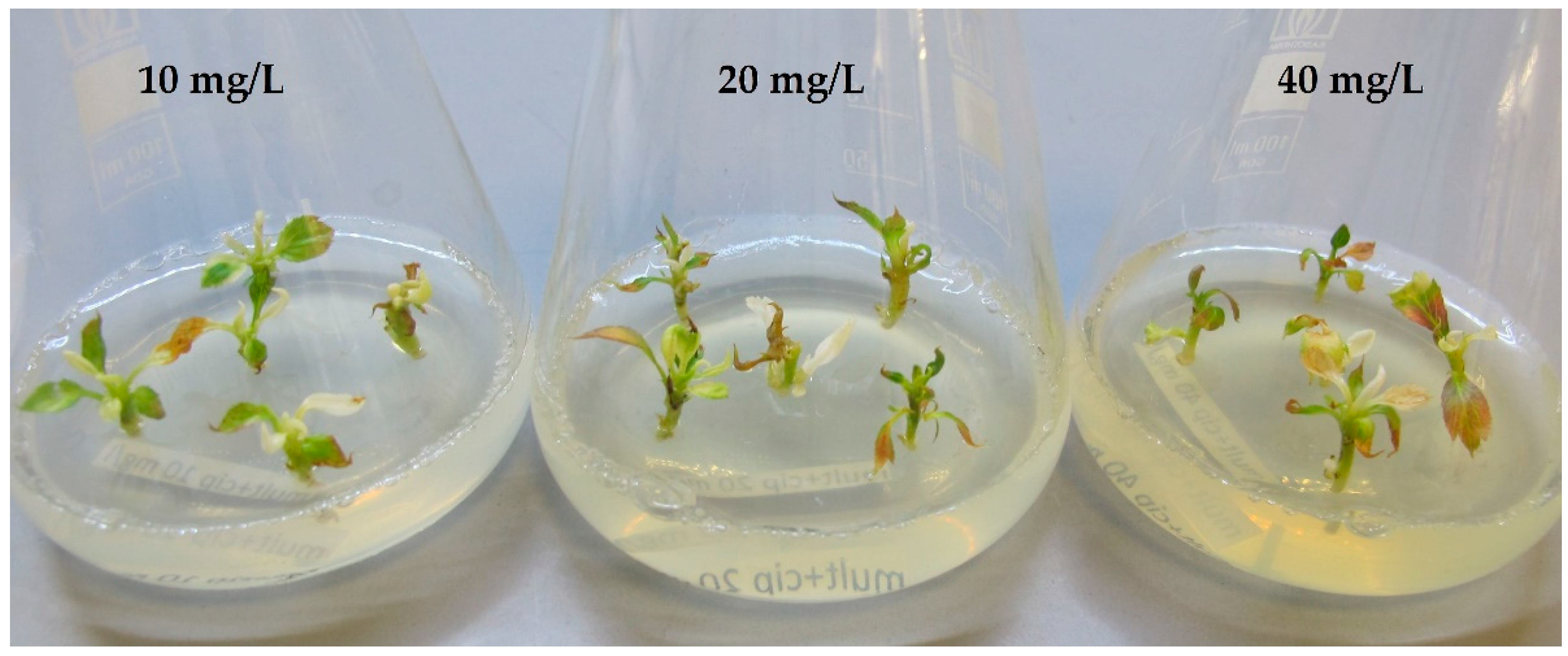
2.1. Phytotoxicity
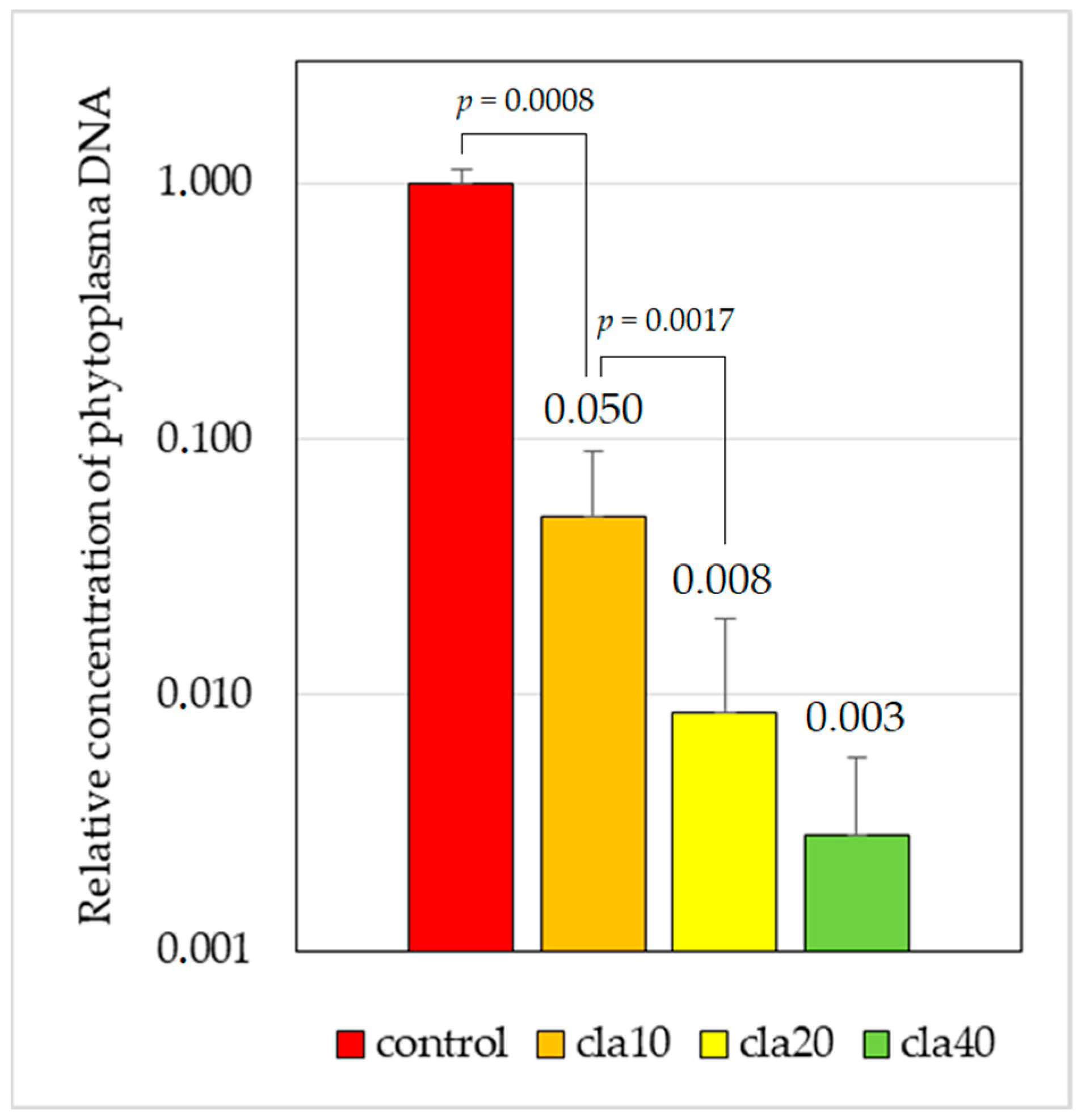
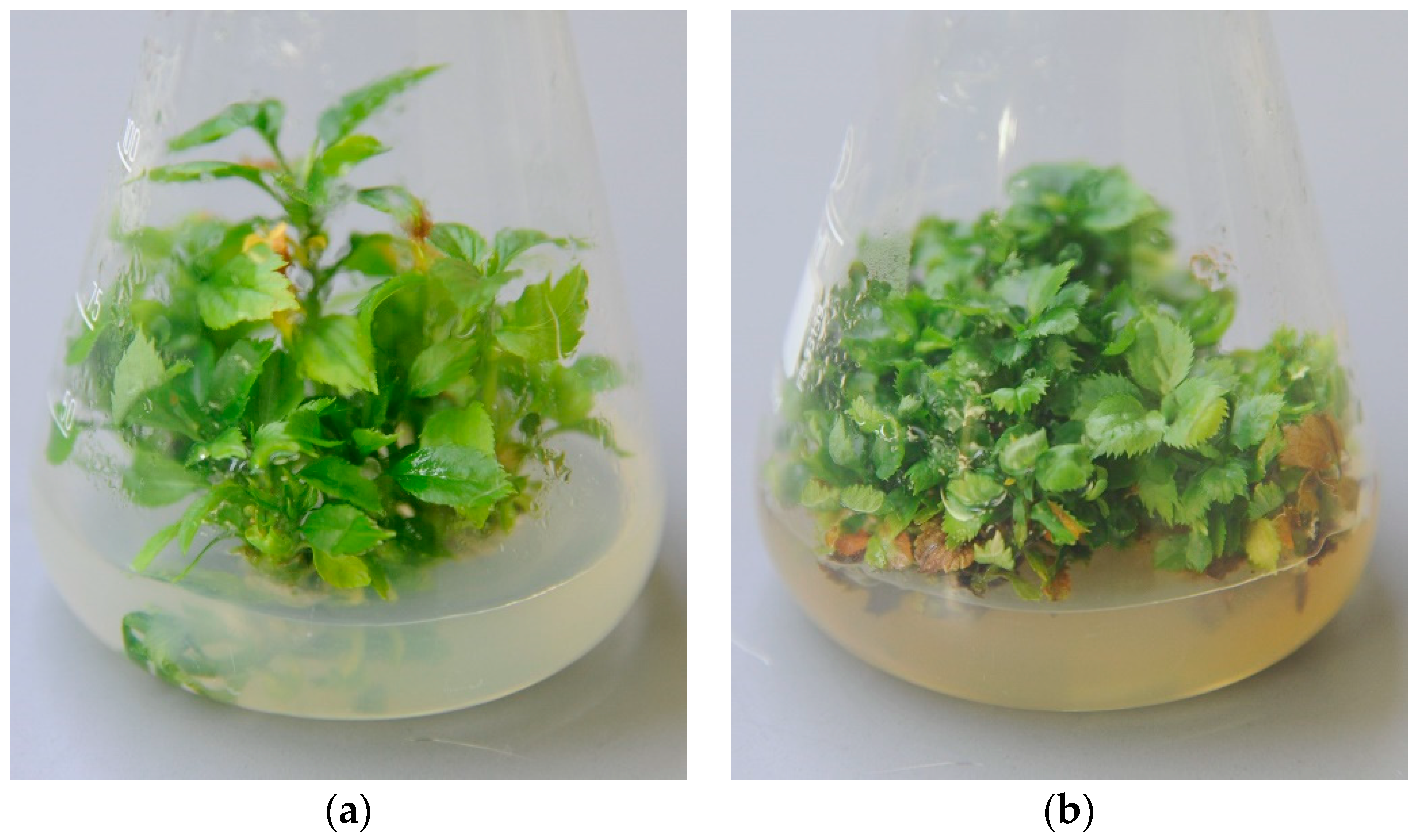
2.2. Efficiency of Chemotherapy
3. Discussion
4. Materials and Methods
- Forward: 5′-GCAGCTGCGGTAATACATGG;
- Reverse: 5′-GAATTCCACTTGCCTCTATCCAA;
- Probe: ROX-5′-AGTTCAACGCTTAACGTTGTGATGCTAT-BHQ2.
- The presence of chloroplast 16S rDNA was used as an internal control:
- Forward: 5′-CGGACGGGAAGTGGTGTTTC;
- Reverse: 5′-ACGCGAGCCCCTCCTCGG;
- Probe: 6-FAM-5′-CCGTAGGCTGAGGAGCAAAAGGAGGAATC-BHQ1.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weintraub, P.; Beanland, L. Insect Vectors of Phytoplasmas. Annu. Rev. Entomol. 2006, 51, 91–111. [Google Scholar] [CrossRef] [PubMed]
- Bertaccini, A. Phytoplasmas: Diversity, Taxonomy, and Epidemiology. Front. Biosci.-Landmark 2007, 12, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Zhao, Y. Phytoplasma Taxonomy: Nomenclature, Classification, and Identification. Biology 2022, 11, 1119. [Google Scholar] [CrossRef] [PubMed]
- Marcone, C.; Ragozzino, A.; Seemuller, E. Dodder Transmission of Alder Yellows Phytoplasma to the Experimental Host Catharanthus Roseus (Periwinkle). Eur. J. For. Pathol. 2007, 27, 347–350. [Google Scholar] [CrossRef]
- Marcone, C.; Valiunas, D.; Salehi, M.; Mondal, S.; Sundararaj, R. Phytoplasma Diseases of Trees. In Forest Microbiology; Asiegbu, F.O., Kovalchuk, A., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 99–120. [Google Scholar]
- Doi, Y.; Teranaka, M.; Yora, K.; Asuyama, H. Mycoplasma- or PLT Group-like Microorganisms Found in the Phloem Elements of Plants Infected with Mulberry Dwarf, Potato Witches’ Broom, Aster Yellows, or Paulownia Witches’ Broom. Jpn. J. Phytopathol. 1967, 33, 259–266. [Google Scholar] [CrossRef]
- Severin, H.H. Infection of Perennial Delphiniums by California-Aster-Yellows Virus. Hilgardia 1942, 14, 411–440. [Google Scholar] [CrossRef]
- Black, L.M. Transmission of Plant Viruses by Cicadellids. In Advances in Virus Research; Maramorosch, K., Murphy, F.A., Eds.; Academic Press: Cambridge, MA, USA, 1953; pp. 69–89. [Google Scholar]
- Bertaccini, A.; Duduk, B. Phytoplasma and Phytoplasma Diseases: A Review of Recent Research. Phytopathol. Mediterr. 2010, 48, 355–378. [Google Scholar] [CrossRef]
- PM 7/62 (3) ‘Candidatus Phytoplasma Mali’, ‘Ca. P. Pyri’ and ‘Ca. P. Prunorum’. EPPO Bull. 2020, 50, 69–85. [CrossRef]
- Seemüller, E.; Schneider, B. ‘Candidatus Phytoplasma Mali’, ‘Candidatus Phytoplasma Pyri’ and ‘Candidatus Phytoplasma Prunorum’, the Causal Agents of Apple Proliferation, Pear Decline and European Stone Fruit Yellows, Respectively. Int. J. Syst. Evol. Microbiol. 2004, 54, 1217–1226. [Google Scholar] [CrossRef]
- Seemüller, E.; Carraro, L.; Jarausch, W.; Schneider, B. Apple Proliferation Phytoplasma. In Virus and Virus-Like Diseases of Pome and Stone Fruits; Hadidi, A., Barba, M., Candresse, T., Jelkmann, W., Eds.; APS Press: St. Paul, MI, USA, 2011; pp. 67–73. [Google Scholar]
- Görg, L.M.; Gallinger, J.; Gross, J. The Phytopathogen ‘Candidatus Phytoplasma Mali’ Alters Apple Tree Phloem Composition and Affects Oviposition Behavior of Its Vector Cacopsylla Picta. Chemoecology 2021, 31, 31–45. [Google Scholar] [CrossRef]
- Nyland, G. Tetracycline Therapy of Pear Decline and X-Desease in Peach and Cherry. In Proceedings of the Acta Horticulturae, International Society for Horticultural Science (ISHS), Leuven, Belgium, 1 February 1975; pp. 17–18. [Google Scholar]
- Křižan, B.; Moravcová, K.; Ondrušiková, E.; Adam, M.; Holleinová, V.; Pidra, M. Thermotherapy of Grapevines and Apricots by Reason of Viruses and Phytoplasma Elimination. Acta Hortic. 2008, 781, 93–96. [Google Scholar] [CrossRef]
- Hot Water Treatment of Grapevine to Control Grapevine Flavescence Dorée Phytoplasma. EPPO Bull. 2012, 42, 490–492. [CrossRef]
- Jarausch, W.; Lansac, M.; Dosba, F. Long-Term Maintenance of Nonculturable Apple-Proliferation Phytoplasmas in Their Micropropagated Natural Host Plant. Plant Pathol. 1996, 45, 778–786. [Google Scholar] [CrossRef]
- Linck, H.; Lankes, C.; Krüger, E.; Reineke, A. Elimination of Phytoplasmas in Rubus Mother Plants by Tissue Culture Coupled with Heat Therapy. Plant Dis. 2019, 103, 1252–1255. [Google Scholar] [CrossRef] [PubMed]
- Laimer, M. Detection and Elimination of Viruses and Phytoplasmas from Pome and Stone Fruit Trees. In Horticultural Reviews; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 187–236. ISBN 978-0-470-65085-1. [Google Scholar]
- Sedlák, J.; Semerák, M.; Rejlová, M. Sanitation of Apple Cultivars from AP Phytoplasma and ApMV and ACLSV Viruses Using In Vitro Culture and Cryo-Knife Therapy in Liquid Nitrogen. Appl. Sci. 2023, 13, 7527. [Google Scholar] [CrossRef]
- Wang, R.; Mou, H.Q.; Gao, X.; Chen, L.; Li, M.; Wang, Q. Cryopreservation for Eradication of Jujube Witches’ Broom Phytoplasma from Chinese Jujube (Ziziphus Jujuba). Ann. Appl. Biol. 2014, 166, 218–228. [Google Scholar] [CrossRef]
- da Vies, D.L.; Clark, M.F. Maintenance of Mycoplasma-like Organisms Occurring in Pyrus Species by Micropropagation and Their Elimination by Tetracycline Therapy. Plant Pathol. 2007, 43, 819–823. [Google Scholar] [CrossRef]
- Wongkaew, P.; Fletcher, J. Sugarcane White Leaf Phytoplasma in Tissue Culture: Long-Term Maintenance, Transmission, and Oxytetracycline Remission. Plant Cell Rep. 2004, 23, 426–434. [Google Scholar] [CrossRef]
- Laimer, M.; Bertaccini, A. Phytoplasma Elimination from Perennial Horticultural Crops: Transmission and Management of Phytoplasma—Associated Diseases. In Phytoplasmas: Plant Pathogenic Bacteria-II Transmission and Management of Phytoplasma Associated Diseases; Bertaccini, A., Weintraub, P.G., Rao, G.P., Mori, N., Eds.; Springer: Singapore, 2019; pp. 185–206. [Google Scholar]
- Bertaccini, A. Containment of Phytoplasma-Associated Plant Diseases by Antibiotics and Other Antimicrobial Molecules. Antibiotics 2021, 10, 1398. [Google Scholar] [CrossRef]
- Tanno, K.; Maejima, K.; Miyazaki, A.; Koinuma, H.; Nozomu, I.; Kitazawa, Y.; Nijo, T.; Hashimoto, M.; Yamaji, Y.; Namba, S. Comprehensive Screening of Antimicrobials to Control Phytoplasma Diseases Using an in Vitro Plant–Phytoplasma Co-Culture System. Microbiology 2018, 164, 1048–1058. [Google Scholar] [CrossRef]
- Plavsic, B.; Krivokapic, K.; Eric, Z.; Buturovic, D. Kinetin Treatment of “Stolbur” Diseased Tomato Plants (Lycopersicum esculentum L.) and the Possibility of Its Application in Chemotherapy. Acta Bot. Croat. 1986, 45, 27–32. [Google Scholar]
- Plavsic, B.; Krivokapic, K.; Eric, Z. Kinetin Treatment of Stolbur Diseased Plants and Possibility of Its Application in Chemotherapy. In Mycoplasma Diseases of Crops: Basic and Applied Aspects; Maramorosch, K., Raychaudhuri, S.P., Eds.; Springer: New York, NY, USA, 1988; pp. 417–430. [Google Scholar]
- Chang, C. Pathogenicity of Aster Yellows Phytoplasma and Spiroplasma Citri on Periwinkle. Phytopathology 1998, 88, 1347–1350. [Google Scholar] [CrossRef] [PubMed]
- Pertot, I.; Musetti, R.; Pressacco, L.; Osler, R. Changes in Indole-3-Acetic Acid Level in Micropropagated Tissues of Catharanthus Roseus Infected by the Agent of the Clover Phyllody and Effect of Exogenous Auxins on Phytoplasma Morphology. Cytobios 1998, 95, 13–23. [Google Scholar]
- Pedrazzoli, F.; Ciccotti, A.; Bianchedi, P.; Salvadori, A.; Zorer, R. Seasonal Colonisation Behaviour of Candidatus Phytoplasma Mali in Apple Trees in Trentino. Acta Hortic. 2008, 781, 483–489. [Google Scholar] [CrossRef]
- Bisognin, C.; Ciccotti, A.; Salvadori, A.; Moser, M.; Grando, M.; Jarausch, W. In Vitro Screening for Resistance to Apple Proliferation in Malus Species. Plant Pathol. 2008, 57, 1163–1171. [Google Scholar] [CrossRef]
- Herranz, M.C.; Niehl, A.; Rosales, M.; Fiore, N.; Zamorano, A.; Granell, A.; Pallas, V. A Remarkable Synergistic Effect at the Transcriptomic Level in Peach Fruits Doubly Infected by Prunus Necrotic Ringspot Virus and Peach Latent Mosaic Viroid. Virol. J. 2013, 10, 164. [Google Scholar] [CrossRef]
- Wright, A.; Cross, A.; Harper, S. A Bushel of Viruses: Identification of Seventeen Novel Putative Viruses by RNA-Seq in Six Apple Trees. PLoS ONE 2020, 15, e0227669. [Google Scholar] [CrossRef]
- Champney, S.; Burdine, R. Macrolide Antibiotics Inhibit 50S Ribosomal Subunit Assembly in Bacillus Subtilis and Staphylococcus Aureus. Antimicrob. Agents Chemother. 1995, 39, 2141–2144. [Google Scholar] [CrossRef]
- Poddighe, D.; Aljofan, M. Clinical Evidences on the Antiviral Properties of Macrolide Antibiotics in the COVID-19 Era and Beyond. Antivir. Chem. Chemother. 2020, 28, 2040206620961712. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The Role of Polyphenols in Abiotic Stress Response: The Influence of Molecular Structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Manso, T.; Lores, M.; de Miguel, T. Antimicrobial Activity of Polyphenols and Natural Polyphenolic Extracts on Clinical Isolates. Antibiotics 2022, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Paprštein, F.; Sedlák, J.; Svobodová, L.; Polák, J.; Gadiou, S. Results of in Vitro Chemotherapy of Apple Cv. Fragrance—Short Communication. Hortic. Sci. 2013, 40, 186–190. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]


| Sample * | DNA Internal Control Ct | AP Phytoplasma Ct | Relative Conc. of Phytopl. DNA |
|---|---|---|---|
| in vivo—mother plant | 11.09 | 15.09 | 1.9593 |
| in vitro control (1) | 11.04 | 15.95 | 1.0427 |
| in vitro control (2) | 10.48 | 15.79 | 0.7902 |
| in vitro control (3) | 10.45 | 15.34 | 1.0573 |
| in vitro control (4) | 12.04 | 16.86 | 1.1098 |
| cla10 (1) | 10.39 | 19.89 | 0.0433 |
| cla10 (2) | 10.26 | 21.49 | 0.0131 |
| cla10 (3) | 10.59 | 19.69 | 0.0571 |
| cla10 (4) | 9.57 | 20.76 | 0.0134 |
| cla10 (5) | 11.97 | 20.64 | 0.0770 |
| cla10 (6) | 10.54 | 18.84 | 0.0995 |
| cla10 (7) | 10.45 | 20.46 | 0.0304 |
| cla10 (8) | 9.71 | 19.73 | 0.0302 |
| cla10 (9) | 10.55 | 19.92 | 0.0474 |
| cla10 (10) | 10.60 | 19.51 | 0.0652 |
| cla10 (11) | 10.74 | 19.78 | 0.0596 |
| cla10 (12) | 10.29 | 22.85 | 0.0052 |
| cla10 (13) | 9.89 | 25.19 | 0.0008 |
| cla10 (14) | 10.41 | 18.03 | 0.1594 |
| cla10 (15) | 10.39 | 19.79 | 0.0464 |
| cla20 (1) | 10.74 | 20.97 | 0.0261 |
| cla20 (2) | 10.41 | 33.99 | 2.5 × 10−6 |
| cla20 (3) | 9.74 | 31.54 | 8.6 × 10−6 |
| cla20 (4) | 10.21 | 19.85 | 0.0393 |
| cla20 (5) | 9.41 | 21.50 | 0.0072 |
| cla20 (6) | 10.06 | 22.61 | 0.0052 |
| cla20 (7) | 9.74 | 20.53 | 0.0177 |
| cla20 (8) | 9.78 | 24.70 | 0.0010 |
| cla20 (9) | 9.75 | 21.37 | 0.0100 |
| cla20 (10) | 10.06 | – | negative |
| cla20 (11) | 9.82 | 24.25 | 0.0014 |
| cla20 (12) | 9.87 | 23.27 | 0.0029 |
| cla20 (13) | 9.92 | 30.81 | 1.6 × 10−5 |
| cla20 (14) | 11.19 | 23.17 | 0.0078 |
| cla40 (1) | 10.05 | 24.13 | 0.0018 |
| cla40 (2) | 10.41 | 22.60 | 0.0067 |
| cla40 (3) | 10.01 | 34.61 | 1.2 × 10−6 |
| Sample | DNA Internal Control Ct | AP Phytoplasma Ct | Relative Conc. of Phytopl. DNA |
|---|---|---|---|
| untreated culture (1) | 13.55 | 17.01 | 2.8487 |
| untreated culture (2) | 13.37 | 17.01 | 2.5146 |
| untreated culture (3) | 12.76 | 16.52 | 2.3139 |
| untreated culture (4) | 12.66 | 16.64 | 1.9866 |
| cla20 (10) | 12.27 | – | negative |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semerák, M.; Sedlák, J.; Čmejla, R. Clarithromycin Suppresses Apple Proliferation Phytoplasma in Explant Cultures. Plants 2023, 12, 3820. https://doi.org/10.3390/plants12223820
Semerák M, Sedlák J, Čmejla R. Clarithromycin Suppresses Apple Proliferation Phytoplasma in Explant Cultures. Plants. 2023; 12(22):3820. https://doi.org/10.3390/plants12223820
Chicago/Turabian StyleSemerák, Matěj, Jiří Sedlák, and Radek Čmejla. 2023. "Clarithromycin Suppresses Apple Proliferation Phytoplasma in Explant Cultures" Plants 12, no. 22: 3820. https://doi.org/10.3390/plants12223820
APA StyleSemerák, M., Sedlák, J., & Čmejla, R. (2023). Clarithromycin Suppresses Apple Proliferation Phytoplasma in Explant Cultures. Plants, 12(22), 3820. https://doi.org/10.3390/plants12223820






