Fruit Quality and Metabolomic Analyses of Fresh Food Accessions Provide Insights into the Key Carbohydrate Metabolism in Blueberry
Abstract
:1. Introduction
2. Results
2.1. Appearance Quality Characteristics of Different Blueberry Fruits
2.2. Flavor Quality Traits of Different Blueberry Fruits
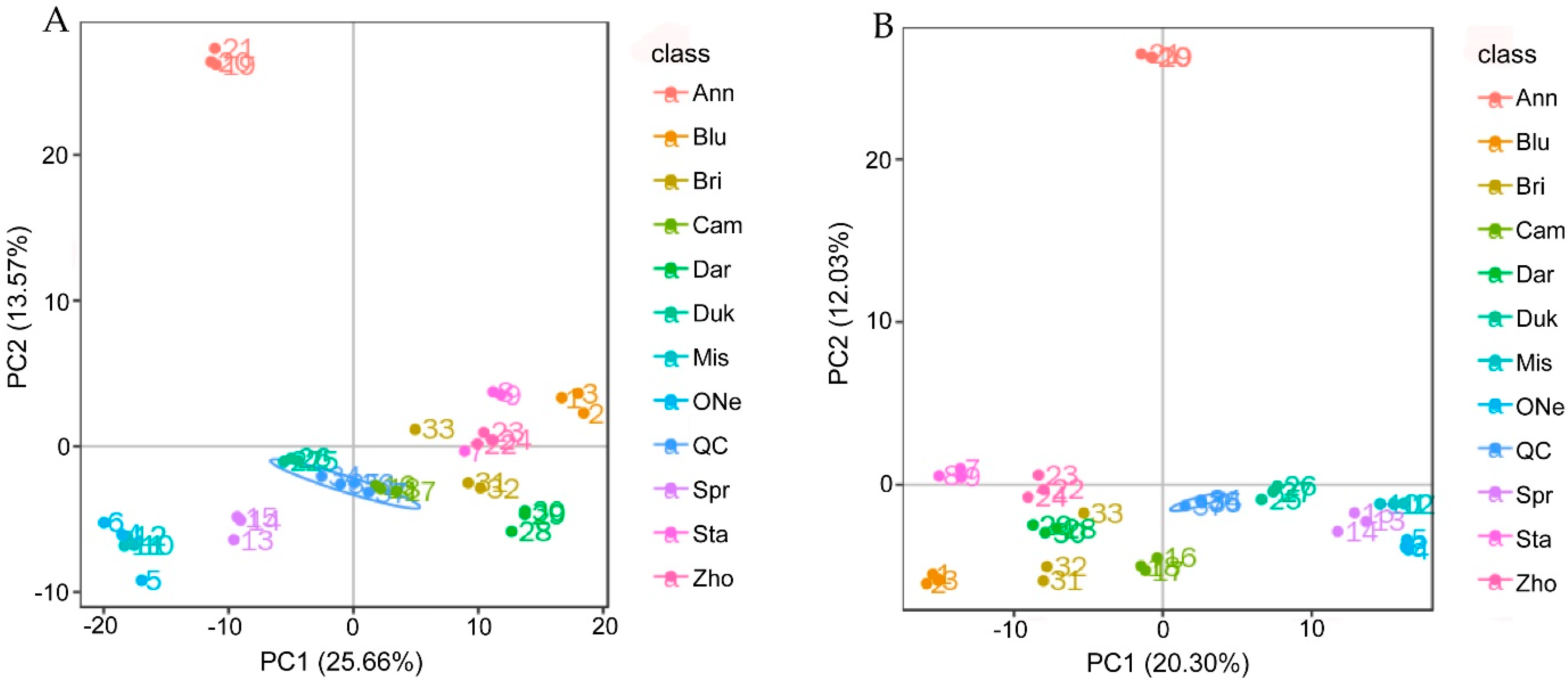
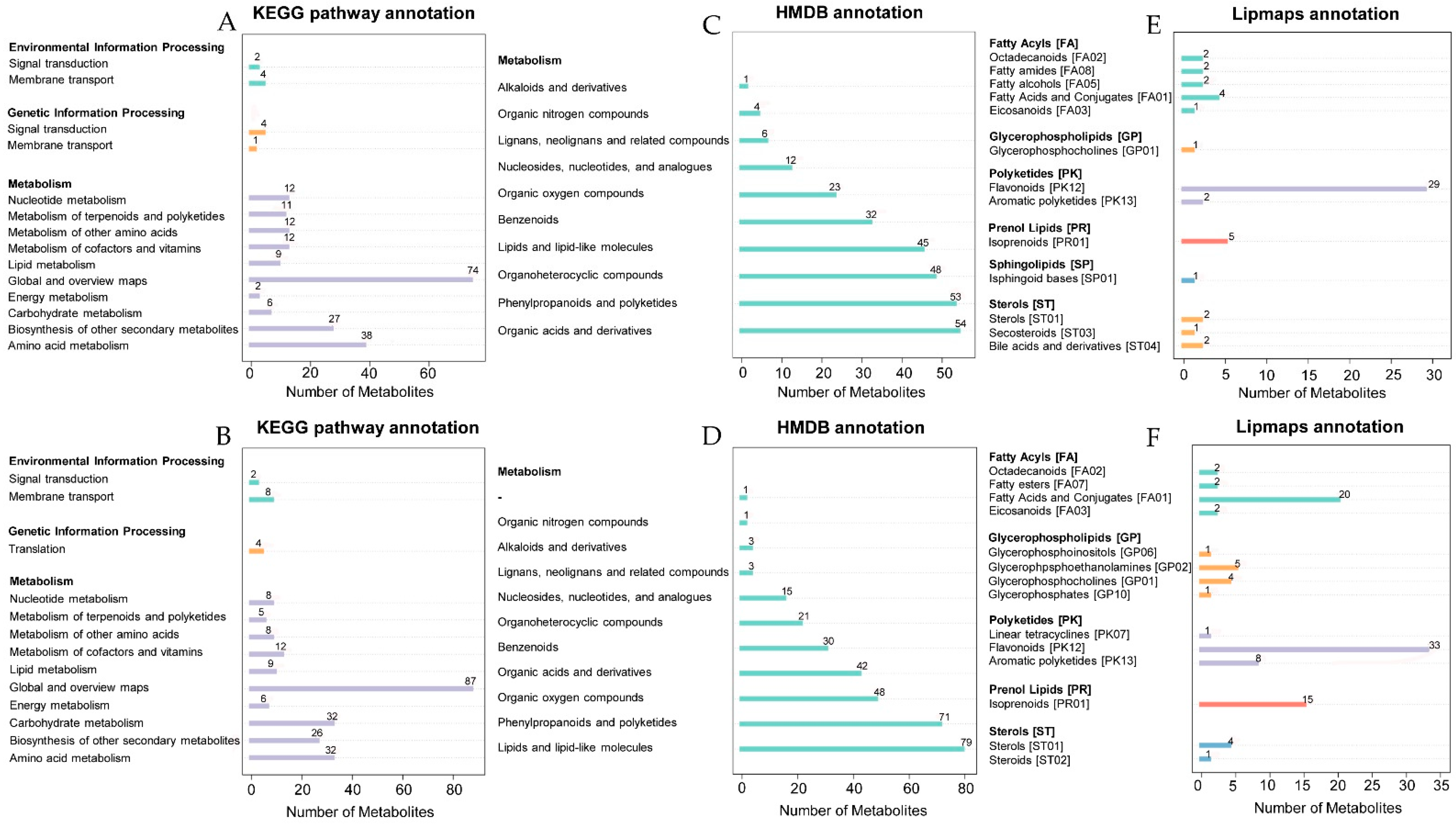
2.3. Non-targeted Metabolomics Annotation Revealed the Allocation of Metabolites
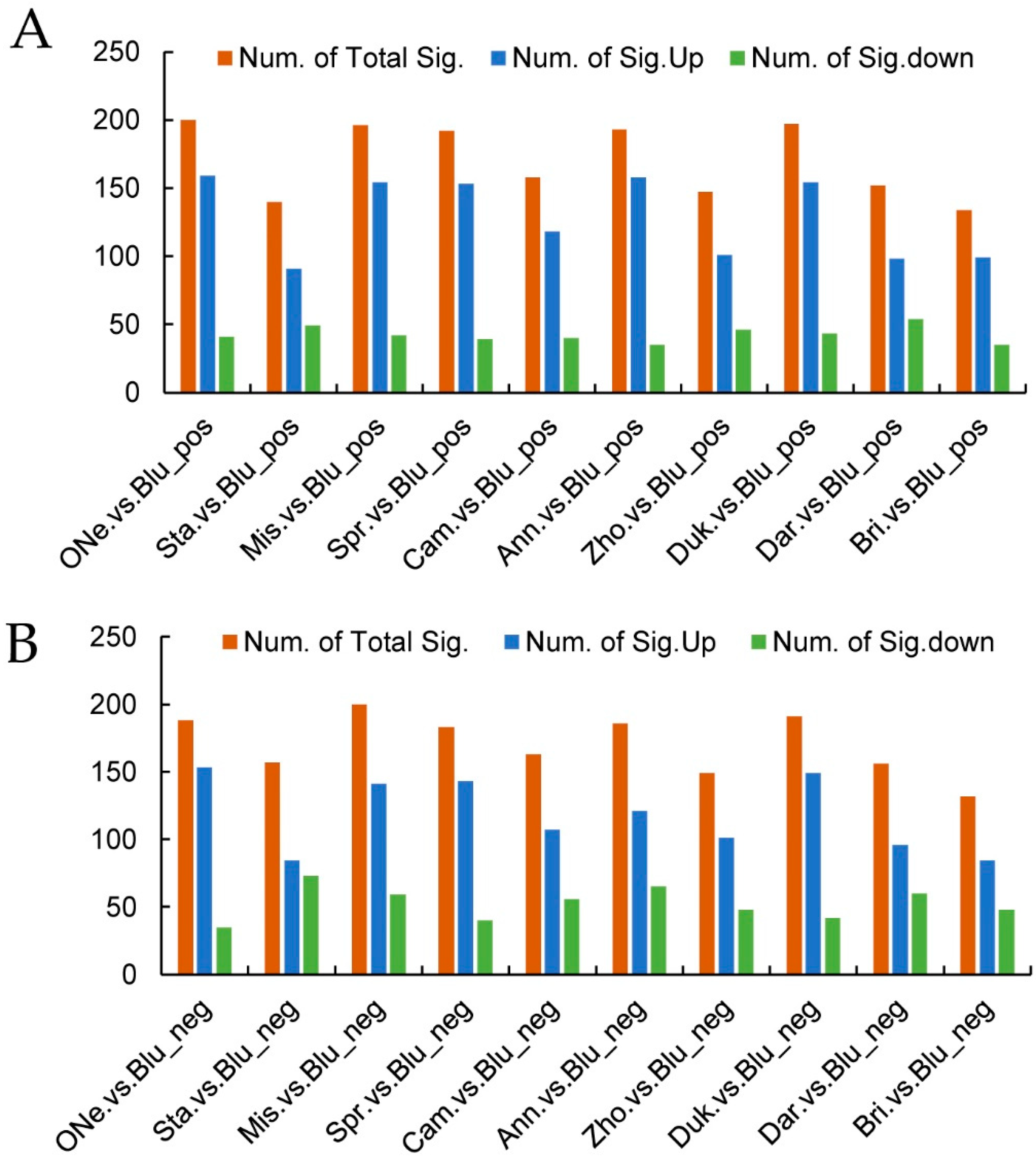
2.4. Differentially Accumulated Metabolites (DAMs) Analysis
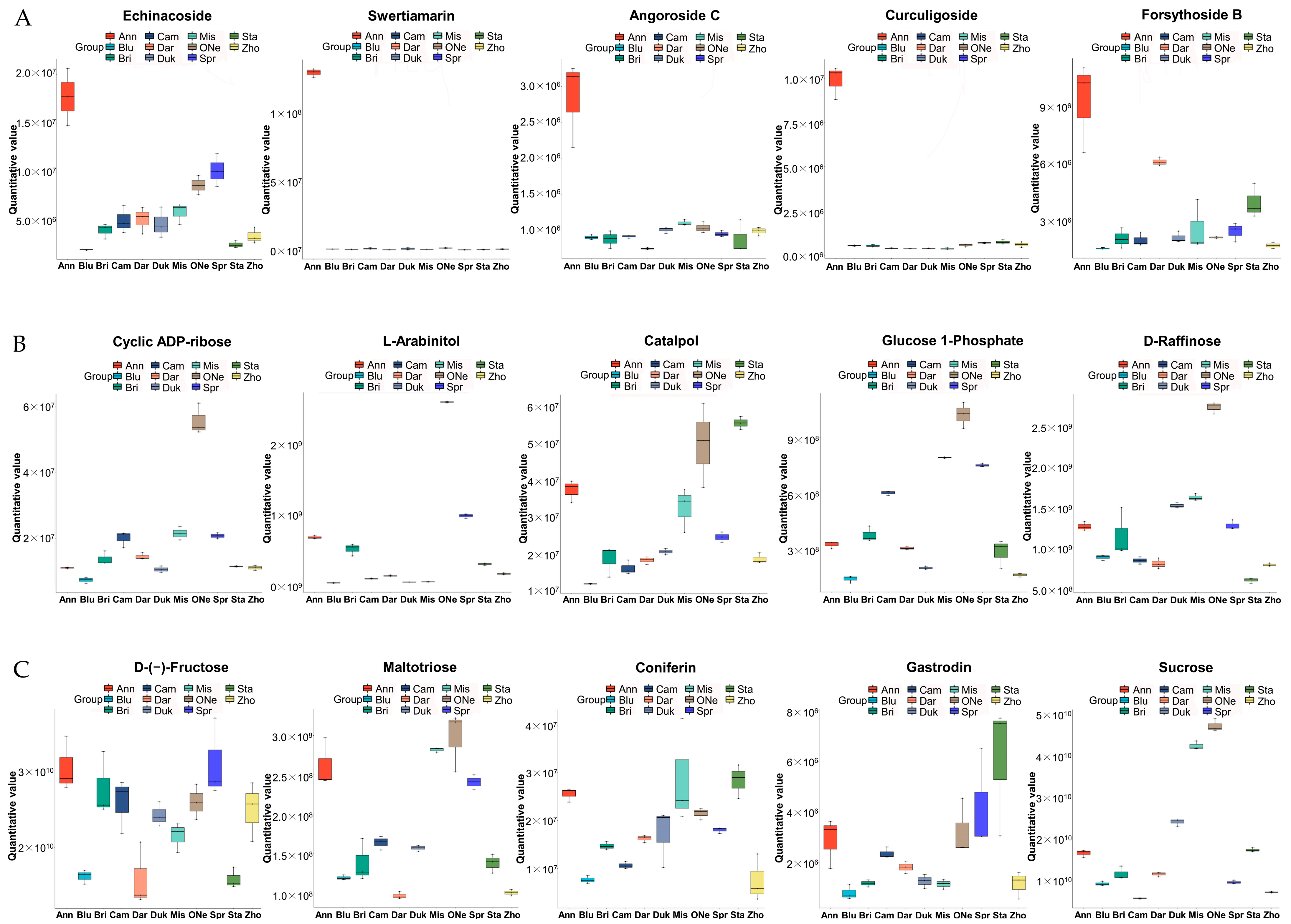
2.5. Analysis of Differentially Accumulated Carbohydrate Profiles
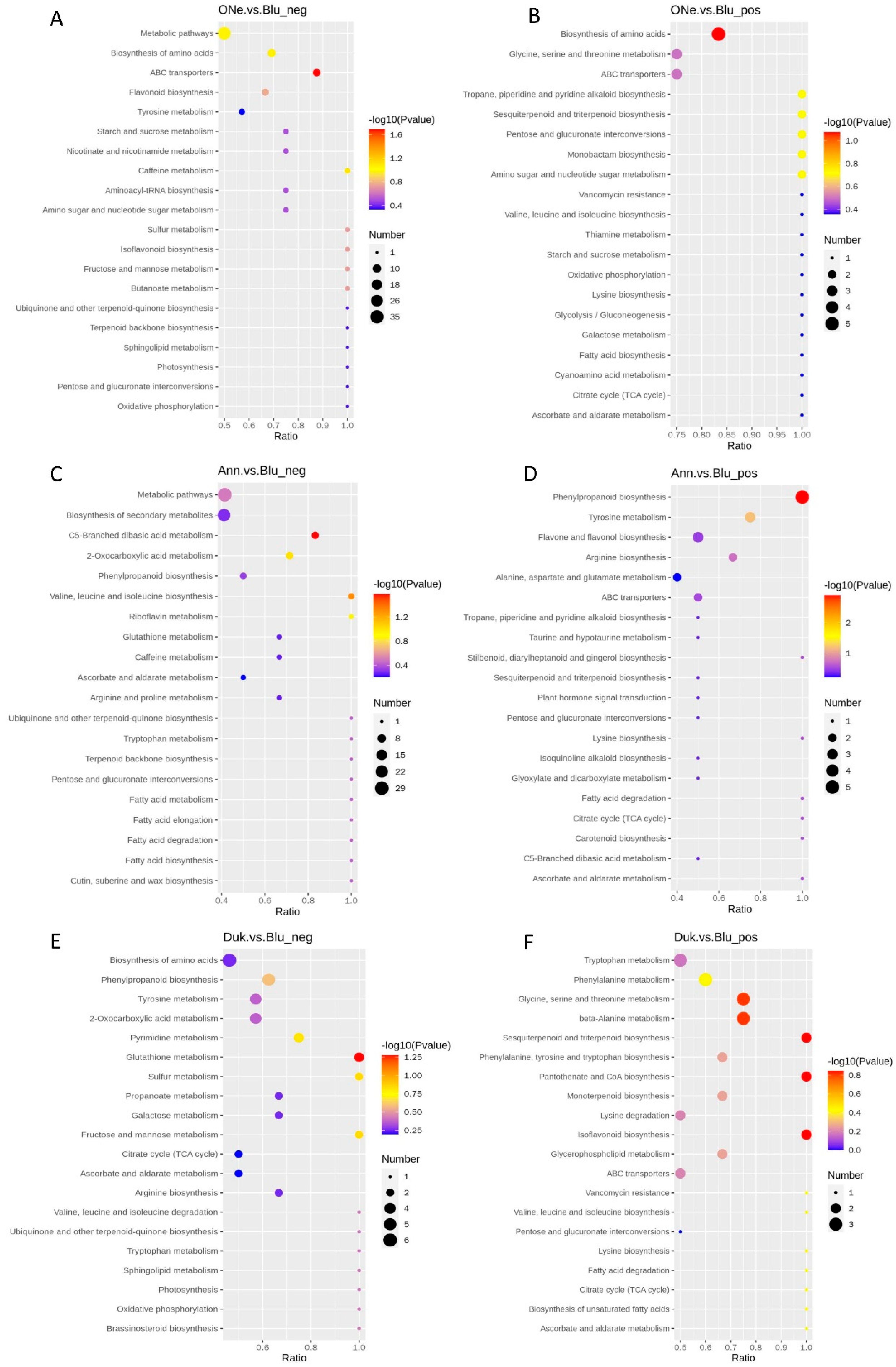
2.6. KEGG Pathway Enrichment Analysis of DAMs
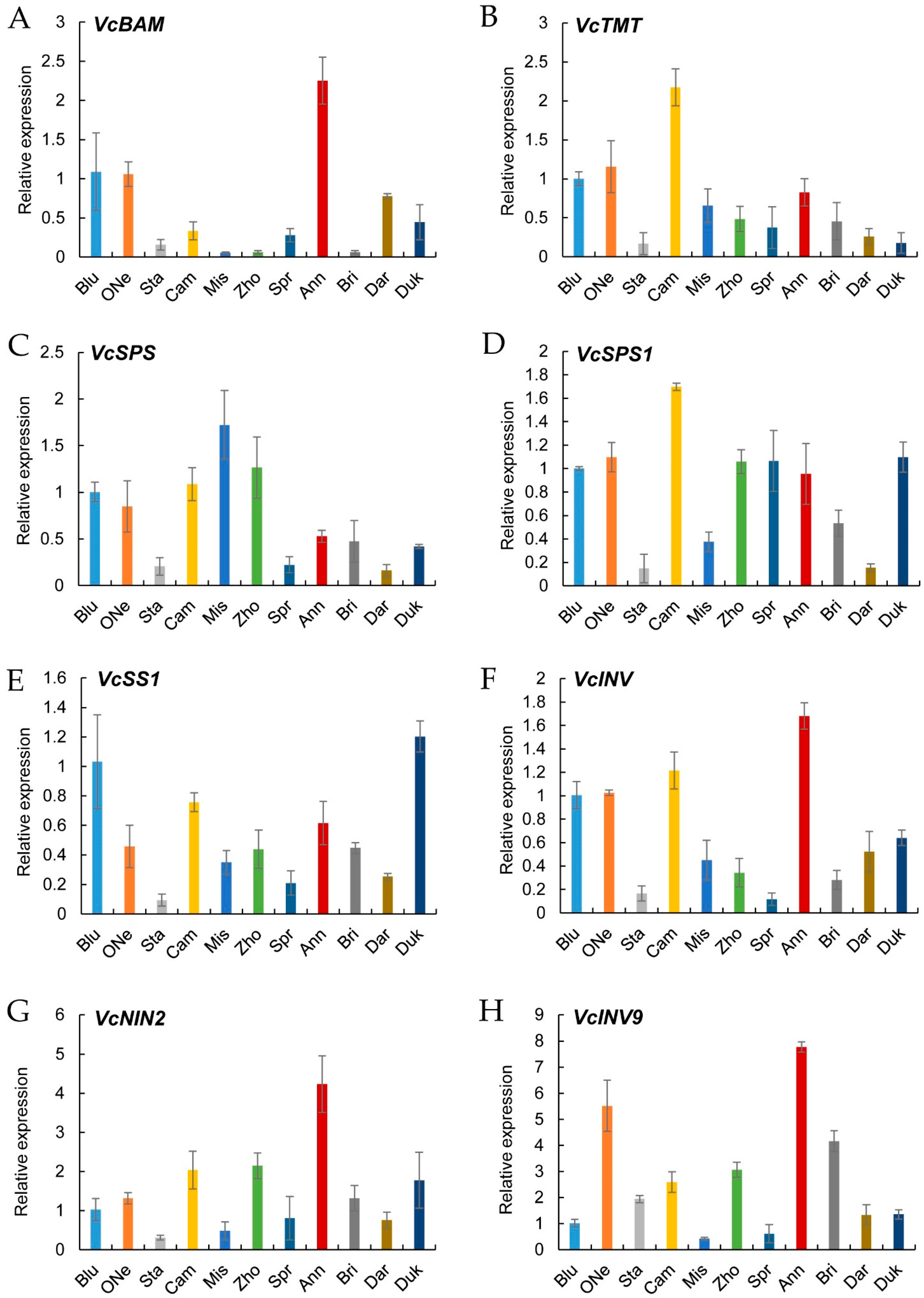
2.7. Verification of Sugar Metabolism Genes by Quantitative Real-Time PCR (qRT-PCR)
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Analysis of the Appearance Quality of Different Blueberry Cultivars
4.3. Analysis of the Fruit Flavor Quality Traits of Different Blueberry Cultivars
4.4. Nontargeted LC-MS-Based Metabolic Profiling and Identification
4.5. QRT-PCR Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gosch, C. Antioxidative effects of blueberries (Vaccinium sp.): An overview. Erwerbs Obstbau 2003, 45, 117–124. [Google Scholar]
- Li, D.N.; Li, B.; Ma, Y.; Sun, X.Y.; Lin, Y.; Meng, X.J. Polyphenols, anthocyanins, and flavonoids contents and the antioxidant capacity of various cultivars of highbush and half-high blueberries. J. Food Compos. Anal. 2017, 62, 84–93. [Google Scholar] [CrossRef]
- Kalt, W.; Ryan, D.A.J.; Duy, J.C.; Prior, R.L.; Ehlenfeldt, M.K.; Vander Kloet, S.P. Interspecific variation in anthocyanins, phenolics, and antioxidant capacity among genotypes of highbush and lowbush blueberries (Vaccinium section cyanococcus spp.). J. Agric. Food Chem. 2001, 49, 4761–4767. [Google Scholar] [CrossRef]
- Saftner, R.; Polashock, J.; Ehlenfeldt, M.; Vinyard, B. Instrumental and sensory quality characteristics of blueberry fruit from twelve cultivars. Postharvest Biol. Technol. 2008, 49, 19–26. [Google Scholar] [CrossRef]
- Gilbert, J.L.; Schwieterman, M.L.; Colquhoun, T.A.; Clark, D.G.; Olmstead, J.W. Potential for increasing southern highbush blueberry flavor acceptance by breeding for major volatile components. HortScience 2013, 48, 835–843. [Google Scholar] [CrossRef]
- Gilbert, J.L.; Olmstead, J.W.; Colquhoun, T.A.; Levin, L.A.; Clark, D.G.; Moskowitz, H.R. Consumer-assisted selection of blueberry fruit quality traits. HortScience 2014, 49, 864–873. [Google Scholar] [CrossRef]
- Gilbert, J.L.; Guthart, M.J.; Gezan, S.A.; de Carvalho, M.P.; Schwieterman, M.L.; Colquhoun, T.A.; Bartoshuk, L.M.; Sims, C.A.; Clark, D.G.; Olmstead, J.W. Identifying breeding priorities for blueberry flavor using biochemical, sensory, and genotype by environment analyses. PLoS ONE 2015, 10, e0138494. [Google Scholar] [CrossRef]
- Barrett, D.M.; Beaulieu, J.C.; Shewfelt, R. Color, flavor, texture, and nutritional quality of fresh-cut fruits and vegetables: Desirable levels, instrumental and sensory measurement, and the effects of processing. Crit. Rev. Food Sci. Nutr. 2010, 50, 369–389. [Google Scholar] [CrossRef]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food; Springer: New York, NY, USA, 2010. [Google Scholar]
- Forney, C.F. Horticultural and other factors affecting aroma volatile composition of small fruit. HortTechnology 2001, 11, 529–538. [Google Scholar] [CrossRef]
- Cebolla-Cornejo, J.; Rosello, S.; Valcarcel, M.; Serrano, E.; Beltran, J.; Nuez, F. Evaluation of genotype and environment effects on taste and aroma flavor components of Spanish fresh tomato varieties. J. Agric. Food Chem. 2011, 59, 2440–2450. [Google Scholar] [CrossRef]
- Forney, C.F.; Qiu, S.S.; Jordan, M.A.; McCarthy, D.; Fillmore, S. Comparison of volatile compounds contributing to flavor of wild lowbush (Vaccinium augustifolium) and cultivated highbush (Vaccinium corymbosum) blueberry fruit using Gas Chromatography-Olfactometry. Foods 2022, 11, 2516. [Google Scholar] [CrossRef] [PubMed]
- Galletta, G.J.; Ballinger, W.E.; Monroe, R.J.; Kushman, L.J. Relationships between fruit acidity and soluble solids levels of highbush blueberry clones and fruit keeping quality. J. Am. Soc. Hortic. Sci. 1971, 96, 758–762. [Google Scholar] [CrossRef]
- Du, X.F.; Plotto, A.; Song, M.; Olmstead, J.; Rouseff, R. Volatile composition of four southern highbush blueberry cultivars and effect of growing location and harvest date. J. Agric. Food Chem. 2011, 59, 8347–8357. [Google Scholar] [CrossRef]
- Forney, C.F.; Kalt, W.; Jordan, M.A.; Vinqvist-Tymchuk, M.R.; Fillmore, S.A.E. Blueberry and cranberry fruit composition during development. J. Berry Res. 2012, 2, 169–177. [Google Scholar] [CrossRef]
- Nookaraju, A.; Upadhyaya, C.P.; Pandey, S.K.; Young, K.E.; Hong, S.J.; Park, S.K.; Park, S.W. Molecular approaches for enhancing sweetness in fruits and vegetables. Sci. Hortic. 2010, 127, 1–15. [Google Scholar] [CrossRef]
- Li, X.B.; Li, C.N.; Sun, J.; Jackson, A. Dynamic changes of enzymes involved in sugar and organic acid level modification during blueberry fruit maturation. Food Chem. 2020, 309, 125617. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Huang, G.H.; Zhang, Q.; Wang, Y.H.; Dia, V.P.; Meng, X.J. Ripening affects the physicochemical properties, phytochemicals and antioxidant capacities of two blueberry cultivars. Postharvest Biol. Technol. 2020, 162, 111097. [Google Scholar] [CrossRef]
- Miron, D.; Schaffer, A.A. Sucrose phosphate synthase, sucrose synthase, and invertase activities in developing fruit of Lycopersicon esculentum Mill. and the sucrose accumulating Lycopersicon hirsutum Humb. and Bonpl. Plant Physiol. 1991, 2, 623–627. [Google Scholar] [CrossRef]
- Lee, J.H.; Yu, D.J.; Kim, S.J.; Choi, D.; Lee, H.J. Intraspecies differences in cold hardiness, carbohydrate content and beta-amylase gene expression of Vaccinium corymbosum during cold acclimation and deacclimation. Tree Physiol. 2012, 32, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Cheng, Y.S.; Lu, J.H.; Chen, J.Q.; Wang, Y.Z.; Zhang, S.L.; Zhang, H.P. The gene PbTMT4 from pear (Pyrus bretschneideri) mediates vacuolar sugar transport and strongly affects sugar accumulation in fruit. Physiol. Plant. 2018, 164, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Reuscher, S.; Akiyama, M.; Yasuda, T.; Makino, H.; Aoki, K.; Shibata, D.; Shiratake, K. The sugar transporter inventory of tomato: Genome-wide identification and expression analysis. Plant Cell Physiol. 2014, 55, 1123–1141. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.J.; Wang, Y.L.; Wang, M.M.; Han, H.Y.; Luo, Y.S.; Ding, W.Q.; Xu, W.L.; Zhong, Y.J.; Huang, H.X.; Qu, S.P. Soluble sugars accumulation and related gene expression during fruit development in Cucurbita maxima Duchesne. Sci. Hortic. 2020, 272, 109520. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, R.L.; Li, B.Q.; Tian, S.P. Characterization of genes encoding key enzymes involved in sugar metabolism of apple fruit in controlled atmosphere storage. Food Chem. 2013, 141, 3323–3328. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.J.; Sun, M.H.; Lu, J.; Liu, Y.J.; Hu, D.G.; Hao, Y.J. Transcription factor AREB2 is involved in soluble sugar accumulation by activating sugar transporter and amylase genes. Plant Physiol. 2017, 174, 2348–2362. [Google Scholar] [CrossRef]
- Nguyen, C.T.T.; Lee, J.H.; Tran, P.T. Accumulation of sugars and associated gene expression in highbush blueberries differ by cultivar, ripening stage, and storage temperature. J. Berry Res. 2021, 11, 511–527. [Google Scholar] [CrossRef]
- Wang, S.Y.; Zhou, Q.; Zhou, X.; Zhang, F.; Ji, S.J. Ethylene plays an important role in the softening and sucrose metabolism of blueberries postharvest. Food Chem. 2020, 310, 125965. [Google Scholar] [CrossRef]
- Nguyen, C.T.T.; Yang, S.O.; Choi, W.S. Changes in sucrose and the VcADR gene induced sugar accumulation in blueberries during ripening and after chitosan treatment. J. Berry Res. 2022, 12, 401–414. [Google Scholar] [CrossRef]
- Xu, W.; Chen, C.; Deng, S.; Liu, Y.; Lyu, Y. Comprehensive evaluation of blueberry quality based on principal component analysis and cluster analysis. Sci. Technol. Food Ind. 2022, 43, 311–319, (in Chinese with English abstract). [Google Scholar]
- Shi, J.Y.; Xiao, Y.H.; Jia, C.L.; Zhang, H.M.; Gan, Z.L.; Li, X.Y.; Yang, M.Q.; Yin, Y.D.; Zhang, G.Q.; Hao, J.Y.; et al. Physiological and biochemical changes during fruit maturation and ripening in highbush blueberry (Vaccinium corymbosum L.). Food Chem. 2023, 410, 135299. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.R.; Liu, C.; Lu, X.C.; Tong, Z.G.; Tao, C.G.; Yuan, X.F. Comprehensive transcriptome analyses of carbohydrate metabolism and flavonoid biosynthesis in blueberry (Vaccinium corymbosum) during fruit maturation. Int. J. Agric. Biol. 2020, 24, 101–111. [Google Scholar]
- Colantonio, V.; Ferra, L.F.V.; Tieman, D.M.; Bliznyuk, N.; Sims, C.; Klee, H.J.; Munoz, P.; Resende, M.F.R. Metabolomic selection for enhanced fruit flavor. Proc. Natl. Acad. Sci. USA 2022, 119, e2115865119. [Google Scholar] [CrossRef]
- Ferrᾶo, L.F.V.; Sater, H.; Lyrene, P.; Amadeu, R.R.; Sims, C.A.; Tieman, D.M.; Munoz, P.R. Terpene volatiles mediates the chemical basis of blueberry aroma and consumer acceptability. Food Res. Int. 2022, 158, 111468. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Peng, B.; Yuan, F. Volatile composition of eight blueberry cultivars and their relationship with sensory attributes. Flavour Frag. J. 2020, 35, 443–453. [Google Scholar] [CrossRef]
- Vrancheva, R.; Ivanov, I.; Dincheva, I.; Badjakov, I.; Pavlov, A. Triterpenoids and other non-polar compounds in leaves of wild and cultivated Vaccinium species. Plants 2021, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Bersaneti, G.T.; Prudencio, S.H.; Mali, S.; Celligoi, M.A.P.C. Assessment of a new edible film biodegradable based on starch-nystose to increase quality and the shelf life of blackberries. Food Biosci. 2021, 42, 101173. [Google Scholar] [CrossRef]
- Olmo, I.D.; Blanch, M.; Romero, I.; Vazquez-Hernandez, M.; Sanchez-Ballesta, M.T.; Escribano, M.I.; Merodio, C. Involvement of oligosaccharides and sucrose-related genes on sucrose retention in strawberries from ripening to shelf-life. Postharvest Biol. Technol. 2020, 169, 111301. [Google Scholar] [CrossRef]
- Hubbard, N.L.; Huber, S.C.; Pharr, D.M. Sucrose phosphate synthase and acid invertase as determinants of sucrose concentration in developing Muskmelon (Cucumis melo L.) fruits. Plant Physiol. 1989, 91, 1527–1534. [Google Scholar] [CrossRef]
- Darnell, R.L.; Cano-Medrano, R.; Koch, K.E.; Avery, M.L. Differences in sucrose metabolism relative to accumulation of bird-deterrent sucrose levels in fruits of wild and domestic Vaccinium species. Physiol. Plant. 2010, 92, 336–342. [Google Scholar] [CrossRef]
- Ferrᾶo, L.F.V.; Johnson, T.S.; Benevenuto, J.; Edger, P.P.; Colquhoun, T.A.; Munoz, P.R. Genome-wide association of volatiles reveals candidate loci for blueberry flavor. New Phytol. 2020, 226, 1725–1737. [Google Scholar] [CrossRef]
- Wang, Y.S.; Luo, Z.S.; Du, R.X. Nitric oxide delays chlorophyll degradation and enhances antioxidant activity in banana fruits after cold storage. Acta Physiol. Plant. 2015, 37, 74–83. [Google Scholar] [CrossRef]

| Cultivars | Transverse Diameter (mm) | Longitudinal Diameter (mm) | Fruit Shape Index * | Fruit Weight (g/per Berry) | Firmness (N) |
|---|---|---|---|---|---|
| Bluerain | 12.83 ± 0.41 e | 12.54 ± 0.48 cd | 0.98 ± 0.04 a | 0.94 ± 0.13 h | 3.75 ± 0.49 a |
| O’Neal | 15.36 ± 0.75 cd | 12.36 ± 0.53 cd | 0.81 ± 0.04 cd | 1.47 ± 0.28 fg | 2.64 ± 0.21 cd |
| Star | 15.77 ± 0.96 bcd | 12.79 ± 0.74 c | 0.81 ± 0.04 cd | 1.95 ± 0.28 cd | 3.18 ± 0.19 b |
| Camellia | 15.01 ± 0.80 d | 11.22 ± 0.87 e | 0.75 ± 0.05 ef | 1.54 ± 0.35 ef | 3.15 ± 0.33 b |
| Misty | 16.08 ± 1.14 bc | 12.45 ± 0.74 cd | 0.78 ± 0.05 de | 1.78 ± 0.39 cde | 3.80 ± 0.27 a |
| Zhongzhi 3 | 15.71 ± 1.14 bcd | 12.18 ± 0.45 cd | 0.78 ± 0.04 cde | 1.73 ± 0.32 def | 3.03 ± 0.29 b |
| Springhigh | 17.40 ± 1.01 a | 14.20 ± 0.74 a | 0.82 ± 0.05 bc | 3.03 ± 0.49 a | 2.72 ± 0.30 c |
| Anna | 13.30 ± 0.93 e | 11.30 ± 0.57 e | 0.85 ± 0.04 b | 1.23 ± 0.15 g | 3.23 ± 0.17 b |
| Brigitta | 17.90 ± 1.04 a | 13.40 ± 0.69 b | 0.75 ± 0.01 ef | 2.60 ± 0.40 b | 3.25 ± 0.15 b |
| Darrow | 15.53 ± 0.86 cd | 10.92 ± 0.73 e | 0.70 ± 0.03 g | 1.85 ± 0.29 cd | 2.41 ± 0.18 d |
| Duke | 16.51 ± 0.61 b | 11.97 ± 0.49 d | 0.73 ± 0.03 fg | 2.06 ± 0.22 c | 2.75 ± 0.21 c |
| Cultivar | Soluble Sugar (mg/g FW) | Titratable Acidity (TA) (%) | Soluble Solids (SS) (%) | SS/TA Ratio | Anthocyanin Content (mg/g FW) | Flavonoids Content (mg/g FW) | Total Polyphenols (mg/g FW) | VC Content (µg/mg FW) |
|---|---|---|---|---|---|---|---|---|
| Bluerain | 151.45 ± 1.03 b | 1.07 ± 0.02 a | 18.42 ± 0.61 a | 17.24 ± 0.29 de | 1.08 ± 0.00 | 4.74 ± 0.07 c | 0.28 ± 0.01 d | 103.52 ± 7.18 a |
| O’Neal | 143.50 ± 6.29 bc | 0.27 ± 0.02 | 16.16 ± 0.06 b | 60.96 ± 4.40 a | 1.83 ± 0.00 c | 4.89 ± 0.05 b | 0.32 ± 0.01 b | 88.58 ± 10.14 b |
| Star | 126.06 ± 6.40 ef | 0.79 ± 0.02 c | 14.68 ± 0.08 cd | 18.57 ± 0.44 d | 1.03 ± 0.00 | 2.86 ± 0.07 g | 0.27 ± 0.02 d | 47.01 ± 1.24 e |
| Camellia | 123.64 ± 1.03 fg | 0.47 ± 0.02 g | 14.92 ± 0.30 cd | 31.84 ± 1.23 b | 1.92 ± 0.00 b | 4.14 ± 0.05 d | 0.35 ± 0.01 a | 104.42 ± 4.25 a |
| Misty | 137.00 ± 1.67 cd | 0.61 ± 0.02 e | 14.30 ± 0.16 de | 23.40 ± 0.67 c | 1.15 ± 0.00 h | 3.85 ± 0.07 e | 0.35 ± 0.01 a | 68.12 ± 1.79 c |
| Zhongzhi 3 | 129.26 ± 0.92 def | 0.72 ± 0.02 d | 12.72 ± 0.13 bf | 17.76 ± 0.43 de | 1.19 ± 0.01 g | 2.85 ± 0.05 g | 0.32 ± 0.01 b | 49.46 ± 3.30 e |
| Springhigh | 133.71 ± 1.93 de | 0.44 ± 0.02 h | 14.80 ± 0.07 cd | 33.96 ± 1.36 b | 1.61 ± 0.00 e | 1.23 ± 0.07 | 0.30 ± 0.01 c | 85.99 ± 4.23 b |
| Anna | 172.01 ± 9.87 a | 0.26 ± 0.02 | 16.50 ± 1.33 b | 63.92 ± 4.42 a | 0.72 ± 0.00 | 0.95 ± 0.07 | 0.23 ± 0.01 e | 26.79 ± 1.29 f |
| Brigitta | 120.84 ± 3.27 fg | 0.86 ± 0.02 b | 13.70 ± 0.61 e | 15.96 ± 0.33 de | 1.34 ± 0.00 f | 2.66 ± 0.05 h | 0.22 ± 0.00 e | 58.08 ± 4.43 d |
| Darrow | 126.36 ± 6.00 ef | 1.03 ± 0.03 a | 15.20 ± 0.10 c | 14.71 ± 0.45 e | 1.76 ± 0.00 d | 3.69 ± 0.07 f | 0.30 ± 0.01 c | 119.52 ± 5.26 a |
| Duke | 115.58 ± 4.34 g | 0.53 ± 0.02 f | 12.70 ± 0.55 f | 23.80 ± 0.98 c | 2.22 ± 0.01 a | 5.56 ± 0.04 a | 0.35 ± 0.01 a | 108.13 ± 7.80 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Li, J.; Wang, J.; Lyu, L.; Wu, W.; Li, W.; Wu, Y. Fruit Quality and Metabolomic Analyses of Fresh Food Accessions Provide Insights into the Key Carbohydrate Metabolism in Blueberry. Plants 2023, 12, 3200. https://doi.org/10.3390/plants12183200
Zhang C, Li J, Wang J, Lyu L, Wu W, Li W, Wu Y. Fruit Quality and Metabolomic Analyses of Fresh Food Accessions Provide Insights into the Key Carbohydrate Metabolism in Blueberry. Plants. 2023; 12(18):3200. https://doi.org/10.3390/plants12183200
Chicago/Turabian StyleZhang, Chunhong, Jie Li, Jialuan Wang, Lianfei Lyu, Wenlong Wu, Weilin Li, and Yaqiong Wu. 2023. "Fruit Quality and Metabolomic Analyses of Fresh Food Accessions Provide Insights into the Key Carbohydrate Metabolism in Blueberry" Plants 12, no. 18: 3200. https://doi.org/10.3390/plants12183200
APA StyleZhang, C., Li, J., Wang, J., Lyu, L., Wu, W., Li, W., & Wu, Y. (2023). Fruit Quality and Metabolomic Analyses of Fresh Food Accessions Provide Insights into the Key Carbohydrate Metabolism in Blueberry. Plants, 12(18), 3200. https://doi.org/10.3390/plants12183200





