Meta-Analysis of Organic Fertilization Effects on Soil Bacterial Diversity and Community Composition in Agroecosystems
Abstract
:1. Introduction
2. Results
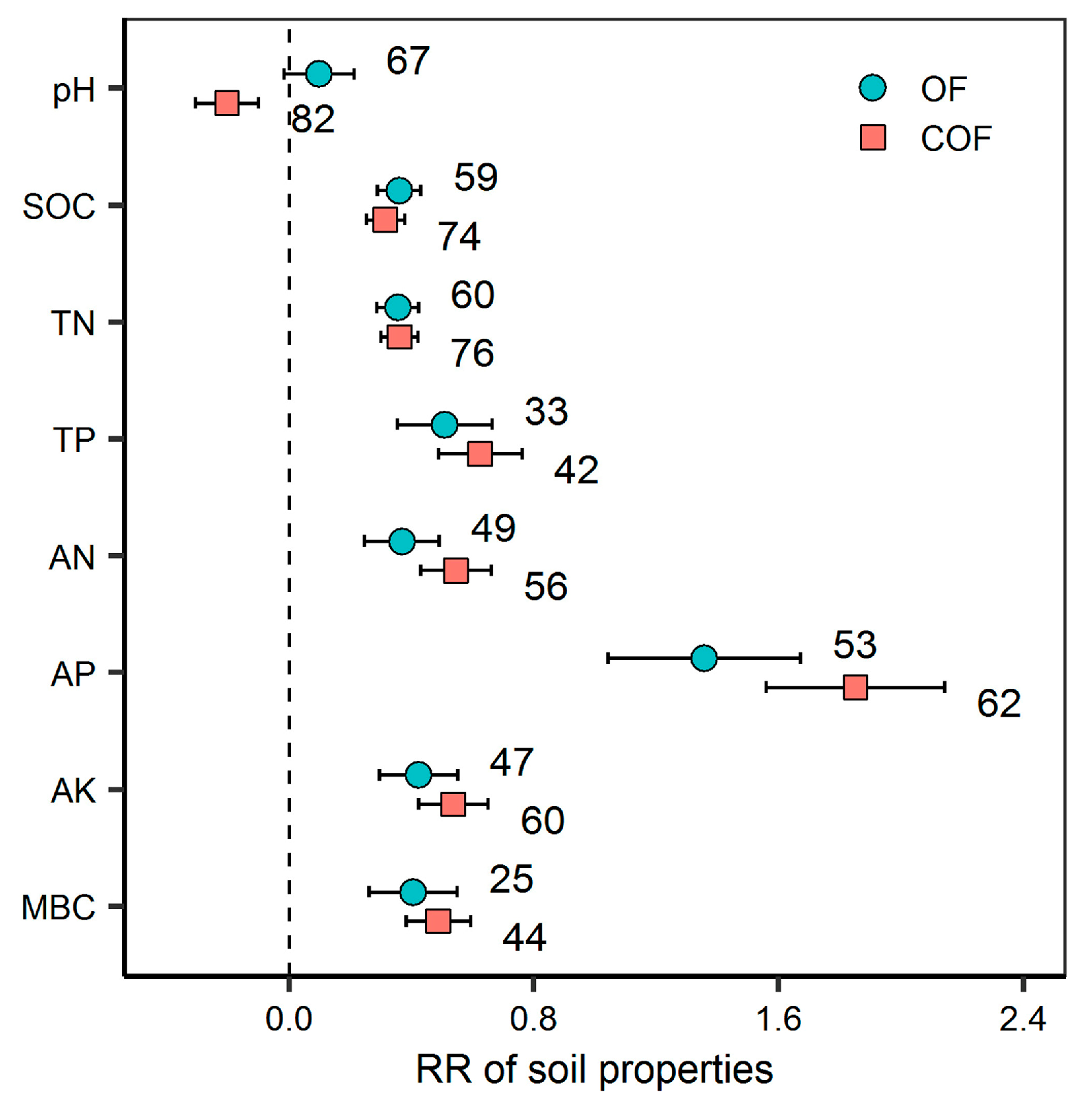
2.1. Response of Soil Properties to Different Fertilizer Regimes
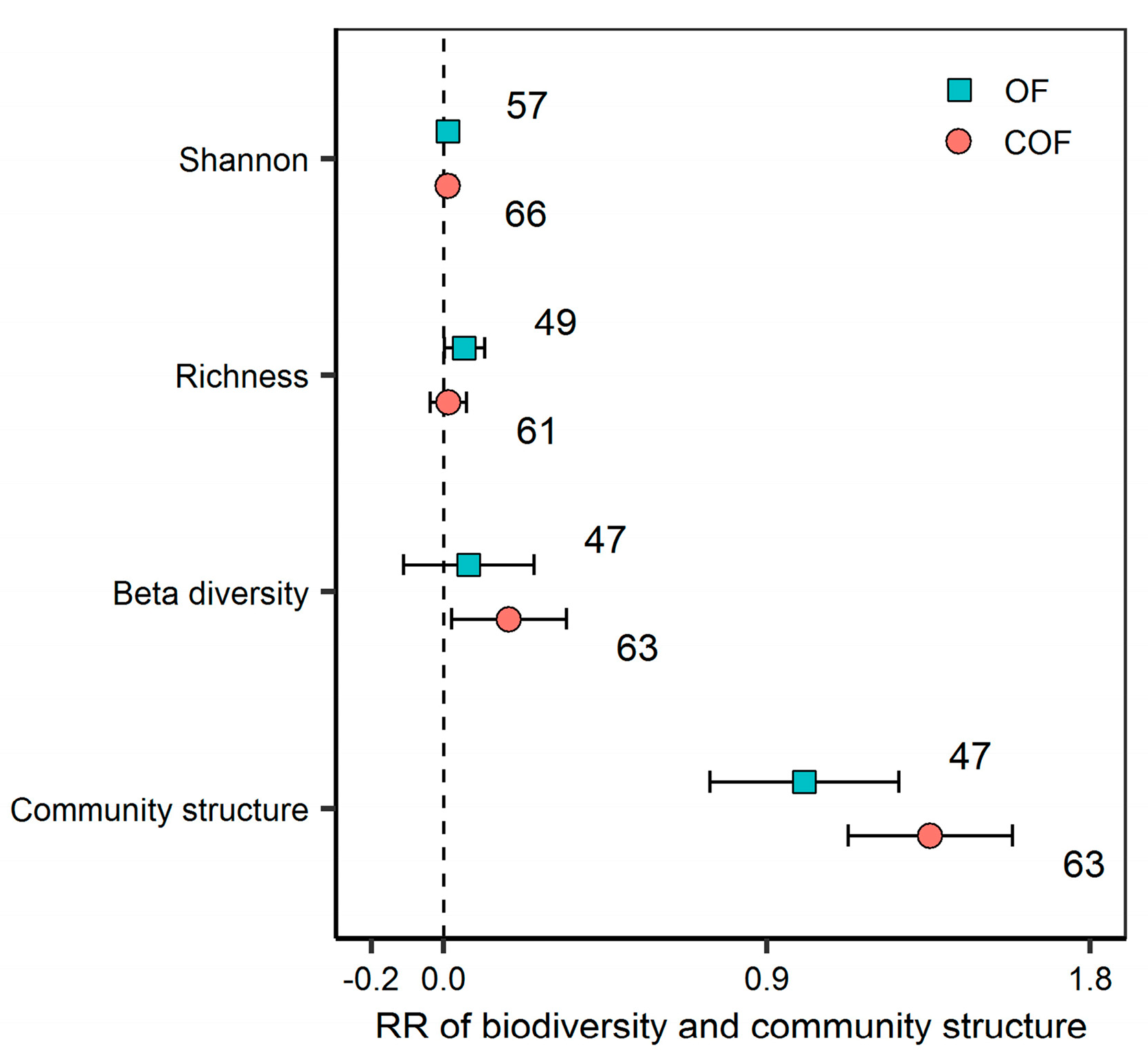
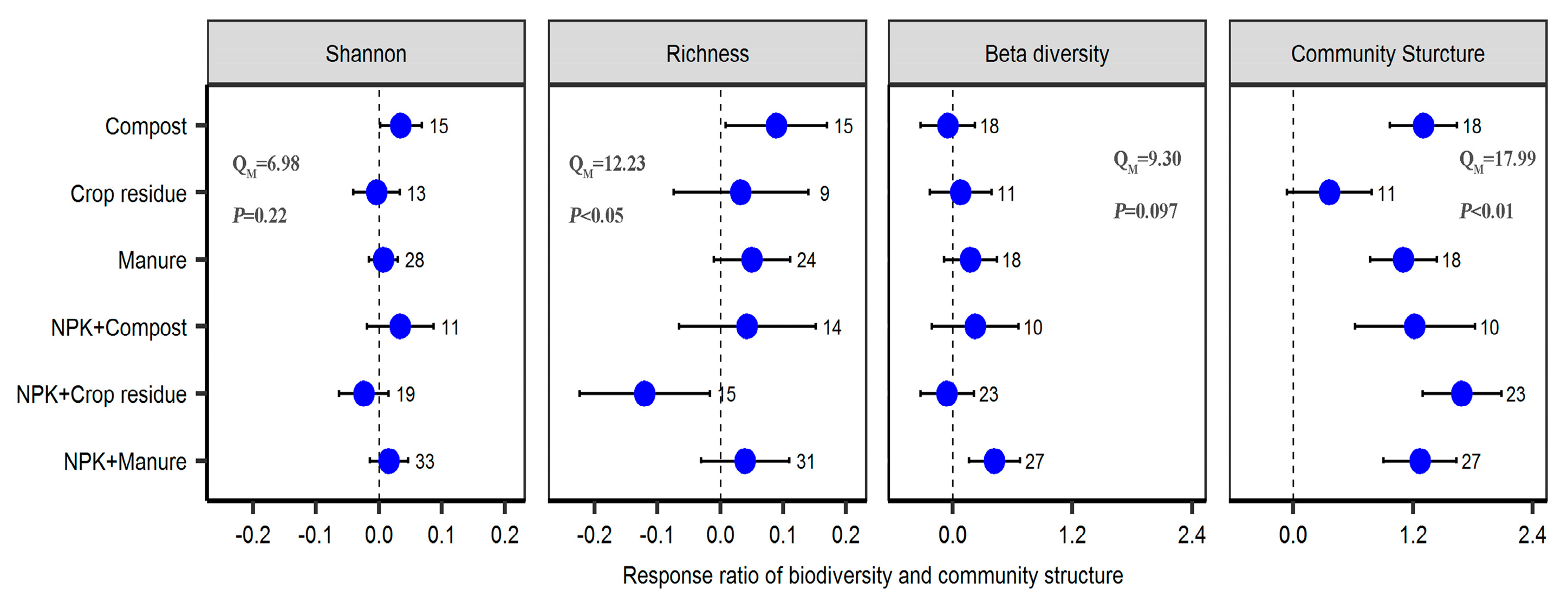
2.2. Response of Bacterial Diversity and Community Structure to Different Fertilizer Regimes
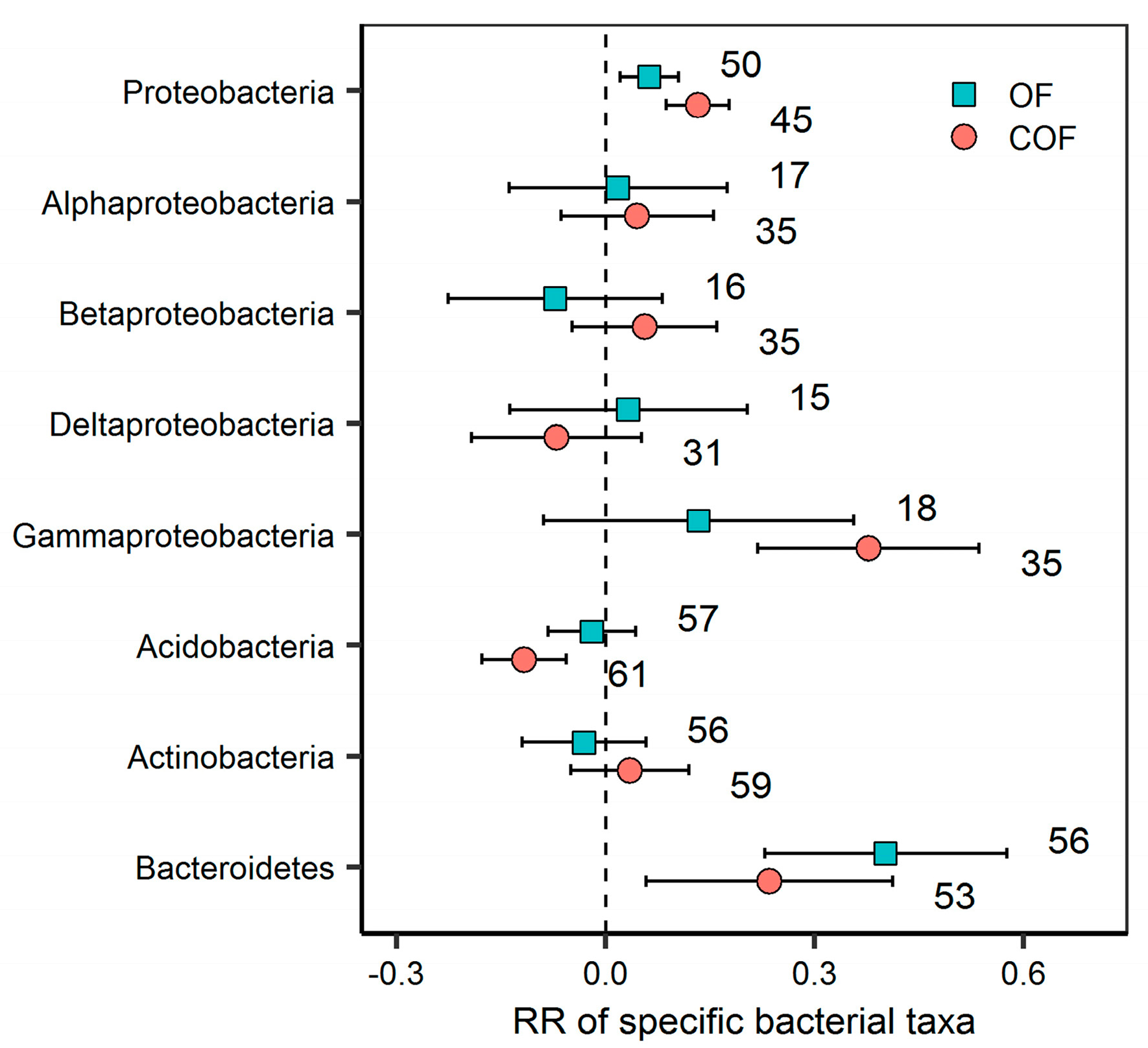
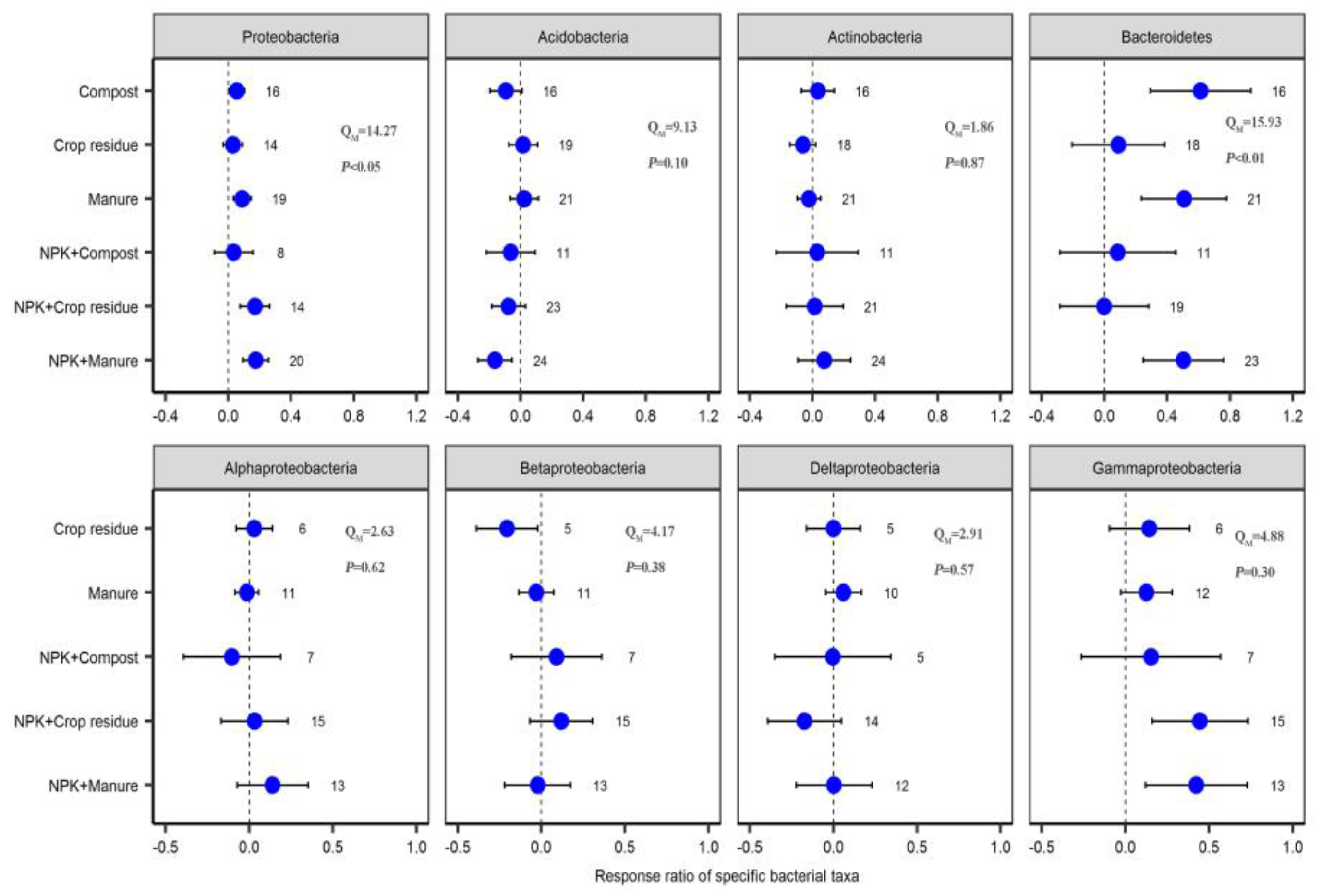
2.3. Response of Specific Bacterial Phyla to Different Fertilizer Regimes
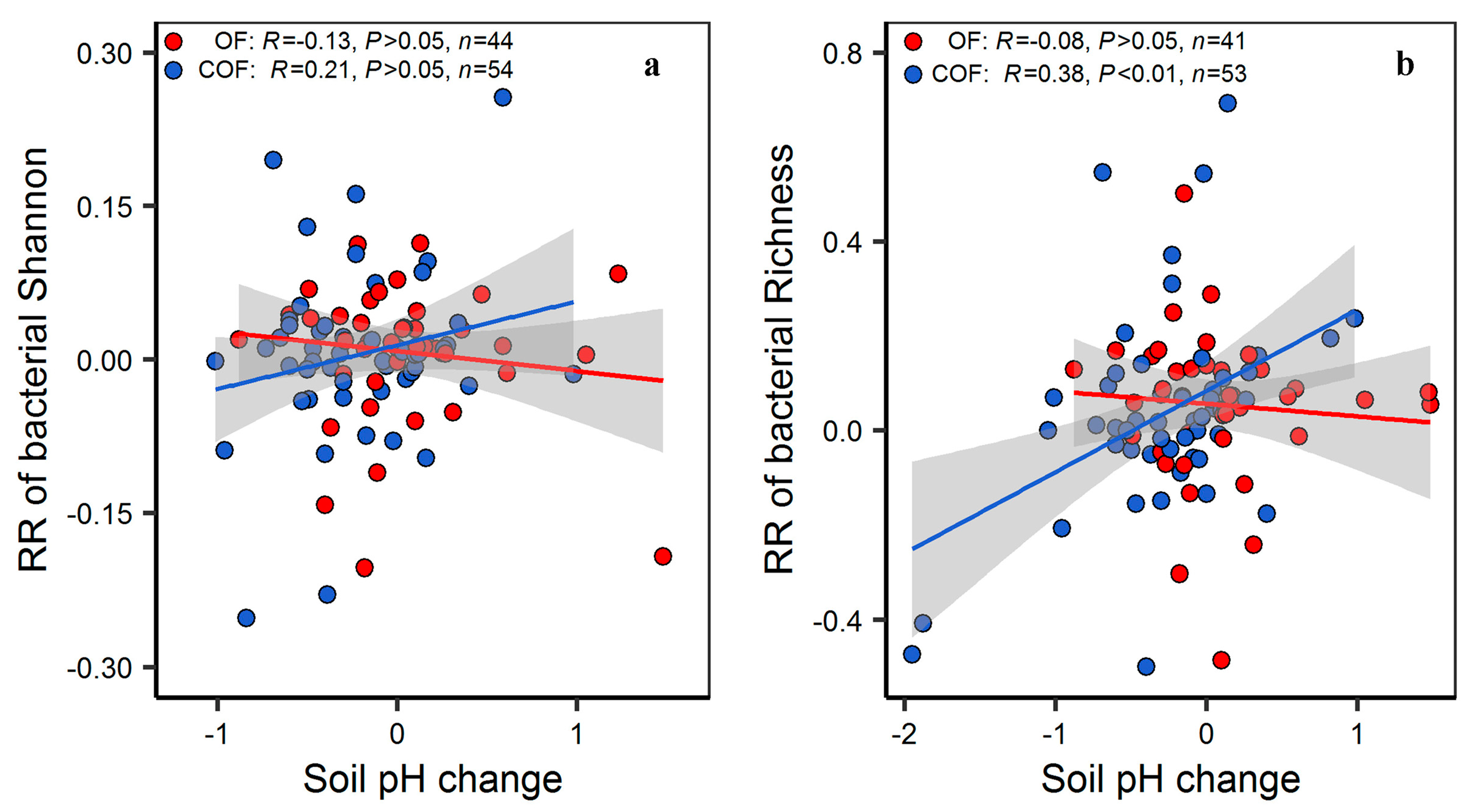
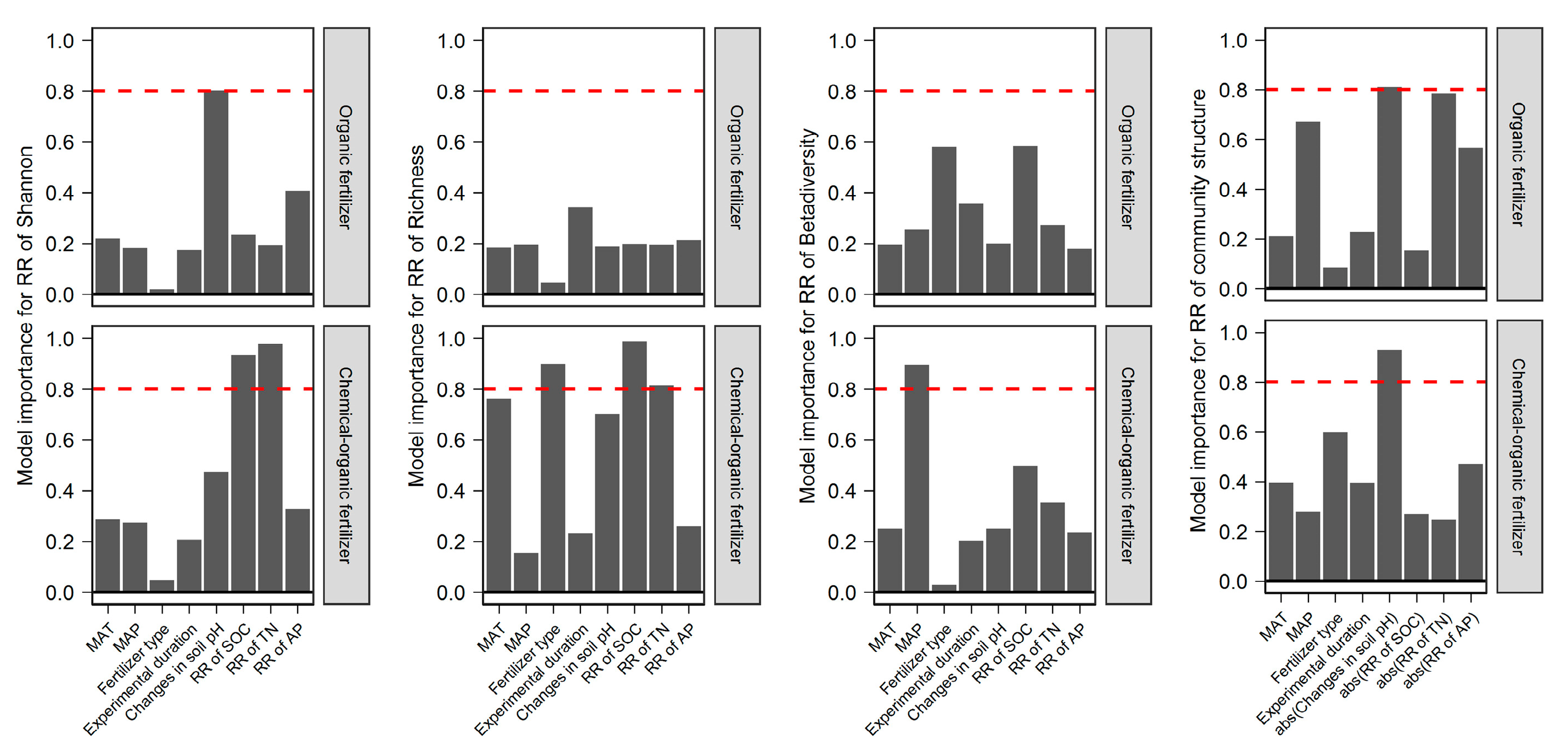
2.4. The Importance of the Predictors of Different Fertilizer Regimes Effect on Bacterial Diversity and Community Structure
3. Discussion
3.1. Response of Specific Bacterial Diversity and Community Structure to Organic and Chemical–Organic Fertilization
3.2. Response of Specific Bacterial Phyla to Organic and Chemical–Organic Fertilization
4. Materials and Methods
4.1. Data Collection
4.2. Meta-Analysis
4.3. Model Selection Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bai, Z.; Caspari, T.; Gonzalez, M.R.; Batjes, N.H.; Mäder, P.; Bünemann, E.K.; de Goede, R.; Brussaard, L.; Xu, M.; Ferreira, C.S.S.; et al. Effects of Agricultural Management Practices on Soil Quality: A Review of Long-Term Experiments for Europe and China. Agric. Ecosyst. Environ. 2018, 265, 1–7. [Google Scholar] [CrossRef]
- Kehoe, L.; Romero-Muñoz, A.; Polaina, E.; Estes, L.; Kreft, H.; Kuemmerle, T. Biodiversity at Risk under Future Cropland Expansion and Intensification. Nat. Ecol. Evol. 2017, 1, 1129–1135. [Google Scholar] [CrossRef]
- Wang, C.; Liu, D.; Bai, E. Decreasing Soil Microbial Diversity Is Associated with Decreasing Microbial Biomass under Nitrogen Addition. Soil Biol. Biochem. 2018, 120, 126–133. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, M.; Petropoulos, E.; Zhang, J.; Nie, J.; Liao, Y.; Li, Z.; Lin, X.; Feng, Y. Responses of Paddy Soil Bacterial Community Assembly to Different Long-Term Fertilizations in Southeast China. Sci. Total Environ. 2019, 656, 625–633. [Google Scholar] [CrossRef]
- Jiang, G.; Zhang, W.; Xu, M.; Kuzyakov, Y.; Zhang, X.; Wang, J.; Di, J.; Murphy, D.V. Manure and Mineral Fertilizer Effects on Crop Yield and Soil Carbon Sequestration: A Meta-Analysis and Modeling across China. Glob. Biogeochem. Cycles 2018, 32, 1659–1672. [Google Scholar] [CrossRef]
- Luo, G.; Li, L.; Friman, V.-P.; Guo, J.; Guo, S.; Shen, Q.; Ling, N. Organic Amendments Increase Crop Yields by Improving Microbe-Mediated Soil Functioning of Agroecosystems: A Meta-Analysis. Soil Biol. Biochem. 2018, 124, 105–115. [Google Scholar] [CrossRef]
- Xia, L.; Lam, S.K.; Wolf, B.; Kiese, R.; Chen, D.; Butterbach-Bahl, K. Trade-Offs between Soil Carbon Sequestration and Reactive Nitrogen Losses under Straw Return in Global Agroecosystems. Glob. Chang. Biol. 2018, 24, 5919–5932. [Google Scholar] [CrossRef]
- Bardgett, R.D.; van der Putten, W.H. Belowground Biodiversity and Ecosystem Functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Larsbrink, J.; McKee, L.S. Bacteroidetes Bacteria in the Soil: Glycan Acquisition, Enzyme Secretion, and Gliding Motility. Adv. Appl. Microbiol. 2020, 110, 63–98. [Google Scholar] [CrossRef]
- Crowther, T.W.; van den Hoogen, J.; Wan, J.; Mayes, M.A.; Keiser, A.D.; Mo, L.; Averill, C.; Maynard, D.S. The Global Soil Community and Its Influence on Biogeochemistry. Science 2019, 365, eaav0550. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Oliverio, A.M.; Brewer, T.E.; Benavent-González, A.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K.; Fierer, N. A Global Atlas of the Dominant Bacteria Found in Soil. Science 2018, 359, 320–325. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Trivedi, P.; Trivedi, C.; Eldridge, D.J.; Reich, P.B.; Jeffries, T.C.; Singh, B.K. Microbial Richness and Composition Independently Drive Soil Multifunctionality. Funct. Ecol. 2017, 31, 2330–2343. [Google Scholar] [CrossRef]
- Hermans, S.M.; Buckley, H.L.; Case, B.S.; Curran-Cournane, F.; Taylor, M.; Lear, G. Bacteria as Emerging Indicators of Soil Condition. Appl. Environ. Microbiol. 2017, 83, e02826-16. [Google Scholar] [CrossRef]
- Dai, Z.; Su, W.; Chen, H.; Barberán, A.; Zhao, H.; Yu, M.; Yu, L.; Brookes, P.C.; Schadt, C.W.; Chang, S.X.; et al. Long-Term Nitrogen Fertilization Decreases Bacterial Diversity and Favors the Growth of Actinobacteria and Proteobacteria in Agro-Ecosystems across the Globe. Glob. Change Biol. 2018, 24, 3452–3461. [Google Scholar] [CrossRef]
- Kim, N.; Zabaloy, M.C.; Guan, K.; Villamil, M.B. Do Cover Crops Benefit Soil Microbiome? A Meta-Analysis of Current Research. Soil Biol. Biochem. 2020, 142, 107701. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Luo, Y. Meta-Analysis of the Impacts of Global Change Factors on Soil Microbial Diversity and Functionality. Nat. Commun. 2020, 11, 3072. [Google Scholar] [CrossRef]
- Li, Y.; Song, D.; Liang, S.; Dang, P.; Qin, X.; Liao, Y.; Siddique, K.H.M. Effect of No-Tillage on Soil Bacterial and Fungal Community Diversity: A Meta-Analysis. Soil Till. Res. 2020, 204, 104721. [Google Scholar] [CrossRef]
- Chen, J.; Guo, Q.; Liu, D.; Hu, C.; Sun, J.; Wang, X.; Liang, G.; Zhou, W. Composition, Predicted Functions, and Co-Occurrence Networks of Fungal and Bacterial Communities_Links to Soil Organic Carbon under Long-Term Fertilization in a Rice-Wheat Cropping System. Eur. J. Soil Biol. 2020, 100, 103226. [Google Scholar] [CrossRef]
- Soman, C.; Li, D.; Wander, M.M.; Kent, A.D. Long-Term Fertilizer and Crop-Rotation Treatments Differentially Affect Soil Bacterial Community Structure. Plant Soil 2017, 413, 145–159. [Google Scholar] [CrossRef]
- Liu, J.; Liu, M.; Wu, M.; Jiang, C.; Chen, X.; Cai, Z.; Wang, B.; Zhang, J.; Zhang, T.; Li, Z. Soil pH Rather than Nutrients Drive Changes in Microbial Community Following Long-Term Fertilization in Acidic Ultisols of Southern China. J. Soils Sediments 2018, 18, 1853–1864. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, G.; Yu, C. A Meta-Analysis of the Effects of Organic and Inorganic Fertilizers on the Soil Microbial Community. J. Resour. Ecol. 2020, 11, 298. [Google Scholar] [CrossRef]
- Ye, G.; Lin, Y.; Luo, J.; Di, H.J.; Lindsey, S.; Liu, D.; Fan, J.; Ding, W. Responses of Soil Fungal Diversity and Community Composition to Long-Term Fertilization: Field Experiment in an Acidic Ultisol and Literature Synthesis. Appl. Soil Ecol. 2020, 145, 103305. [Google Scholar] [CrossRef]
- Kamaa, M.; Mburu, H.; Blanchart, E.; Chibole, L.; Chotte, J.-L.; Kibunja, C.; Lesueur, D. Effects of Organic and Inorganic Fertilization on Soil Bacterial and Fungal Microbial Diversity in the Kabete Long-Term Trial, Kenya. Biol. Fertil. Soils 2011, 47, 315–321. [Google Scholar] [CrossRef]
- Xun, W.; Zhao, J.; Xue, C.; Zhang, G.; Ran, W.; Wang, B.; Shen, Q.; Zhang, R. Significant Alteration of Soil Bacterial Communities and Organic Carbon Decomposition by Different Long-Term Fertilization Management Conditions of Extremely Low-Productivity Arable Soil in South China: Bacterial Structure and Functionality Alteration. Environ. Microbiol. 2016, 18, 1907–1917. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Ge, T.; Zhou, P.; Liu, S.; Roberts, P.; Zhu, H.; Zou, Z.; Tong, C.; Wu, J. Soil Microbial Biomass and Bacterial and Fungal Community Structures Responses to Long-Term Fertilization in Paddy Soils. J. Soils Sediment 2013, 13, 877–886. [Google Scholar] [CrossRef]
- Ye, G.; Lin, Y.; Liu, D.; Chen, Z.; Luo, J.; Bolan, N.; Fan, J.; Ding, W. Long-Term Application of Manure over Plant Residues Mitigates Acidification, Builds Soil Organic Carbon and Shifts Prokaryotic Diversity in Acidic Ultisols. Appl. Soil Ecol. 2019, 133, 24–33. [Google Scholar] [CrossRef]
- Ge, Y.; Zhang, J.; Zhang, L.; Yang, M.; He, J. Long-Term Fertilization Regimes Affect Bacterial Community Structure and Diversity of an Agricultural Soil in Northern China. J. Soils Sediment 2008, 8, 43–50. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, H.; Gao, C. Differential Responses of Soil Bacterial Taxa to Long-Term P, N, and Organic Manure Application. J. Soils Sediment 2016, 16, 1046–1058. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, P.; Zeng, Z. Dynamics of Bacterial Communities in a 30-Year Fertilized Paddy Field under Different Organic–Inorganic Fertilization Strategies. Agronomy 2019, 9, 14. [Google Scholar] [CrossRef]
- Tilman, D.; Isbell, F.; Cowles, J.M. Biodiversity and Ecosystem Functioning. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 471–493. [Google Scholar] [CrossRef]
- Saleem, M.; Hu, J.; Jousset, A. More Than the Sum of Its Parts: Microbiome Biodiversity as a Driver of Plant Growth and Soil Health. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 145–168. [Google Scholar] [CrossRef]
- Pan, H.; Chen, M.; Feng, H.; Wei, M.; Song, F.; Lou, Y.; Cui, X.; Wang, H.; Zhuge, Y. Organic and Inorganic Fertilizers Respectively Drive Bacterial and Fungal Community Compositions in a Fluvo-Aquic Soil in Northern China. Soil Till. Res. 2020, 198, 104540. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, J.; Li, Y.; Jin, J.; Liu, X.; Wang, G. Impact of Land Use, Fertilization and Seasonal Variation on the Abundance and Diversity of nirS-Type Denitrifying Bacterial Communities in a Mollisol in Northeast China. Eur. J. Soil Biol. 2018, 85, 4–11. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, L.; Yang, S.; Wang, Z.; Tian, R.; Peng, Z.; Chen, Y.; Zhang, X.; Kuang, J.; Ling, N.; et al. Critical Transition of Soil Bacterial Diversity and Composition Triggered by Nitrogen Enrichment. Ecology 2020, 101, e03053. [Google Scholar] [CrossRef] [PubMed]
- Ratzke, C.; Barrere, J.; Gore, J. Strength of Species Interactions Determines Biodiversity and Stability in Microbial Communities. Nat. Ecol. Evol. 2020, 4, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Karimi, B.; Terrat, S.; Dequiedt, S.; Saby, N.P.A.; Horrigue, W.; Lelièvre, M.; Nowak, V.; Jolivet, C.; Arrouays, D.; Wincker, P.; et al. Biogeography of Soil Bacteria and Archaea across France. Sci. Adv. 2018, 4, eaat1808. [Google Scholar] [CrossRef]
- Tripathi, B.M.; Stegen, J.C.; Kim, M.; Dong, K.; Adams, J.M.; Lee, Y.K. Soil pH Mediates the Balance between Stochastic and Deterministic Assembly of Bacteria. ISME J. 2018, 12, 1072–1083. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, X.-X.; Guo, X.; Wang, D.; Chu, H. Bacterial Diversity in Soils Subjected to Long-Term Chemical Fertilization Can Be More Stably Maintained with the Addition of Livestock Manure than Wheat Straw. Soil Biol. Biochem. 2015, 88, 9–18. [Google Scholar] [CrossRef]
- Bao, Y.; Feng, Y.; Stegen, J.C.; Wu, M.; Chen, R.; Liu, W.; Zhang, J.; Li, Z.; Lin, X. Straw Chemistry Links the Assembly of Bacterial Communities to Decomposition in Paddy Soils. Soil Biol. Biochem. 2020, 148, 107866. [Google Scholar] [CrossRef]
- Bell, T.; Newman, J.A.; Silverman, B.W.; Turner, S.L.; Lilley, A.K. The Contribution of Species Richness and Composition to Bacterial Services. Nature 2005, 436, 1157–1160. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the Unknown: Disentangling the Complexities of the Soil Microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.; Lonardo, D.P.D.; Bodelier, P.L.E. Revisiting Life Strategy Concepts in Environmental Microbial Ecology. FEMS Microbiol. Ecol. 2017, 93, fix006. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Huang, X.; Tan, W.; Di, H.; Xu, J.; Li, Y. High Manure Load Reduces Bacterial Diversity and Network Complexity in a Paddy Soil under Crop Rotations. Soil Ecol. Lett. 2020, 2, 104–119. [Google Scholar] [CrossRef]
- Jones, R.T.; Robeson, M.S.; Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. A Comprehensive Survey of Soil Acidobacterial Diversity Using Pyrosequencing and Clone Library Analyses. ISME J. 2009, 3, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Samaddar, S.; Chatterjee, P.; Truu, J.; Anandham, R.; Kim, S.; Sa, T. Long-Term Phosphorus Limitation Changes the Bacterial Community Structure and Functioning in Paddy Soils. Appl. Soil Ecol. 2019, 134, 111–115. [Google Scholar] [CrossRef]
- Tan, H.; Barret, M.; Mooij, M.J.; Rice, O.; Morrissey, J.P.; Dobson, A.; Griffiths, B.; O’Gara, F. Long-Term Phosphorus Fertilisation Increased the Diversity of the Total Bacterial Community and the phoD Phosphorus Mineraliser Group in Pasture Soils. Biol. Fertil. Soils 2013, 49, 661–672. [Google Scholar] [CrossRef]
- Spohn, M.; Pötsch, E.M.; Eichorst, S.A.; Woebken, D.; Wanek, W.; Richter, A. Soil Microbial Carbon Use Efficiency and Biomass Turnover in a Long-Term Fertilization Experiment in a Temperate Grassland. Soil Biol. Biochem. 2016, 97, 168–175. [Google Scholar] [CrossRef]
- Shu, X.; He, J.; Zhou, Z.; Xia, L.; Hu, Y.; Zhang, Y.; Zhang, Y.; Luo, Y.; Chu, H.; Liu, W.; et al. Organic Amendments Enhance Soil Microbial Diversity, Microbial Functionality and Crop Yields: A Meta-Analysis. Sci. Total Environ. 2022, 829, 154627. [Google Scholar] [CrossRef]
- Biederman, L.A.; Harpole, W.S. Biochar and Its Effects on Plant Productivity and Nutrient Cycling: A Meta-Analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Geisseler, D.; Linquist, B.A.; Lazicki, P.A. Effect of Fertilization on Soil Microorganisms in Paddy Rice Systems–A Meta-Analysis. Soil Biol. Biochem. 2017, 115, 452–460. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, X.; Liu, W.; Huang, H.; Ye, Q.; Zhu, S.; Peng, Z.; Li, Y.; Deng, L.; Yang, Z.; Chen, H.; et al. Meta-Analysis of Organic Fertilization Effects on Soil Bacterial Diversity and Community Composition in Agroecosystems. Plants 2023, 12, 3801. https://doi.org/10.3390/plants12223801
Shu X, Liu W, Huang H, Ye Q, Zhu S, Peng Z, Li Y, Deng L, Yang Z, Chen H, et al. Meta-Analysis of Organic Fertilization Effects on Soil Bacterial Diversity and Community Composition in Agroecosystems. Plants. 2023; 12(22):3801. https://doi.org/10.3390/plants12223801
Chicago/Turabian StyleShu, Xiangyang, Weijia Liu, Han Huang, Qinxin Ye, Shunxi Zhu, Zhaohui Peng, Yiding Li, Liangji Deng, Zepeng Yang, Honglin Chen, and et al. 2023. "Meta-Analysis of Organic Fertilization Effects on Soil Bacterial Diversity and Community Composition in Agroecosystems" Plants 12, no. 22: 3801. https://doi.org/10.3390/plants12223801
APA StyleShu, X., Liu, W., Huang, H., Ye, Q., Zhu, S., Peng, Z., Li, Y., Deng, L., Yang, Z., Chen, H., Liu, D., & Shi, J. (2023). Meta-Analysis of Organic Fertilization Effects on Soil Bacterial Diversity and Community Composition in Agroecosystems. Plants, 12(22), 3801. https://doi.org/10.3390/plants12223801




