Abstract
Zirconium (Zr) is one of the toxic metals that are heavily incorporated into the ecosystem due to intensive human activities. Their accumulation in the ecosystem disrupts the food chain, causing undesired alterations. Despite Zr’s phytotoxicity, its impact on plant growth and redox status remains unclear, particularly if combined with elevated CO2 (eCO2). Therefore, a greenhouse pot experiment was conducted to test the hypothesis that eCO2 can alleviate the phytotoxic impact of Zr upon oat (Avena sativa) plants by enhancing their growth and redox homeostasis. A complete randomized block experimental design (CRBD) was applied to test our hypothesis. Generally, contamination with Zr strikingly diminished the biomass and photosynthetic efficiency of oat plants. Accordingly, contamination with Zr triggered remarkable oxidative damage in oat plants, with concomitant alteration in the antioxidant defense system of oat plants. Contrarily, elevated levels of CO2 (eCO2) significantly mitigated the adverse effect of Zr upon both fresh and dry weights as well as the photosynthesis of oat plants. The improved photosynthesis consequently quenched the oxidative damage caused by Zr by reducing the levels of both H2O2 and MDA. Moreover, eCO2 augmented the total antioxidant capacity with the concomitant accumulation of molecular antioxidants (e.g., polyphenols, flavonoids). In addition, eCO2 not only improved the activities of antioxidant enzymes such as peroxidase (POX), superoxide dismutase (SOD) and catalase (CAT) but also boosted the ASC/GSH metabolic pool that plays a pivotal role in regulating redox homeostasis in plant cells. In this regard, our research offers a novel perspective by delving into the previously unexplored realm of the alleviative effects of eCO2. It sheds light on how eCO2 distinctively mitigates oxidative stress induced by Zr, achieving this by orchestrating adjustments to the redox balance within oat plants.
1. Introduction
Growing environmental and health apprehensions have been raised regarding toxic metals, primarily due to their extensive dissemination throughout the ecosystem. The accumulation of such toxic metals in the ecosystem imposes rigorous imperilment on both human health and agricultural productivity. There are several reasons for the introduction of such toxic metals to the environment. Anthropogenic endeavors such as mining activities, the application of pesticides and industrial wastes are the common sources of such toxic metals [1]. Once they have been introduced to the ecosystem, they are incorporated into the food chain, causing undesired alterations [2]. Although plants need some of these metals for their growth and development, the existence of these metals in high levels causes a tremendous disruption in plant physiology and metabolism [3]. Like other toxic metals, Zirconium (Zr) is widely distributed in the Earth’s crust [4]. It is considered a reactive metal but is more resistant to corrosion than many other metals. This is due to the formation of a thin layer of zirconium dioxide (ZrO2, also known as zirconia) that acts as a protective layer for the underlying metal against further oxidation [5]. Zr and its alloys are widely used in different human activities. It is commonly applied in the nuclear industry due to its high resistance to corrosion, in addition to being used as cladding for nuclear fuel rods in reactors [5]. Furthermore, Zr compounds can be utilized in various sectors of industries such as ceramics, refractories, and electronics. More interestingly, ZrO2 is a material that can be utilized in various applications, including dental implants, catalysts, and solid oxide fuel cells. The widespread occurrence of Zr in various activities has led to its extensive presence in our surroundings. This situation has raised alarm within the scientific community due to the potential detrimental effects it poses on human health and agricultural productivity. Zr and its compounds are toxic to human health, so proper handling and safety precautions should be taken to prevent its dispersion [6]. Concerning plants, although Zr is not an essential element for plant growth, excessive exposure to Zr and its compounds may slow plant growth and development and change the soil structure [7]. Nonetheless, when it comes to understanding the effects of Zr on plants, there has been relatively limited research compared to more extensively studied elements. The excessive buildup of Zr within plant tissues has the potential to interfere with nutrient absorption and crucial metabolic processes, ultimately resulting in a significant decline in plant growth [6]. Shaid et al. [6] also declared that Zr can interfere with the availability of some essential nutrients, such as Ca, Mg, and K which are vital for most plant metabolic processes, something that can hinder overall plant growth and development. Moreover, excessive exposure to Zr has been reported to alter the photosynthetic pigments of Chlorella pyrenoidosa, leading to a negative impact on culture mass and yield [8]. Both root function and development can also be affected by higher exposure to Zr. The disturbance in root function and growth, in turn, can impact the plant’s capacity to absorb nutrients and water, diminishing its ability to acquire vital resources, leading to stunted growth and decreased vitality [9]. Furthermore, Fodor et al. [9] declared that plant exposure to Zr may trigger plant oxidative stress that causes an imbalance between the production of ROS and the ability to detoxify them. This oxidative damage can disrupt the cellular components and slow different metabolic pathways in plants [10]. It is crucial to understand that the detrimental impacts of Zr on plants can be influenced by various factors, including the type and the concentration of Zr in the soil, soil conditions and the specific plant species involved. Nonetheless, the precise mechanisms through which Zr influences plant metabolism remain incompletely elucidated, underscoring the need for further research to comprehensively assess the impact on plant growth and metabolic processes. To this end, researchers should explore environmentally friendly and economically viable agricultural approaches to mitigate the potential harmful effects of excessive Zr accumulation. One of these tools is by enriching the atmosphere of plants with carbon dioxide.
The intensive increase in atmospheric CO2 levels is a major environmental challenge. This elevation is expected to further modify soil properties, altering the growth and development of economically important crops. Progressive industrial activities have caused a rapid increase in CO2 concentration from 280 ppm to 400 ppm [11]. Prognostications have shown that this increment will continue to increase as a consequence of immense human industrial impact [12]. In point of fact, elevation in CO2 within a physiological range has been proven to enrich plant growth, enhancing photosynthetic carbon metabolism with consequent boosting in assimilation [10]. The enhancement in carbon metabolism will accordingly be reflected in the partitioning of plant carbon and nitrogen [11]. Furthermore, several studies showed that eCO2 could alleviate the detrimental effects of a variety of environmental constraints upon plant growth and metabolism [10,13,14]. This potential of eCO2 is attributed to its effective changes in stomatal conductance, which play a crucial role in enhancing plant water uptake [15]. More interestingly, eCO2 could mitigate the imposed stress by modulating the redox homeostasis through regulating ROS production and scavenging [16]. Therefore, the scientific community should pay attention to the impact of eCO2 in boosting plant tolerance under different environmental challenges, especially for economically important crops such as oat plants. In this regard, eCO2 raised the tolerance level of two barley cultivars grown under salinity stress [17]. Furthermore, Mhamdi and Noctor [18] suggested that Arabidopsis subjected to eCO2 treatment exhibits increased resistance against bacterial and fungal invasions. Likewise, it was found that treatment with eCO2 enhanced the defense system of Arabidopsis against different leaf and root pathogenic fungi [19]. Furthermore, tomato plants treated with eCO2 can cope with heat stress by enhancing photosynthesis and redox homeostasis [20]. Overall, these investigations and more were directed to study the combined effect of both eCO2 and different biotic and abiotic stresses. Nevertheless, the effect of raising atmospheric CO2 upon a toxic metal like Zr has not yet been considered. Therefore, our study was conducted to closely investigate the impact of two levels of CO2 (ambient, 420 ppm, and elevated, 710 ppm) on the growth and redox status of oat (Avena sativa) grown under the curb of Zr pollution. We further aim to verify the hypothesis that eCO2 amplifies carbohydrate portioning by bolstering photosynthetic efficiency. This, in turn, fortifies the antioxidant defense system, enabling it to counteract the oxidative damage caused by Zr.
2. Material and Methods
2.1. Plant Materials and Treatment
In order to gain a deeper insight into the impact of Zr on the plants, this study was performed with conditions that approached environmentally realistic conditions that closely mimic the environmental scenarios. The seeds of Avena sativa were homogeneously selected and sterilized with Na-hypochlorite (5% v/v, 20 min) and then washed thoroughly with distilled water. The sterilized seeds were sown in 20 cm diameter, 30 cm height polyvinyl chloride (PVC) tubes containing sandy soil with adjusted pH (7.6). The soil was irrigated daily to maintain soil capacity at 68%. The cultivation of plants took place within chambers that were illuminated by sunlight and maintained under controlled temperature and CO2 levels. The upper parts of these sunlit chambers were constructed with a transparent polycarbonate plate to permit light entry. The temperature was set to 26 °C during the day and 20 °C at night, and Photosynthetic Active Radiation was detected using an SDEC, type JYP1000 quantum sensor. The pots were divided into four groups, each with 4 replicates: (1) ambient CO2 (aCO2, 420 ppm); (2) aCO2 + Zr (400 mg/kg soil); (3) future climate CO2 (eCO2, 710 ppm); and (4) eCO2 + Zr (400 mg/kg soil). Zr was introduced to the soil as ZrSO4.4H2O (Sigma-Aldrich, Germany). The soil was consistently mixed with the desired amount of Zr. After 8 weeks, the rhizosphere soil as well as plant tissues were collected and kept in −80 °C for further biological analyses. The fresh and dry weights of oat plants were recorded.
2.2. Determination of Zr in Plant Tissues
To extract Zr, the plant samples, which had been dried at 70 °C, were subjected to a treatment involving 13 M nitric acid at a temperature of 185 °C for a duration of 25 min. Subsequently, the concentration of elements was determined using a quadrupole inductively coupled plasma mass spectrometry (ICP-MS; model 820-MS) setup, linked with a glass nebulizer operating at a flow rate of 0.4 mL/min. To generate calibration curves, external standards were prepared at concentrations ranging from 1 to 600 μg/L. Additionally, yttrium was included as an internal standard during the extraction process to calibrate the efficiency of the nebulizer. Standard mineral samples were prepared using 0.23 M nitric acid.
2.3. Photosynthesis and Photosynthetic Related Parameters
Photosynthetic and stomatal conductance measurements were carried out following the methodologies detailed in Ainsworht and Rogers [15]. To determine photosynthesis under saturating light conditions (Asat, μmol CO2 m−2 s−1), a LI-COR LI-6400 instrument (LI-COR Inc., Lincoln, NE, USA) was used. Each step of the measurement included a leaf equilibration period of at least 5 min before recording data. The LI-COR leaf chamber was configured with conditions set at either 400 or 620 ppm CO2, a block temperature of 22 °C, and a saturated photon flux density of 1500 μmol m−2 s−1. Stomatal conductance (gs, mol CO2 m−2 s−1) was assessed on the abaxial surface of fully developed leaves utilizing a leaf porometer (Model SC-1, Decagon Devices, Inc., Hopkins, Pullman, WA, USA). The average vapor pressure deficit and leaf temperature were maintained at 0.37 ± 0.02 and 20 ± 2.02, respectively. Chlorophyll fluorescence was gauged on fully expanded leaves that had been dark-acclimated, using an FMS-2 pulse-modulated fluorometer (Hansatech Instruments, Norfolk, UK). For leaves adapted to darkness for 30 min, minimal fluorescence (F0) and maximal fluorescence (Fm) were recorded. The photochemical efficiency of Photosystem II (PSII) (Fv/Fm) for these dark-adapted leaves was computed, where Fv (maximal variable fluorescence) was calculated as Fm−F0. Chlorophyll a, chlorophyll b and carotenoid concentrations were assessed in oat shoots homogenized using acetone [21]. RuBisCo activity was quantified using a non-radioactive microplate-based method that involved the enzymatic cycle between glycerol-3-phosphate dehydrogenase and glycerol-3-phosphate oxidase to measure the product, 3-phosphoglycerate (3-PGA) [22].
2.4. Determination of Oxidative Stress Markers
Lipid peroxidation was assessed using the thiobarbituric acid–malondialdehyde (TBA–MDA) assay according to the method outlined by Senthilkumar et al. [23]. For this, 0.3 g of frozen oat tissue was homogenized in 80% ethanol using a mortar and pestle. Centrifuge the extract (10,000× g for 10 min). Take 0.5 mL of the clear supernatant then add 2.5 mL of the TBA and 0.5 mL butylated hydroxytoluene (BHT) to prevent further oxidation during heating. Incubate in a boiling water bath for 30 min, centrifuge and measure the absorbance at 532 nm. MDA concentration was calculated using the molar extinction coefficient of MDA (1.53 mM−1cm−1). The quantification of H2O2 levels was performed using the xylenol orange-based FOX1 technique [22]. Mix 0.1 mL of plant extract with 0.9 mL FOX1 reagent (4.4 mM xylenol orange, 2.6 mM sorbitol, 25 mM sulfuric acid, and 0.2 mM ferrous ammonium sulfate) then add 0.05 mL of ascorbic acid. Incubate the mixture at room temperature for 30 min in the dark, then centrifuge (10,000× g for 5 min). Measure the absorbance of the colored complex at 560 nm. H2O2 levels were calculated using a standard curve with known concentrations of H2O2.
2.5. Determination of Antioxidants’ Secondary Metabolites
Polyphenols and flavonoids were measured employing the Folin–Ciocalteu and aluminum chloride assays, respectively, as outlined in the reference [24]. The separation and quantification of tocopherols were achieved through High-Performance Liquid Chromatography (HPLC) using normal phase conditions with a Shimadzu system based in Hertogenbosch, Netherlands. The HPLC setup involved a Particil Pac column (5 μm column material, length 250 mm, i.d. 4.6 mm). Total Antioxidant Capacity (TAC) was evaluated following the Ferric Reducing Antioxidant Power (FRAP) method described by Benzie and Strain [25], wherein Trolox (Sigma-Aldrich, St. Louis, MO, USA) was employed as an internal standard.
2.6. Estimation of Enzymatic Antioxidants
For the determination of antioxidant enzyme activity, proteins were extracted using K-phosphate extraction buffer (50 mM, pH 7.0) supplemented with 10% PVPP (w/v), 0.25% Triton X-100 (v/v) and 1 mM PMSF. Peroxidase (POX) activity was assessed through the oxidation of pyrogallol at 430 nm [26], while superoxide dismutase (SOD) enzyme activities were determined by monitoring the inhibition of NBT reduction at 560 nm [27]. A known weight of frozen tissue (0.5 g) was homogenized in 1 ml extraction buffer (0.1 M phosphate buffer, pH 6.0), then centrifuged (4000× g, for 10 min at 4 °C). Then, 1 ml clear extract was taken and mixed with 1 ml assay mixture (20 M pyrogallol; 20 mM guaiacol; 30% H2O2). After mixing well, the produced color was measured at 420 nm. Dehydroascorbate reductase (DHAR), glutathione reductase (GR), ascorbate peroxidase (APX), and monodehydroascorbate reductase (MDHAR) were evaluated spectrophotometrically based on the method described by Murshed et al., [28] by utilizing 0.05 M MES/KOH as the buffer. Catalase (CAT) activity was quantified by observing the rate of H2O2 decomposition at 240 nm [29]. A known fresh weight of plant tissue was homogenized in 0.1 M potassium phosphate buffer (pH 7.0), then centrifuged at low speed (3000 g) for 10 min at 4 °C. Then, 100 µL clear extract was mixed with 2 mL assay mixture (0.1 M KH2PO4 buffer, pH 7.0; 30% H2O2). The change in color at 240 nm was measured over time. Glutathione peroxidase (GPX) activity was measured by monitoring the reduction of NADPH at 340 nm [30]. For GPX activity, a known weight of powdered fresh tissue was homogenized in ice-cold 0.1 M KH2PO4 buffer (pH 7.0). After centrifugation, 50 µL of the clear extract was mixed with the assay mixture (0.1 M KH2PO4; 1 M GSH; 1 mM NADPH). The change in color was monitored at 340 nm, after adding 30% H2O2 to initiate the reaction. The total soluble protein concentration was determined using the Lowry technique [31].
2.7. Determination of AsA/GSH Metabolites
Reduced ascorbate (ASC) and reduced glutathione (GSH) levels were measured using High-Performance Liquid Chromatography (HPLC) with a Shimadzu SIL10-ADvp system and a C18 column (Spherisorb ODS2, 5 μm particle diameter, 4.6 × 250 mm, Waters). The concentrations of total ascorbate (ASC + DHA) and glutathione (GSH + GSSG) were determined after reduction with dithiothreitol (DTT), following the methodology outlined by Sinha et al. [32]. Enzyme activities including superoxide dismutase (SOD), glutathione peroxidase (GPX), ascorbate peroxidase (APX), glutathione reductase (GR), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), and glutathione-S-transferase (GST) were assessed according to the procedures detailed in our previous research [33].
2.8. Statistical Analysis
The outcomes of the measured parameters are presented as the average of four biological replicates (n = 4). Statistical evaluation was executed utilizing one-way ANOVA and two-way ANOVA within the SPSS 21 software (Inc., Chicago, IL, USA). Mean distinctions were ascertained through Fisher’s least significance difference (LSD) test. The normality of data distribution and the equality of variances were assessed using the Kolmogorov–Smirnov test and Levene’s test, respectively. Furthermore, a comparative evaluation of pollution severity between Zr and Zr + eCO2 was conducted through LSD, with a significance threshold set at p < 0.05.
3. Results
3.1. Effect of eCO2 on Zr Cumulation and the Growth of Oat Plants
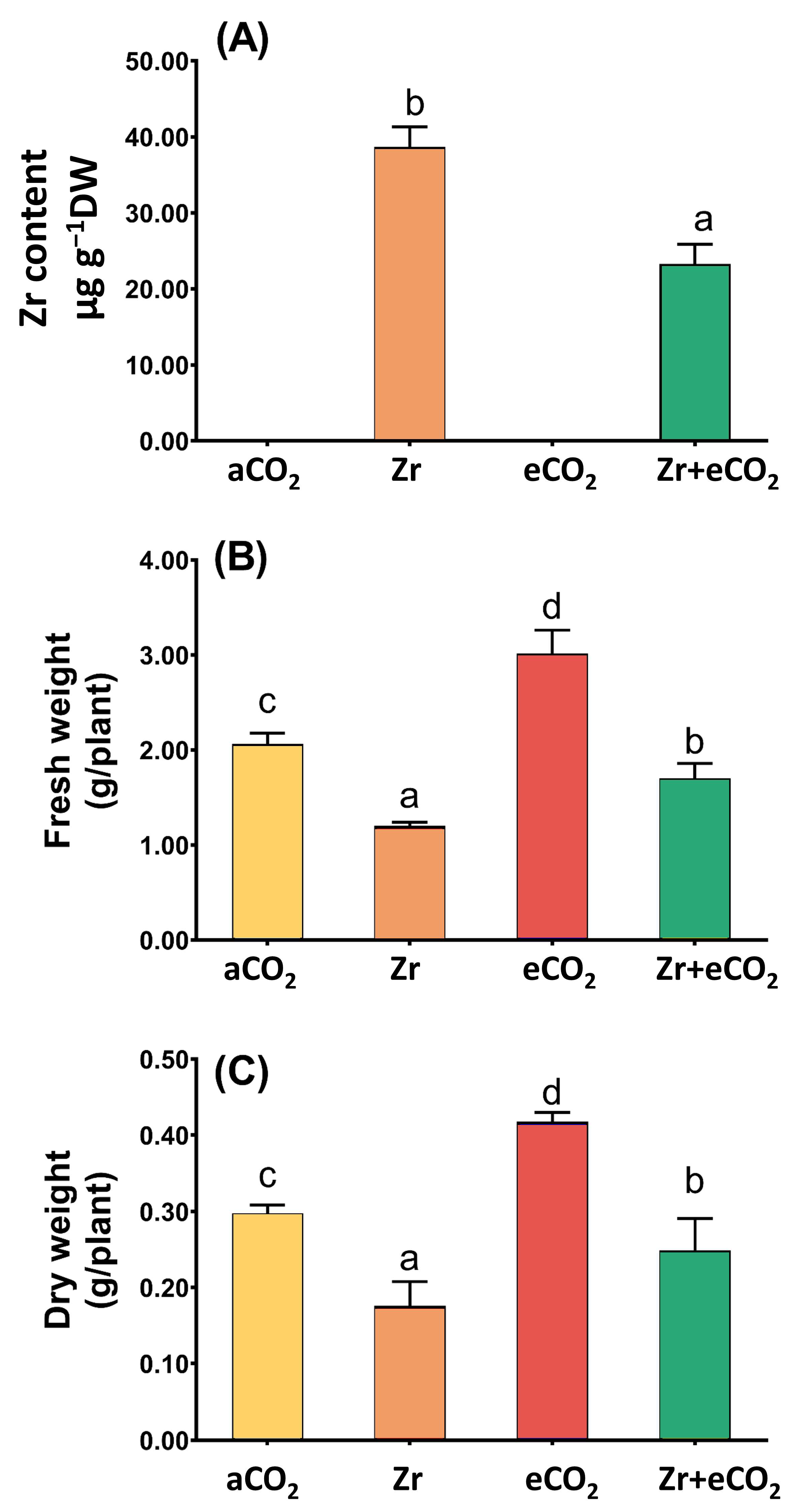
Our results showed that the levels of Zr in contaminated oat plants was very high; however, the cotreatment of oat plants with both Zr and elevated levels of eCO2 caused a remarkable reduction in the levels of Zr (~40% reduction) when compared with oat plants grown in contaminated soils (Figure 1A). This significant reduction in the levels of Zr due to the application of eCO2 prompted us to measure other growth parameters stand on the mitigative impact of eCO2. In this regard, oat plants experienced a noticeable reduction in the biomass (fresh and dry weights) in response to Zr pollution when compared with uncontaminated control plants (Figure 1B,C). On the other hand, treatment with eCO2 significantly enriched the biomass of oat plants (p < 0.05) as compared with normal control plants. Further, the co-application of both Zr and CO2 noticeably enhanced both fresh and dry weights of oat relative to plants contaminated with Zr. A two-way ANOVA revealed that both Zr and eCO2 significantly interacted to affect both fresh (p < 0.001) and dry (p < 0.05) weights of oat plans (Table 1).
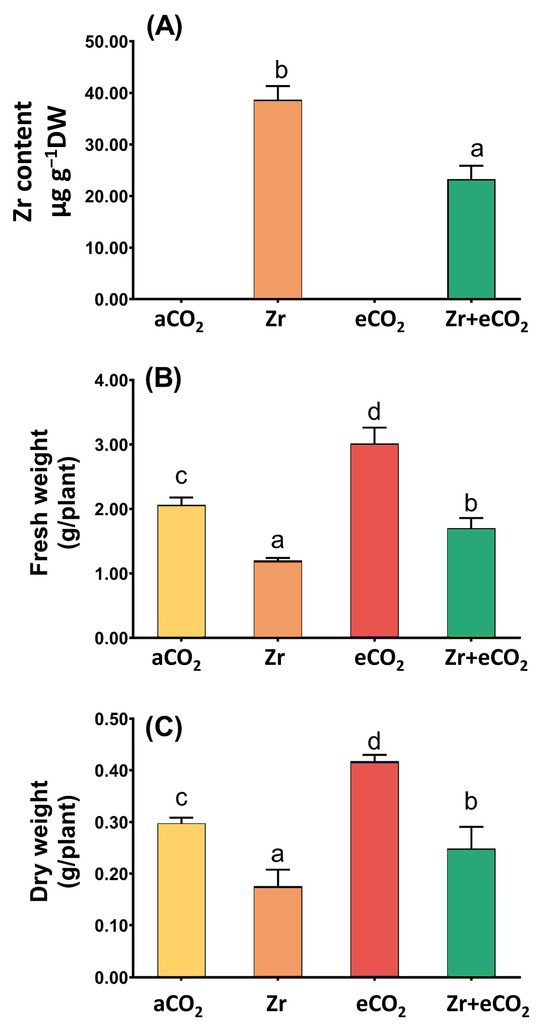
Figure 1.
The combined effect of both eCO2 and/or Zr upon (A) Zr accumulation, (B) fresh weight and (C) dry weight of oat plants. Four biological replicates were used to investigate the response. The vertical error bars are the standard error (SE). Fisher’s LSD test (p < 0.05, n = 4) was used for pairwise comparison between groups. The different letters indicate significant differences between the means of each group.

Table 1.
Two-way ANOVA on the significance of interaction between the two independent variables (Zr and eCO2) upon Zr content and the biomass and photosynthesis in oat plants (ns = non-significant; * = p < 0.05; ** = p < 0.01; *** = p < 0.001).
3.2. Photosynthesis of Oats as Affected by Zr and/or eCO2
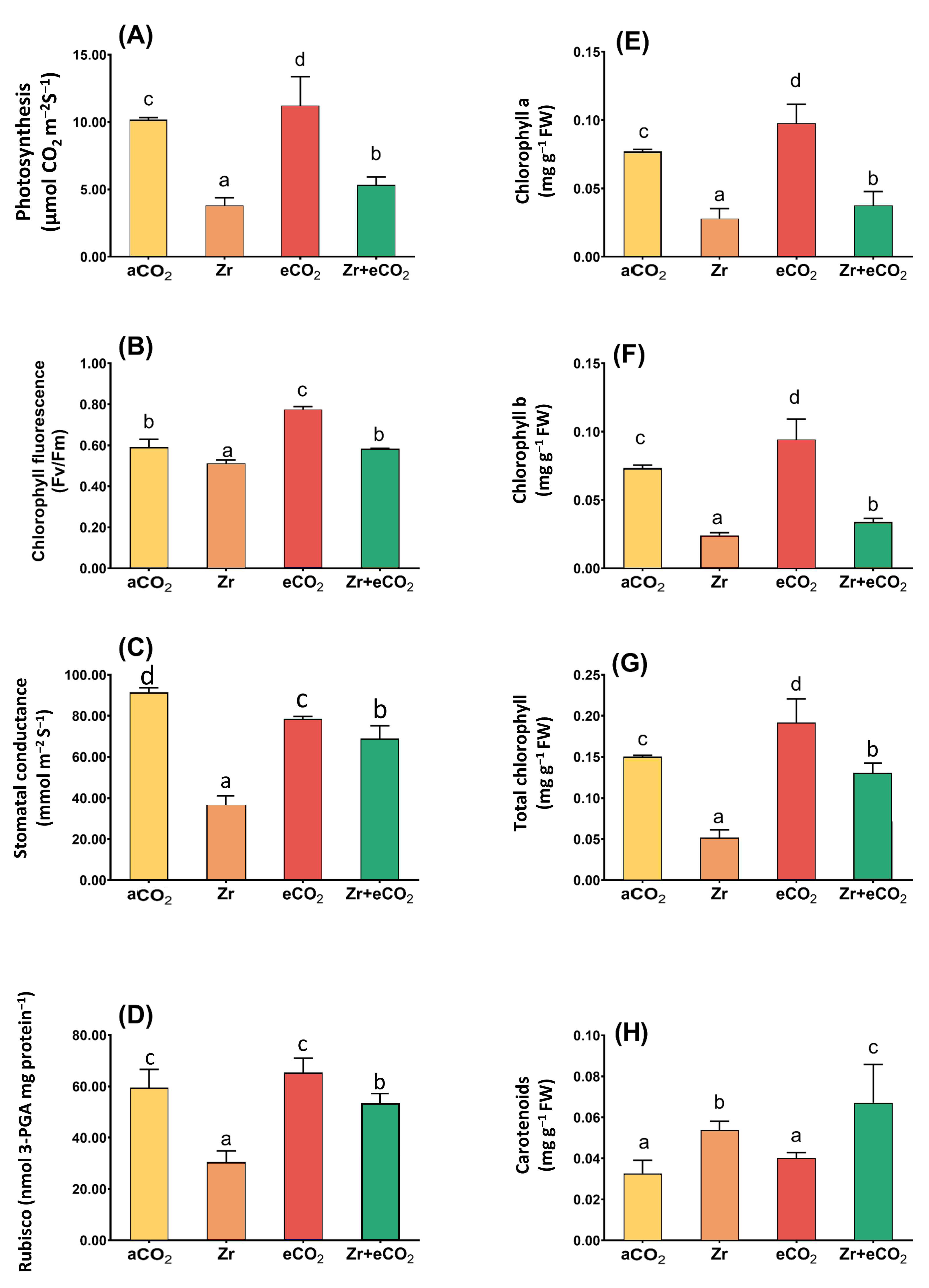
The enrichment in oat growth due to the application of eCO2 in combination with Zr encouraged us to investigate its effect upon the photosynthetic efficiency (Figure 2). As intended, the delay in the biomass of oat in response to Zr pollution was associated with a concomitant reduction in the photosynthetic machinery. This reduction was more obvious in the levels of Chl a, Chl b and Chl (a + b), and accordingly the rate of photosynthesis, which decreased by 61%, 71%, 50%, and 62%, respectively, in contaminated oat as compared with uncontaminated control plants (Figure 2A,E–G). This could be attributed to the significant decline in both stomatal and non-stomatal parameters such as stomatal conductance and RuBisco that exhibited about 50% reduction relative to uncontaminated control plants (Figure 2C,D). On the other hand, the individual treatment with eCO2 significantly affected the rate of photosynthesis in oat plants. More interestingly, the application of eCO2 along with Zr recovered the adverse effect of Zr upon photosynthetic parameters. For instance, stomatal conductance and RuBisco increased by about 60% and 40%, respectively, when compared with oat grown under aCO2 and Zr conditions (Figure 2C,D). More obviously, total chlorophyll content experienced a three-fold increase relative to plants grown in aCO2 and Zr conditions (Figure 2G). The two-way ANOVA showed different interactive responses between the two independent variables (eCO2 and Zr) for all photosynthetic parameters (Table 1). The co-application of eCO2 and Zr (eCO2 + Zr) significantly enhanced the chlorophyll (a + b) and carotenoids (p < 0.05) as well as chlorophyll fluorescence (p < 0.001).
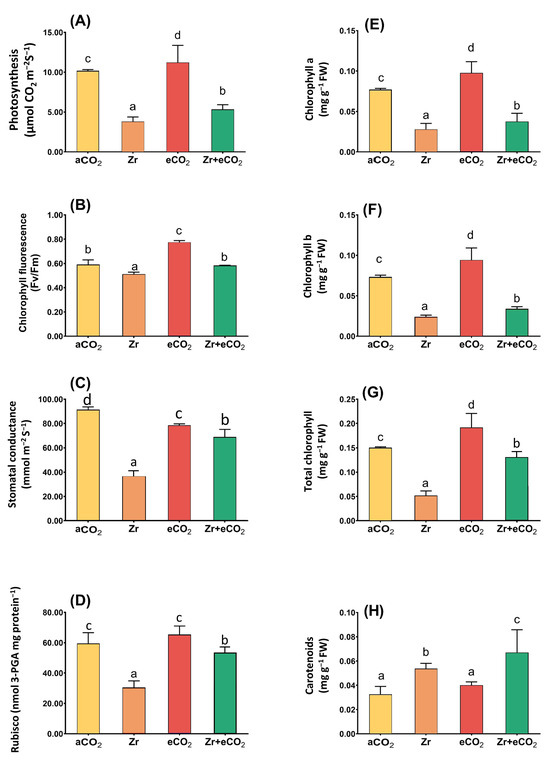
Figure 2.
The combined effect of eCO2 and/or Zr upon photosynthetic efficiency of oat plants. (A) rate of photosynthesis, (B) chlorophyll fluorescence, (C) stomatal conductance, (D) RuBisco activity, (E) chlorophyll a, (F) chlorophyll b, (G) total chlorophyll, and (H) carotenoids. Four biological replicates were used to investigate the response. The vertical error bars are the standard error (SE). Fisher’s LSD test (p < 0.05, n = 4) was used for pairwise comparison between groups. The different letters indicate significant differences between the means of each group.
3.3. Influence of eCO2 on the Zr-Induced Oxidative Damage in Oat Plants
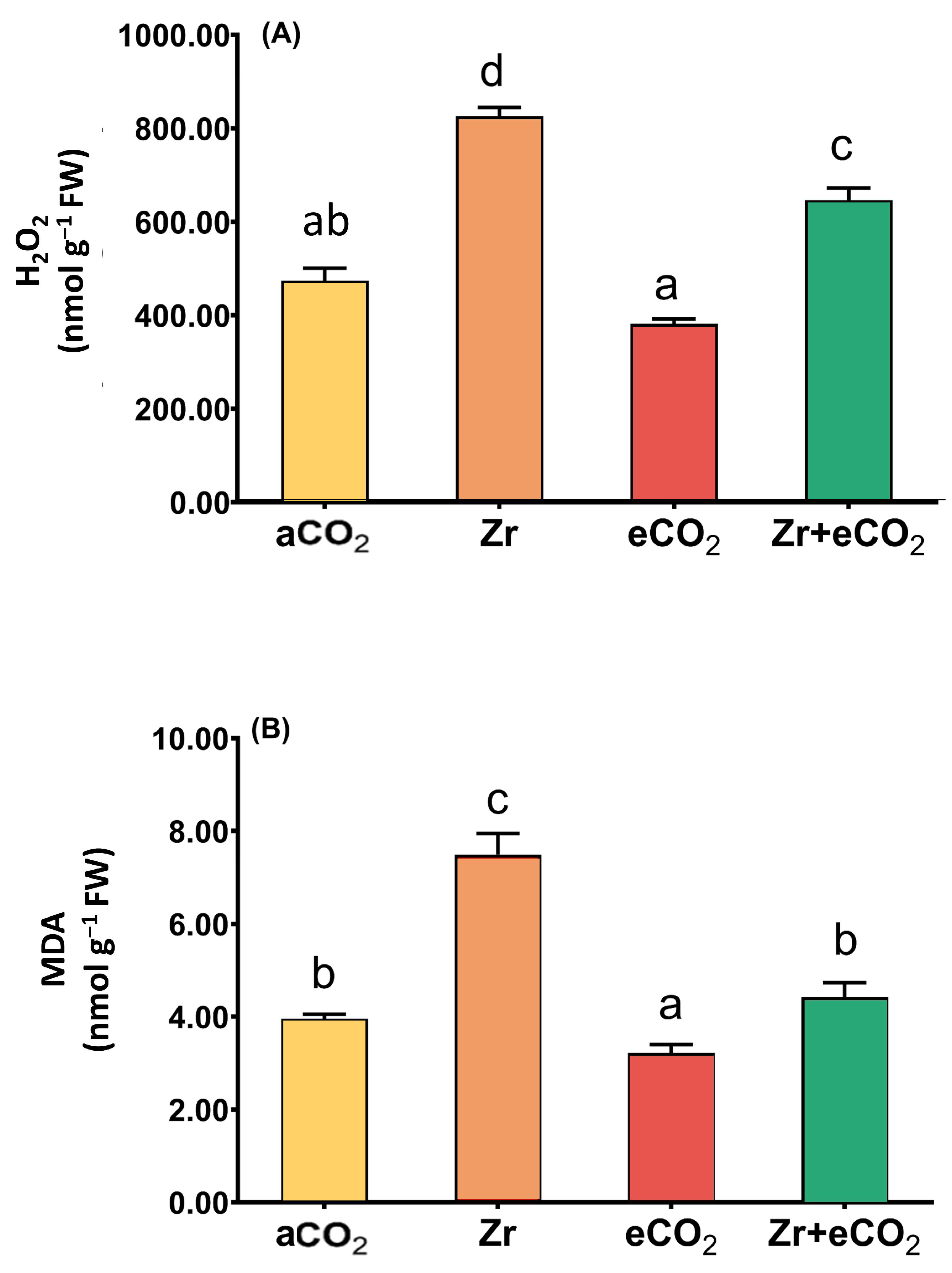
The accumulation of lipid peroxidation (MDA) and H2O2 is considered one of the main markers for oxidative damage in plants [34]. In this context, the individual application of Zr strikingly increased the levels of H2O2 (a two-fold increase) in oat plants (Figure 3A). It is likely that the levels of MDA exhibited a noticeable accumulation in oat plants in response to treatment with Zr (87% increase) relative to plants grown in eCO2-uncontaminated conditions. Additionally, individual treatment with eCO2 reduced the levels of both H2O2 and MDA a little bit. The results showed that eCO2 alone reduced the levels of H2O2 and MDA in oat by about 15% and 25%, respectively, as compared with the counter control plants treated with aCO2 (Figure 3). More interestingly, the application of eCO2 in combination with Zr caused a tremendous reduction in the levels of H2O2 and MDA. The reduction was more pronounced in the levels of MDA that exhibited a 47% reduction relative to Zr-treated control plants (Figure 3B). Overall, a significant interaction was found between eCO2 and Zr for both H2O2 and MDA (p < 0.01 and p < 0.001, respectively) (Table 2).
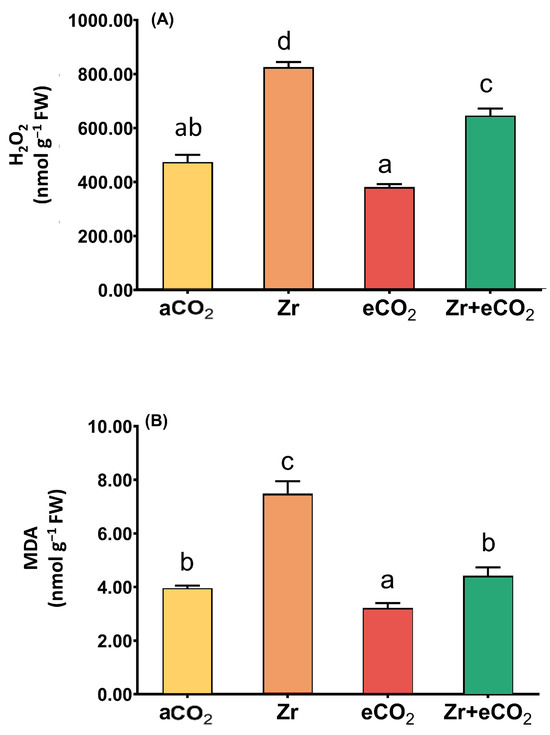
Figure 3.
The combined effect of eCO2 and/or Zr on the levels of oxidative stress markers of oat plants. (A) hydrogen peroxide (H2O2) and (B) malondialdehyde (MDA). Four biological replicates are used to investigate the response. The vertical error bars are the standard error (SE). Fisher’s LSD test (p < 0.05, n = 4) was used for pairwise comparison between groups. The different letters indicate significant differences between the means of each group.

Table 2.
Two-way ANOVA for the significance of interaction between the two independent variables (Zr and eCO2) upon oxidative damage, as well as the enzymatic and non-enzymatic antioxidants in oat plants (ns = non-significant; * = p < 0.05; ** = p < 0.01; *** = p < 0.001).
3.4. eCO2 and Non-Enzymatic Antioxidants in Zr-Contaminated Oat Plants
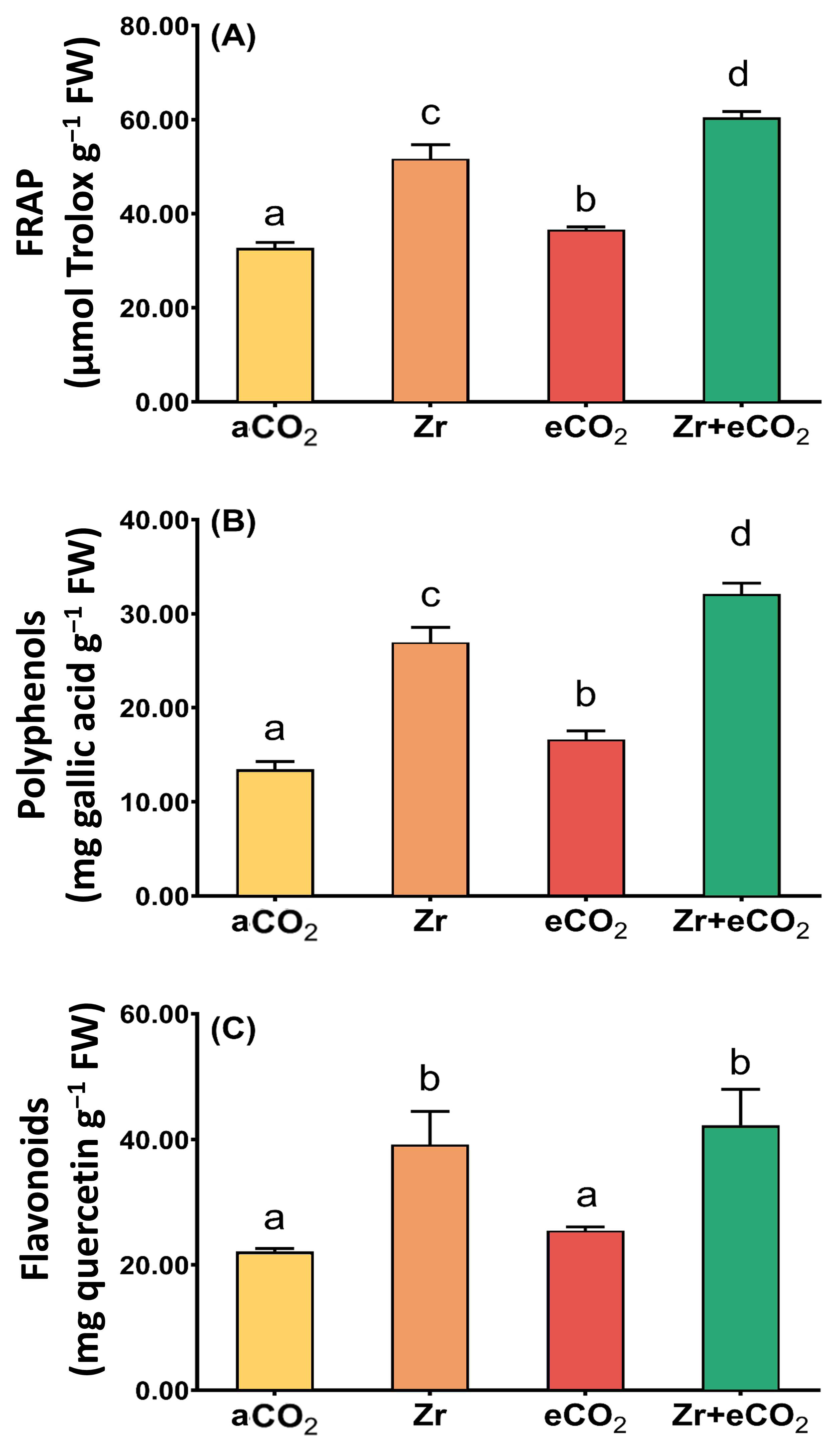
To contend with the oxidative damage, plants augmented their antioxidant defense arsenal to keep cell viability against the phytotoxic impact of Zr. Therefore, we aimed to measure the total antioxidant capacity (FRAP) as well as some non-enzymatic antioxidants (polyphenols and flavonoids) in oat plants treated with eCO2 and/or Zr (Figure 4). Our results revealed that Zr significantly increased the levels of FRAP in oat (~36% increase) compared to aCO2-treated oat plants (Figure 4A). This increment was associated with a remarkable increase in the levels of both polyphenols and flavonoids. Polyphenols experienced a two-fold increase in response to individual treatment with Zr relative to aCO2-treated control plants (Figure 4B). Similarly, treatment with Zr increased the levels of flavonoids of oat plants by about 72% as compared with aCO2-treated control plants (Figure 4C). Except for flavonoids, eCO2 caused a significant elevation in FRAP as well as polyphenols (Figure 4A,B). More interestingly, the application of both Zr and eCO2 FRAP caused a two-fold increase in both FRAP and flavonoids of oat plants relative to those grown under aCO2. To a greater extent, polyphenols exhibited about a three-fold increment in oat plants grown under both Zr and eCO2 if they were compared with those grown under aCO2. According to the two-way ANOVA, the interaction between Zr and eCO2 had varying significance on the responses. The significance of interaction between factors in polyphenols was higher (p < 0.001) than that of both FRAP and flavonoids (p < 0.05) (Table 2).
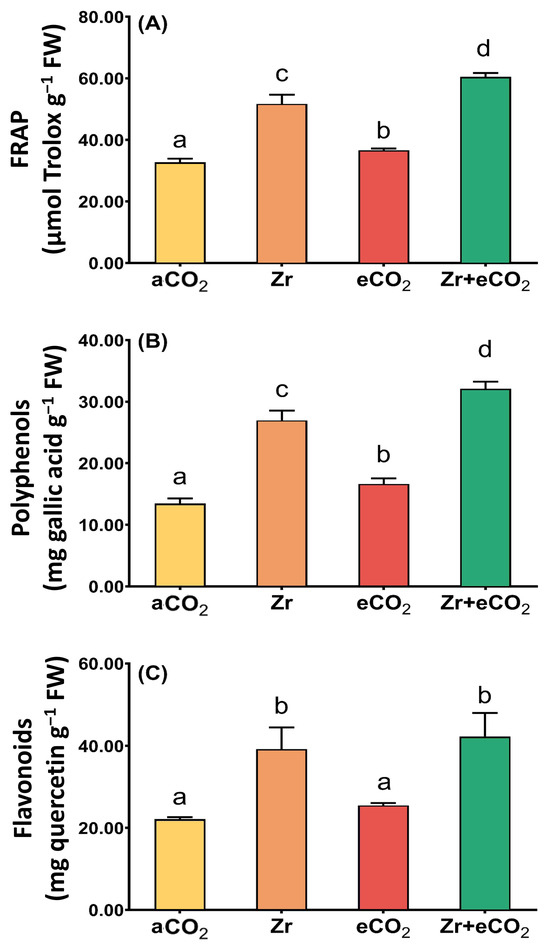
Figure 4.
The combined effect of eCO2 and/or Zr upon the levels of total antioxidant capacity and molecular antioxidants of oat plants. (A) total antioxidant capacity (FRAP), (B) polyphenols, (C) flavonoids. Four biological replicates were used to investigate the response. The vertical error bars are the standard error (SE). Fisher’s LSD test (p < 0.05, n = 4) was used for pairwise comparison between groups. The different letters indicate significant differences between the means of each group.
3.5. eCO2 Affecting the Enzymatic Antioxidants of Zr-Contaminated Oat Plants
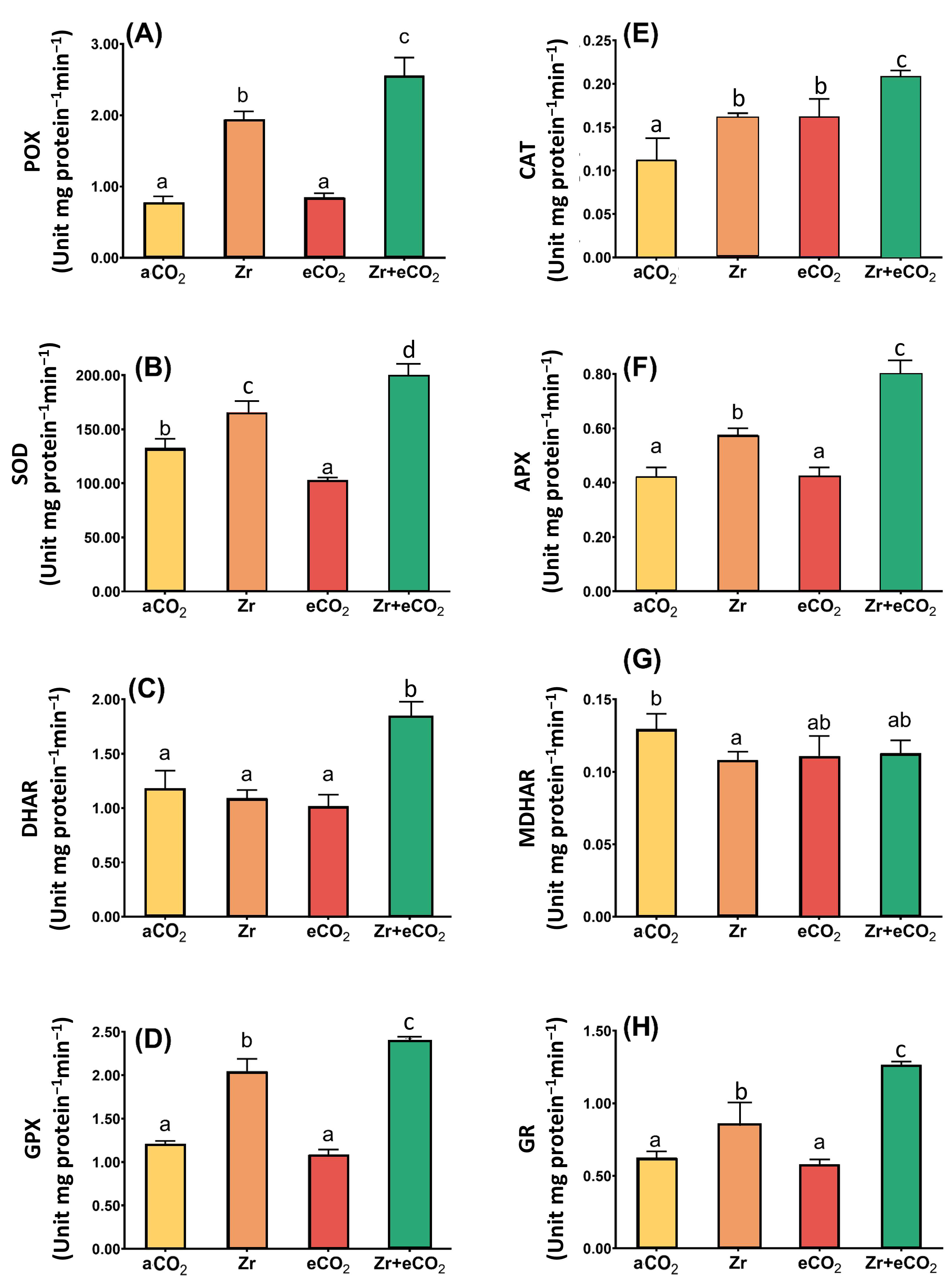
Antioxidant enzymes with those of the ASC/GSH cycle play a pivotal role in ROS homeostasis. In our study, we were concerned with investigating the activities of peroxidase (POX), superoxide dismutase (SOD), GSH-peroxidase (GPX) and catalase (CAT), in addition to the enzymes involved in ASC/GSH metabolism in oat plants treated with Zr and eCO2, either alone or in combination (Figure 5). Although the treatment of oat plants with Zr alone had no significant effect upon DHAR, and MDHAR, it caused a slight increase in the activities of some other enzymes. For example, SOD activity increased by 39% in response to the treatment of oats with Zr (Figure 5B). Moreover, the application of Zr upon oat plants increased the activity of both CAT and GPX up to 45% and 54%, respectively, when compared with control (aCO2) uncontaminated plants (Figure 5D). It is likely that the activity of glutathione reductase (GR) in oat plants exhibited a 33% increase due to the contamination with Zr if compared with plants grown under aCO2 conditions (Figure 5H). To a greater extent, POX activity showed an about two-fold increment in response to Zr treatment relative to control plants grown under aCO2 conditions (Figure 5A). Compared to aCO2, eCO2 had no significant impact upon the activities of the enzymatic antioxidants, except for CAT, whose activity increased by 46% relative to plants grown under aCO2 conditions (Figure 5E). Contrarily, eCO2 highly enhanced the enzymatic antioxidants in oat plants grown in Zr pollution conditions. In this regard, the activities of SOD and DHAR were augmented (30% and 50% increase, respectively) in oat plants in response to the co-application of both eCO2 and Zr if compared with aCO2-uncontaminated control plants (Figure 5B,C). A further increase was observed in the activities of both GPX and CAT (91% increase for each) due to the treatment of oat with both eCO2 and Zr (Figure 5D,E). Additionally, the treatment of oat plants with both eCO2 and Zr caused a two-fold enhancement in the activities of both APX and GR in comparison with aCO2 uncontaminated control plants (Figure 5F,H). More interestingly, POX activity was increased by about four-fold in oat plants treated with eCO2 and Zr relative to aCO2-uncontaminated control plants (Figure 5A). A two-way ANOVA declared that a differential interactive effect existed between the two independent variables (Zr and eCO2) upon SOD (p < 0.001), DHAR (p < 0.01) and GPX (p < 0.05) (Table 2).
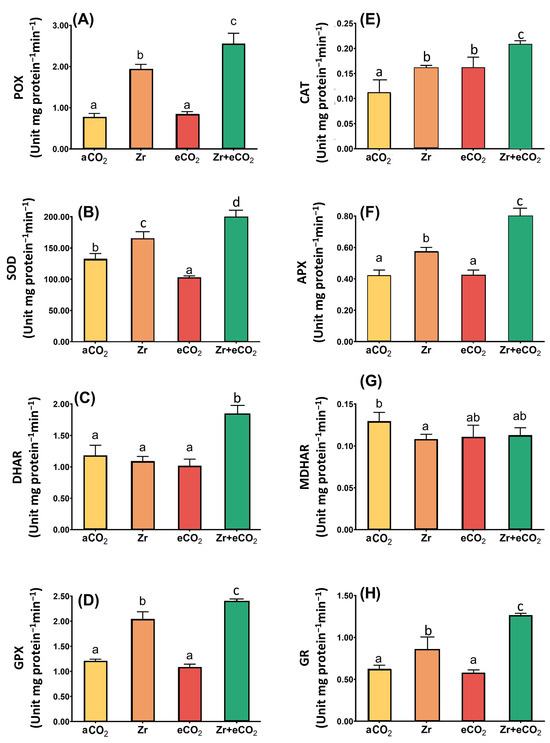
Figure 5.
The combined effect of eCO2 and/or Zr upon the activities of antioxidant oat plants. (A) peroxidase, (B) superoxide dismutase, (C) dehydroascorbate reductase, (D) glutathione peroxidase, (E) catalase, (F) ascorbate peroxidase, (G) monodehydroascorbate reductase, and (H) glutathione reductase. Four biological replicates were used to investigate the response. The vertical error bars are the standard error (SE). Fisher’s LSD test (p < 0.05, n = 4) was used for pairwise comparison between groups. The different letters indicate significant differences between the means of each group.
3.6. Effect of Zr and/or eCO2 on the AsA/GSH Metabolic Pool of Oat Plants
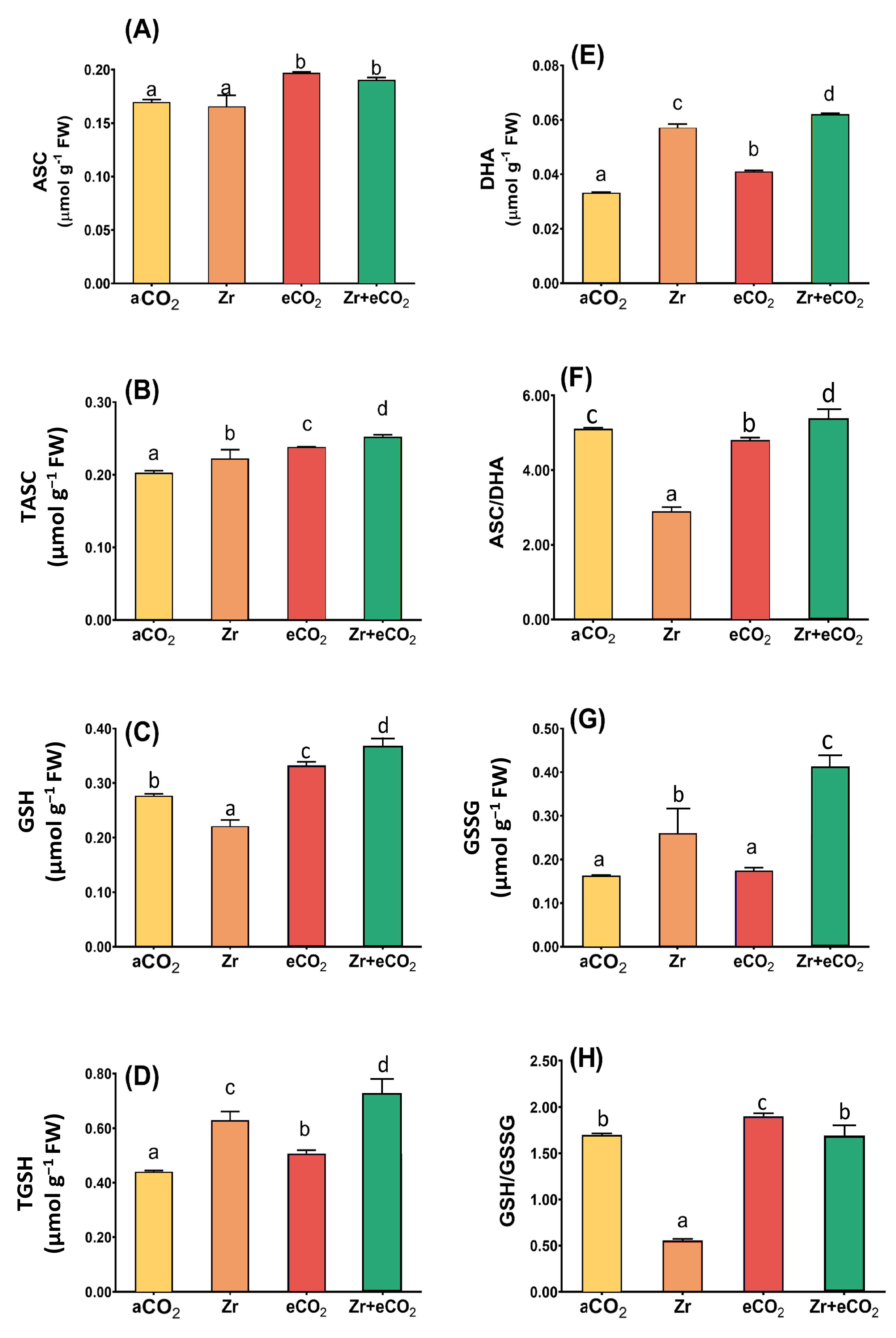
The ascorbate/glutathione metabolic pathway is involved in different plant metabolic reactions, including those elicited by environmental cues. Therefore, measuring its metabolic components is of great importance for the effectiveness of eCO2 in mitigating the phytotoxic effect of Zr. Although Zr had no significant effect on the levels of ascorbate (Figure 6A), it greatly reduced the glutathione levels in oat plants (Figure 6C). In addition, Zr caused a three-fold reduction in the GSH/GSSG ratio in polluted oat relative to aCO2-unpolluted control plants (Figure 6H). Contrarily, oat plants grown in soils polluted with Zr exhibited a noticeable increment in the levels of tASC and tGSH (Figure 6B,D). A further elevation was observed in the levels of DHA and GSSG, which increased by 65% and 59% higher than aCO2-uncontaminated control plants (Figure 6E,G). Moreover, GSSG experienced a more than two-fold increase in oats due to contamination with Zr that could explain the remarkable reduction in GSH/GSSG ratio (Figure 6G,H). Except for the GSSG and ASC/DHA ratio, the individual application of eCO2 significantly enhanced the components of ascorbate/glutathione metabolic pathway in oat plants relative to aCO2 control plants (Figure 6). For instance, tGSH showed a 25% increase in oats treated with eCO2 alone when compared with aCO2 control plants (Figure 6C). More interestingly, eCO2 further enhanced the levels of ascorbate/glutathione metabolites in oats when grown under Zr-contamination conditions. In this context, the treatment of oat plants with both eCO2 and Zr enhanced the levels of tGSH by about 28% higher than those treated with Zr alone (Figure 6D). Moreover, eCO2 improved GSH and DHA by about 66% and 65% in oat plants grown in Zr compared with those grown in Zr alone (Figure 6C,E). A further increase was observed in GSSG, as well as in the ratio of both ASC/DHA and GSH/GSSG (two-fold and three-fold increase, respectively) in response to the treatment of oat plants with both eCO2 and Zr, as compared with those treated with Zr alone (Figure 6F–H). Two-way ANOVA indicates presence of interactive effect of both Zr contamination and eCO2 treatment that was apparent on the activities of DHA, GSH, TGSH (p < 0.05, 0.01, 0.001), in addition to the levels of GSH and GSSG (p < 0.01, 0.001) (Table 3).
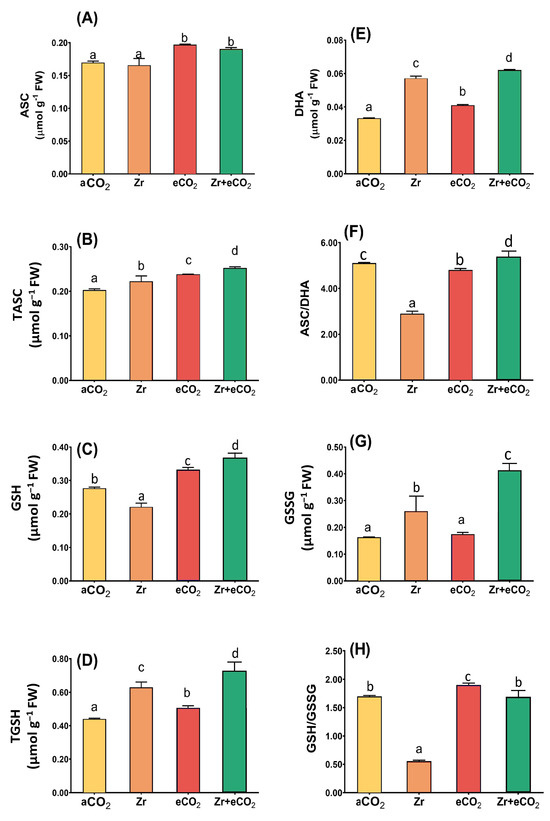
Figure 6.
The combined effect of eCO2 and/or Zr upon the ascorbate/glutathione metabolic pool of oat plants. (A) ascorbic acid, (B) total ascorbate, (C) glutathione, (D) total glutathione, (E) dehydroascorbate, (F) ascorbate/dehydroascorbate ratio, (G) glutathione disulfide and (H) glutathione/glutathione disulfide ratio. Four biological replicates were used to investigate the response. The vertical error bars are the standard error (SE). Fisher’s LSD test (p < 0.05, n = 4) was used for pairwise comparison between groups. The different letters indicate significant differences between the means of each group.

Table 3.
Two-way ANOVA for the significance of interaction between the two independent variables (Zr and eCO2) upon ascorbate/glutathione metabolic pool in oat plants (ns = non-significant; * = p < 0.05; ** = p < 0.01; *** = p < 0.001).
4. Discussion
Upon being incorporated into the food chain, Zr becomes a threat to human health as it can be easily taken in by the human body through food or water. Concerning plants, Zr was found to inhibit the germination of seeds and the growth of plant shoots and roots [35]. Nevertheless, there has been a lack of information about its impact upon plant physiology and metabolism. Therefore, our study aims to investigate the phytotoxic impact of Zr upon the growth, photosynthesis and ROS homeostasis of oat plants (Avena sativa) and how elevated CO2 could ameliorate this phytotoxic effect.
4.1. eCO2 Ameliorated the Growth Reduction and Oxidative Burst in Oat That Was Initiated by Zr
Our results showed a remarkable reduction in the growth as well as photosynthesis of oat plants in response to contamination with Zr. These findings highlight the phytotoxic impact of Zr upon plant growth and development. Comparable findings were noted in the case of algae by Simon et al. [8], who reported an adverse impact of Zr on the growth and photosynthesis of Chlorella pyrenoidosa. Moreover, the dry matter of maize shoots and roots were dramatically decreased by increasing the concentration of Zr [35]. It is likely that treatment with Zr not only inhibits the germination of Triticum aestivum but also adversely inhibits the growth of their shoots and roots [9]. Generally, soil contamination with toxic metals adversely delays the growth and development of plants. In this context, the growth of Oryza sativa was highly delayed when grown in soils contaminated with Indium [36]. A similar observation was opined by Kopittke et al. [37], who found that contamination with trace metals such as Ga, In, Hg and Ru significantly reduced the growth of cowpea roots and caused cell rupture. This phytotoxic effect could be explained by the high binding tendency of these elements to the cell wall, increasing the cell rigidity, delaying the cell growth and finally causing the rupturing of cells [33]. It is important to recognize that the biogeochemical behavior of toxic elements like Zr in the environment and their phytotoxic impact on plants are primarily contingent on their speciation. Speciation refers to the existence of metals in diverse chemical forms, which is greatly influenced by environmental factors such as soil pH and interactions with soil organic matter [6]. Regarding Zr, it exists in the soil in different chemical forms that have a wide variety of solubility and bioavailability [38]. This speciation could explain the adverse effect of Zr upon plant growth and biomass.
On the other hand, our study revealed that the phytotoxic effect of Zr was obviously mitigated by the application of eCO2. This ameliorative effect was embodied in the recovery of the plant biomass and photosynthetic efficiency relative to contaminated plants. In line with our findings, the growth of both maize and barley contaminated with As2O3 and HgO, respectively, exhibited a noticeable recovery in both growth and photosynthetic efficiency in response to treatment with eCO2 [39]. This advantageous effect might be ascribed to the ability of eCO2, within the physiological range, to enhance plant biomass by stimulating its photosynthetic machinery [33]. This, in turn, will modulate the photosynthetic carbon metabolism and so improve carbohydrate partitioning [10]. Furthermore, the enhancement in photosynthesis due to eCO2 can also be attributed to the elevated levels of CO2 at the RuBisco site, which reduces the availability of NADPH and ATP for photorespiration, thus facilitating CO2 assimilation in plants [40]. Kaiser et al. [41] reported that eCO2 increased the relative carbon gain with concomitant enhancements in the photosynthesis of tomato by initiating carboxylation reaction and slowing oxygenation reaction at RuBisco. Likewise, the photosynthesis in lettuce was improved in response to eCO2, the thing that reflected on its growth due to the availability of the carbon skeleton, resulting in higher levels of carbohydrates [42]. In conclusion, it is hypothesized that eCO2 could provide protection to significant crops against various environmental threats, including metal pollution like Zr, by enhancing their growth and photosynthesis.
4.2. eCO2 Highly Quenched the Oxidative Damage Caused by Zr in Oat Plants
Improvement in plant growth and photosynthesis is associated with modulating the plant redox homeostasis, including the regulation of reactive oxygen species under various environmental cues [33]. Our results declared that Zr triggered oxidative stress in oat plants by increasing the levels of H2O2 and MDA. In this regard, the treatment of wheat seedlings with Zr caused an oxidative damage by altering the activities of the antioxidant enzymes [9]. Similarly, Urtica dioica grown in heavy-metal-contaminated soils experienced remarkable oxidative stress with concomitant DNA damage [43]. Likewise, soil contamination with Zn and Pd provoked noticeable oxidative damage in sorghum plants [44]. Oxidative stress was triggered in both wheat and soybean plants treated with As [45]. Moreover, cereal crops treated with tungsten nanoparticles exhibited a remarkable accumulation in both H2O2 and MDA, the main cause of oxidative damage in plants [46]. Plants respond to metal stress by triggering diverse signaling pathways, including those related to ROS metabolism, as tools to cope with the different environmental challenges [47]. The adverse effects of toxic metals, one of the environmental challenges, on the growth and oxidative status of plants could be due to direct or indirect reasons. The direct toxic impact lies in the ability of heavy metals to inhibit the cytoplasmic enzymes and the destruction of cell structures in response to oxidative damage [48]. Conversely, toxic metals can indirectly influence plant growth and oxidative balance by inhibiting the functions of soil microorganisms. This, in turn, impacts the decomposition of organic matter, leading to adverse effects on soil nutrient levels [49]. Furthermore, essential metabolic enzymes may be hampered due to the interference of the toxic metals with the soil microorganisms [50]. Toxic metals may also accumulate H2O2 by impairing photorespiration, one of most important H2O2-generating pools in plants [51]. Additionally, toxic metals caused ABA accumulation, leading to the hyperaccumulation of H2O2 and the slowing of cell division [52]. These detrimental effects, when combined, can impede plant growth and initiate plant oxidative damage. On the other hand, the co-application of eCO2 with Zr obviously reduced the oxidative damage caused by Zr alone by reducing the levels of H2O2 and MDA in oat plants. Similar results were obtained by AbdElgawad et al. [53], who proclaimed that the growth of both wheat and soybean in an atmosphere enriched with CO2 reduced the oxidative damage caused by As stress. The authors also reported that eCO2 enhanced the antioxidative defense system in cereals grown in As-contaminated soils [45]. Moreover, eCO2 obviously quenched the oxidative damage triggered in both wheat and maize treated with both NiO and HgO by diminishing the accumulation of H2O2 and MDA [13,54]. This mitigative impact of eCO2 could be attributed to its potency to reduce the chance of oxygenation reaction on RuBisco [55]. This will increase the rate of carboxylation reaction that, in turn, will increase the carbon assimilation [11]. Moreover, high levels of CO2 can reduce the activity of the enzymes of photorespiration, leading to a reduction in the accumulated H2O2, accordingly [56].
4.3. eCO2 Raised Oat Tolerance to Zr Contamination by Regulating the Antioxidant Defense System
In plants, the antioxidant defense system includes low molecular weight non-enzymatic antioxidants (ascorbate, glutathione, polyphenols, flavonoids, etc.) and antioxidant enzymes that function in a coordinated manner to restrain the over-production of ROS in plants [57]. Superoxide dismutase (SOD) is the key enzyme in the conversion of superoxide ions into H2O2, which is further turned into H2O by CAT, GPX, or APX [34]. Therefore, the elevation in the activities of such enzymes in response to Zr treatments is an attempt by the plant to rein in the overproduction of H2O2, one of the main causes of oxidative damage. We also opined an elevation in the ascorbate/glutathione pathway that functions mainly in scavenging H2O2 in oat plants under contamination conditions [58]. Pollution with Zr, like other environmental stresses, causes oxidative damage in oat plants. Likewise, we previously found that tungsten nanoparticles caused an increment in the components of both enzymatic and non-enzymatic antioxidants [46]. Moreover, the growth of Camellia sinensis in media containing Cd enhanced the accumulation of phenolics including flavans [59]. Similarly, oilseed rape treated with Cd showed a noticeable increase in the levels of non-enzymatic antioxidants [60]. Also, the application of Zn caused a noticeable increase in lipid peroxidation in Brassica juncea due to the hyperaccumulation of ROS [61]. Analogous findings were documented in the moss Taxithelium nepalense, wherein exposure to Cr and Pd led to the generation of reactive oxygen species (ROS), accompanied by a simultaneous reduction in the antioxidant defense system [62]. In rice, the application of Cu caused an over-accumulation of ROS and an observable decline in the antioxidant defense arsenal [63]. In this context, ascorbic acid was induced in wheat shoots subjected to nickel stress, indicating its pivotal role in ROS manipulation [64]. Moreover, the redox statuses of ASC (ASA/DHA) and GSH (GSH/GSSG) in oat plants were reduced in response to Zr contamination, the thing that delayed the antioxidant defense system in oat plants. Overall, plants activate the production of secondary metabolites such as phenylpropanoids in response to different environmental cues [65]. This process provides the cell with a wide array of phenolic metabolites by activating the phenylpropanoid pathway [66]. By activating the phenylpropanoid pathway, non-enzymatic antioxidants were synthesized to enhance plant tolerance against environmental stresses such as toxic metals [67].
On the other hand, eCO2 not only augmented the detoxification of ROS, but also kept the balance of both GSH/GSSG and ASC/DHA in Zr-polluted oat plants. In line with our findings, AbdElgawad et al. [68] declared that eCO2 caused a remarkable improvement in the growth of cereals grown in soils contaminated with As via augmenting the ascorbate/glutathione metabolism. It is worth noting that the ASC/DHA metabolic pool keeps the redox homeostasis in the photosystem by maintaining high ratios of GSH/GSSG and ASC/DHA, which is important for plant tolerance against different environmental challenges [69]. Therefore, the concurrent suppression of photorespiration and improvement of photosynthesis highlights the crucial contribution of eCO2 in mitigating the phytotoxic effect of Zr-contaminated oats. In other words, the mitigative role of eCO2 could be mainly due to its ability to increase carboxylation at the expense of the oxygenation reaction in RuBisco [10]. More interestingly, eCO2 can provide the cells with the C skeleton and energy needed for the growth of stressed plants [70]. This increment in carbon availability will supply the cells with antioxidant molecules, so raising the plant tolerance against the stress-induced oxidative damage [40]. In our study, the observed elevation in total antioxidant capacity (FRAP) due to treatment with eCO2 indicates an awakening within the plant to tolerate the ROS generated by Zr. In accordance, we opined a noticeable increment in the levels of polyphenols and flavonoids in response to eCO2 in plants contaminated with Zr (Figure 4). Indeed, this increment could quench the toxicity induced by Zr pollution as an adaptive response to environmental stresses [71]. Another perspective is that the sugars accumulated in response to enhanced photosynthesis can scavenge free radicals generated by ROS leading to alleviation of the oxidative damage caused by environmental stresses [72]. To this end, our study, for the first time, shed light on the positive role of increasing atmospheric CO2 in modulating the ROS homeostasis in oat plants to cope with pollution with Zr.
5. Conclusions
This investigation improves our knowledge about how eCO2 differentially alleviates the phytotoxic effect of Zr on the growth, photosynthesis, and redox status of Avena sativa plants. Contamination with Zr greatly reduced the photosynthesis of oat plants with concomitant reduction in their biomass. Moreover, contamination with Zr triggered remarkable oxidative stress in oats. On the other hand, eCO2 significantly recovered the adverse effect of Zr on the biomass and redox status of oats. Furthermore, eCO2 modulated the redox homeostasis of oat plants by enhancing their photosynthetic efficiency. Accordingly, enhanced photosynthesis can improve the plant biomass under both clean and contaminated conditions. This beneficial effect of eCO2 extends beyond photosynthesis and biomass; it also mitigates the severe oxidative damage in oats by decreasing the levels of H2O2 and MDA induced by Zr pollution. Moreover, eCO2 ameliorated the negative impact of Zr upon the non-enzymatic antioxidants by improving the total antioxidant capacity (FRAP) and then the levels of the non-enzymatic antioxidants (polyphenols and flavonoids). Moreover, eCO2 caused a noticeable activation in all measured antioxidant enzymes that were inhibited in oats contaminated with Zr. In conclusion, our study, for the first time, introduces a new insight on the ability of eCO2 in harnessing the redox homeostasis of oat plants to cope with the phytotoxic effect imposed by toxic metals, particularly Zr.
Author Contributions
M.M.Y.M. and H.A.: conceptualization, methodology, resources, data curation, and writing—original draft preparation. M.M.Y.M., H.A. and A.M.S.: software and data validation. A.M.S. and H.A.: formal analysis. A.M.S., H.A., M.M.Y.M. and A.M.A.K.: investigation. M.M.Y.M. and A.M.S.: writing—review and editing. A.M.A.K. and D.A.G.: visualization. A.M.S., M.M.Y.M. and H.A.: project administration. A.M.S. and M.M.Y.M.: funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia [grant number 445-9-379].
Data Availability Statement
Data presented in this study is included in this article.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research and Innovation, Ministry of Education, in Saudi Arabia for funding this research work through the project number 445-9-379.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yadav, S.K. Heavy Metals Toxicity in Plants: An Overview on the Role of Glutathione and Phytochelatins in Heavy Metal Stress Tolerance of Plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Shanmugaraj, B.M.; Malla, A.; Ramalingam, S. Cadmium Stress and Toxicity in Plants: An Overview. In Cadmium Toxicity and Tolerance in Plants: From Physiology to Remediation; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 1–17. ISBN 9780128148655. [Google Scholar]
- Goyal, D.; Yadav, A.; Prasad, M.; Singh, T.B.; Shrivastav, P.; Ali, A.; Dantu, P.K.; Mishra, S. Effect of Heavy Metals on Plant Growth: An Overview. In Contaminants in Agriculture; Springer International Publishing: Cham, Switzerland, 2020; pp. 79–101. [Google Scholar]
- Perks, C.; Mudd, G. Titanium, Zirconium Resources and Production: A State of the Art Literature Review. Ore Geol. Rev. 2019, 107, 629–646. [Google Scholar] [CrossRef]
- Song, T.; Xia, C.; Ding, Y.; Liu, S.; Chen, B.; Feng, Z.; Yang, T.; Li, Q. Improvement of Corrosion and Wear Resistance of Novel Zr-Ti-Al-V Alloy with High Strength and Toughness by Thermal Nitridation Treatment. Corros. Sci. 2022, 208, 110685. [Google Scholar] [CrossRef]
- Shahid, M.; Ferrand, E.; Schreck, E.; Dumat, C. Behavior and Impact of Zirconium in the Soil-Plant System: Plant Uptake and Phytotoxicity. Rev. Environ. Contam. Toxicol. 2013, 221, 107–127. [Google Scholar] [CrossRef]
- Parveen, A.; Siddiqui, Z.A. Zirconium Dioxide Nanoparticles Affect Growth, Photosynthetic Pigments, Defense Enzymes Activities and Mitigate Severity of Bacterial and Fungal Diseases of Tomato. Gesunde Pflanz. 2022, 74, 615–628. [Google Scholar] [CrossRef]
- Simon, L.; Fodor, M.; Pais, I. Effects of Zirconium on the Growth and Photosynthetic Pigment Composition of Chlorella Pyrenoidosa Green Algae. J. Plant Nutr. 2001, 24, 159–174. [Google Scholar] [CrossRef]
- Fodor, M.; Hegedüs, A.; Stefanovits-Bányai, É. Zirconium Induced Physiological Alterations in Wheat Seedlings. Biol. Plant. 2005, 49, 633–636. [Google Scholar] [CrossRef]
- Shabbaj, I.; AbdElgawad, H.; Tammar, A.; Alsiary, W.A.; Madany, M.M.Y. Future Climate CO2 Can Harness ROS Homeostasis and Improve Cell Wall Fortification to Alleviate the Hazardous Effect of Phelipanche Infection in Pea Seedlings. Plant Physiol. Biochem. 2021, 166, 1131–1141. [Google Scholar] [CrossRef]
- Thompson, M.; Gamage, D.; Hirotsu, N.; Martin, A.; Seneweera, S. Effects of Elevated Carbon Dioxide on Photosynthesis and Carbon Partitioning: A Perspective on Root Sugar Sensing and Hormonal Crosstalk. Front. Physiol. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Dong, J.; Gruda, N.; Lam, S.K.; Li, X.; Duan, Z. Effects of Elevated CO2 on Nutritional Quality of Vegetables: A Review. Front. Plant Sci. 2018, 9, 924. [Google Scholar] [CrossRef]
- Saleh, A.M.; Hassan, Y.M.; Selim, S.; AbdElgawad, H. NiO-Nanoparticles Induce Reduced Phytotoxic Hazards in Wheat (Triticum aestivum L.) Grown under Future Climate CO2. Chemosphere 2019, 220, 1047–1057. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Farfan-Vignolo, E.R.; de Vos, D.; Asard, H. Elevated CO2 Mitigates Drought and Temperature-Induced Oxidative Stress Differently in Grasses and Legumes. Plant Sci. 2015, 231, 1–10. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A. The Response of Photosynthesis and Stomatal Conductance to Rising [CO2]: Mechanisms and Environmental Interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef]
- Abdelgawad, H.; Sheteiwy, M.S.; Saleh, A.M.; Mohammed, A.E.; Alotaibi, M.O.; Beemster, G.T.S.; Madany, M.M.Y.; Dijk, J.R. Van Elevated CO2 Differentially Mitigates Chromium (VI) Toxicity in Two Rice Cultivars by Modulating Mineral Homeostasis and Improving Redox Status. Chemosphere 2022, 307, 135880. [Google Scholar] [CrossRef]
- Pérez-López, U.; Robredo, A.; Lacuesta, M.; Sgherri, C.; Muñoz-Rueda, A.; Navari-Izzo, F.; Mena-Petite, A. The Oxidative Stress Caused by Salinity in Two Barley Cultivars Is Mitigated by Elevated CO2. Physiol. Plant. 2009, 135, 29–42. [Google Scholar] [CrossRef]
- Mhamdi, A.; Noctor, G. High CO2 Primes Plant Biotic Stress Defences through Redox-Linked Pathways. Plant Physiol. 2016, 172, 929–942. [Google Scholar] [CrossRef]
- Zhou, Y.; Van Leeuwen, S.K.; Pieterse, C.M.J.; Bakker, P.A.H.M.; Van Wees, S.C.M. Effect of Atmospheric CO2 on Plant Defense against Leaf and Root Pathogens of Arabidopsis. Eur. J. Plant Pathol. 2019, 154, 31–42. [Google Scholar] [CrossRef]
- Pan, C.; Ahammed, G.J.; Li, X.; Shi, K. Elevated CO2 Improves Photosynthesis under High Temperature by Attenuating the Functional Limitations to Energy Fluxes, Electron Transport and Redox Homeostasis in Tomato Leaves. Front. Plant Sci. 2018, 871, 1739. [Google Scholar] [CrossRef]
- Hemphill, J.K.; Venketeswaran, S. Chlorophyll and Carotenoid Accumulation in Three Chlorophyllous Callus Phenotypes of Glycine Max. Am. J. Bot. 1978, 65, 1055–1063. [Google Scholar] [CrossRef]
- Jiang, Z.-Y.; Woollard, A.C.S.; Wolff, S.P. Hydrogen Peroxide Production during Experimental Protein Glycation. FEBS Lett. 1990, 268, 69–71. [Google Scholar] [CrossRef]
- Senthilkumar, M.; Amaresan, N.; Sankaranarayanan, A. Estimation of Malondialdehyde (MDA) by Thiobarbituric Acid (TBA) Assay; Springer: Berlin/Heidelberg, Germany, 2021; pp. 103–105. [Google Scholar]
- Mohamed, M.S.M.; Saleh, A.M.; Abdel-Farid, I.B.; El-Naggar, S.A. Growth, Hydrolases and Ultrastructure of Fusarium Oxysporum as Affected by Phenolic Rich Extracts from Several Xerophytic Plants. Pestic. Biochem. Physiol. 2017, 141, 57–64. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “ Antioxidant Power ”: The FRAP Assay. Anal. Biochem. 1996, 76, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.B.; Khan, P.A. Peroxidase & Polyphenol Oxidase in Excised Ragi (Eleusine Corocana Cv PR 202) Leaves during Senescence. Indian J. Exp. Biol. 1982, 20, 412–416. [Google Scholar]
- Dhindsa, R.S.; Plumb-dhindsa, P.; Thorpe, T.A. Leaf Senescence: Correlated with Increased Levels of Membrane Permeability and Lipid Peroxidation, and Decreased Levels of Superoxide Dismutase and Catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Murshed, R.; Lopez-Lauri, F.; Sallanon, H. Microplate Quantification of Enzymes of the Plant Ascorbate–Glutathione Cycle. Anal. Biochem. 2008, 383, 320–322. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in Vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Drotar, A.; Phelps, P.; Fall, R. Evidence for Glutathione Peroxidase Activities in Cultured Plant Cells. Plant Sci. 1985, 42, 35–40. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.K.; AbdElgawad, H.; Giblen, T.; Zinta, G.; De Rop, M.; Asard, H.; Blust, R.; De Boeck, G. Anti-Oxidative Defences Are Modulated Differentially in Three Freshwater Teleosts in Response to Ammonia-Induced Oxidative Stress. PLoS ONE 2014, 9, e95319. [Google Scholar] [CrossRef]
- Shabbaj, I.I.; Abdelgawad, H.; Balkhyour, M.A.; Tammar, A.; Madany, M.M.Y. Elevated CO2 Differentially Mitigated Oxidative Stress Induced by Indium Oxide Nanoparticles in Young and Old Leaves of C3 and C4 Crops. Antioxidants 2022, 11, 308. [Google Scholar] [CrossRef]
- Madany, M.M.Y.; Zinta, G.; Abuelsoud, W.; Hozzein, W.N.; Selim, S.; Asard, H.; Elgawad, H.A. Hormonal Seed-Priming Improves Tomato Resistance against Broomrape Infection. J. Plant Physiol. 2020, 250, 153184. [Google Scholar] [CrossRef]
- Masalem, T.; Daher, A.; Mahmoud, A.; Al-Shobaki, M.; Shosha, A. Effect of Zirconium in Nutrient Solution on Growth and Uptake of Some Elements By Maize Plant. J. Soil Sci. Agric. Eng. 2010, 1, 613–619. [Google Scholar] [CrossRef][Green Version]
- Syu, C.H.; Chien, P.H.; Huang, C.C.; Jiang, P.Y.; Juang, K.W.; Lee, D.Y. The Growth and Uptake of Ga and In of Rice (Oryza sative L.) Seedlings as Affected by Ga and In Concentrations in Hydroponic Cultures. Ecotoxicol. Environ. Saf. 2017, 135, 32–39. [Google Scholar] [CrossRef]
- Kopittke, P.M.; McKenna, B.A.; Blamey, F.P.C.; Wehr, J.B.; Menzies, N.W. Metal-Induced Cell Rupture in Elongating Roots Is Associated with Metal Ion Binding Strengths. Plant Soil 2009, 322, 303–315. [Google Scholar] [CrossRef]
- Wilke, M.; Schmidt, C.; Dubrail, J.; Appel, K.; Borchert, M.; Kvashnina, K.; Manning, C.E. Zircon Solubility and Zirconium Complexation in H2O+Na2O+SiO2±Al2O3 Fluids at High Pressure and Temperature. Earth Planet. Sci. Lett. 2012, 349–350, 15–25. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Hassan, Y.M.; Alotaibi, M.O.; Mohammed, A.E.; Saleh, A.M. C3 and C4 Plant Systems Respond Differently to the Concurrent Challenges of Mercuric Oxide Nanoparticles and Future Climate CO2. Sci. Total Environ. 2020, 749, 142356. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, S.; Sidhu, G.P.S. Climate Change Regulated Abiotic Stress Mechanisms in Plants: A Comprehensive Review; Springer: Berlin/Heidelberg, Germany, 2022; Volume 41, ISBN 0029902102759. [Google Scholar]
- Kaiser, E.; Zhou, D.; Heuvelink, E.; Harbinson, J.; Morales, A.; Marcelis, L.F.M. Elevated CO2 Increases Photosynthesis in Fluctuating Irradiance Regardless of Photosynthetic Induction State. J. Exp. Bot. 2017, 68, 5629–5640. [Google Scholar] [CrossRef]
- Pérez-López, U.; Miranda-Apodaca, J.; Muñoz-Rueda, A.; Mena-Petite, A. Lettuce Production and Antioxidant Capacity Are Differentially Modified by Salt Stress and Light Intensity under Ambient and Elevated CO2. J. Plant Physiol. 2013, 170, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Gjorgieva, D.; Kadifkova Panovska, T.; Ruskovska, T.; Bačeva, K.; Stafilov, T. Influence of Heavy Metal Stress on Antioxidant Status and DNA Damage in Urtica Dioica. Biomed Res. Int. 2013, 2013, 276417. [Google Scholar] [CrossRef]
- Ali, A.; Guo, D.; Mahar, A.; Ma, F.; Li, R.; Shen, F.; Wang, P.; Zhang, Z. Streptomyces Pactum Assisted Phytoremediation in Zn/Pb Smelter Contaminated Soil of Feng County and Its Impact on Enzymatic Activities. Sci. Rep. 2017, 7, 46087. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Schoenaers, S.; Zinta, G.; Hassan, Y.M.; Abdel-Mawgoud, M.; Alkhalifah, D.H.M.; Hozzein, W.N.; Asard, H.; Abuelsoud, W. Soil Arsenic Toxicity Differentially Impacts C3 (Barley) and C4 (Maize) Crops under Future Climate Atmospheric CO2. J. Hazard. Mater. 2021, 414, 125331. [Google Scholar] [CrossRef]
- Selim, S.; AbdElgawad, H.; Reyad, A.M.; Alowaiesh, B.F.; Hagagy, N.; Al-Sanea, M.M.; Alsharari, S.S.; Madany, M.M.Y. Potential Use of a Novel Actinobacterial Species to Ameliorate Tungsten Nanoparticles Induced Oxidative Damage in Cereal Crops. Plant Physiol. Biochem. 2022, 171, 226–239. [Google Scholar] [CrossRef]
- Kumar, S.; Trivedi, P.K. Heavy Metal Stress Signaling in Plants. In Plant Metal Interaction; Elsevier: Amsterdam, The Netherlands, 2016; pp. 585–603. ISBN 9780128031582. [Google Scholar]
- Rizvi, A.; Khan, M.S. Heavy Metal Induced Oxidative Damage and Root Morphology Alterations of Maize (Zea mays L.) Plants and Stress Mitigation by Metal Tolerant Nitrogen Fixing Azotobacter Chroococcum. Ecotoxicol. Environ. Saf. 2018, 157, 9–20. [Google Scholar] [CrossRef]
- Chibuike, G.U.; Obiora, S.C. Heavy Metal Polluted Soils: Effect on Plants and Bioremediation Methods. Appl. Environ. Soil Sci. 2014, 2014, 752708. [Google Scholar] [CrossRef]
- Hasan, S.A.; Fariduddin, Q.; Ali, B.; Hayat, S.; Ahmad, A. Cadmium: Toxicity and Tolerance in Plants. J. Environ. Biol. 2009, 30, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Černý, M.; Habánová, H.; Berka, M.; Luklová, M.; Brzobohatý, B. Hydrogen Peroxide: Its Role in Plant Biology and Crosstalk with Signalling Networks. Int. J. Mol. Sci. 2018, 19, 2812. [Google Scholar] [CrossRef]
- Shu, S.; Gao, P.; Li, L.; Yuan, Y.; Sun, J.; Guo, S. Abscisic Acid-Induced H2O2 Accumulation Enhances Antioxidant Capacity in Pumpkin-Grafted Cucumber Leaves under Ca(NO3)2 Stress. Front. Plant Sci. 2016, 7, 1489. [Google Scholar] [CrossRef] [PubMed]
- AbdElgawad, H.; El-Sawah, A.M.; Mohammed, A.E.; Alotaibi, M.O.; Yehia, R.S.; Selim, S.; Saleh, A.M.; Beemster, G.T.S.; Sheteiwy, M.S. Increasing Atmospheric CO2 Differentially Supports Arsenite Stress Mitigating Impact of Arbuscular Mycorrhizal Fungi in Wheat and Soybean Plants. Chemosphere 2022, 296, 134044. [Google Scholar] [CrossRef]
- Saleh, A.M.; Hassan, Y.M.; Habeeb, T.H.; Alkhalaf, A.A.; Hozzein, W.N.; Selim, S.; AbdElgawad, H. Interactive Effects of Mercuric Oxide Nanoparticles and Future Climate CO2 on Maize Plant. J. Hazard. Mater. 2021, 401, 123849. [Google Scholar] [CrossRef]
- Voss, I.; Sunil, B.; Scheibe, R.; Raghavendra, A.S. Emerging Concept for the Role of Photorespiration as an Important Part of Abiotic Stress Response. Plant Biol. 2013, 15, 713–722. [Google Scholar] [CrossRef]
- Bräutigam, A.; Gowik, U. Photorespiration Connects C3 and C4 Photosynthesis. J. Exp. Bot. 2016, 67, 2953–2962. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.J. The Role of the Plant Antioxidant System in Drought Tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in Plants: Biosynthesis and Physiological Role in Environmental Stress Tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Zubova, M.Y.; Nechaeva, T.L.; Kartashov, A.V.; Zagoskina, N.V. Regulation of the Phenolic Compounds Accumulation in the Tea-Plant Callus Culture with a Separate and Combined Effect of Light and Cadmium Ions. Biol. Bull. 2020, 47, 593–604. [Google Scholar] [CrossRef]
- Yan, H.; Filardo, F.; Hu, X.; Zhao, X.; Fu, D.H. Cadmium Stress Alters the Redox Reaction and Hormone Balance in Oilseed Rape (Brassica napus L.) Leaves. Environ. Sci. Pollut. Res. 2016, 23, 3758–3769. [Google Scholar] [CrossRef] [PubMed]
- Bortoloti, G.A.; Baron, D. Phytoremediation of Toxic Heavy Metals by Brassica Plants: A Biochemical and Physiological Approach. Environ. Adv. 2022, 8, 100204. [Google Scholar] [CrossRef]
- Choudhury, S.; Panda, S.K. Toxic Effects, Oxidative Stress and Ultrastructural Changes in Moss Taxithelium Nepalense (Schwaegr.) Broth. Under Chromium and Lead Phytotoxicity. Water. Air. Soil Pollut. 2005, 167, 73–90. [Google Scholar] [CrossRef]
- Thounaojam, T.C.; Panda, P.; Choudhury, S.; Patra, H.K.; Panda, S.K. Zinc Ameliorates Copper-Induced Oxidative Stress in Developing Rice (Oryza sativa L.) Seedlings. Protoplasma 2014, 251, 61–69. [Google Scholar] [CrossRef]
- Gajewska, E.; Skłodowska, M.; Słaba, M.; Mazur, J. Effect of Nickel on Antioxidative Enzyme Activities, Proline and Chlorophyll Contents in Wheat Shoots. Biol. Plant. 2006, 50, 653–659. [Google Scholar] [CrossRef]
- Upadhyay, S.; Dixit, M. Role of Polyphenols and Other Phytochemicals on Molecular Signaling. Oxid. Med. Cell. Longev. 2015, 2015, 504253. [Google Scholar] [CrossRef]
- Siemińska-Kuczer, A.; Szymańska-Chargot, M.; Zdunek, A. Recent Advances in Interactions between Polyphenols and Plant Cell Wall Polysaccharides as Studied Using an Adsorption Technique. Food Chem. 2022, 373, 131487. [Google Scholar] [CrossRef]
- Christophe, H.; Duangjai, T. Plant Polyphenols, More than Just Simple Natural Antioxidants: Oxidative Stress, Aging and Age-Related Diseases. Medicines 2020, 7, 26. [Google Scholar]
- AbdElgawad, H.; Zinta, G.; Abuelsoud, W.; Hassan, Y.M.; Alkhalifah, D.H.M.; Hozzein, W.N.; Zrieq, R.; Beemster, G.T.; Schoenaers, S. An Actinomycete Strain of Nocardiopsis Lucentensis Reduces Arsenic Toxicity in Barley and Maize. J. Hazard. Mater. 2021, 417, 126055. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Sirhindi, G.; Bhardwaj, R.; Alyemeni, M.N.; Siddique, K.H.M.; Ahmad, P. 28-Homobrassinolide Regulates Antioxidant Enzyme Activities and Gene Expression in Response to Salt- and Temperature-Induced Oxidative Stress in Brassica Juncea. Sci. Rep. 2018, 8, 8735. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Wang, Y.; Wang, L.; Ding, J.; Cao, Y.; Qin, G.; Yan, L.; Xi, L.; Zhang, J.; Zou, Z. Increased CO2 and Light Intensity Regulate Growth and Leaf Gas Exchange in Tomato. Physiol. Plant. 2020, 168, 694–708. [Google Scholar] [CrossRef]
- Dawood, M.F.A.; Azooz, M.M. Insights into the Oxidative Status and Antioxidative Responses of Germinating Broccoli (Brassica oleracea Var. Italica L.) Seeds in Tungstate Contaminated Water. Chemosphere 2020, 261, 127585. [Google Scholar] [CrossRef] [PubMed]
- Couée, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of Soluble Sugars in Reactive Oxygen Species Balance and Responses to Oxidative Stress in Plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).