Comparative Analysis of Cd Uptake and Tolerance in Two Mangrove Species (Avicennia marina and Rhizophora stylosa) with Distinct Apoplast Barriers
Abstract
:1. Introduction
2. Results
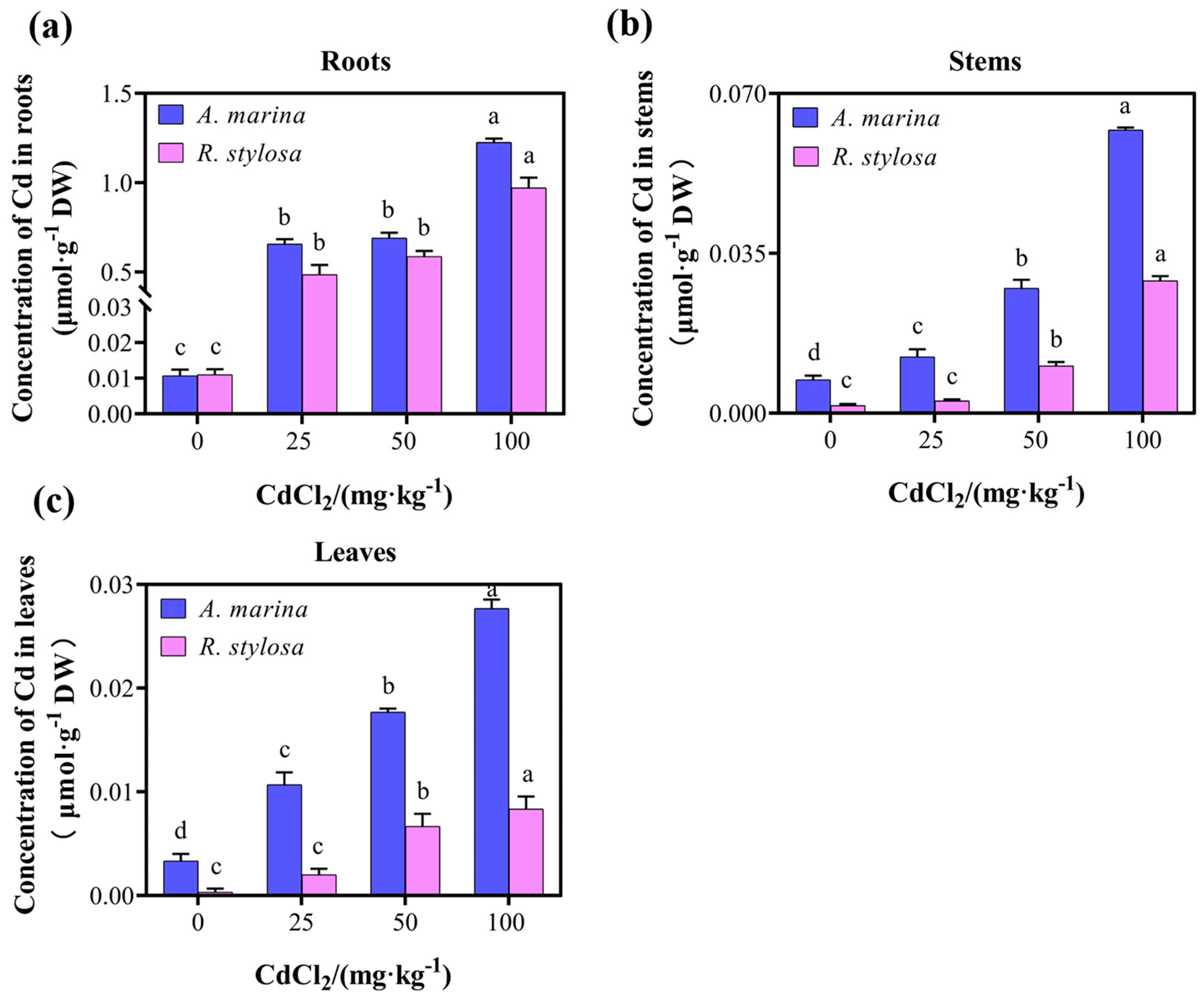
2.1. Cd Uptake and Distribution in the Two Mangrove Cultivars
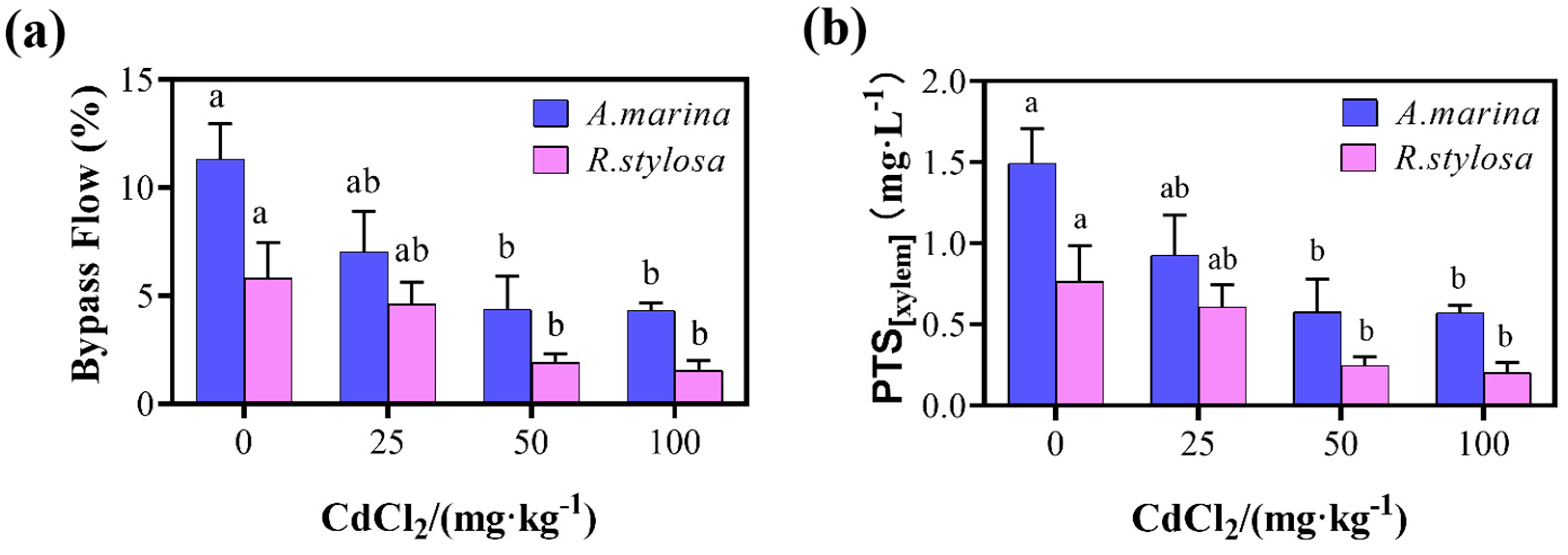
2.2. Apoplastic Bypass Flow and Apparent PTS Content in the Two Mangrove Cultivars
2.3. Root Anatomical Characteristics of Suberin Lamellae in Response to Cd Treatment
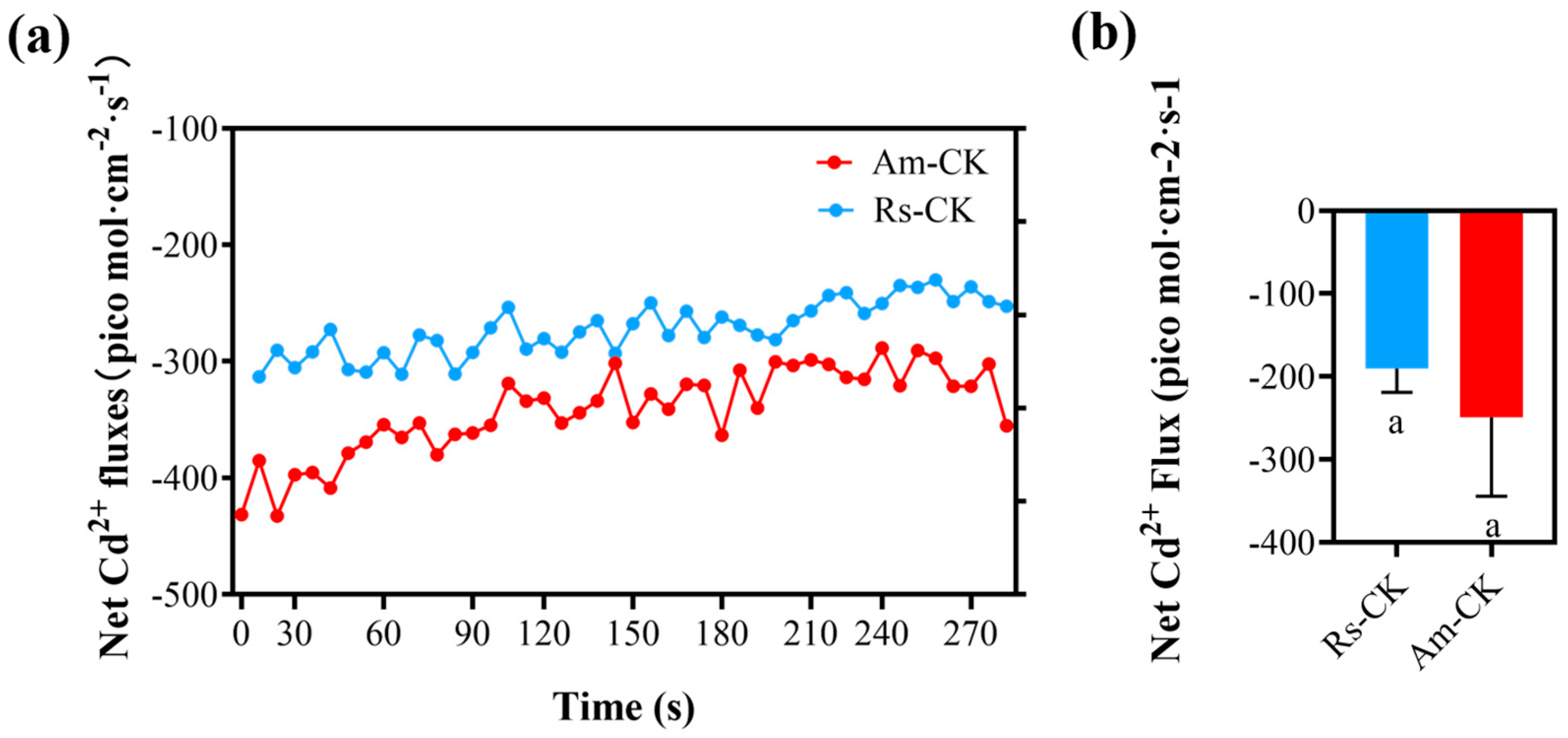
2.4. Net Fluxes of Cd2+ in Roots Surface
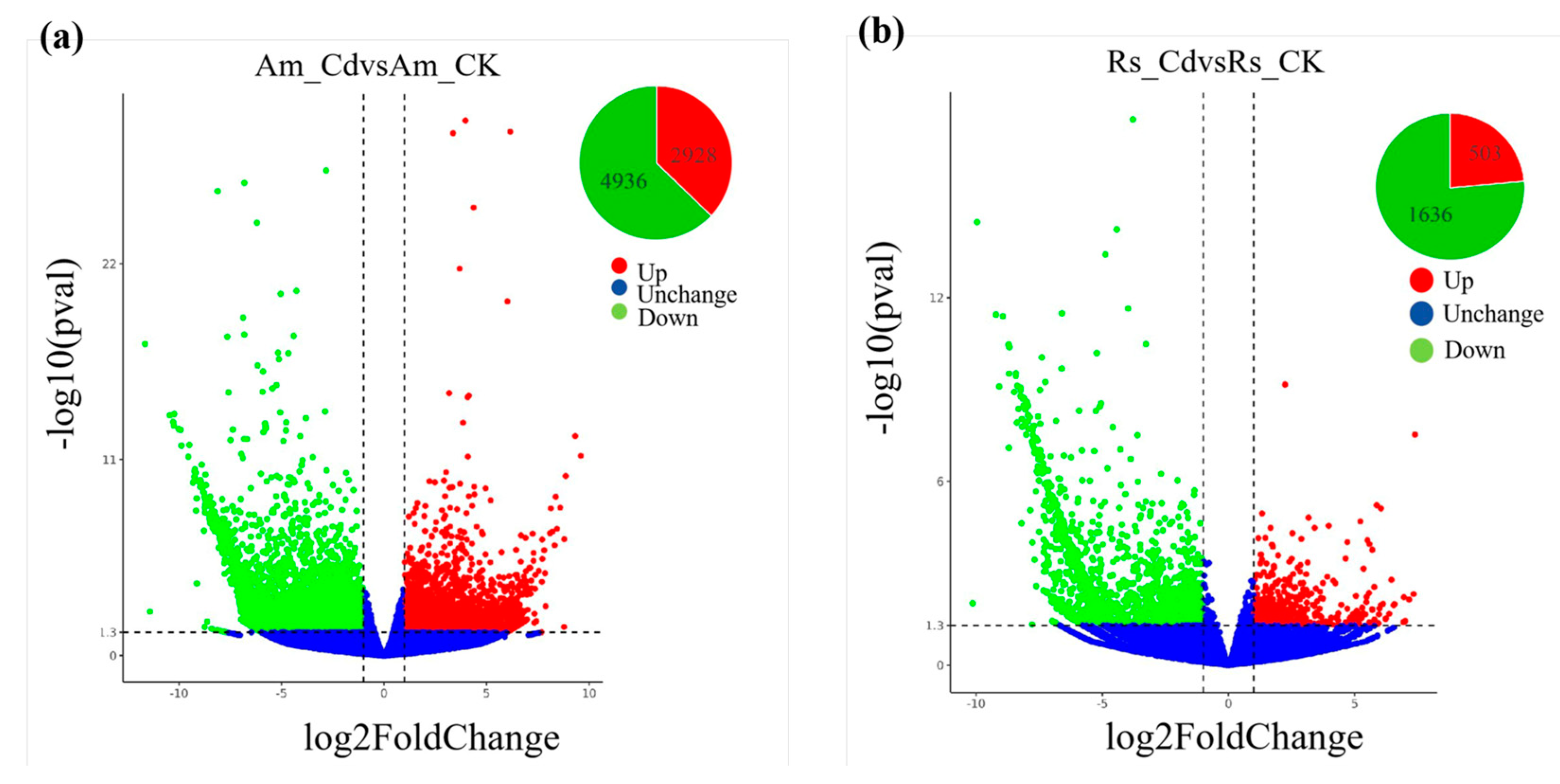
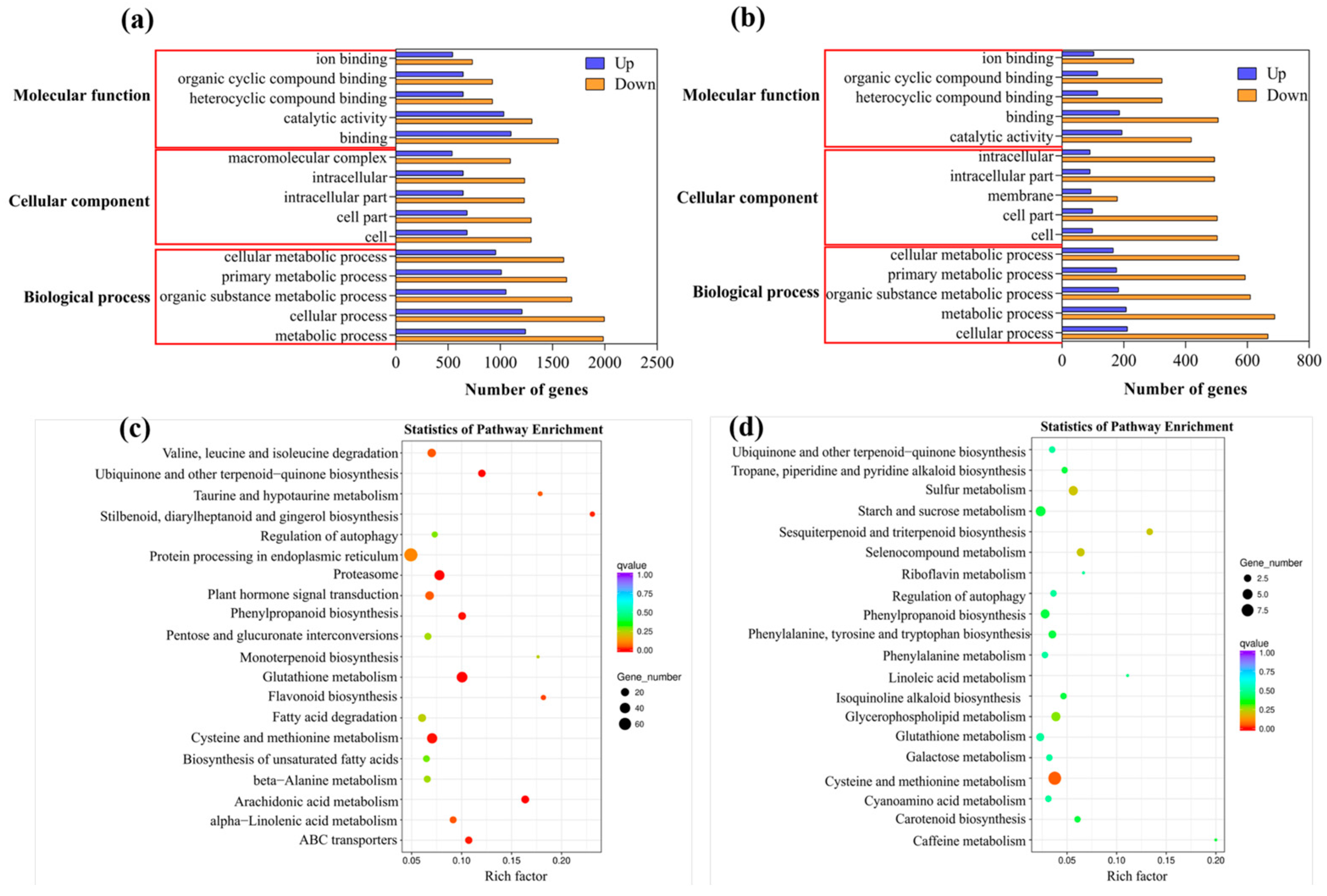
2.5. Identification and Functional Classification of DEGs
2.6. Candidate Genes for Suberin Biosynthesis and Regulation
3. Discussion
3.1. Role of Suberin on Cd2+ Uptake, Transportation, and Tolerance in Mangrove Seedlings
3.2. Effects of Cd2+ Stress on Suberin Biosynthesis in Mangrove Plants
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Measurement of Total Ion Concentration (Cd2+) from Plants
4.3. Measurement of Apoplastic Bypass Flow of Different Cultivars
4.4. Histochemical Detection of Suberin Lamellae (SL) in Roots
4.5. Measurement of Cd2+ Fluxes
4.6. RNA-Seq Library RNA Preparation, Sequencing, and Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ximenes, A.C.; Cavanaugh, K.C.; Arvor, D.; Murdiyarso, D.; Thomas, N.; Arcoverde, G.F.B.; Bispo, P.D.C.; Van der Stocken, T. A comparison of global mangrove maps: Assessing spatial and bioclimatic discrepancies at poleward range limits. Sci. Total Environ. 2023, 860, 160380. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-S.; Gu, J.-D. Ecological responses, adaptation and mechanisms of mangrove wetland ecosystem to global climate change and anthropogenic activities. Int. Biodeterior. Biodegrad. 2021, 162, 105248. [Google Scholar] [CrossRef]
- Ouyang, X.; Guo, F. Intuitionistic fuzzy analytical hierarchical processes for selecting the paradigms of mangroves in municipal wastewater treatment. Chemosphere 2018, 197, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Mahdavian, K. Evaluating the Ability of Mangrove Plants in the Asalouyeh Region for Heavy Metals Removal. Russ. J. Plant Physiol. 2023, 70, 103. [Google Scholar] [CrossRef]
- Liang, F.; Hu, J.; Liu, B.; Li, L.; Yang, X.; Bai, C.; Tan, X. New Evidence of Semi-Mangrove Plant Barringtonia racemosa in Soil Clean-Up: Tolerance and Absorption of Lead and Cadmium. Int. J. Environ. Res. Public Health 2022, 19, 12947. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Yan, C.; Du, D.; Lu, H. The alleviation effect of iron on cadmium phytotoxicity in mangrove A. marina. Alleviation effect of iron on cadmium phytotoxicity in mangrove Avicennia marina (Forsk.) Vierh. Chemosphere 2019, 226, 413–420. [Google Scholar] [CrossRef]
- Kaur, H.; Hussain, S.J.; Al-Huqail, A.A.; Siddiqui, M.H.; Al-Huqail, A.A.; Khan, M.I.R. Hydrogen sulphide and salicylic acid regulate antioxidant pathway and nutrient balance in mustard plants under cadmium stress. Plant Biol. 2022, 24, 660–669. [Google Scholar] [CrossRef]
- Li, J.; Yan, C.; Du, D.; Lu, H.; Liu, J. Accumulation and speciation of Cd in Avicennia marina tissues. Int. J. Phytoremediation 2017, 19, 1000–1006. [Google Scholar] [CrossRef]
- Zhang, L.D.; Song, L.Y.; Dai, M.J.; Liu, J.Y.; Li, J.; Xu, C.Q.; Guo, Z.J.; Song, S.W.; Liu, J.W.; Zhu, X.Y.; et al. Inventory of cadmium-transporter genes in the root of mangrove plant Avicennia marina under cadmium stress. J. Hazard Mater. 2023, 459, 132321. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, Y.S.; Liu, Y.; Ye, Z.H.; Wu, M.L.; Sun, C.C. Pb uptake and tolerance in the two selected mangroves with different root lignification and suberization. Ecotoxicology 2015, 24, 1650–1658. [Google Scholar] [CrossRef]
- Cheng, H.; Jiang, Z.Y.; Liu, Y.; Ye, Z.H.; Wu, M.L.; Sun, C.C.; Sun, F.L.; Fei, J.; Wang, Y.S. Metal (Pb, Zn and Cu) uptake and tolerance by mangroves in relation to root anatomy and lignification/suberization. Tree Physiol. 2014, 34, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Wang, Y.S.; Ye, Z.H.; Chen, D.T.; Wang, Y.T.; Peng, Y.L.; Wang, L.Y. Influence of N deficiency and salinity on metal (Pb, Zn and Cu) accumulation and tolerance by Rhizophora stylosa in relation to root anatomy and permeability. Environ. Pollut. 2012, 164, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Tam, N.F.; Li, W.C.; Ye, Z. The role of root apoplastic barriers in cadmium translocation and accumulation in cultivars of rice (Oryza sativa L.) with different Cd-accumulating characteristics. Environ. Pollut. 2020, 264, 114736. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yu, H.; Wang, S.; Bayouli, I.T.; Huang, H.; Ye, D.; Zhang, X.; Liu, T.; Wang, Y.; Zheng, Z.; et al. Root radial apoplastic transport contributes to shoot cadmium accumulation in a high cadmium-accumulating rice line. J. Hazard Mater. 2023, 460, 132276. [Google Scholar] [CrossRef] [PubMed]
- Peralta Ogorek, L.L.; Takahashi, H.; Nakazono, M.; Pedersen, O. The barrier to radial oxygen loss protects roots against hydrogen sulphide intrusion and its toxic effect. New Phytol. 2023, 238, 1825–1837. [Google Scholar] [CrossRef] [PubMed]
- Andersen, T.G.; Barberon, M.; Geldner, N. Suberization—The second life of an endodermal cell. Curr. Opin. Plant Biol. 2015, 28, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Franke, R.; Schreiber, L. Suberin—A biopolyester forming apoplastic plant interfaces. Curr. Opin. Plant Biol. 2007, 10, 252–259. [Google Scholar] [CrossRef]
- Bernards, M.A. Demystifying suberin. Can. J. Bot. 2002, 80, 227–240. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Jyothi-Prakash, P.A.; Qin, L.; He, J.; Lin, Q.; Loh, C.S.; Kumar, P.P. Role of root hydrophobic barriers in salt exclusion of a mangrove plant Avicennia officinalis. Plant Cell Environ. 2014, 37, 1656–1671. [Google Scholar] [CrossRef]
- Ye, J.; Yan, C.; Liu, J.; Lu, H.; Liu, T.; Song, Z. Effects of silicon on the distribution of cadmium compartmentation in root tips of Kandelia obovata (S., L.) Yong. Environ. Pollut. 2012, 162, 369–373. [Google Scholar] [CrossRef]
- Cheng, H.; Mai, Z.; Wang, Y.; Liu, D.; Sun, Y. Role of extracellular polymeric substances in metal sequestration during mangrove restoration. Chemosphere 2022, 306, 135550. [Google Scholar] [CrossRef] [PubMed]
- Saoussen, B.; Helmi, H.; Shino, M.; Yoshiro, O. Hyperaccumulator Thlaspi caerulescens (Ganges ecotype) response to increasing levels of dissolved cadmium and zinc. Chem. Ecol. 2012, 28, 561–573. [Google Scholar] [CrossRef]
- Gao, Y.; An, T.; Kuang, Q.; Wu, Y.; Liu, S.; Liang, L.; Yu, M.; Macrae, A.; Chen, Y. The role of arbuscular mycorrhizal fungi in the alleviation of cadmium stress in cereals: A multilevel meta-analysis. Sci. Total Environ. 2023, 902, 166091. [Google Scholar] [CrossRef]
- Wang, X.; Du, H.; Ma, M.; Rennenberg, H. The dual role of nitric oxide (NO) in plant responses to cadmium exposure. Sci. Total Environ. 2023, 892, 164597. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, X.; Kang, J.; Korpelainen, H.; Li, C. Are males and females of Populus cathayana differentially sensitive to Cd stress? J. Hazard Mater. 2020, 393, 122411. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Inyang, A.; Li, C.D.; Fei, J.; Zhou, Y.W.; Wang, Y.S. Salt tolerance and exclusion in the mangrove plant Avicennia marina in relation to root apoplastic barriers. Ecotoxicology 2020, 29, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Barberon, M.; Vermeer, J.E.; De Bellis, D.; Wang, P.; Naseer, S.; Andersen, T.G.; Humbel, B.M.; Nawrath, C.; Takano, J.; Salt, D.E.; et al. Adaptation of Root Function by Nutrient-Induced Plasticity of Endodermal Differentiation. Cell 2016, 164, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Salas-Gonzalez, I.; Reyt, G.; Flis, P.; Custodio, V.; Gopaulchan, D.; Bakhoum, N.; Dew, T.P.; Suresh, K.; Franke, R.B.; Dangl, J.L.; et al. Coordination between microbiota and root endodermis supports plant mineral nutrient homeostasis. Science 2021, 371, eabd0695. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, H.; Li, P.; Li, H.; Xu, C.; Cohen, H.; Aharoni, A.; Wu, S. Developmental programs interact with abscisic acid to coordinate root suberization in Arabidopsis. Plant J. 2020, 104, 241–251. [Google Scholar] [CrossRef]
- Tao, Q.; Jupa, R.; Liu, Y.; Luo, J.; Li, J.; Kovac, J.; Li, B.; Li, Q.; Wu, K.; Liang, Y.; et al. Abscisic acid-mediated modifications of radial apoplastic transport pathway play a key role in cadmium uptake in hyperaccumulator Sedum alfredii. Plant Cell Environ. 2019, 42, 1425–1440. [Google Scholar] [CrossRef]
- Xiao, Z.; Ye, M.; Gao, Z.; Jiang, Y.; Zhang, X.; Nikolic, N.; Liang, Y. Silicon Reduces Aluminum-Induced Suberization by Inhibiting the Uptake and Transport of Aluminum in Rice Roots and Consequently Promotes Root Growth. Plant Cell Physiol. 2022, 63, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Woolfson, K.N.; Esfandiari, M.; Bernards, M.A. Suberin Biosynthesis, Assembly, and Regulation. Plants 2022, 11, 555. [Google Scholar] [CrossRef]
- Ursache, R.; De Jesus Vieira Teixeira, C.; Denervaud Tendon, V.; Gully, K.; De Bellis, D.; Schmid-Siegert, E.; Grube Andersen, T.; Shekhar, V.; Calderon, S.; Pradervand, S.; et al. GDSL-domain proteins have key roles in suberin polymerization and degradation. Nat. Plants 2021, 7, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Rains, M.K.; Gardiyehewa de Silva, N.D.; Molina, I. Reconstructing the suberin pathway in poplar by chemical and transcriptomic analysis of bark tissues. Tree Physiol. 2018, 38, 340–361. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Song, T.; Kosma, D.K.; Parsons, E.P.; Rowland, O.; Jenks, M.A. Arabidopsis CER8 encodes LONG-CHAIN ACYL-COA SYNTHETASE 1 (LACS1) that has overlapping functions with LACS2 in plant wax and cutin synthesis. Plant J. 2009, 59, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.; Post-Beittenmiller, D.; Jaworski, J.G. KCS1 encodes a fatty acid elongase 3-ketoacyl-CoA synthase affecting wax biosynthesis in Arabidopsis thaliana. Plant J. 1999, 17, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Bernard, A.; Domergue, F.; Pascal, S.; Jetter, R.; Renne, C.; Faure, J.D.; Haslam, R.P.; Napier, J.A.; Lessire, R.; Joubes, J. Reconstitution of plant alkane biosynthesis in yeast demonstrates that Arabidopsis ECERIFERUM1 and ECERIFERUM3 are core components of a very-long-chain alkane synthesis complex. Plant Cell 2012, 24, 3106–3118. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, K.; Geeta, R.; Panjabi, P. Origin and diversification of ECERIFERUM1 (CER1) and ECERIFERUM3 (CER3) genes in land plants and phylogenetic evidence that the ancestral CER1/3 gene resulted from the fusion of pre-existing domains. Mol. Phylogenetics Evol. 2021, 159, 107101. [Google Scholar] [CrossRef]
- Hofer, R.; Briesen, I.; Beck, M.; Pinot, F.; Schreiber, L.; Franke, R. The Arabidopsis cytochrome P450 CYP86A1 encodes a fatty acid omega-hydroxylase involved in suberin monomer biosynthesis. J. Exp. Bot. 2008, 59, 2347–2360. [Google Scholar] [CrossRef]
- Beisson, F.; Li, Y.; Bonaventure, G.; Pollard, M.; Ohlrogge, J.B. The acyltransferase GPAT5 is required for the synthesis of suberin in seed coat and root of Arabidopsis. Plant Cell 2007, 19, 351–368. [Google Scholar] [CrossRef]
- Waschburger, E.; Kulcheski, F.R.; Veto, N.M.; Margis, R.; Margis-Pinheiro, M.; Turchetto-Zolet, A.C. Genome-wide analysis of the Glycerol-3-Phosphate Acyltransferase (GPAT) gene family reveals the evolution and diversification of plant GPATs. Genet. Mol. Biol. 2018, 41, 355–370. [Google Scholar] [CrossRef]
- Zhang, B.; Lewis, K.M.; Abril, A.; Davydov, D.R.; Vermerris, W.; Sattler, S.E.; Kang, C. Structure and Function of the Cytochrome P450 Monooxygenase Cinnamate 4-hydroxylase from Sorghum bicolor. Plant Physiol. 2020, 183, 957–973. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Li, M.; Li, D.; Zhang, J.; Jin, Q.; Sheng, L.; Cai, Y.; Lin, Y. Characterization and analysis of CCR and CAD gene families at the whole-genome level for lignin synthesis of stone cells in pear (Pyrus bretschneideri) fruit. Biol. Open 2017, 6, 1602–1613. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, R.; Smolka, U.; Altmann, S.; Eschen-Lippold, L.; Senning, M.; Sonnewald, S.; Weigel, B.; Frolova, N.; Strehmel, N.; Hause, G.; et al. The ABC transporter ABCG1 is required for suberin formation in potato tuber periderm. Plant Cell 2014, 26, 3403–3415. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Molina, I.; Ranathunge, K.; Castillo, I.Q.; Rothstein, S.J.; Reed, J.W. ABCG transporters are required for suberin and pollen wall extracellular barriers in Arabidopsis. Plant Cell 2014, 26, 3569–3588. [Google Scholar] [CrossRef] [PubMed]
- Bird, D.; Beisson, F.; Brigham, A.; Shin, J.; Greer, S.; Jetter, R.; Kunst, L.; Wu, X.; Yephremov, A.; Samuels, L. Characterization of Arabidopsis ABCG11/WBC11, an ATP binding cassette (ABC) transporter that is required for cuticular lipid secretion. Plant J. 2007, 52, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Shiono, K.; Ando, M.; Nishiuchi, S.; Takahashi, H.; Watanabe, K.; Nakamura, M.; Matsuo, Y.; Yasuno, N.; Yamanouchi, U.; Fujimoto, M.; et al. RCN1/OsABCG5, an ATP-binding cassette (ABC) transporter, is required for hypodermal suberization of roots in rice (Oryza sativa). Plant J. 2014, 80, 40–51. [Google Scholar] [CrossRef]
- Debono, A.; Yeats, T.H.; Rose, J.K.; Bird, D.; Jetter, R.; Kunst, L.; Samuels, L. Arabidopsis LTPG is a glycosylphosphatidylinositol-anchored lipid transfer protein required for export of lipids to the plant surface. Plant Cell 2009, 21, 1230–1238. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S.B.; Kim, H.J.; Min, M.K.; Hwang, I.; Suh, M.C. Characterization of glycosylphosphatidylinositol-anchored lipid transfer protein 2 (LTPG2) and overlapping function between LTPG/LTPG1 and LTPG2 in cuticular wax export or accumulation in Arabidopsis thaliana. Plant Cell Physiol. 2012, 53, 1391–1403. [Google Scholar] [CrossRef]
- Rojas-Murcia, N.; Hematy, K.; Lee, Y.; Emonet, A.; Ursache, R.; Fujita, S.; De Bellis, D.; Geldner, N. High-order mutants reveal an essential requirement for peroxidases but not laccases in Casparian strip lignification. Proc. Natl. Acad. Sci. USA 2020, 117, 29166–29177. [Google Scholar] [CrossRef]
- Quiroga, M.; Botella, G.C.; Barcelo, M.A.; Amaya, A.; Medina, I.; Alonso, M.I.; Forchetti, F.J.; de Tigie, S.M. A tomato peroxidase involved in the synthesis of lignin and suberin. Plant Physiol. 2000, 122, 8. [Google Scholar] [CrossRef] [PubMed]
- Barros, J.; Serk, H.; Granlund, I.; Pesquet, E. The cell biology of lignification in higher plants. Ann. Bot. 2015, 115, 1053–1074. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Perez, F.; Vivar, T.; Pomar, F.; Pedreno, M.A.; Novo-Uzal, E. Peroxidase 4 is involved in syringyl lignin formation in Arabidopsis thaliana. J. Plant Physiol. 2015, 175, 86–94. [Google Scholar] [CrossRef]
- Shafi, A.; Chauhan, R.; Gill, T.; Swarnkar, M.K.; Sreenivasulu, Y.; Kumar, S.; Kumar, N.; Shankar, R.; Ahuja, P.S.; Singh, A.K. Expression of SOD and APX genes positively regulates secondary cell wall biosynthesis and promotes plant growth and yield in Arabidopsis under salt stress. Plant Mol. Biol. 2015, 87, 615–631. [Google Scholar] [CrossRef] [PubMed]
- Ghosh Dasgupta, M.; George, B.S.; Bhatia, A.; Sidhu, O.P. Characterization of Withania somnifera leaf transcriptome and expression analysis of pathogenesis-related genes during salicylic acid signaling. PLoS ONE 2014, 9, e94803. [Google Scholar] [CrossRef]
- Soliman, A.; Adam, L.R.; Rehal, P.K.; Daayf, F. Overexpression of Solanum tuberosum Respiratory Burst Oxidase Homolog A (StRbohA) Promotes Potato Tolerance to Phytophthora infestans. Phytopathology 2021, 111, 1410–1419. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.; Han, J.P.; Cleard, F.; Lefebvre-Legendre, L.; Gully, K.; Flis, P.; Berhin, A.; Andersen, T.G.; Salt, D.E.; Nawrath, C.; et al. Suberin plasticity to developmental and exogenous cues is regulated by a set of MYB transcription factors. Proc. Natl. Acad. Sci. USA 2021, 118, e2101730118. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.; Fedyuk, V.; Wang, C.; Wu, S.; Aharoni, A. SUBERMAN regulates developmental suberization of the Arabidopsis root endodermis. Plant J. 2020, 102, 431–447. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Vishal, B.; Ho, W.J.; Lok, F.C.J.; Lee, F.S.M.; Kumar, P.P. Regulation of a Cytochrome P450 Gene CYP94B1 by WRKY33 Transcription Factor Controls Apoplastic Barrier Formation in Roots to Confer Salt Tolerance. Plant Physiol. 2020, 184, 2199–2215. [Google Scholar] [CrossRef]
- Marhavy, P.; Siddique, S. Histochemical Staining of Suberin in Plant Roots. Bio Protoc. 2021, 11, e3904. [Google Scholar] [CrossRef]

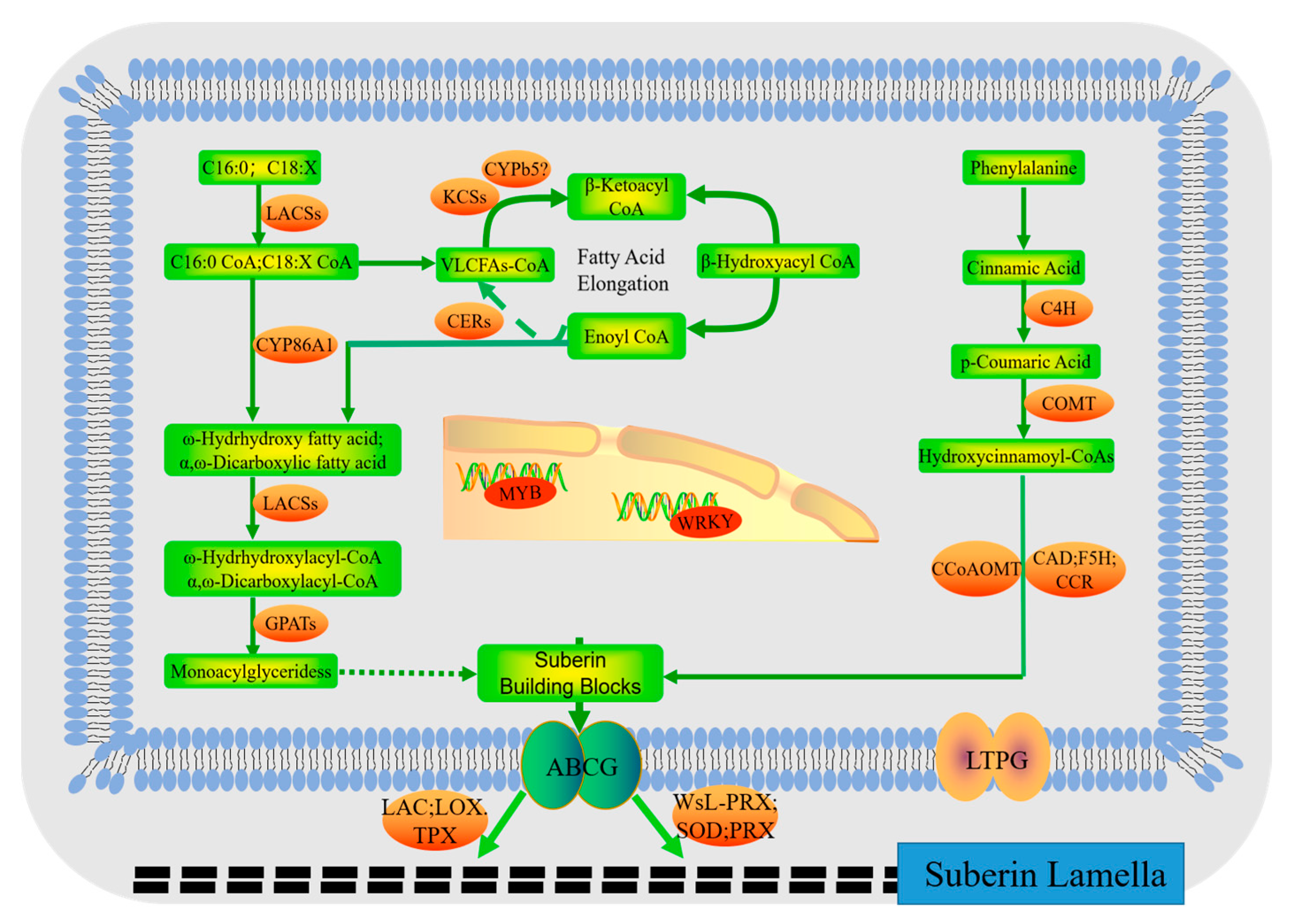
| Gene | Corresponding Enzyme Function 1 | Gene ID | Plant Species |
|---|---|---|---|
| Synthesis of suberin monomers | |||
| LACS6 | Long-chain acyl-CoA synthetase | Cluster-29888.0 | A. marina |
| KCS1 | 3-ketoacyl-CoA synthase | Cluster-14650.20981 | A. marina |
| CER1 | Very-long-chain aldehyde decarbonylase | Cluster-14650.23822 | A. marina |
| CER3 | Very-long-chain aldehyde decarbonylase | Cluster-12850.10147 | R. stylosa |
| GPAT5 | Glycerol-3-phosphate acyltransferase | Cluster-14650.34157 | A. marina |
| CYP86A1 | Cytochrome P450-dependent fatty acid ω -hydroxylase | Cluster-14650.26258 | A. marina |
| CYTB5 | Cytochrome b5 | Cluster-14650.28547 | A. marina |
| CCR1 | Cinnamoyl-CoA reductase | Cluster-14650.33944 | A. marina |
| CCR | Cinnamoyl-CoA reductase | Cluster-12850.9370 | R. stylosa |
| F5H/CYP84A | Ferulate 5-hydroxlyase | Cluster-14650.33388 | A. marina |
| COMT | Caffeic acid O-methyltransferase | Cluster-14650.15351 | A. marina |
| COMT | Caffeic acid O-methyltransferase | Cluster-3477.0 | R. stylosa |
| CAD | Cinnamyl alcohol dehydrogenase | Cluster-55339.0 | A. marina |
| CAD | Cinnamyl alcohol dehydrogenase | Cluster-55339.0 | R. stylosa |
| C4H/CYP73A | Cinnamic acid 4-hydroxylase | Cluster-14650.32547 | A. marina |
| CCoAOMT | Caffeoyl-CoA-O-methyltransferase | Cluster-14650.32082 | A. marina |
| Polymerization and assembly of suberin monomer | |||
| LTPG2 | Nonspecific lipid transfer protein GPI-anchored 2 | Cluster-14650.39736 | A. marina |
| ABCG11 | ATP-binding cassette subfamily G transporter | Cluster-14650.27512 | A. marina |
| PRX4 | Peroxygenase | Cluster-14650.16427 | A. marina |
| TPX1 | Cationic peroxidase | Cluster-14650.35143 | A. marina |
| TPX1 | Cationic peroxidase | Cluster-12850.633 | R. stylosa |
| WsL-PRX | Lignin-forming anionic peroxidase | Cluster-14650.24652 | A. marina |
| WsL-PRX | Lignin-forming anionic peroxidase | Cluster-12850.8199 | R. stylosa |
| LOX1 | Lipoxygenase | Cluster-12850.12299 | R. stylosa |
| LAC7 | Laccase | Cluster-14650.28143 | A. marina |
| LAC14 | Laccase | Cluster-14650.29125 | A. marina |
| SOD2 | Superoxide dismutase, Fe-Mn family | Cluster-52365.0 | A. marina |
| SOD1 | Superoxide dismutase, Cu-Zn family | Cluster-14650.35925 | A. marina |
| Transcription factors (TF) | |||
| MYB53 | Transcription factor MYB | Cluster-14650.15097 | A. marina |
| MYB39 | Transcription factor MYB | Cluster-14650.18329 | A. marina |
| WRKY33 | Transcription factor WRKY | Cluster-12850.13359 | R. stylosa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, L.-F.; Fei, J.; Wang, Y.-S.; Ma, X.-Y.; Zhao, Y.; Cheng, H. Comparative Analysis of Cd Uptake and Tolerance in Two Mangrove Species (Avicennia marina and Rhizophora stylosa) with Distinct Apoplast Barriers. Plants 2023, 12, 3786. https://doi.org/10.3390/plants12223786
Chang L-F, Fei J, Wang Y-S, Ma X-Y, Zhao Y, Cheng H. Comparative Analysis of Cd Uptake and Tolerance in Two Mangrove Species (Avicennia marina and Rhizophora stylosa) with Distinct Apoplast Barriers. Plants. 2023; 12(22):3786. https://doi.org/10.3390/plants12223786
Chicago/Turabian StyleChang, Li-Fang, Jiao Fei, You-Shao Wang, Xiao-Yu Ma, Yan Zhao, and Hao Cheng. 2023. "Comparative Analysis of Cd Uptake and Tolerance in Two Mangrove Species (Avicennia marina and Rhizophora stylosa) with Distinct Apoplast Barriers" Plants 12, no. 22: 3786. https://doi.org/10.3390/plants12223786
APA StyleChang, L.-F., Fei, J., Wang, Y.-S., Ma, X.-Y., Zhao, Y., & Cheng, H. (2023). Comparative Analysis of Cd Uptake and Tolerance in Two Mangrove Species (Avicennia marina and Rhizophora stylosa) with Distinct Apoplast Barriers. Plants, 12(22), 3786. https://doi.org/10.3390/plants12223786






