Fusarium Head Blight Infection Induced Responses of Six Winter Wheat Varieties in Ascorbate–Glutathione Pathway, Photosynthetic Efficiency and Stress Hormones
Abstract
:1. Introduction
2. Results
2.1. Disease Severity
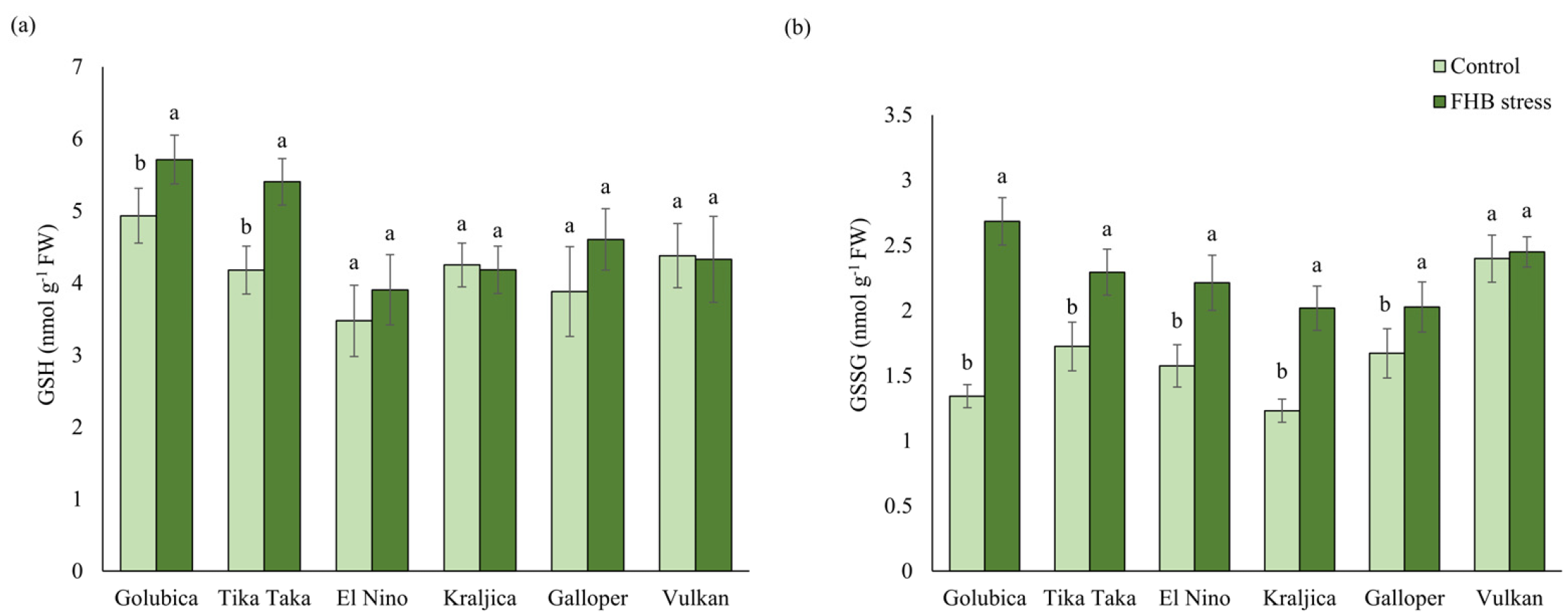
2.2. GSH and GSSG Content
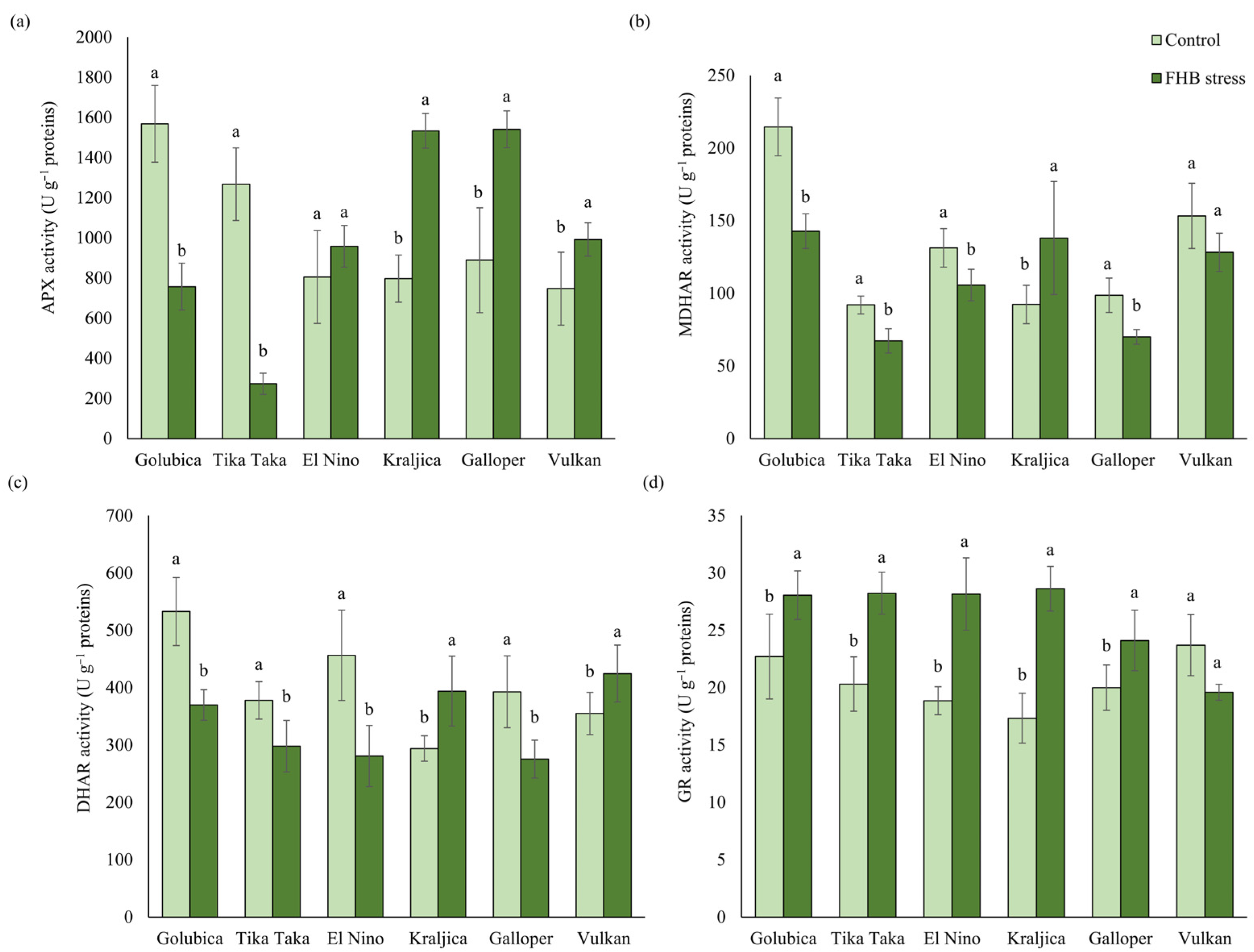
2.3. Activities of the AsA-GSH Cycle Enzymes
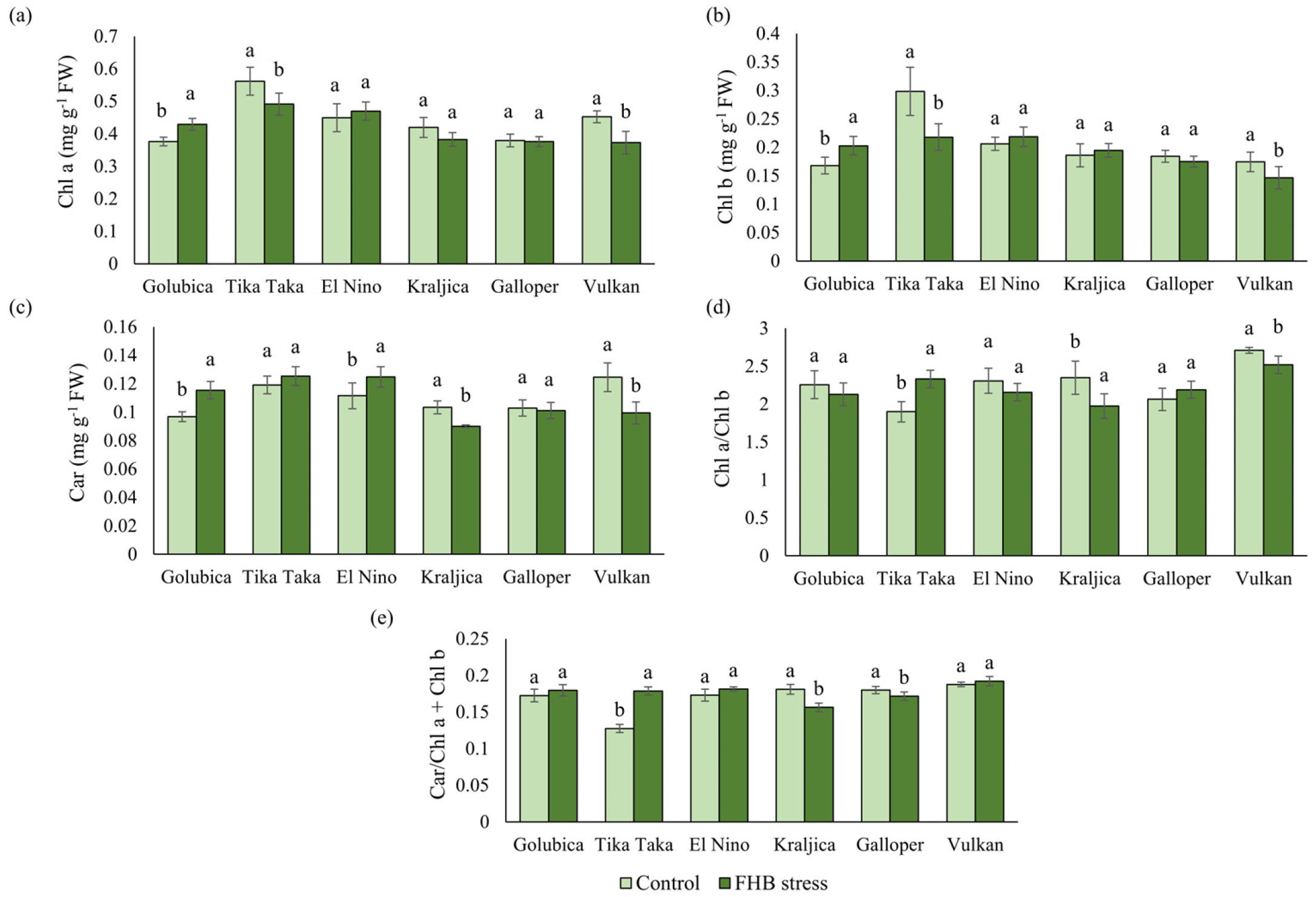
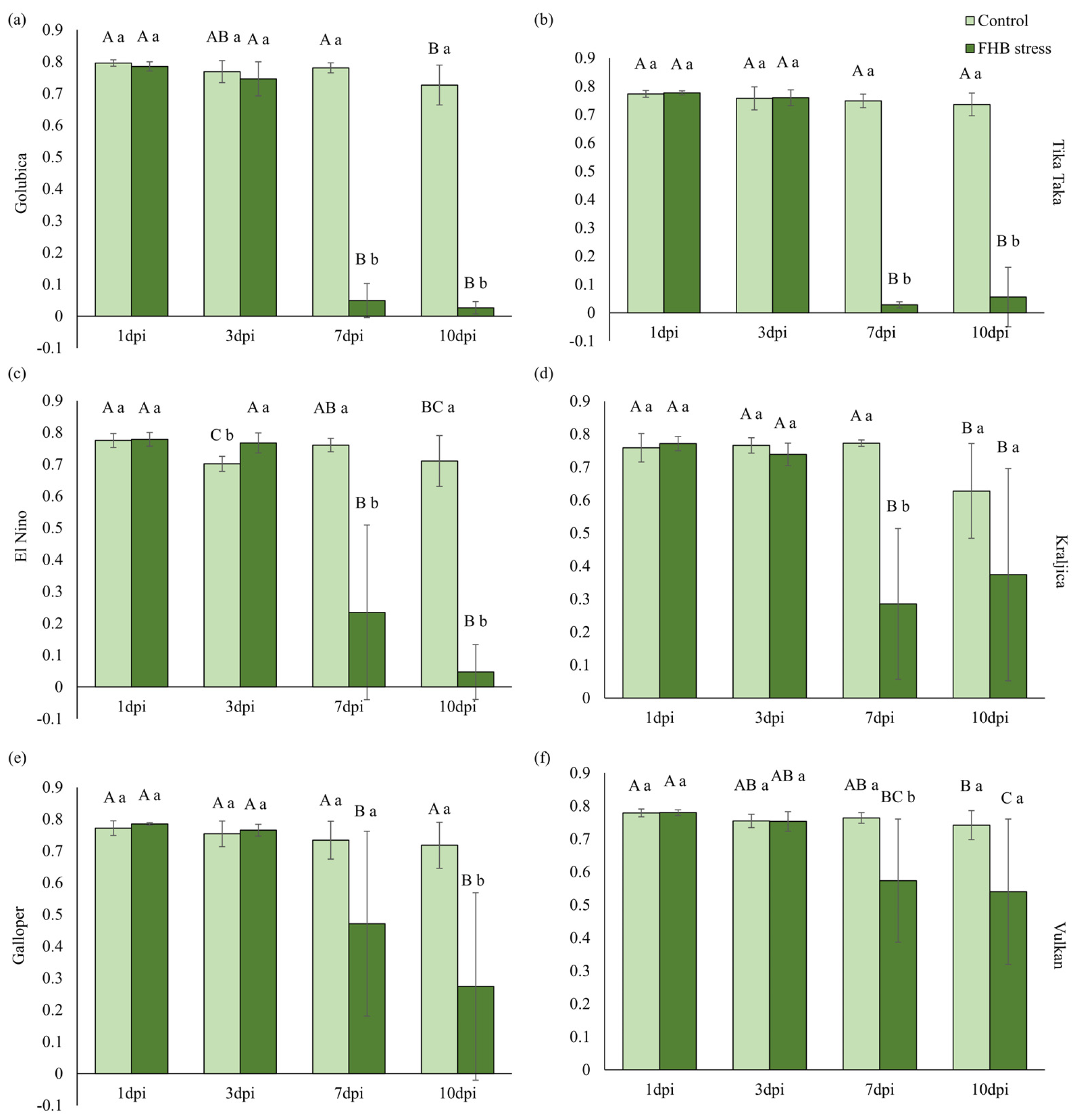
2.4. Pigments and Photosynthetic Efficiency
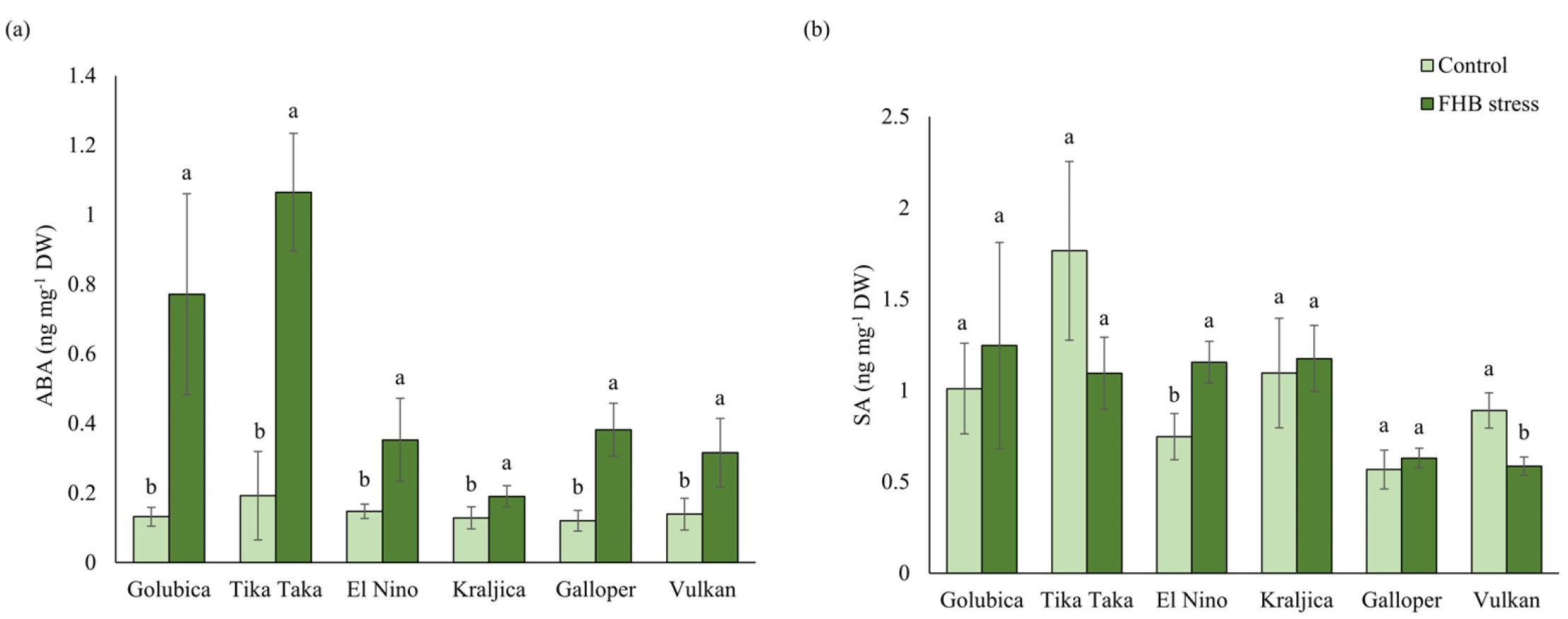
2.5. ABA and SA Content
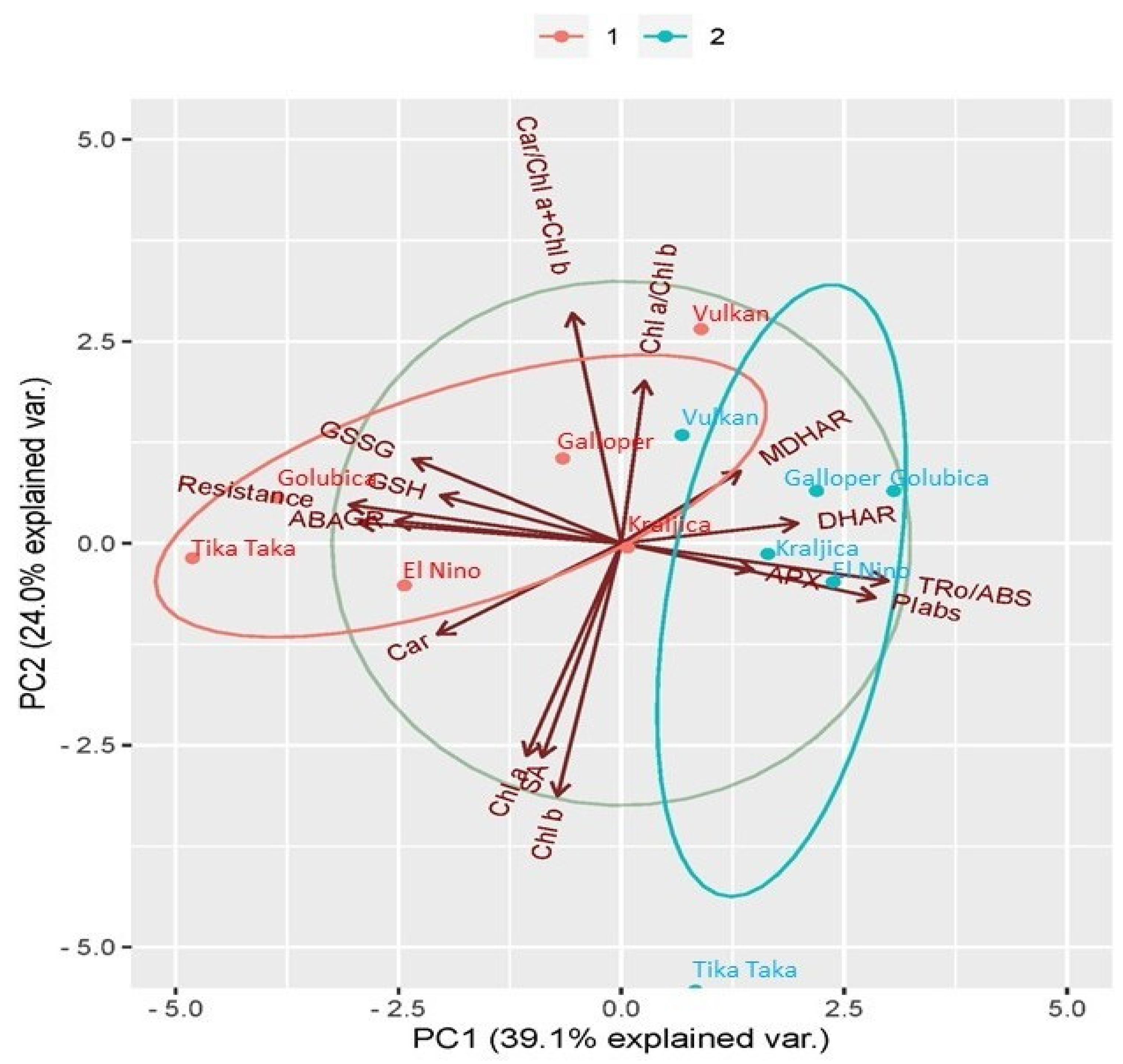
2.6. Principal Component Analysis (PCA) Analysis between Investigated Traits
3. Discussion
3.1. AsA-GSH Metabolism Response to FHB Stress
3.2. Pigments and Photosynthetic Efficiency of Wheat Spikes in Response to FHB Stress
3.3. ABA and SA Response to FHB Stress
3.4. Relations between Analyzed Traits in FHB Treatment of Six Winter Wheat Varieties and Their Respective Controls
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Inoculation of Wheat Spikes and Disease Severity Assessment
4.3. Determination of the Glutathione Content
4.4. Activities of the Enzymes of Ascorbate–Glutathione Cycle
4.5. Photosynthetic Pigments
4.6. Measurement and Analysis of Fast Chlorophyll a Fluorescence
4.7. Abscisic Acid and Salicylic Acid Analysis
4.8. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Serfling, A.; Kopahnke, D.; Habekuss, A.; Novakazi, F.; Ordon, F. Wheat Diseases: An Overview. In Achieving Sustainable Cultivation of Wheat; Burleigh Dodds Series in Agricultural Science; Langridge, P., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2017; Volume 1, pp. 263–294. [Google Scholar] [CrossRef]
- Różewicz, M.; Wyzińska, M.; Grabiński, J. The Most Important Fungal Diseases of Cereals—Problems and Possible Solutions. Agronomy 2021, 11, 714. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 Fungal Pathogens in Molecular Plant Pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Buerstmayr, M.; Steiner, B.; Buerstmayr, H. Breeding for Fusarium Head Blight Resistance in Wheat—Progress and Challenges. Plant Breed. 2020, 139, 429–454. [Google Scholar] [CrossRef]
- Sunic, K.; D’Auria, J.C.; Sarkanj, B.; Spanic, V. Metabolic Profiling Identifies Changes in the Winter Wheat Grains Following Fusarium Treatment at Two Locations in Croatia. Plants 2023, 12, 911. [Google Scholar] [CrossRef] [PubMed]
- Spanic, V.; Maricevic, M.; Ikic, I.; Sulyok, M.; Sarcevic, H. Three-Year Survey of Fusarium Multi-Metabolites/Mycotoxins Contamination in Wheat Samples in Potentially Epidemic FHB Conditions. Agronomy 2023, 13, 805. [Google Scholar] [CrossRef]
- Czaban, J.; Wróblewska, B.; Sułek, A.; Mikos, M.; Boguszewska, E.; Podolska, G.; Nieróbca, A. Colonisation of Winter Wheat Grain by Fusarium spp. and Mycotoxin Content as Dependent on a Wheat Variety, Crop Rotation, a Crop Management System and Weather Conditions. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2015, 32, 874–910. [Google Scholar] [CrossRef]
- Vaughan, M.; Backhouse, D.; Del Ponte, E.M. Climate Change Impacts on the Ecology of Fusarium graminearum Species Complex and Susceptibility of Wheat to Fusarium Head Blight: A Review. World Mycotoxin J. 2016, 9, 685–700. [Google Scholar] [CrossRef]
- Juroszek, P.; von Tiedemann, A. Climate Change and Potential Future Risks through Wheat Diseases: A Review. Eur. J. Plant Pathol. 2013, 136, 21–33. [Google Scholar] [CrossRef]
- Shah, D.A.; De Wolf, E.D.; Paul, P.A.; Madden, L.V. Predicting Fusarium Head Blight Epidemics with Boosted Regression Trees. Phytopathology 2014, 104, 702–714. [Google Scholar] [CrossRef]
- Khan, M.K.; Pandey, A.; Athar, T.; Choudhary, S.; Deval, R.; Gezgin, S.; Hamurcu, M.; Topal, A.; Atmaca, E.; Santos, P.A.; et al. Fusarium Head Blight in Wheat: Contemporary Status and Molecular Approaches. 3 Biotech 2020, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Spanic, V.; Zdunic, Z.; Drezner, G.; Viljevac Vuletic, M. Differences in Physiological Traits at the Initial Stage of Fusarium Head Blight Infection in Wheat. Biol. Plant. 2020, 64, 174–181. [Google Scholar] [CrossRef]
- Wang, L.; Li, Q.; Liu, Z.; Surendra, A.; Pan, Y.; Li, Y.; Irina Zaharia, L.; Ouellet, T.; Fobert, P.R. Integrated Transcriptome and Hormone Profiling Highlight the Role of Multiple Phytohormone Pathways in Wheat Resistance against Fusarium Head Blight. PLoS ONE 2018, 13, e0207036. [Google Scholar] [CrossRef]
- Makandar, R.; Nalam, V.J.; Lee, H.; Trick, H.N.; Dong, Y.; Shah, J. Salicylic Acid Regulates Basal Resistance to Fusarium Head Blight in Wheat. Mol. Plant-Microbe Interact. 2012, 25, 431–439. [Google Scholar] [CrossRef]
- Chen, Y.E.; Cui, J.M.; Su, Y.Q.; Yuan, S.; Yuan, M.; Zhang, H.Y. Influence of Stripe Rust Infection on the Photosynthetic Characteristics and Antioxidant System of Susceptible and Resistant Wheat Cultivars at the Adult Plant Stage. Front. Plant Sci. 2015, 6, 779. [Google Scholar] [CrossRef] [PubMed]
- Khaledi, N.; Taheri, P.; Falahati-Rastegar, M. Reactive Oxygen Species and Antioxidant System Responses in Wheat Cultivars during Interaction with Fusarium Species. Australas. Plant Pathol. 2016, 45, 653–670. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS Signaling: The New Wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Taheri, P. Cereal Diseases Caused by Fusarium graminearum: From Biology of the Pathogen to Oxidative Burst-Related Host Defense Responses. Eur. J. Plant Pathol. 2018, 152, 1–20. [Google Scholar] [CrossRef]
- García-Caparrós, P.; De Filippis, L.; Gul, A.; Hasanuzzaman, M.; Ozturk, M.; Altay, V.; Lao, M.T. Oxidative Stress and Antioxidant Metabolism under Adverse Environmental Conditions: A Review. Bot. Rev. 2021, 87, 421–466. [Google Scholar] [CrossRef]
- Kuźniak, E.; Kopczewski, T.; Chojak-Koźniewska, J. Ascorbate-Glutathione Cycle and Biotic Stress Tolerance in Plants. In Ascorbic Acid in Plant Growth, Development and Stress Tolerance, 1st ed.; Hossain, M., Munné-Bosch, S., Burritt, D., Diaz-Vivancos, P., Fujita, M., Lorence, A., Eds.; Springer: Cham, Switzerland, 2017; pp. 201–231. [Google Scholar]
- Vuković, R.; Čamagajevac, I.Š.; Vuković, A.; Šunić, K.; Begović, L.; Mlinarić, S.; Sekulić, R.; Sabo, N.; Španić, V. Physiological, Biochemical and Molecular Response of Different Winter Wheat Varieties under Drought Stress at Germination and Seedling Growth Stage. Antioxidants 2022, 11, 693. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, B.N. Role of Ascorbic Acid in Photosynthesis. Biochemistry 2014, 79, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.; Sinha, A.K.; Roitsch, T. Plant Physiology Meets Phytopathology: Plant Primary Metabolism and Plant-Pathogen Interactions. J. Exp. Bot. 2007, 58, 4019–4026. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Luo, P. Changes in Photosynthesis Could Provide Important Insight into the Interaction between Wheat and Fungal Pathogens. Int. J. Mol. Sci. 2021, 22, 8865. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yao, J. Chloroplasts at the Crossroad of Photosynthesis, Pathogen Infection and Plant Defense. Int. J. Mol. Sci. 2018, 19, 3900. [Google Scholar] [CrossRef]
- Colasuonno, P.; Lozito, M.L.; Marcotuli, I.; Nigro, D.; Giancaspro, A.; Mangini, G.; De Vita, P.; Mastrangelo, A.M.; Pecchioni, N.; Houston, K.; et al. The Carotenoid Biosynthetic and Catabolic Genes in Wheat and Their Association with Yellow Pigments. BMC Genom. 2017, 18, 122. [Google Scholar] [CrossRef]
- Spanic, V.; Sarcevic, H. Evaluation of Effective System for Tracing FHB Resistance in Wheat: An Editorial Commentary. Agronomy 2023, 13, 2116. [Google Scholar] [CrossRef]
- Ding, L.; Xu, H.; Yi, H.; Yang, L.; Kong, Z.; Zhang, L.; Xue, S.; Jia, H.; Ma, Z. Resistance to Hemi-Biotrophic F. graminearum Infection Is Associated with Coordinated and Ordered Expression of Diverse Defense Signaling Pathways. PLoS ONE 2011, 6, e19008. [Google Scholar] [CrossRef]
- Sorahinobar, M.; Niknam, V.; Ebrahimzadeh, H.; Soltanloo, H.; Behmanesh, M.; Enferadi, S.T. Central Role of Salicylic Acid in Resistance of Wheat Against Fusarium graminearum. J. Plant Growth Regul. 2016, 35, 477–491. [Google Scholar] [CrossRef]
- Qi, P.F.; Johnston, A.; Balcerzak, M.; Rocheleau, H.; Harris, L.J.; Long, X.Y.; Wei, Y.M.; Zheng, Y.L.; Ouellet, T. Effect of Salicylic Acid on Fusarium graminearum, the Major Causal Agent of Fusarium Head Blight in Wheat. Fungal Biol. 2012, 116, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Klessig, D.F.; Choi, H.W.; Dempsey, D.A. Systemic Acquired Resistance and Salicylic Acid: Past, Present, and Future. Mol. Plant-Microbe Interact. 2018, 31, 871–888. [Google Scholar] [CrossRef]
- Tripathi, D.; Raikhy, G.; Kumar, D. Chemical Elicitors of Systemic Acquired Resistance—Salicylic Acid and Its Functional Analogs. Curr. Plant Biol. 2019, 17, 48–59. [Google Scholar] [CrossRef]
- Bektas, Y.; Eulgem, T. Synthetic Plant Defense Elicitors. Front. Plant Sci. 2015, 5, 804. [Google Scholar] [CrossRef]
- Gordon, C.S.; Rajagopalan, N.; Risseeuw, E.P.; Surpin, M.; Ball, F.J.; Barber, C.J.; Buhrow, L.M.; Clark, S.M.; Page, J.E.; Todd, C.D.; et al. Characterization of Triticum aestivum Abscisic Acid Receptors and a Possible Role for These in Mediating Fusarium Head Blight Susceptibility in Wheat. PLoS ONE 2016, 11, e0164996. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.F.; Balcerzak, M.; Rocheleau, H.; Leung, W.; Wei, Y.M.; Zheng, Y.L.; Ouellet, T. Jasmonic Acid and Abscisic Acid Play Important Roles in Host-Pathogen Interaction between Fusarium graminearum and Wheat during the Early Stages of Fusarium Head Blight. Physiol. Mol. Plant Pathol. 2016, 93, 39–48. [Google Scholar] [CrossRef]
- Gietler, M.; Fidler, J.; Labudda, M.; Nykiel, M. Review Abscisic Acid—Enemy or Savior in the Response of Cereals to Abiotic and Biotic Stresses? Int. J. Mol. Sci. 2020, 21, 4607. [Google Scholar] [CrossRef] [PubMed]
- Asselbergh, B.; De Vleesschauwer, D.; Höfte, M. Global Switches and Fine-Tuning-ABA Modulates Plant Pathogen Defense. Mol. Plant-Microbe Interact. 2008, 21, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Spence, C.A.; Lakshmanan, V.; Donofrio, N.; Bais, H.P. Crucial Roles of Abscisic Acid Biogenesis in Virulence of Rice Blast Fungus Magnaporthe oryzae. Front. Plant Sci. 2015, 6, 1082. [Google Scholar] [CrossRef] [PubMed]
- Balmer, D.; Flors, V.; Glauser, G.; Mauch-Mani, B. Metabolomics of Cereals under Biotic Stress: Current Knowledge and Techniques. Front. Plant Sci. 2013, 4, 82. [Google Scholar] [CrossRef] [PubMed]
- Leslie, J.F.; Moretti, A.; Mesterh, Á.; Ameye, M.; Audenaert, K.; Singh, P.K.; Richard-forget, F.; Chulze, N.; Del Ponte, E.M.; Chala, A.; et al. Key Global Actions for Mycotoxin Management in Wheat and Other Small Grains. Toxins 2021, 13, 725. [Google Scholar] [CrossRef]
- Bai, G.; Shaner, G. Management and Resistance in Wheat and Barley to Fusarium Head Blight. Annu. Rev. Phytopathol. 2004, 42, 135–161. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; Su, Z.; Cai, J. Wheat Resistance to Fusarium Head Blight. Can. J. Plant Pathol. 2018, 40, 336–346. [Google Scholar] [CrossRef]
- Spanic, V.; Vukovic, A.; Cseplo, M.; Vukovic, R.; Buchvaldt Amby, D.; Cairo Westergaard, J.; Puskas, K.; Roitsch, T. Early Leaf Responses of Cell Physiological and Sensor-Based Signatures Reflect Susceptibility of Wheat Seedlings to Infection by Leaf Rust. Physiol. Plant. 2023, 175, e13990. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Oxidant and Antioxidant Signalling in Plants: A Re-Evaluation of the Concept of Oxidative Stress in a Physiological Context. Plant Cell Environ. 2005, 28, 1056–1071. [Google Scholar] [CrossRef]
- Pandey, P.; Singh, J.; Achary, V.M.M.; Mallireddy Reddy, K. Redox Homeostasis via Gene Families of Ascorbate-Glutathione Pathway. Front. Environ. Sci. 2015, 3, 25. [Google Scholar] [CrossRef]
- Gallie, D.R. The Role of L-Ascorbic Acid Recycling in Responding to Environmental Stress and in Promoting Plant Growth. J. Exp. Bot. 2013, 64, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Motallebi, P.; Niknam, V.; Ebrahimzadeh, H.; Enferadi, S.T.; Hashemi, M. The Effect of Methyl Jasmonate on Enzyme Activities in Wheat Genotypes Infected by the Crown and Root Rot Pathogen Fusarium culmorum. Acta Physiol. Plant. 2015, 37, 237. [Google Scholar] [CrossRef]
- Gherbawy, Y.; El-Tayeb, M.; Maghraby, T.; Shebany, Y.; El-Deeb, B. Response of Antioxidant Enzymes and Some Metabolic Activities in Wheat to Fusarium spp. Infections. Acta Agron. Hung. 2012, 60, 319–333. [Google Scholar] [CrossRef]
- Sorahinobar, M.; Niknam, V.; Ebrahimzadeh, H.; Soltanloo, H. Differential Antioxidative Responses of Susceptible and Resistant Wheat Cultivars against Fusarium Head Blight. Int. J. Farming Allied Sci. 2015, 4, 239–243. [Google Scholar]
- Spanic, V.; Viljevac Vuletic, M.; Abicic, I.; Marcek, T. Early Response of Wheat Antioxidant System with Special Reference to Fusarium Head Blight Stress. Plant Physiol. Biochem. 2017, 115, 34–43. [Google Scholar] [CrossRef]
- Agathokleous, E.; Feng, Z.Z.; Peñuelas, J. Chlorophyll Hormesis: Are Chlorophylls Major Components of Stress Biology in Higher Plants? Sci. Total Environ. 2020, 726, 138637. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. Preconditioning Is Hormesis Part II: How the Conditioning Dose Mediates Protection: Dose Optimization within Temporal and Mechanistic Frameworks. Pharmacol. Res. 2016, 110, 265–275. [Google Scholar] [CrossRef]
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. Hormesis: A Compelling Platform for Sophisticated Plant Science. Trends Plant Sci. 2019, 24, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Zabala, J.; González-Murua, C.; Marino, D. Mild Ammonium Stress Increases Chlorophyll Content in Arabidopsis thaliana. Plant Signal. Behav. 2015, 10, e991596. [Google Scholar] [CrossRef] [PubMed]
- Fini, A.; Brunetti, C.; Di Ferdinando, M.; Ferrini, F.; Tattini, M. Stress-Induced Flavonoid Biosynthesis and the Antioxidant Machinery of Plants. Plant Signal. Behav. 2011, 6, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.; Mittra, B. Alleviation of Fusarium oxysporum Induced Oxidative Stress in Wheat by Trichoderma Viride. Arch. Phytopathol. Plant Prot. 2017, 50, 84–96. [Google Scholar] [CrossRef]
- Atanasova-Penichon, V.; Barreau, C.; Richard-Forget, F. Antioxidant Secondary Metabolites in Cereals: Potential Involvement in Resistance to Fusarium and Mycotoxin Accumulation. Front. Microbiol. 2016, 7, 566. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Michalek, W.; Jamiolkowska, A. Chlorophyll Fluorescence Measurements as Indicators of Fusariosis Severity in Tomato Plants. Agron. Res. 2006, 4, 461–464. [Google Scholar]
- Francesconi, S.; Balestra, G.M. The Modulation of Stomatal Conductance and Photosynthetic Parameters Is Involved in Fusarium Head Blight Resistance in Wheat. PLoS ONE 2020, 15, e0235482. [Google Scholar] [CrossRef] [PubMed]
- Katanić, Z.; Mlinarić, S.; Katanić, N.; Ćosić, J.; Španić, V. Photosynthetic Efficiency in Flag Leaves and Ears of Winter Wheat during Fusarium Head Blight Infection. Agronomy 2021, 11, 2415. [Google Scholar] [CrossRef]
- Seemann, J.R.; Sharkey, T.D. The Effect of Abscisic Acid and Other Inhibitors on Photosynthetic Capacity and the Biochemistry of CO2 Assimilation. Plant Physiol. 1987, 84, 696–700. [Google Scholar] [CrossRef]
- Duvnjak, J.; Lončarić, A.; Brkljačić, L.; Šamec, D.; Šarčević, H.; Salopek-Sondi, B.; Španić, V. Morpho-Physiological and Hormonal Response of Winter Wheat Varieties to Drought Stress at Stem Elongation and Anthesis Stages. Plants 2023, 12, 418. [Google Scholar] [CrossRef]
- Mauch-Mani, B.; Mauch, F. The Role of Abscisic Acid in Plant-Pathogen Interactions. Curr. Opin. Plant Biol. 2005, 8, 409–414. [Google Scholar] [CrossRef]
- Qi, P.F.; Jiang, Y.F.; Guo, Z.R.; Chen, Q.; Ouellet, T.; Zong, L.J.; Wei, Z.Z.; Wang, Y.; Zhang, Y.Z.; Xu, B.J.; et al. Transcriptional Reference Map of Hormone Responses in Wheat Spikes. BMC Genom. 2019, 20, 390. [Google Scholar] [CrossRef] [PubMed]
- Dodge, A.G.; Wackett, L.P. Metabolism of Bismuth Subsalicylate and Intracellular Accumulation of Bismuth by Fusarium Sp. Strain BI. Appl. Environ. Microbiol. 2005, 71, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.F.; Zhang, Y.Z.; Liu, C.H.; Chen, Q.; Guo, Z.R.; Wang, Y.; Xu, B.J.; Jiang, Y.F.; Zheng, T.; Gong, X.; et al. Functional Analysis of FgNahG Clarifies the Contribution of Salicylic Acid to Wheat (Triticum aestivum) Resistance against Fusarium Head Blight. Toxins 2019, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yen, Y. Jasmonate and Ethylene Signaling Pathway May Mediate Fusarium Head Blight Resistance in Wheat. Crop Sci. 2008, 48, 1888–1896. [Google Scholar] [CrossRef]
- Dat, J.; Vandenabeele, S.; Vranová, E.; Van Montagu, M.; Inzé, D.; Van Breusegem, F. Dual Action of the Active Oxygen Species during Plant Stress Responses. Cell. Mol. Life Sci. 2000, 57, 779–795. [Google Scholar] [CrossRef]
- Rao, M.V.; Paliyath, G.; Ormrod, D.P.; Murr, D.P.; Watkins, C.B. Influence of Salicylic Acid on H2O2 Production, Oxidative Stress, and H2O2-Metabolizing Enzymes. Plant Physiol. 1997, 115, 137–149. [Google Scholar] [CrossRef]
- Ameye, M.; Audenaert, K.; De Zutter, N.; Steppe, K.; Van Meulebroek, L.; Vanhaecke, L.; De Vleesschauwer, D.; Haesaert, G.; Smagghe, G. Priming of Wheat with the Green Leaf Volatile Z-3-Hexenyl Acetate Enhances Defense against Fusarium graminearum but Boosts Deoxynivalenol Production. Plant Physiol. 2015, 167, 1671–1684. [Google Scholar] [CrossRef]
- Owen, W. Griffith Determination of Glutathione and Glutathione Disulfide Using Glutathione Reductase and 2-Vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Hossain, M.A.; Nakano, Y.; Asada, K. Monodehydroascorbate Reductase in Spinach Chloroplasts and Its Participation in Regeneration of Ascorbate for Scavenging Hydrogen Peroxide. Plant Cell Physiol. 1984, 25, 385–395. [Google Scholar] [CrossRef]
- Wang, M.F.; Cheng, L. Exposure of the Shaded Side of Apple Fruit to Full Sun Leads to Up-Regulation of Both the Xanthophyll Cycle and the Ascorbate-Glutathione Cycle. HortScience 2004, 39, 887A–887. [Google Scholar] [CrossRef]
- Murshed, R.; Lopez-Lauri, F.; Sallanon, H. Microplate Quantification of Enzymes of the Plant Ascorbate-Glutathione Cycle. Anal. Biochem. 2008, 383, 320–322. [Google Scholar] [CrossRef]
- Racker, E. Glutathione Reductase from Bakers’ Yeast and Beef Liver. J. Biol. Chem. 1955, 217, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Yusuf, M.A.; Kumar, D.; Rajwanshi, R.; Strasser, R.J.; Tsimilli-Michael, M.; Govindjee; Sarin, N.B. Overexpression of γ-Tocopherol Methyl Transferase Gene in Transgenic Brassica juncea Plants Alleviates Abiotic Stress: Physiological and Chlorophyll a Fluorescence Measurements. Biochim. Biophys. Acta Bioenerg. 2010, 1797, 1428–1438. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient. In Chlorophyll a Fluorescence; Springer Nature: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]


| Variety | Number of Infected Spikelets on 10 dpi |
|---|---|
| Golubica | 3.7 ± 0.47 a |
| Tika Taka | 3.5 ± 0.76 ab |
| El Nino | 2.5 ± 0.96 bc |
| Kraljica | 2 ± 0.58 cd |
| Galloper | 1.8 ± 1.46 cd |
| Vulkan | 1 ± 0.82 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sunic, K.; Brkljacic, L.; Vukovic, R.; Katanic, Z.; Salopek-Sondi, B.; Spanic, V. Fusarium Head Blight Infection Induced Responses of Six Winter Wheat Varieties in Ascorbate–Glutathione Pathway, Photosynthetic Efficiency and Stress Hormones. Plants 2023, 12, 3720. https://doi.org/10.3390/plants12213720
Sunic K, Brkljacic L, Vukovic R, Katanic Z, Salopek-Sondi B, Spanic V. Fusarium Head Blight Infection Induced Responses of Six Winter Wheat Varieties in Ascorbate–Glutathione Pathway, Photosynthetic Efficiency and Stress Hormones. Plants. 2023; 12(21):3720. https://doi.org/10.3390/plants12213720
Chicago/Turabian StyleSunic, Katarina, Lidija Brkljacic, Rosemary Vukovic, Zorana Katanic, Branka Salopek-Sondi, and Valentina Spanic. 2023. "Fusarium Head Blight Infection Induced Responses of Six Winter Wheat Varieties in Ascorbate–Glutathione Pathway, Photosynthetic Efficiency and Stress Hormones" Plants 12, no. 21: 3720. https://doi.org/10.3390/plants12213720
APA StyleSunic, K., Brkljacic, L., Vukovic, R., Katanic, Z., Salopek-Sondi, B., & Spanic, V. (2023). Fusarium Head Blight Infection Induced Responses of Six Winter Wheat Varieties in Ascorbate–Glutathione Pathway, Photosynthetic Efficiency and Stress Hormones. Plants, 12(21), 3720. https://doi.org/10.3390/plants12213720








