Abstract
Drought is an important environmental factor that influences physiological processes in plants; however, few studies have examined the physiological mechanisms underlying plants’ responses to continuous drought. In this study, the seedlings and younger individuals of Haloxylon ammodendron were experimentally planted in the southern part of the Gurbantunggut Desert. We measured their photosynthetic traits, functional traits and non-structural carbohydrate contents (NSCs) in order to assess the effects of continuous drought (at 15-day and 30-day drought points) on the plants’ physiological responses. The results showed that at the 15-day (15 d) drought point, the leaf light-saturated net photosynthetic rate (An) values of both the seedlings and the younger individuals were decreased (by −68.9% and −45.2%, respectively). The intrinsic water use efficiency (iWUE) of the seedlings was significantly lower than that of the control group (−52.2%), but there was no diffenrence of iWUE observed in younger individuals. At the 30-day (30 d) drought point, a decrease in the An (−129.8%) of the seedlings was induced via biochemical inhibition, with a lower potential maximum photochemical rate (Fv/Fm, 0.42) compared with the control group, while a decrease in the An (−52.3%) of the younger individuals was induced due to lower stomatal conductance (gs, −50.5%). Our results indicated that prolonged drought induced a greater risk of seedling mortality as the relatively limited ability of stomatal regulation may increase the possibility of massive embolism, resulting in hydraulic failure.
1. Introduction
The high and unexpected frequency of extreme climatic events and the effects of human activities have strongly affected the amount of water available for the growth of vegetation [1,2,3,4,5]. Drought is the principal limiting factor for plant physiological processes and growth in a natural ecosystem [6,7,8]. Vegetation in the desert is strongly constrained by low soil water availability induced by frequent global climatic changes and increasing human activities [9,10]. In particular, limited water availability can strongly reduce plant growth and even affect the survival of plant seedlings [11]. Plants in deserts are extremely sensitive to environmental changes and highly vulnerable to extreme drought events [12,13], and they exhibit markedly variable physiological and morphological strategies for withstanding drought [14]. Many studies have reported the different adaptation strategies of species [15,16,17]; however, few studies have investigated the different physiological response strategies of plants at different developmental stages to drought.
Plant response strategies to drought are complex and differ among ages and species, thereby influencing plant survival after drought events [4,18,19]. Many previous studies reported the eco-physiological responses of plants to drought stress, including morphological traits (increasing the leaf area per mass and reducing the leaf size, stem length and leaf length/width) and physiological traits (reducing net photosynthetic rates, stomatal conductance and leaf water potential) [11,20]. In addition, a plant’s physiological response to drought is markedly related to its mortality risk, species composition and ecosystem functions [15,21,22]. The leaf gas exchange, moreover, is an essential process in an ecosystem’s carbon cycle and also an important physiological trait which can be easily impacted by environmental factors [23,24,25]. A large number of studies have reported that the leaf light-saturated net photosynthetic rate (An) decreases with decreasing water availability due to stomatal closure to save water and maintain hydraulic structural integrity [16,26]. With long-term drought, biochemical limitations to photosynthesis are generated via severe injury in biochemical processes, which further reduce the An value [27]. Additionally, a plant’s uptake and transportation of water are closely related to its acquisition of nutrients from soil [28]. Thus, low soil water availability could inhibit plant nutrient acquisition, which would further impair photosynthetic biochemical processes [29]. Aside from stomatal regulation, leaf desiccation and canopy wilt are two common means of reducing the transpiration area which affect carbon fixation as well [9]. Furthermore, positive stomatal regulation can reduce water loss but also limits CO2 availability, while negative stomatal regulation can maintain high CO2 availability but may lead to higher risks of stem cavitation and hydraulic failure [30,31]. According to the stomatal response to drought stress, the water use strategies of species can be divided into risky and conservative strategies [32]. Stomatal regulation strategies can reflect the plant water use theory that stomatal optimization can be framed in terms of maximizing marginal carbon gain through the intrinsic water use efficiency (iWUE) [33]. Water use strategies are closely related to living environmental factors [34,35]; for instance, Wright et al. [36] reported that dry-site species have a conservative strategy that leads to a lower stomatal conductance at a given photosynthetic rate. According to different stomatal regulating strategies, there are two hypotheses to explain the risk of mortality induced by drought: the risk of hydraulic failure related to low hydraulic status with negative stomatal regulation and the risk of carbon starvation induced by long-term low photosynthetic rate with positive stomatal regulation [21,37,38]. Furthermore, stomatal closure can maintain a relatively high midday leaf water potential to avoid or reduce embolism and subsequent hydraulic failure [39].
Regarding water potential, there is increasing recognition that it is an inherent hydraulic trait which can be strongly influenced not only by environment factors but also by a plant’s developmental stages [6]. Additionally, the leaf water potential of trees decreases with declining soil moisture and clearly affects the gas exchange process [20,40]. A low leaf water potential can maintain normal water uptake with little soil water availability to support leaf transpiration but also would increase the risk of xylem embolism and decrease hydraulic conductance [34]. A water potential that is too low also represents low levels of water transportation in stems and further influences biomass allocation and metabolism in organs [41]. Moreover, a leaf water potential below a critical threshold may result in hydraulic failure that can lead to plant mortality [42]. Additionally, leaf water potential is a key regulator of transpiration and a critical predictor of plant water status and mortality [19]; therefore, plants must regulate their water potential to maintain the integrity of the hydraulic net along the soil–plant–atmosphere continuum [34].
Apart from water potential, the response of non-structural carbohydrates (NSCs) content is a critical indicator of plant physiological status and can reflect the dual risks of hydraulic failure and carbon starvation [43]. NSC content in tree tissues is considered to be a measure of carbon supply for growth and a foundation of stress resistance [39,44]. An unexpected change in the NSC pools may lead to an unbalance between carbon demand and concurrent supply which may induce carbon starvation [45]. Indeed, NSCs serve multiple roles in plant tissues including in growth and metabolism [46,47], and also signaling, osmotic regulation and defense [48]. As reported by David et al. [49], the growth of trees is strongly associated with NSC storage when constrained by photosynthesis. In addition, NSC plays a crucial role in phloem transportation when refilling embolisms, which always occur under limited soil water availability conditions [44,50,51]. Starch and soluble sugars are considered as the main fractions of NSC [52]. However, they show different functions in plant tissues. For instance, starch plays a role in storage, defined as the resources that can be used in the future for growth or embolism repair after extreme drought stress in the xylem [43]; in contrast to starch, soluble sugars are important to osmotic regulation to maintain stable hydraulic conductance under drought stress [47]. The reduction in NSCs also reduces the biosynthesis of photosynthase, which could further inhibit photosynthesis [53]. A recent meta-analysis showed that starch and soluble sugars were differentially altered in organs as drought time increased and implied the possibility of NSC depletion, which may cause carbon starvation [54]. Changes in NSCs are variable among species and complex with different influencing factors, such as drought, heat and pests [44]. However, few studies have evaluated NSCs’ variable responses over extended drought duration with seedlings at different developmental stages.
Many studies have reported plant physiological responses to drought stress, whereas few studies have compared the different continuous drought stress effects on seedlings at different developmental stages. Haloxylon ammodendron is a dominant species in arid regions in Central Asia and the main vegetation used for restoration in desert areas, such as the Gurbantunggut Desert [55]. H. ammodendron acclimates to drought mainly with a safe strategy dominated by positive stomatal closure and morphological regulation, including thinner and shorter leaves, suggesting it could be used in the sustainable development of ecological restoration areas [56]. Additionally, H. ammodendron is characterized by well-developed vertical roots of more than 10 m caused by its strong dependence on groundwater [57,58]. H. ammodendron seedlings and younger individuals are the two main objects for restoring vegetation [59]. Previous studies have investigated the eco-physiological adaption of H. ammodendron to drought in situ [56], but there is limited information about the impact of the developmental stage on H. ammodendron seedlings’ responses to drought stress. In this study, we compared seedlings and younger individuals at two different developmental stages of H. ammodendron to investigate whether the developmental stage impacts the physiological responses to extreme continuous drought. We hypothesized that: 1. There would be different limited factors of leaf photosynthesis during different drought periods, 2. Younger individuals respond to continuous drought stress better than seedlings due to a more stable physiological regulation ability.
2. Results
2.1. Leaf Photosynthetic Traits
Both drought treatment and the developmental stage had significant effects on An and R. While drought treatment alone did not significantly affect gs, its interaction with the developmental stage did (p = 0.015, Table 1). In addition, the developmental stage and its interaction with treatment duration had significant effects on An and R, especially on R values (p < 0.001, Table 1).

Table 1.
Summary of three-way repeated-measures ANOVA p-values for treatment effects on plant water status, leaf gas exchange and NSC content in H. ammodendron. An, net photosynthesis rate (μmol m−2 s−1); R, dark respiration rate (μmol m−2 s−1); gs, stomatal conductance (mol m−2 s−1); iWUE, intrinsic water efficiency (mmol m−2 s−1); Fv/Fm, potential maximum photochemical rate; LDMC, leaf dry mass content (g g−1); RWC, relative water content (g g−1); L-sugar, leaf soluble sugars content (mg g−1); L-starch, leaf starch content (mg g−1); L-NSC, leaf total non-structural carbohydrates (mg g−1); R-sugar, root soluble sugars content (mg g−1); R-starch, root starch content (mg g−1); R-NSC, root total non-structural carbohydrates (mg g−1). p-values in bold are significant (α = 0.05).
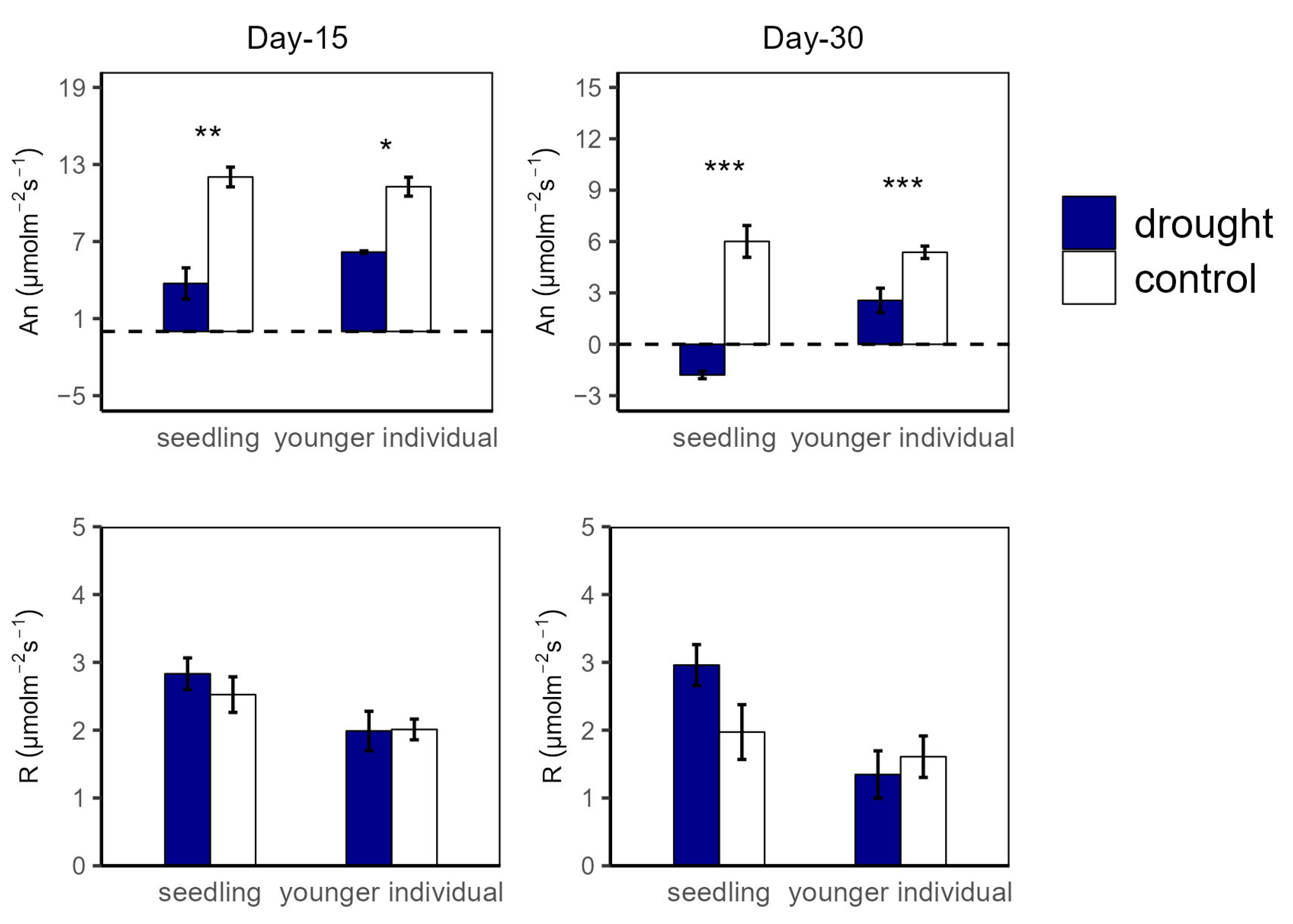
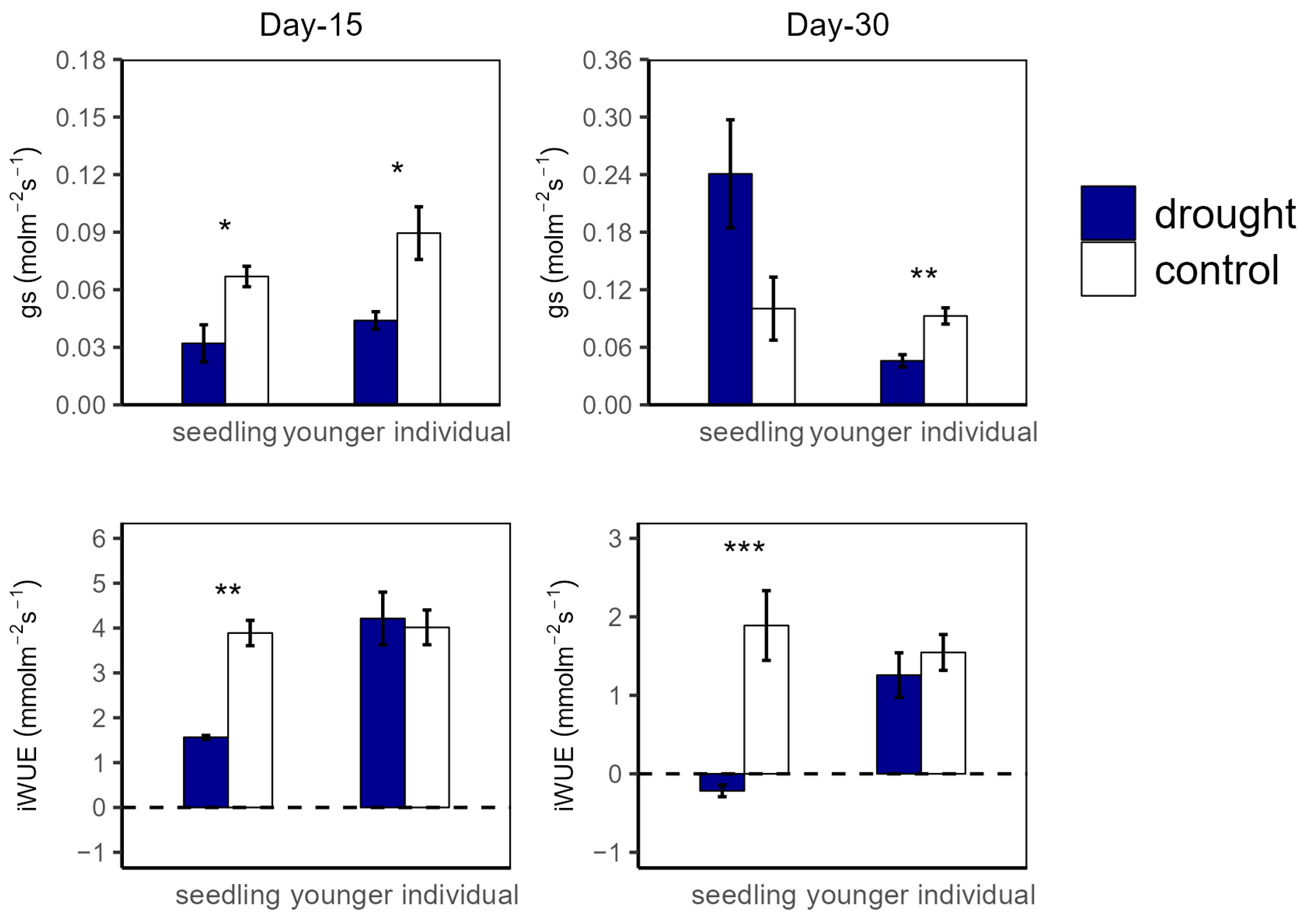
At the 15 d drought point, An values were significantly higher in the control group compared to in the drought group for both seedlings (12.04 vs. 3.74 μmol m−2 s−1 and 11.27 vs. 6.18 μmol m−2 s−1 for seedlings and younger individuals, respectively), with no significant difference between the two developmental stages. For R, there was no significant difference between all groups (Figure 1). Additionally, both for seedlings and younger individuals under drought stress, gs values were significantly lower than for the control group (p < 0.05, −52.2% and −50.5%, respectively) (Figure 2).
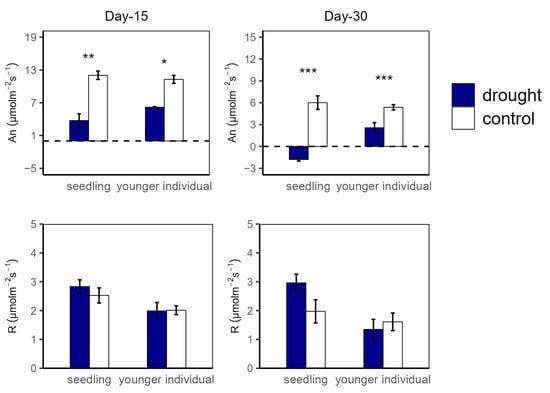
Figure 1.
Net photosynthesis rate (An) and dark respiration rate (R) of seedlings and younger individuals at the 15 d drought point (left) and the 30 d drought point (right). Bars indicate standard errors (n = 5). *, ** and *** represent significant difference at p < 0.05, p < 0.01 and p < 0.001, respectively. The dashed lines indicate the zero tick mark.
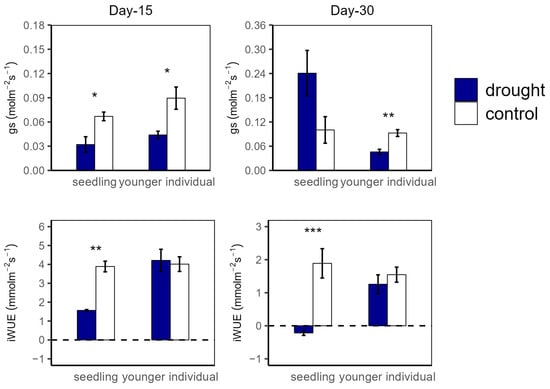
Figure 2.
Stomatal conductance (gs) and intrinsic water use efficiency (iWUE) of seedlings and younger individuals at 15 d drought point (left) and 30 d drought point (right). Bars indicate standard errors (n = 5). *, ** and *** represent significant difference at p < 0.05, p < 0.01 and p < 0.001, respectively. The dashed lines indicate the zero tick mark.
At the 30 d drought point, the drought groups still had lower An values than the control groups; especially for the seedlings, the total photosynthetic rate was lower than the dark respiration rate (An < 0 μmol m−2 s−1) (Figure 1). Moreover, younger individuals had a lower gs (0.05 μmol m−2 s−1) than the control group (0.09 μmol m−2 s−1), and seedlings showed a much higher gs average value (0.24 μmol m−2 s−1) and a large standard deviation (SD = 0.13) (Figure 2).
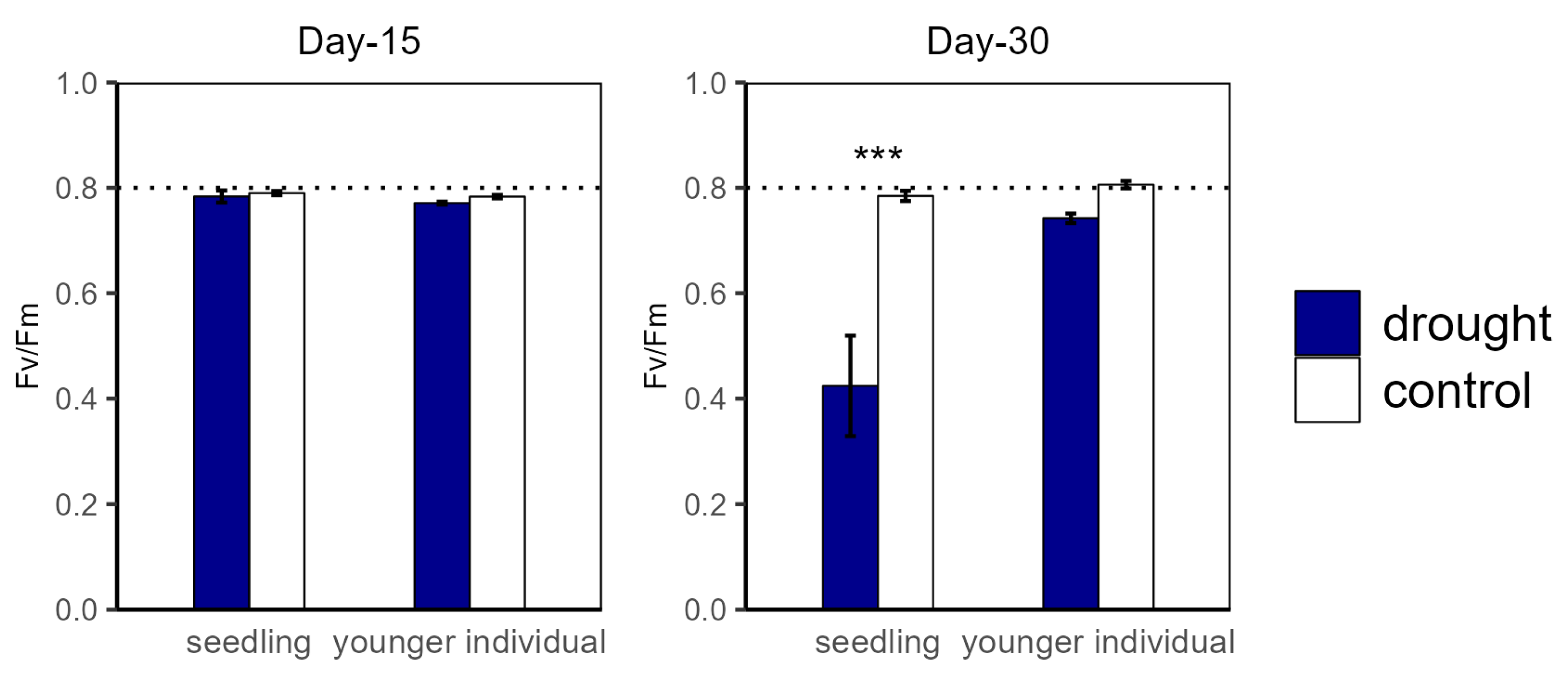
For the drought groups, the iWUE of seedlings was significantly lower than for the control groups, registering values of 1.57 vs. 4.21 μmol m−2 s−1 and −0.22 vs. 1.26 μmol m−2 s−1 at the 15 d and 30 d drought points, respectively. However, younger individuals showed no significant difference between the control and drought groups (Figure 2). Additionally, there was no significant difference in Fv/Fm at the 15 d drought point; nevertheless, the Fv/Fm (0.42) of seedlings was significantly lower compared with that of the control group at the 30 d drought point (Figure 3).
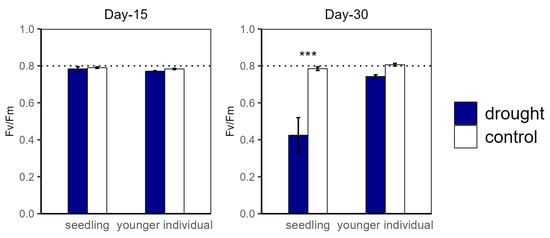
Figure 3.
Potential maximum biochemical rate of PS II (Fv/Fm) of seedlings and younger individuals at 15 d drought point (left) and 30 d drought point (right). Bars indicate standard errors (n = 5). *** represents significant difference p < 0.001. The dashed lines indicate Fv/Fm = 0.8.
2.2. Plant Water Status
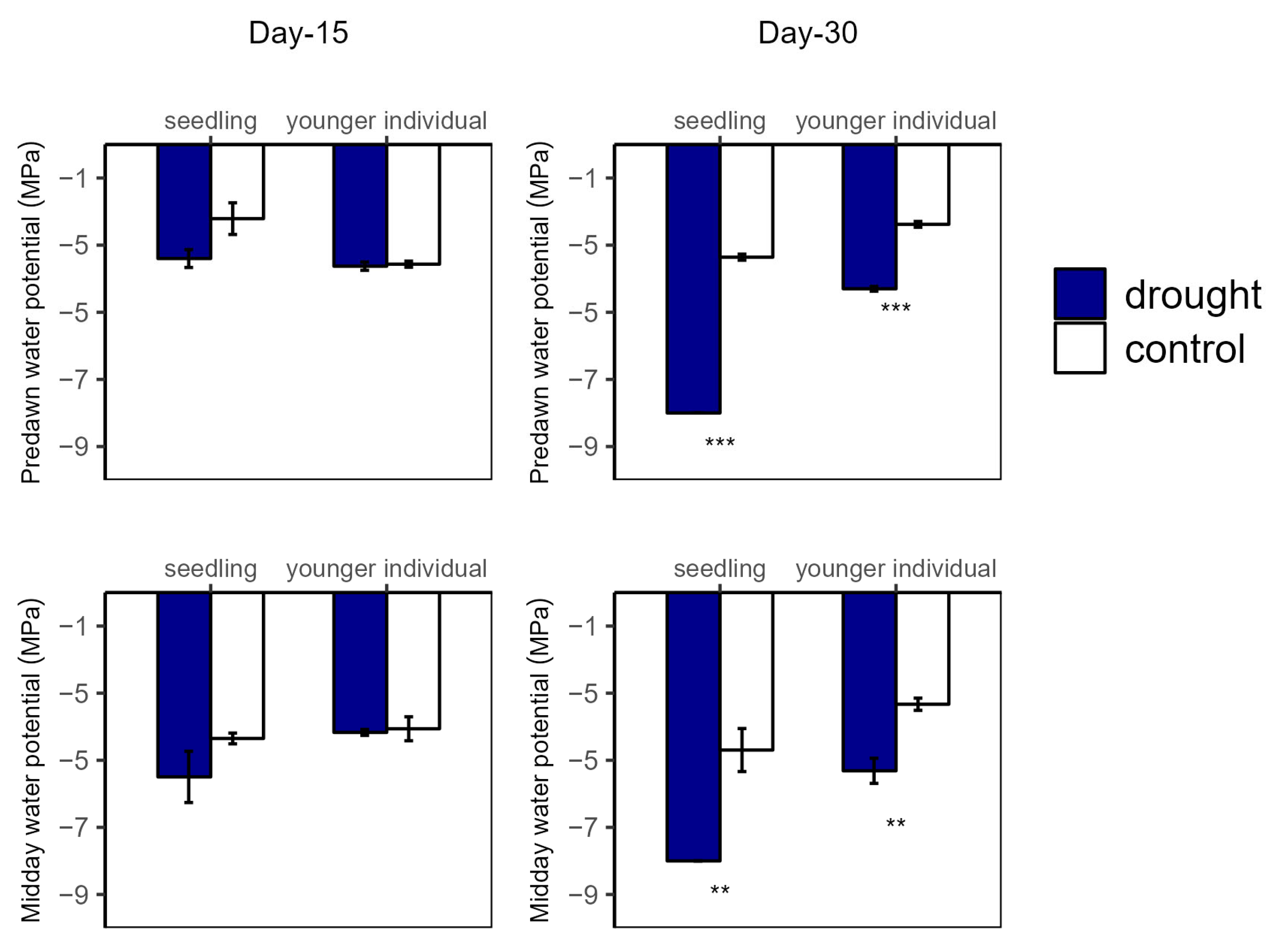
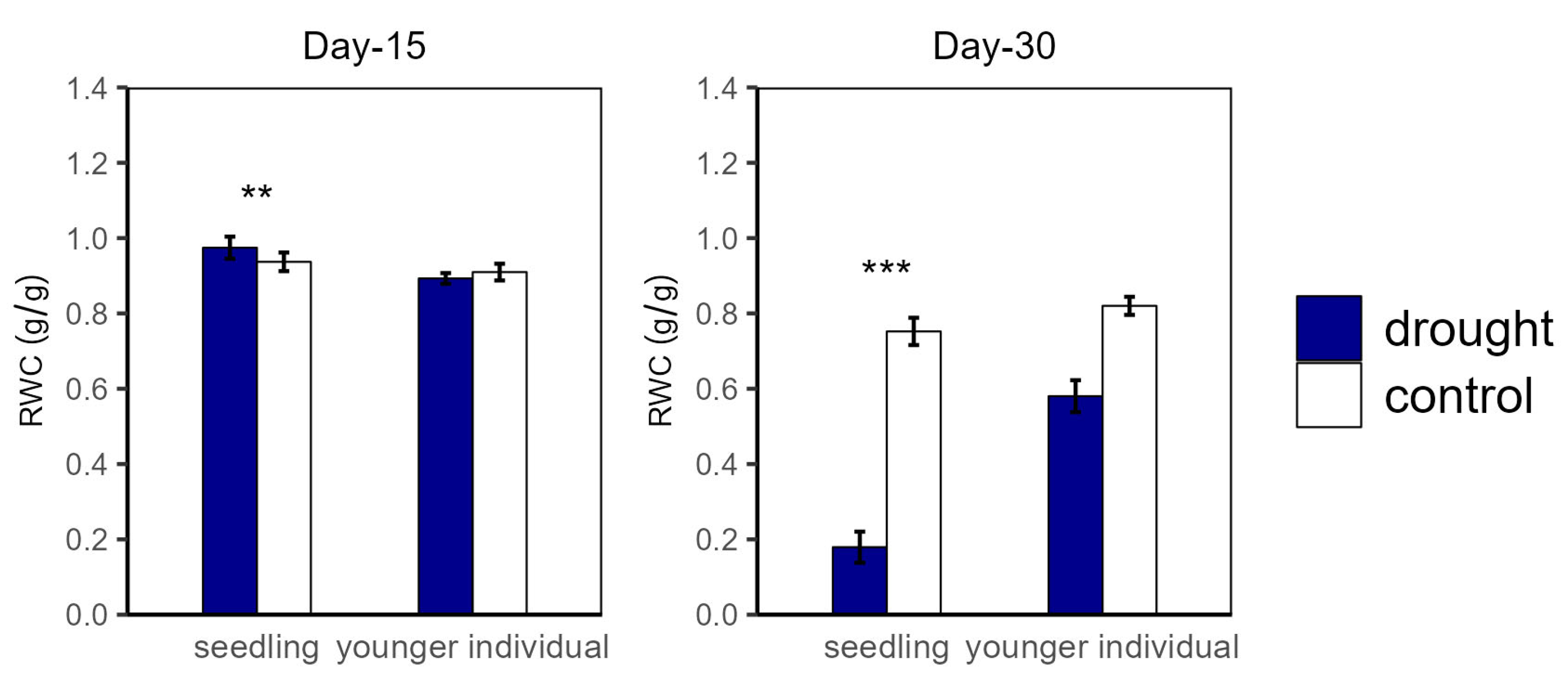
Over the drought duration, all of the groups showed a similar tendency (Figure 4 and Figure 5). As shown in Figure 4 and Figure 5, for both seedlings and younger individuals at the 15 d drought point, there was no significant difference in leaf water potential and RWC between control and drought groups. It should be noted that, at the 30 d drought point, the seedlings’ leaf water potential in the drought group was lower than −8 MPa, which exceeded our scaling range; however, that of the control group (average values for ΨPD and ΨMD were −3.35 MPa and −4.70 MPa, respectively) was still significantly higher than −8 MPa. Therefore, we confirmed that leaf water potential of drought groups was lower than that of the control groups (p < 0.05) (Figure 4). In addition, the RWC of drought groups was significantly lower than that of control groups at the 30 d drought point, with values of 0.58 vs. 0.82 g·g−1 (−29.27%) and 0.18 vs. 0.75 g·g−1 (−79.17%) for younger individuals and seedlings, respectively (Figure 5).
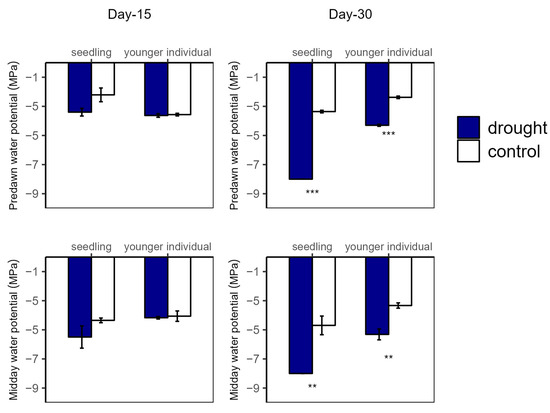
Figure 4.
Predawn (left) and midday (right) leaf water potential of seedlings and younger individuals at 15 d drought point (left) and 30 d drought point (right). Bars indicate standard errors (n = 5). ** and *** represent significant difference at p < 0.01 and p < 0.001, respectively.
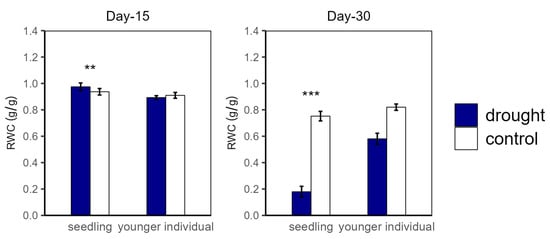
Figure 5.
Relative water content (RWC) of seedlings and younger individuals at 15 d drought point (left) and 30 d drought point (right). Bars indicate standard errors (n = 5). ** and *** represent significant difference at p < 0.01 and p < 0.001, respectively.
2.3. Non-Structural Carbohydrate
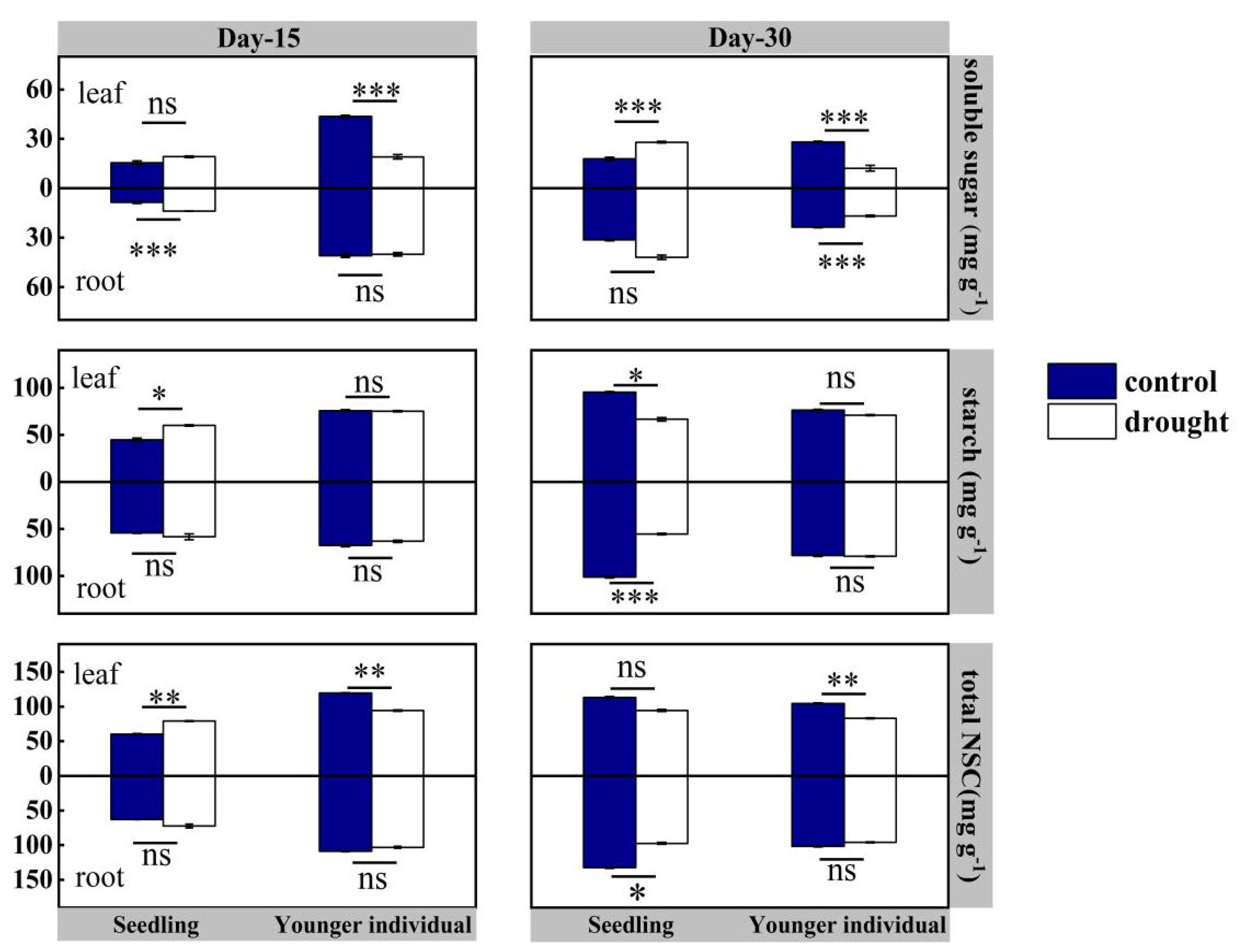
For seedlings, at the 15 d drought point, there was no significant difference in NSCs in roots between the control and drought group (p > 0.05), but leaf total NSC and starch contents of the drought group were higher than those of the control group, registering values of 79.14 vs. 60.28 mg·g−1 (+31.28%) and 60.00 vs. 44.84 mg·g−1 (+33.81%), respectively. At the 30 d drought point, both leaf and root total NSC and starch contents were lower than those of the control group, with leaf total content 94.48 vs. 113.17 mg·g−1 (−16.5%) and root total content 97.30 vs. 123.40 mg·g−1 (−21.2%), respectively (Figure 6).
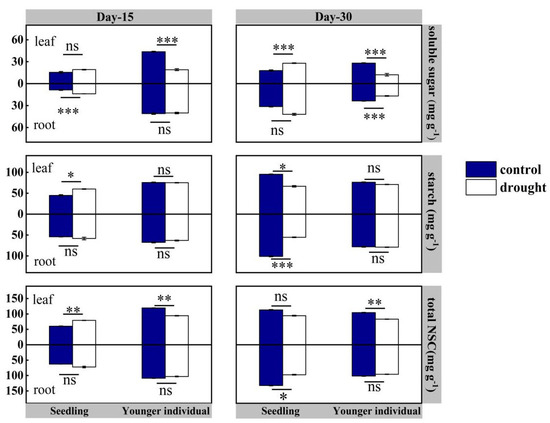
Figure 6.
Leaf and root non-structural carbohydrates (NSC is soluble sugar plus starch) of seedlings and younger individuals of H. ammodendron at 15 d drought point (left) and 30 d drought point (right). The vertical bars represent the standard errors (n = 5). *, ** and *** represent significant difference at p < 0.05, p < 0.01 and p < 0.001, respectively. ns indicates no significant difference.
For younger individuals, at both the 15 d and 30 d drought points, the leaf soluble sugars content of the drought group was significantly lower than that of the control group (−52.5% and −65.9%, respectively), while no significant difference in starch content was observed. Additionally, a significant reduction in leaf total NSC content was observed at 15 d and 30 d drought points (−21.0% and −20.3%, respectively). However, no significant difference in root NSC content was observed between the drought and control groups, except the lower soluble sugars content of the drought group at the 30 d drought point (−28.7%) (Figure 6).
3. Materials and Methods
3.1. Plant Material and Experimental Design
The experiment was conducted at Fukang Station of Desert Ecology, Chinese Academy of Sciences, in the southern Gurbantunggut Desert (44°13′ N, 87°33′ E, 475 m altitude, mean annual temperature 6.6 °C, mean annual precipitation 100~150 mm). We selected Haloxylon ammodendron for our research as it is a dominant species in this area. We investigated how developmental stages, specifically, the seedling and younger individual stages, of H. ammodendron influence physiological responses to continuous drought. At the beginning of the experiment, H. ammodendron seeds and one-year-old individuals were cultivated in pots (each pot with one individual) filled with 9.5 kg dry sandy soil from the southern Gurbantunggut Desert in March 2022. The seedlings and younger individuals were grown for about three months before imposing drought. We randomly assigned 80 individuals (40 seedlings and 40 younger individuals) to two groups: a well-watered control group and a drought group, with 20 of each type in both groups The control groups were watered normally (2 L water every three days), while drought groups underwent water restriction starting from 1 June. The leaf photosynthetic traits were measured at 15-day (15 d) and 30-day (30 d) points after drought treatments; accompanying this, twigs and roots were collected to measure water potential and non-structural carbon contents (n = 10 per treatment of seedlings and younger individuals).
3.2. Leaf Photosynthetic Traits
Photosynthetic traits were measured using a LI-6800 portable open gas exchange system (LI-COR, Lincoln, NE, USA) on twigs (leaves of H. ammodendron degenerate into twigs) of five individuals per treatment during each measurement period. Light-saturated net photosynthetic rates were measured between 9:00 and 13:00 h in a standard leaf chamber (6 cm2) equipped with a light source at PPFD of 1500 μmol m−2, an ambient CO2 concentration of 400 μmol mol−1 and a flow rate of 500 m s−1. The dark respiration rates were measured between 24:00 and 1:00 h after sufficient acclimation with no light, an ambient CO2 concentration of 400 μmol mol−1 and a flow rate of 500 m s−1. Following these measurements, the diameter and length of the twigs within the chamber were measured to calculate the net light-saturated photosynthetic rate per area (An), dark respiration rate per area (R) and stomatal conductance (gs). Instantaneous intrinsic water use efficiency (iWUE) was calculated as the ratio of net photosynthetic rate to stomatal conductance (An/gs). In addition, chlorophyll fluorescence measurements were performed using a LI-6800 Multi-Phase Flash Fluorometer (LI-COR, Lincoln, NE, USA). Measurements were performed using 250 ms duration multi-phase flashes of 10,000 μmol photons m−2 s−1 intensity and 250 kHz flash modulation rate. To ensure that plants were adequately dark-adapted, we determined the potential maximum photochemical rate of photosystem II (Fv/Fm) between 24:00 and 1:00 h.
3.3. Water Status
Five healthy twigs were cut per treatment before sunrise (5:00–6:00 h) to measure predawn water potential (ΨPD) using a Scholander pressure chamber (PMS Instrument Company, Corvallis, OR, USA). Another five twigs were chosen for the midday water potential (ΨMD) measurements, which were taken between 12:00 and 1:00 h. Five healthy twigs were cut from 3~5 individuals per treatment in a container full of ice to reduce water loss. The fresh weight of the twigs (Wfresh) was measured as soon as they reached the laboratory; after measuring the fresh weights, the twigs were soaked in pure water under shade for over 24 h to obtain the water-saturated weight (Wsat). The twigs were oven-dried at 75 °C until they reached a constant weight to obtain the dry weight (Wdry). Leaf dry mass content (LDMC) and relative water content (RWC) were calculated as follows:
3.4. Non-Structural Carbohydrates
The low-molecular-weight sugars (glucose, fructose and sucrose), starch and total NSC contents (starch plus low-molecular-weight sugars) were measured [59]. The twigs and roots were collected and oven-dried for 72 h at 75 °C to avoid enzymatic activity. Then, the dry samples were ground to a powder. Soluble sugar content reagent boxes (BC0030, Solarbio Science & Technology, Beijing, China) and starch content reagent boxes (BC0700, Solarbio Science & Technology, Beijing, China) were used to determine the soluble sugars and starch contents from their solutions, respectively. The free glucose concentration was determined photometrically using a microplate photometer based on the absorbance of the sample solution at 620 nm according to the absorbance of the standard glucose reference solution. Starch content was determined by extracting the glucose content of sample solutions following the hydrolysis with sulfuric acid. Total NSC content was defined as starch plus soluble sugars contents, and all NSC values are presented as unit dry mass.
3.5. Data Analysis
A three-way repeated-measures analysis of variance (ANOVA) was used to test the effects of drought and developmental stage on gas exchange, water status and NSC content. Drought treatment, developmental stage and their interactions were analyzed as between-subjects factors, and treatment time, drought treatment, developmental stage and their interactions were analyzed as within-subjects factors. The values of some variables were transformed to meet the assumptions of normality and homogeneity. Then, differences in each variable between drought treatment and developmental stage were tested for each treatment time using a two-way ANOVA. Finally, a t test was used to analyze the effects of drought at each treatment time for developmental stage and drought treatment. All analyses were performed using R version 4.2.2 [60].
4. Discussion
This study reveals the different physiological strategies and gas exchange processes of H. ammodendron seedlings and younger individuals when responding to an extended duration of drought. For seedlings, the stomatal conductance decreased at the 15 d drought point and increased at the 30 d drought point (Figure 2). This result is consistent with many previous studies, where the photosynthetic rate under drought stress was significantly decreased due to stomatal closure to reduce transpiration [14]. Coupled with photosynthetic rate, stomatal conductance substantially determines iWUE, which sufficiently reflects water use strategies in plant tissue [61]. Compared with that of the control group, the iWUE of seedlings significantly decreased under drought stress, but this was not observed in younger individuals. The results suggest that seedlings close stomata partly or entirely to reduce water losses from the leaf, which may result in a further distance from optimizing water use efficiency [62,63]. A larger standard deviation in gs for seedlings at the 30 d drought point suggests a precarious ability to regulate stomatal conductance, which further suggests that the developmental stage is an important factor influencing drought response strategies and further influences survival after drought events [19]. Our findings revealed that H. ammodendron utilizes conservative stomatal regulation strategies, presenting positive stomatal regulation at the 15 d drought point. Similar to in previous studies, the stomatal conductance of younger individuals significantly decreased under drought stress to reduce water losses [30,31]. However, the gs of seedlings decreased at the 15 d drought point but increased at the 30 d drought point. We speculated that the extended drought duration may lead to stomatal regular failure. According to the simultaneous decline in Fv/Fm at the 30 d drought point, extreme drought stress may induce a stronger photosynthetic inhibition of PSII and a further decrease in photosynthetic rate [62]. Due to the combined large reduction of water status with a significant reduction of Fv/Fm at the 30 d drought point, we considered that the severe injury of PSII may result in hydraulic failure because the reduction in hydraulic traits can inhibit the biosynthesis of chloroplast proteins [64]. Our result that the gs reduced earlier than leaf water potential indicates that plants can close stomata to avoid hydraulic failure in response to little soil available water [63]. We inferred that the photosynthetic rates of seedlings were constrained by stomatal closure at the 15 d drought point and then constrained by biochemical inhibition at the 30 d drought point.
NSCs present multiple cofunctions in plant tissues, including growth, storage, osmotic regulation of hydraulically vulnerable leaves and recovery post drought [42,44]. At the 15 d drought point, there was no significant reduction in NSCs except in the leaf total NSC of younger individuals caused mainly by the reduction in soluble sugars. This result supports the point that carbon starvation takes time because plant carbon reserves appear resilient to extreme disturbance in the short term [38]. The earlier increase and later decrease in the total NSC content of seedlings demonstrated the time-logged impact of drought stress on carbohydrate metabolism [53]. Previous studies reported that stored carbohydrates can keep tissues alive for an extended drought period even with a reduction of photosynthetic rate [65,66]. Therefore, there is little possibility that H. ammodendron would experience carbon starvation under drought stress. Starch and soluble sugars take different roles in plant tissues. Starch is the main storage resource that can be mobilized to support growth and other functions [44,46]. In contrast, soluble sugars play an important role in osmotic regulation [67]. For seedlings under drought stress, starch may be hydrolyzed to increase soluble sugars concentration, which can increase osmotic potentials in the leaf [44,67]. On the contrary, younger individuals prefer to reduce NSCs in leaves as they can reduce carbon loss by foliage loss [68]. The stable root NSC content of younger individuals can maintain alive roots, which are an important foundation of regrowth in the absence of drought [47,69].
According to our result, we confirm that seedlings choose to take a risky strategy to try to maintain carbon assimilation rather than invest in roots to obtain the little water, which may need more investment. By the way, we noticed the strong susceptibility of NSC content to environmental factors in all organs. Previous research on mature H. ammondrendon in situ revealed a significant increase in starch content and decrease in soluble sugars content compared with the control groups [56]; in our study, no significant decrease in starch content was observed in H. ammondrendon individuals, except seedlings during severe drought stress. Previous research has revealed the gene expression of a drought-tolerant cultivate of flax at different developmental stages responding to drought stress [70,71]. We appeal for further study to reveal the potential mechanism underlying the multiple changes of NSCs among organs to enhance the understanding of product regulation at different ages of plants under drought stress.
5. Conclusions
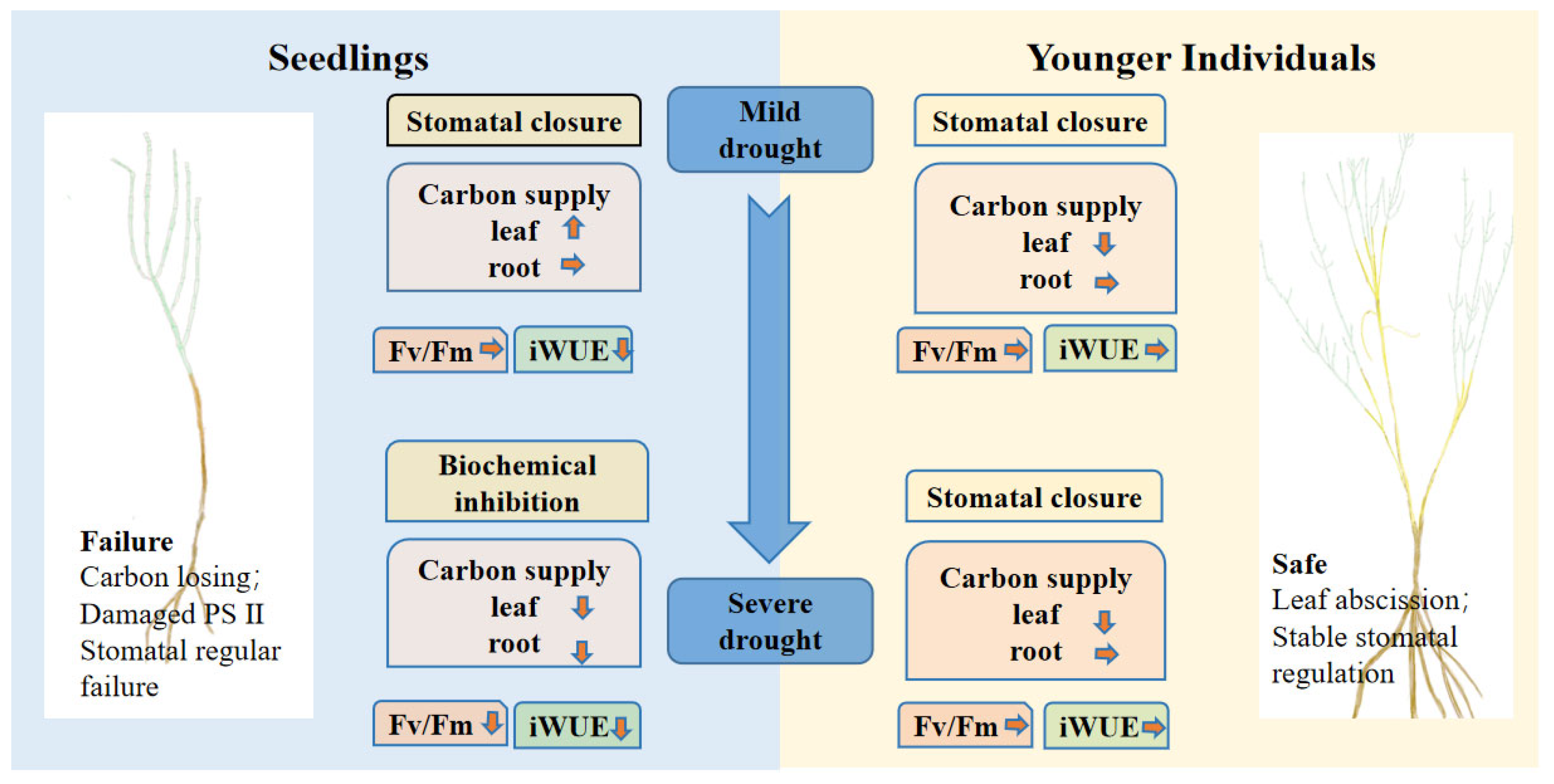
Our results suggest that the effects of a plant’s developmental stage on stomatal conductance strongly depend on drought duration, which indicates the limitation of the regular ability of seedlings under severe drought stress. According to the physiological response of seedlings to drought, we affirm that seedlings prefer risky survival strategies because the seedlings during severe drought periods were characterized by dysfunctional physiological regulation (Figure 7). More importantly, re-opening stomata under extreme water conditions may result in high hydraulic failure risk. Considering the changes of NSCs under different drought duration, NSC content of seedlings experienced firstly an increase and, following, a decrease over the duration of drought due to biochemical inhibition. Combining leaf photosynthetic traits and NSC contents, we can conclude that seedlings have limited ability when responding to reduced soil moisture compared to younger individuals under a prolonged drought period, suggesting that younger individuals can be widely used in vegetation restoration of the desert ecosystem.
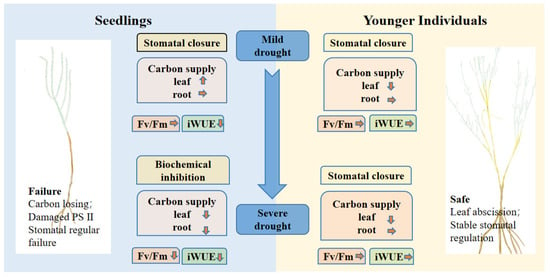
Figure 7.
Conceptual diagram of seedlings’ and younger individuals’ responses to drought stress. The direction of the little arrows indicate the changes of NSC contents of the drought group compared with the control group, the up arrow represents an increase, the down arrow represents a decrease and the right arrow represents no change.
Author Contributions
Conceptualization, L.C.; methodology, R.L.; software, M.L.; validation, L.C., R.L. and C.L.; formal analysis, W.Z.; data curation, M.L.; writing—original draft preparation, L.C.; writing—review and editing, R.L.; visualization, M.L.; supervision, R.L.; project administration, R.L. and C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the “Western Light” program of the Chinese Academy of Sciences grant numbers 2020-XBQNXZ-013 and 2021-XNQNXZ-002, the Outstanding Youth Foundation of Xinjiang Natural Science Foundation 2023D01E19, the Tianshan Talent Program of Xinjiang Uygur Autonomous Region grant numbers 2022TSYCCX0002 and 2022TSYCCX0004 and the National Natural Science Foundation of China grant number 31971731.
Data Availability Statement
Data will be made available on request.
Acknowledgments
We thank all staff of the Fukang National Station of Observation and Research for Desert Ecology for their excellent field and laboratory assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barber, V.A.; Juday, G.P.; Finney, B.P. Reduced Growth of Alaskan White Spruce in the Twentieth Century from Temperature-Induced Drought Stress. Nature 2000, 405, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Simeone, C.; Maneta, M.P.; Holden, Z.A.; Sapes, G.; Sala, A.; Dobrowski, S.Z. Coupled Ecohydrology and Plant Hydraulics Modeling Predicts Ponderosa Pine Seedling Mortality and Lower Treeline in the US Northern Rocky Mountains. New Phytol. 2019, 221, 1814–1830. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, H.; Adams, D.H.; Anderegg, R.L.W.; Jansen, S.; Zeppel, J.B.M. Research Frontiers in Drought-Induced Tree Mortality Crossing Scales and Disciplines. New Phytol. 2015, 205, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, H.; Moura, C.F.; Anderegg, R.L.W.; Ruehr, K.N.; Salmon, Y.; Allen, D.C.; Arndt, K.S.; Breshears, D.D.; Davi, H.; Galbraith, D.; et al. Research Frontiers for Improving Our Understanding of Drought-Induced Tree and Forest. New Phytol. 2018, 218, 15–28. [Google Scholar] [CrossRef]
- Babst, F.; Bouriaud, O.; Poulter, B.; Trouet, V.; Girardin, M.P.; Frank, D.C. Twentieth Century Redistribution in Climatic Drivers of Global Tree Growth. Sci. Adv. 2019, 5, eaat4313. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Nie, Y.; Chen, H.; Wang, K.; Querejeta, J.I. Water Uptake Depth Is Coordinated with Leaf Water Potential, Water-Use Efficiency and Drought Vulnerability in Karst Vegetation. New Phytol. 2021, 229, 1339–1353. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.M.R.; Lopez, R.; Nolf, M.; Hutley, L.B.; Wardlaw, T.; Cernusak, L.A.; Choat, B. Living on the Edge: A Continental-Scale Assessment of Forest Vulnerability to Drought. Glob. Chang. Biol. 2021, 27, 3620–3641. [Google Scholar] [CrossRef]
- Bennett, A.C.; Dargie, G.C.; Cuni-Sanchez, A.; Tshibamba, M.J.; Hubau, W.; Mukinzi, J.M.; Phillips, O.L.; Malhi, Y.; Sullivan, M.J.P.; Cooper, D.L.M.; et al. Resistance of African Tropical Forests to an Extreme Climate Anomaly. Proc. Natl. Acad. Sci. USA 2021, 118, e2003169118. [Google Scholar] [CrossRef] [PubMed]
- Breshears, D.D.; Cobb, N.S.; Rich, P.M.; Price, K.P.; Allen, C.D.; Balice, R.G.; Romme, W.H.; Kastens, J.H.; Floyd, M.L.; Belnap, J.; et al. Regional Vegetation Die-Off in Response to Global-Change-Type Drought. Proc. Natl. Acad. Sci. USA 2005, 102, 15144–15148. [Google Scholar] [CrossRef]
- De Kauwe, M.G.; Medlyn, B.E.; Ukkola, A.M.; Mu, M.; Sabot, M.E.B.; Pitman, A.J.; Meir, P.; Cernusak, L.A.; Rifai, S.W.; Choat, B.; et al. Identifying Areas at Risk of Drought-Induced Tree Mortality across South-Eastern Australia. Glob. Chang. Biol. 2020, 26, 5716–5733. [Google Scholar] [CrossRef]
- Blackman, C.J.; Creek, D.; Maier, C.; Aspinwall, M.J.; Drake, J.E.; Pfautsch, S.; O’Grady, A.; Deizon, S.; Medlyn, B.E.; Tissue, D.T.; et al. Drought response strategies and hydraulic traits contribute to mechanistic understanding of plant dry-down to hydraulic failure. Tree Physiol. 2019, 39, 910–924. [Google Scholar] [CrossRef] [PubMed]
- Nolan, R.H.; Gauthey, A.; Losso, A.; Medlyn, B.E.; Smith, R.; Chhajed, S.S.; Fuller, K.; Song, M.; Li, X.; Beaumont, L.J.; et al. Hydraulic Failure and Tree Size Linked with Canopy Die-Back in Eucalypt Forest During Extreme Drought. New Phytol. 2021, 230, 1354–1365. [Google Scholar] [CrossRef] [PubMed]
- Barbeta, A.; Mejia-Chang, M.; Ogaya, R.; Voltas, J.; Dawson, T.E.; Penuelas, J. The Combined Effects of a Long-Term Experimental Drought and an Extreme Drought on the Use of Plant-Water Sources in a Mediterranean Forest. Glob. Chang. Biol. 2015, 21, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Tan, N.D.; Tissue, D.T.; Huang, J.; Duan, H.L.; Su, W.; Song, Y.T.; Liu, X.J.; Liu, Y.; Li, X.; et al. Physiological Traits and Response Strategies of Four Subtropical Tree Species Exposed to Drought. Environ. Exp. Bot. 2022, 203, 105046. [Google Scholar] [CrossRef]
- Li, X.; Piao, S.; Wang, K.; Wang, X.; Wang, T.; Ciais, P.; Chen, A.; Lian, X.; Peng, S.; Penuelas, J. Temporal Trade-Off between Gymnosperm Resistance and Resilience Increases Forest Sensitivity to Extreme Drought. Nat. Ecol. Evol. 2020, 8, 1075–1083. [Google Scholar] [CrossRef]
- Isaac-Renton, M.; Montwe, D.; Hamann, A.; Spiecker, H.; Cherubini, P.; Treydte, K. Northern Forest Tree Populations Are Physiologically Maladapted to Drought. Nat. Commun. 2018, 8, 5254. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Forner, N.; Adams, H.D.; Sevanto, S.; Collins, A.D.; Dickman, L.T.; Hudson, P.J.; Zeppel, M.J.; Jenkins, M.W.; Powers, H.; Martinez-Vilalta, J.; et al. Responses of Two Semiarid Conifer Tree Species to Reduced Precipitation and Warming Reveal New Perspectives for Stomatal Regulation. Plant Cell Environ. 2016, 39, 38–49. [Google Scholar] [CrossRef]
- Peltier, D.M.P.; Ogle, K. Tree Growth Sensitivity to Climate Is Temporally Variable. Ecol. Lett. 2020, 23, 1561–1572. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of Plant Survival and Mortality During Drought: Why Do Some Plants Survive While Others Succumb to Drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Bhusal, N.; Lee, M.; Reum Han, A.; Han, A.; Kim, H.S. Responses to Drought Stress in Prunus Sargentii and Larix Kaempferi Seedlings Using Morphological and Physiological Parameters. For. Ecol. Manag. 2020, 465, 118099. [Google Scholar] [CrossRef]
- DeSoto, L.; Cailleret, M.; Sterck, F.; Jansen, S.; Kramer, K.; Robert, E.M.R.; Aakala, T.; Amoroso, M.M.; Bigler, C.; Camarero, J.J.; et al. Low Growth Resilience to Drought Is Related to Future Mortality Risk in Trees. Nat. Commun. 2020, 11, 545. [Google Scholar] [CrossRef]
- Arani, B.M.S.; Carpenter, S.R.; Lahti, L.; van Nes, E.H.; Scheffer, M. Exit Time as a Measure of Ecological Resilience. Science 2021, 372, eaay4895. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Kumar, M.; Katul, G.G.; Porporato, A. Reduced Resilience as an Early Warning Signal of Forest Mortality. Nat. Clim. Change 2019, 9, 880–885. [Google Scholar] [CrossRef]
- Taylor, S.H.; Orr, D.J.; Carmo-Silva, E.; Long, S.P. During Photosynthetic Induction, Biochemical and Stomatal Limitations Differ between Brassica Crops. Plant Cell Environ. 2020, 43, 2623–2636. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rodriguez, J.; Perez, P.; Martinez-Carrasco, R. Photosynthesis, Carbohydrate Levels and Chlorophyll Fluorescence-Estimated Intercellular CO2 in Water-Stressed Casuarina Equisetifolia Forst. & Forst. Plant Cell Environ. 1999, 22, 867–873. [Google Scholar] [CrossRef]
- Woodruff, D.R.; Meinzer, F.C.; Marias, D.E.; Sevanto, S.; Jenkins, M.W.; McDowell, N.G. Linking Nonstructural Carbohydrate Dynamics to Gas Exchange and Leaf Hydraulic Behavior in Pinus edulis and Juniperus monosperma. New Phytol. 2015, 206, 411–421. [Google Scholar] [CrossRef]
- Damour, G.; Vandame, M.; Urban, L. Long-term drought results in a reversible decline in photosynthetic capacity in mango leaves, not just a decrease in stomatal conductance. Tree Physiol. 2009, 29, 675–684. [Google Scholar] [CrossRef]
- Cernusak, L.A.; Hutley, L.B.; Beringer, J.; Holtum, J.A.M.; Turner, B.L. Photosynthetic physiology of eucalypts along a sub-continental rainfall gradient in northern Australia. Agric. For. Meteorol. 2011, 151, 1462–1470. [Google Scholar] [CrossRef]
- Arp, W.J.; Van Mierlo, J.E.M.; Berendse, F.; Snijders, W. Interactions between Elevated CO2 Concentration, Nitrogen and Water: Effects on Growth and Water Use of Six Perennial Plant Species. Plant Cell Environ. 1998, 21, 1–11. [Google Scholar] [CrossRef]
- Buckley, T.N. How Do Stomata Respond to Water Status? New Phytol. 2019, 224, 21–36. [Google Scholar] [CrossRef]
- Yang, Y.J.; Bi, M.H.; Nie, Z.F.; Jiang, H.; Liu, X.D.; Fang, X.W.; Brodribb, T.J. Evolution of Stomatal Closure to Optimize Water-Use Efficiency in Response to Dehydration in Ferns and Seed Plants. New Phytol. 2021, 230, 2001–2010. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.G. Mechanisms Linking Drought, Hydraulics, Carbon Metabolism, and Vegetation Mortality. Plant Physiol. 2011, 155, 1051–1059. [Google Scholar] [CrossRef]
- Cowan, I.R.; Farquhar, G.D. Stomatal function in relation to leaf metabolism and environment. Symp. Soc. Exp. Biol. 1977, 31, 471–505. [Google Scholar]
- Guo, J.S.; Hultine, K.R.; Koch, G.W.; Kropp, H.; Ogle, K. Temporal Shifts in Iso/Anisohydry Revealed from Daily Observations of Plant Water Potential in a Dominant Desert Shrub. New Phytol. 2020, 225, 713–726. [Google Scholar] [CrossRef]
- Huang, G.; Li, Y.; Padilla, F.M. Ephemeral Plants Mediate Responses of Ecosystem Carbon Exchange to Increased Precipitation in a Temperate Desert. Agric. For. Meteorol. 2015, 201, 141–152. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M. Strategy Shifts in Leaf Physiology, Structure and Nutrient Content between Species of High- and Low-Rainfall and High- and Low-Nutrient Habitats. Funct. Ecol. 2001, 15, 423–434. [Google Scholar] [CrossRef]
- McDowell, N.G.; Beerling, D.J.; Breshears, D.D.; Fisher, R.A.; Raffa, K.F.; Stitt, M. The Interdependence of Mechanisms Underlying Climate-Driven Vegetation Mortality. Trends Ecol. Evol. 2011, 26, 523–532. [Google Scholar] [CrossRef]
- Arend, M.; Link, R.M.; Patthey, R.; Hoch, G.; Schuldt, B.; Kahmen, A. Rapid Hydraulic Collapse as Cause of Drought-Induced Mortality in Conifers. Proc. Natl. Acad. Sci. USA 2021, 118, e2025251118. [Google Scholar] [CrossRef] [PubMed]
- Peltier, D.M.P.; Carbone, M.S.; McIntire, C.D.; Robertson, N.; Thompson, R.A.; Malone, S.; LeMoine, J.; Richardson, A.D.; McDowell, N.G.; Adams, H.D.; et al. Carbon Starvation Following a Decade of Experimental Drought Consumes Old Reserves in Pinus Edulis. New Phytol. 2023, 240, 92–104. [Google Scholar] [CrossRef]
- Ambrose, A.R.; Baxter, W.L.; Wong, C.S.; Naesborg, R.R.; Williams, C.B.; Dawson, T.E. Contrasting Drought-Response Strategies in California Redwoods. Tree Physiol. 2015, 35, 453–469. [Google Scholar] [CrossRef]
- Wu, J.J.; Wang, J.Y.; Hui, W.K.; Zhao, F.Y.; Wang, P.Y.; Su, C.Y.; Gong, W. Physiology of Plant Responses to Water Stress and Related Genes: A Review. Forests 2022, 13, 324. [Google Scholar] [CrossRef]
- Urli, M.; Porte, A.J.; Cochard, H.; Guengant, Y.; Burlett, R.; Delzon, S. Xylem embolism threshold for catastrophic hydraulic failure in angiosperm trees. Tree Physiol. 2013, 33, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Tomasella, M.; Petrussa, E.; Petruzzellis, F.; Nardini, A.; Casolo, V. The Possible Role of Non-Structural Carbohydrates in the Regulation of Tree Hydraulics. Int. J. Mol. Sci. 2019, 21, 144. [Google Scholar] [CrossRef] [PubMed]
- Piper, F.I.; Paula, S. The Role of Nonstructural Carbohydrates Storage in Forest Resilience under Climate Change. Curr. For. Rep. 2020, 6, 1–13. [Google Scholar] [CrossRef]
- Korner, C. Carbon Limitation in Trees. J. Ecol. 2003, 91, 4–17. [Google Scholar] [CrossRef]
- Chapin, F.S.; Schulze, E.D.; Mooney, H.A. The ecology and economics of storage in plants. Annu. Rev. Ecol. Syst. 1990, 21, 423–447. [Google Scholar] [CrossRef]
- Martinez-Vilalta, J.; Sala, A.; Asensio, D.; Galiano, L.; Hoch, G.; Palacio, S.; Piper, F.I.; Lloret, F. Dynamics of non-structural carbohydrates in terrestrial plants: A global synthesis. Ecol. Monogr. 2014, 86, 495–516. [Google Scholar] [CrossRef]
- Kozlowski, T.T. Carbohydrate Sources and Sinks in Woody-plants. Bot. Rev. 1992, 58, 107–222. [Google Scholar] [CrossRef]
- Woodruff, D.R.; Meinzer, F.C. Water Stress, Shoot Growth and Storage of Non-Structural Carbohydrates Along a Tree Height Gradient in a Tall Conifer. Plant Cell Environ. 2011, 34, 1920–1930. [Google Scholar] [CrossRef]
- Ryan, M.; Epron, D.; Dannoura, M.; Hölttä, T.; Sevanto, S.; Dietrich, L.; Salmon, Y. Drought Impacts on Tree Phloem: From Cell-Level Responses to Ecological Significance. Tree Physiol. 2019, 39, 173–191. [Google Scholar] [CrossRef]
- Salleo, S.; Trifilò, P.; Esposito, S.; Nardini, A.; LoGullo, M.A. Starch-to-Sugar Conversion in Wood Parenchyma of Field-Growing Laurus nobilis Plants: A Component of the Signal Pathway for Embolism Repair? Funct. Plant Biol. 2009, 36, 815–825. [Google Scholar] [CrossRef]
- Adams, H.D.; Germino, M.J.; Breshears, D.D.; Barron-Gafford, G.A.; Guardiola-Claramonte, M.; Zou, C.B.; Huxman, T.E. Nonstructural Leaf Carbohydrate Dynamics of Pinus Edulis During Drought-Induced Tree Mortality Reveal Role for Carbon Metabolism in Mortality Mechanism. New Phytol. 2013, 197, 1142–1151. [Google Scholar] [CrossRef]
- He, W.Q.; Liu, H.Y.; Qi, Y.; Liu, F.; Zhu, X.R. Patterns in Nonstructural Carbohydrate Contents at the Tree Organ Level in Response to Drought Duration. Glob. Chang. Biol. 2020, 26, 3627–3638. [Google Scholar] [CrossRef]
- Xu, G.Q.; Yu, D.D.; Xie, J.B.; Tang, L.S.; Li, Y. What Makes Haloxylon persicum Grow on Sand Dunes While H. Ammodendron Grows on Interdune Lowlands: A Proof from Reciprocal Transplant Experiments. J. Arid Land 2014, 6, 581–591. [Google Scholar] [CrossRef]
- Li, C.J.; Han, H.; Ablimiti, M.; Liu, R.; Zhang, H.; Fan, J.L. Morphological and Physiological Responses of Desert Plants to Drought Stress in a Man-Made Landscape of the Taklimakan Desert Shelter Belt. Ecol. Indic. 2022, 10, 109037. [Google Scholar] [CrossRef]
- Xu, H.; Li, Y. Water-Use Strategy of Three Central Asian Desert Shrubs and Their Responses to Rain Pulse Events. Plant Soil 2006, 285, 5–17. [Google Scholar] [CrossRef]
- Dai, Y.; Zheng, X.J.; Tang, L.S.; Li, Y. Stable Oxygen Isotopes Reveal Distinct Water Use Patterns of Two Haloxylon Species in the Gurbantonggut Desert. Plant Soil 2014, 389, 73–87. [Google Scholar] [CrossRef]
- Li, C.J.; Abulimiti, M.; Fan, J.L.; Wang, H.F. Ecologic Service, Economic Benefits, and Sustainability of the Man-Made Ecosystem in the Taklamakan Desert. Front. Environ. Sci. 2022, 10, 813932. [Google Scholar] [CrossRef]
- Hoch, G.; Richter, A.; Körner, C. Non-Structural Carbon Compounds in Temperate Forest Trees. Plant Cell Environ. 2003, 26, 1067–1081. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environmentfor Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 21 August 2022).
- Beer, C.; Ciais, P.; Reichstein, M.; Baldocchi, D.; Law, B.E.; Papale, D.; Soussana, J.F.; Ammann, C.; Buchmann, N.; Frank, D.; et al. Temporal and among-Site Variability of Inherent Water Use Efficiency at the Ecosystem Level. Global Biogeochem. Cycles 2009, 23, GB2018. [Google Scholar] [CrossRef]
- Metcalfe, D.B.; Meir, P.; Aragao, L.E.; Lobo-do-Vale, R.; Galbraith, D.; Fisher, R.A.; Chaves, M.M.; Maroco, J.P.; da Costa, A.C.; de Almeida, S.S.; et al. Shifts in Plant Respiration and Carbon Use Efficiency at a Large-Scale Drought Experiment in the Eastern Amazon. New Phytol. 2010, 187, 608–621. [Google Scholar] [CrossRef] [PubMed]
- Trueba, S.; Pan, R.; Scoffoni, C.; John, G.P.; Davis, S.D.; Sack, L. Thresholds for Leaf Damage Due to Dehydration: Declines of Hydraulic Function, Stomatal Conductance and Cellular Integrity Precede Those for Photochemistry. New Phytol. 2019, 223, 134–149. [Google Scholar] [CrossRef] [PubMed]
- Bhusal, N.; Park, I.H.; Jeong, S.; Choi, B.H.; Han, S.G.; Yoon, T.M. Photosynthetic Traits and Plant Hydraulic Dynamics in Gamhong Apple Cultivar under Drought, Waterlogging, and Stress Recovery Periods. Sci. Hortic. 2023, 321, 112276. [Google Scholar] [CrossRef]
- Peltier, D.M.P.; Guo, J.; Nguyen, P.; Bangs, M.; Gear, L.; Wilson, M.; Jefferys, S.; Samuels-Crow, K.; Yocom, L.L.; Liu, Y.; et al. Temporal Controls on Crown Nonstructural Carbohydrates in Southwestern US Tree Species. Tree Physiol. 2021, 41, 388–402. [Google Scholar] [CrossRef] [PubMed]
- Aubrey, D.P.; Teskey, R.O. Stored Root Carbohydrates Can Maintain Root Respiration for Extended Periods. New Phytol. 2018, 218, 142–152. [Google Scholar] [CrossRef]
- Pagliarani, C.; Boccacci, P.; Chitarra, W.; Cosentino, E.; Sandri, M.; Perrone, I.; Mori, A.; Cuozzo, D.; Nerva, L.; Rossato, M.; et al. Distinct Metabolic Signals Underlie Clone by Environment Interplay in “Nebbiolo” Grapes Over Ripening. Front. Plant Sci. 2019, 10, 1575. [Google Scholar] [CrossRef]
- Jiménez-Castillo, M.; Fajardo, A.; Lobos-Catalán, P.; Torres-Morales, P.; Piper, F.I. No Carbon Shortage in Declining Trees of the Isohydric Species Araucaria araucana (Molina) K. Koch under Drought. Ann. For. Sci. 2022, 79, 10. [Google Scholar] [CrossRef]
- Dash, P.K.; Cao, Y.G.; Jailani, A.K.; Gupta, P.; Venglat, P.; Xiang, D.Q.; Rai, R.; Sharma, R.; Thirunavukkarasu, N.; Abdin, M.Z.; et al. Genome-Wide Analysis of Drought Induced Gene Expression Changes in Flax (Linum usitatissimum). GM Crops Food 2014, 5, 106–119. [Google Scholar] [CrossRef]
- Dash, P.K.; Rai, R.; Pradhan, S.K.; Shivaraj, S.M.; Deshmukh, R.; Sreevathsa, R.; Singh, N.K. Drought and Oxidative Stress in Flax (Linum usitatissimum L.) Entails Harnessing Non-Canonical Reference Gene for Precise Quantification of Qrt-Pcr Gene Expression. Antioxidants 2023, 12, 950. [Google Scholar] [CrossRef]
- Dash, P.K.; Rai, R.; Mahato, A.K.; Gaikwad, K.; Singh, N.K. Transcriptome Landscape at Different Developmental Stages of a Drought Tolerant Cultivar of Flax (Linum usitatissimum). Front. Chem. 2017, 5, 82. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).