Anti-Melanogenic Effects of Lilium lancifolium Root Extract via Downregulation of PKA/CREB and MAPK/CREB Signaling Pathways in B16F10 Cells
Abstract
1. Introduction
2. Results
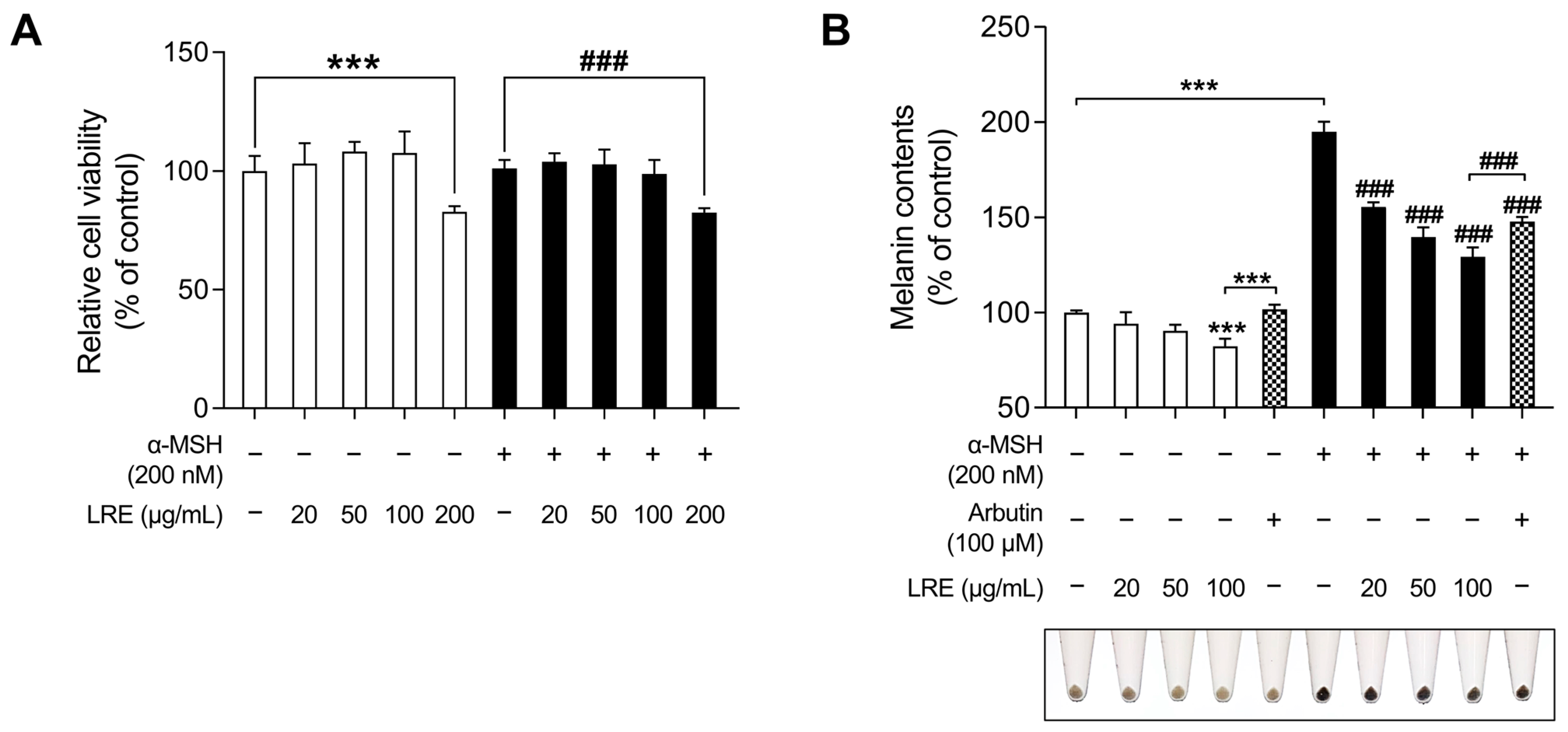
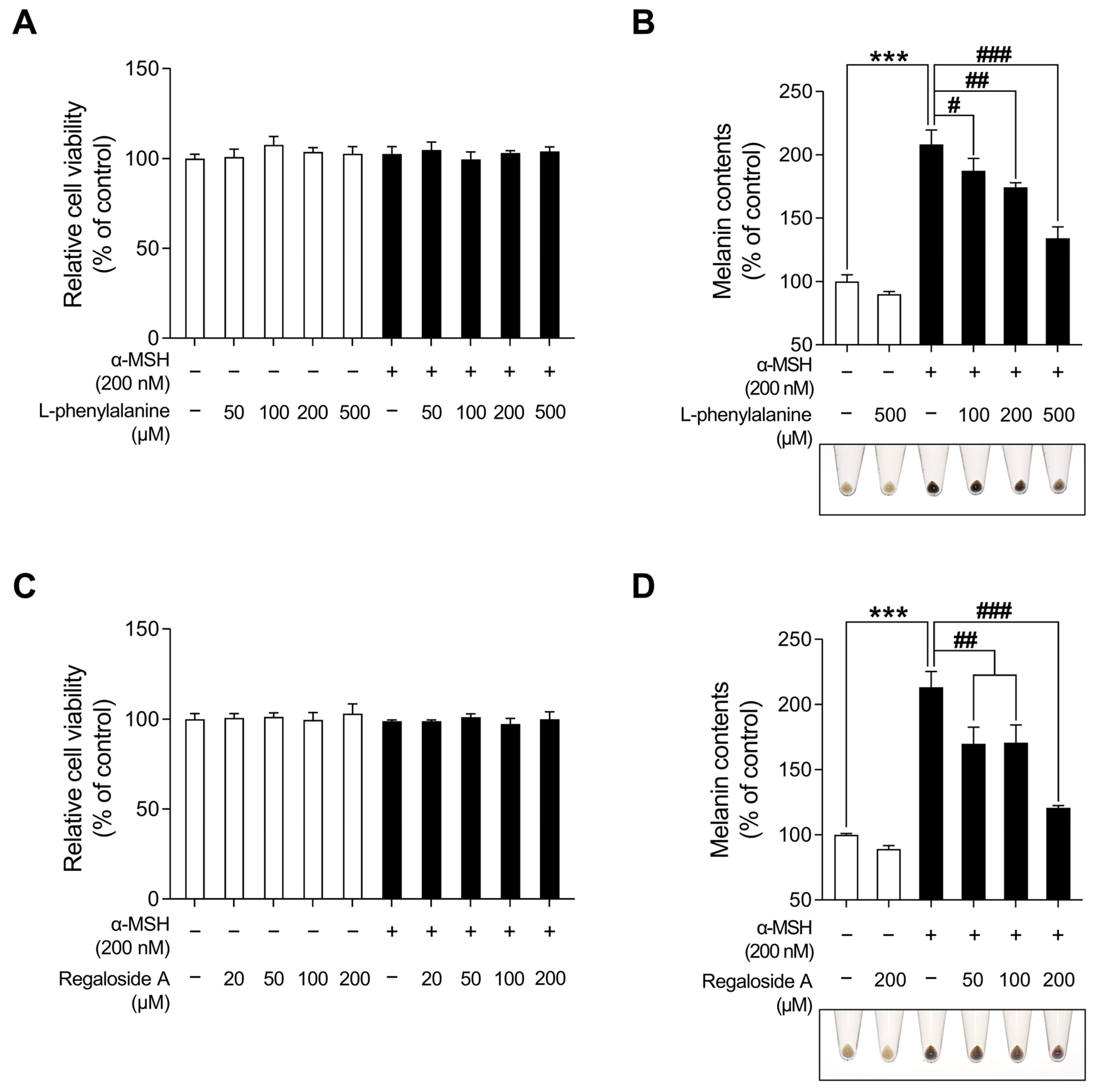
2.1. Effects of LRE on Cell Viability and Melanogenesis in B16F10 Cells
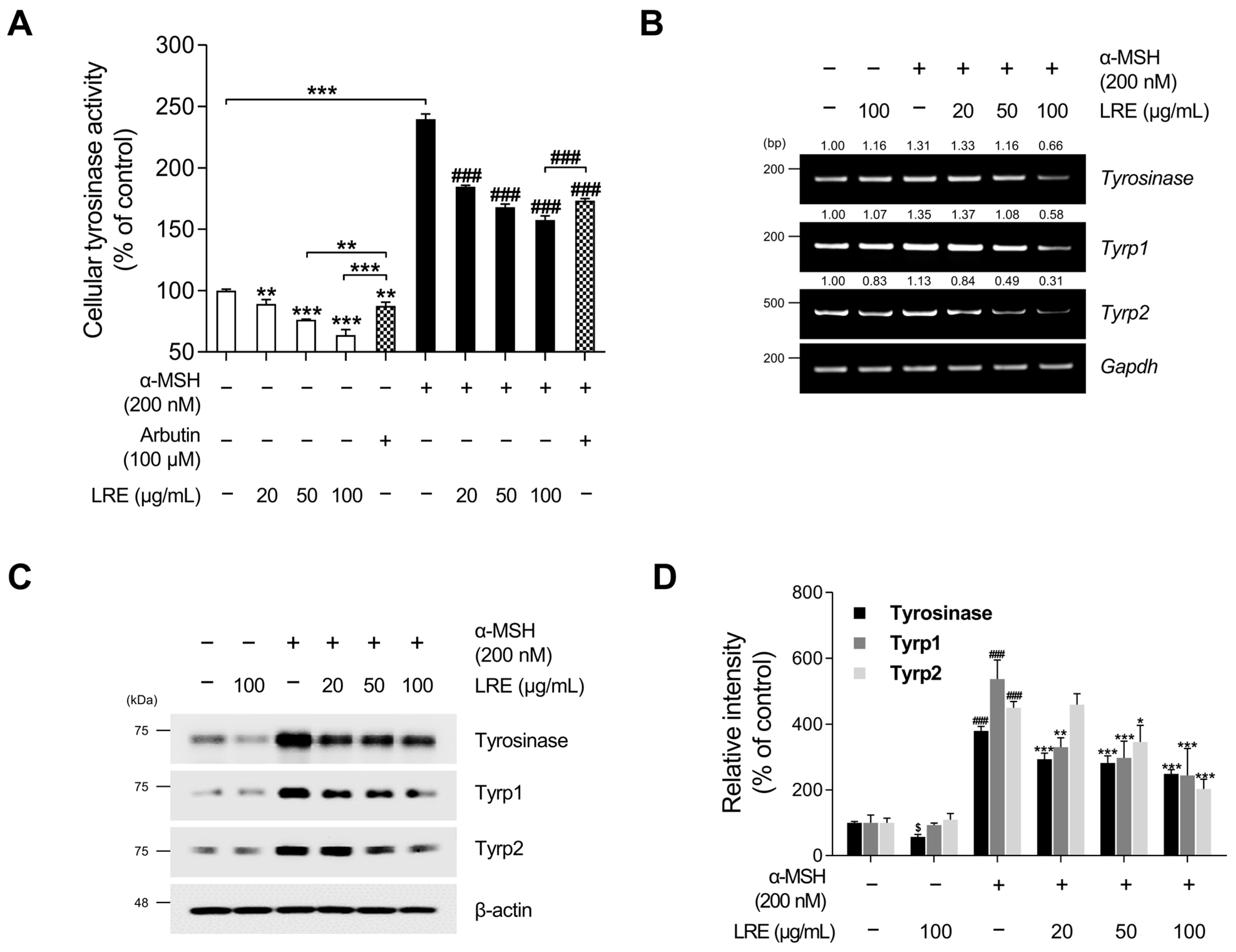
2.2. Inhibitory Effects of LRE on the Expression of Melanogenic Enzymes
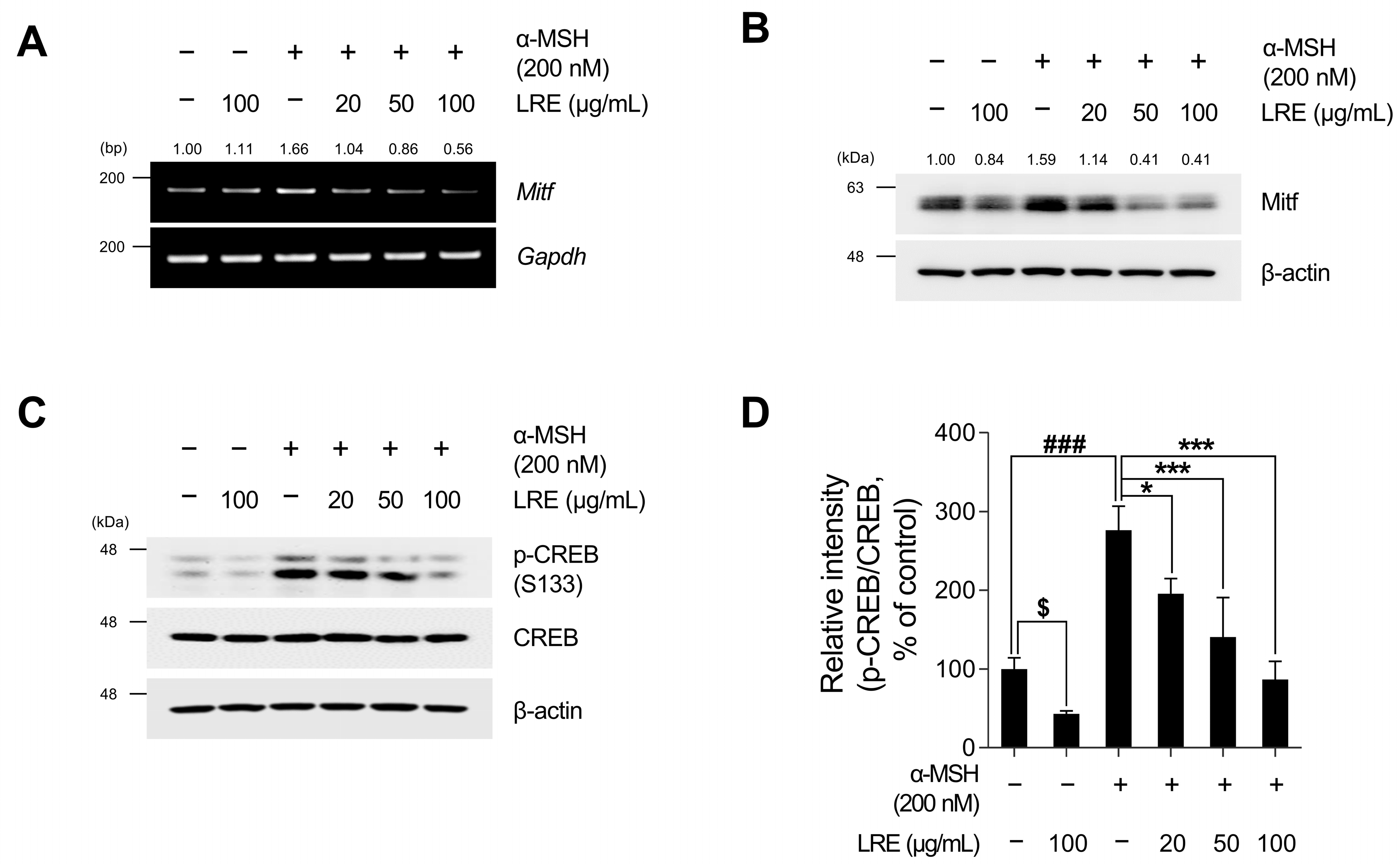
2.3. Inhibitory Effects of LRE on Cyclic Adenosine Monophosphate (cAMP) Response Element-Binding Protein (CREB)/Microphthalmia-Associated Transcription Factor (Mitf) Signaling Pathway
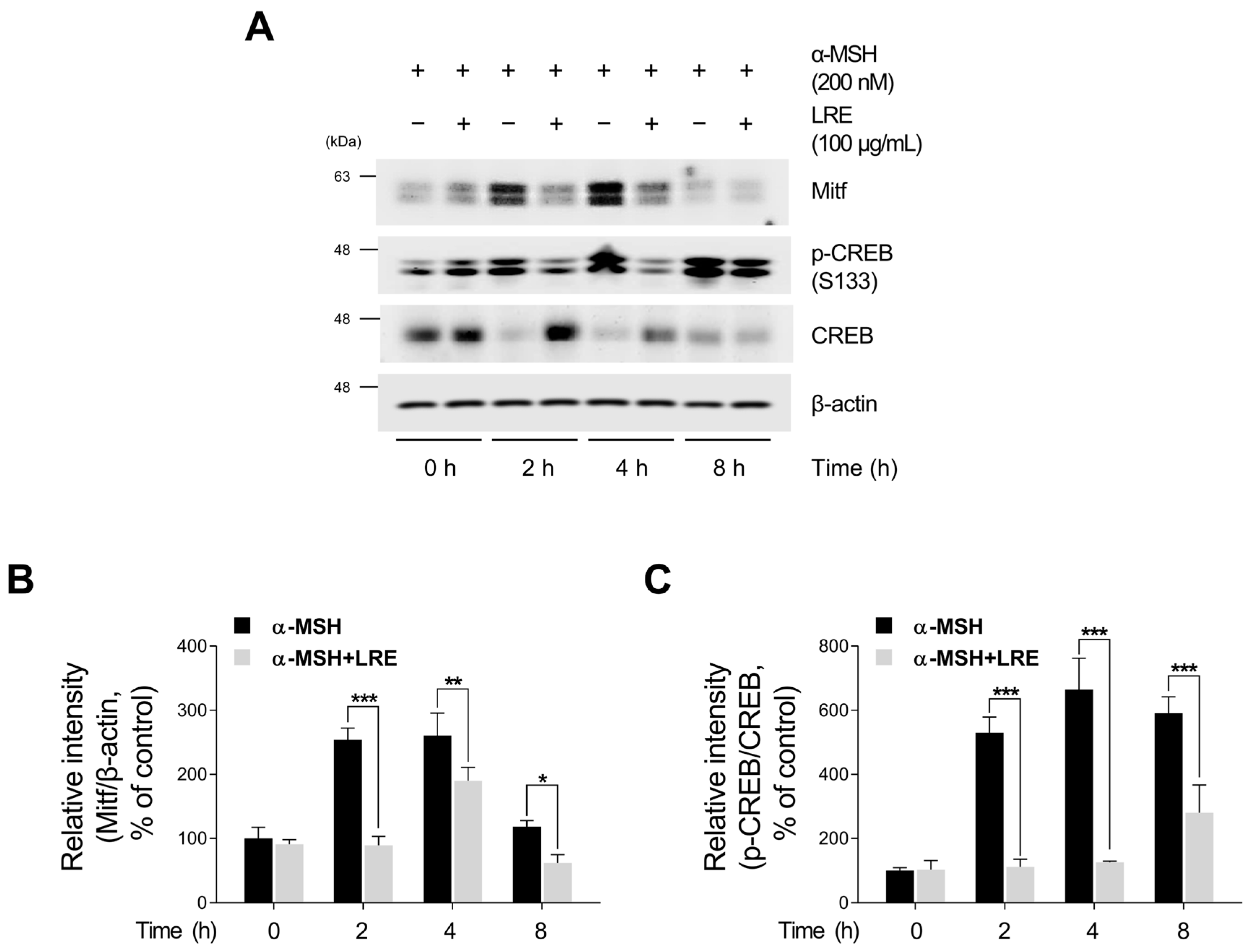
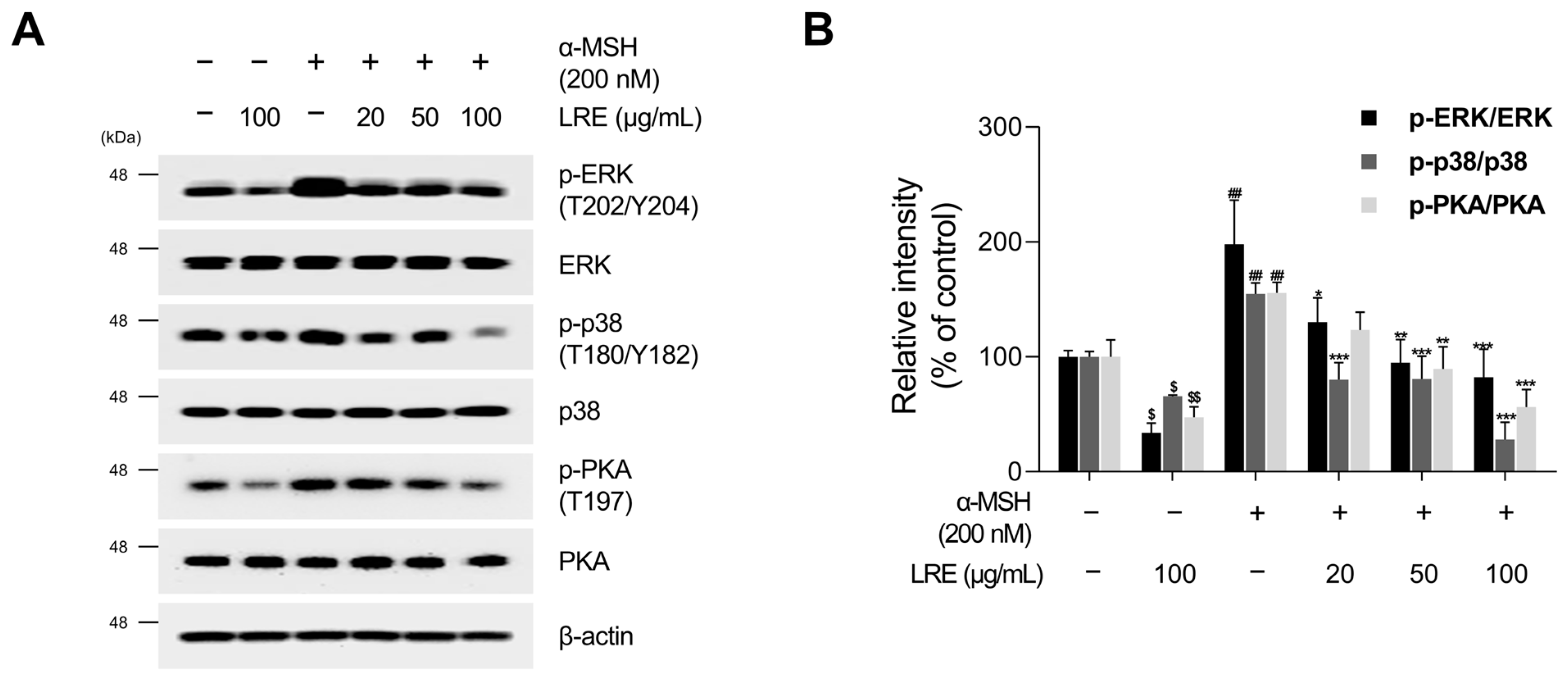
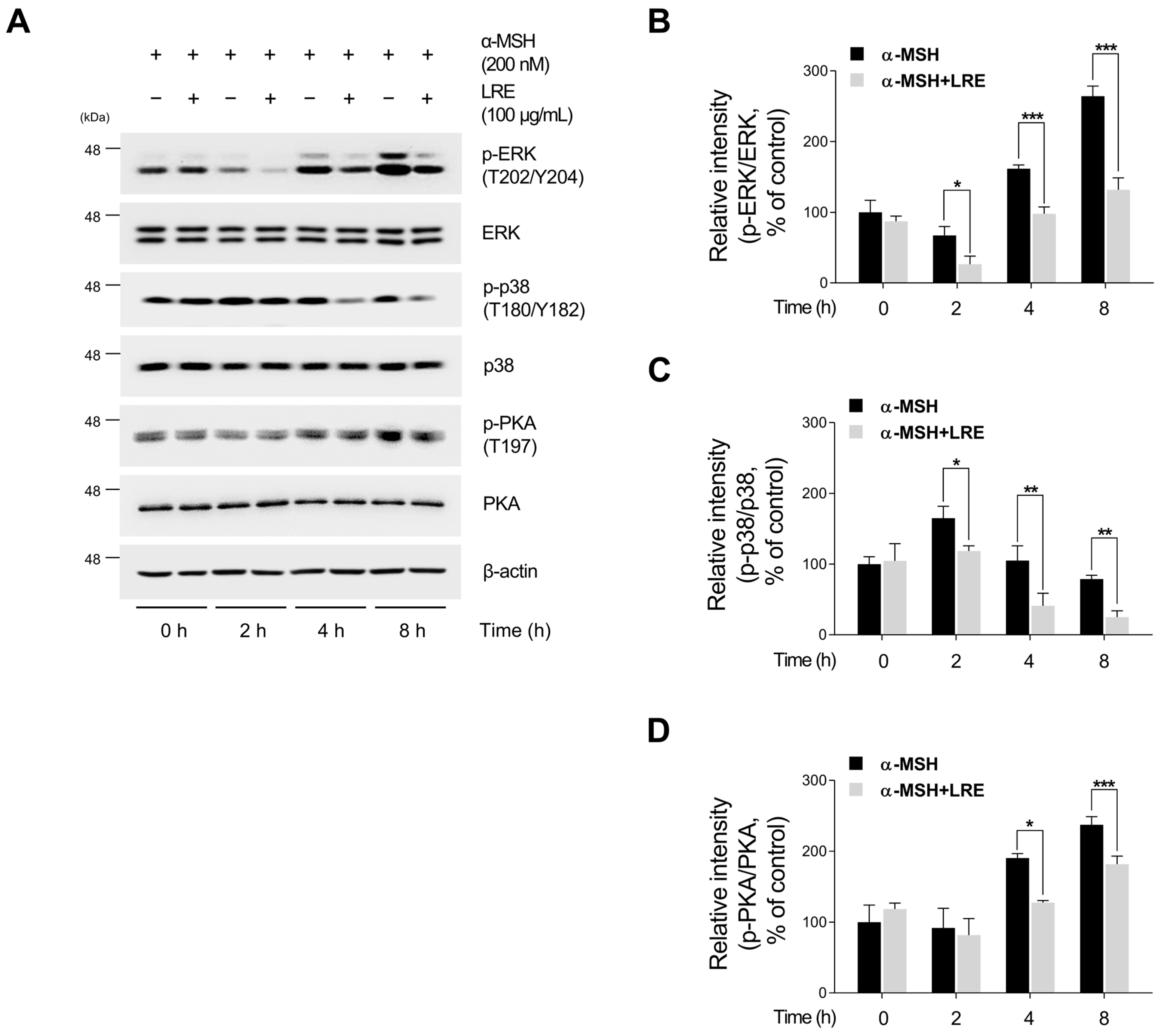
2.4. Inhibitory Effects of LRE on Protein Kinase A (PKA)/CREB and Mitogen-Activated Protein Kinase/CREB Signaling Pathways
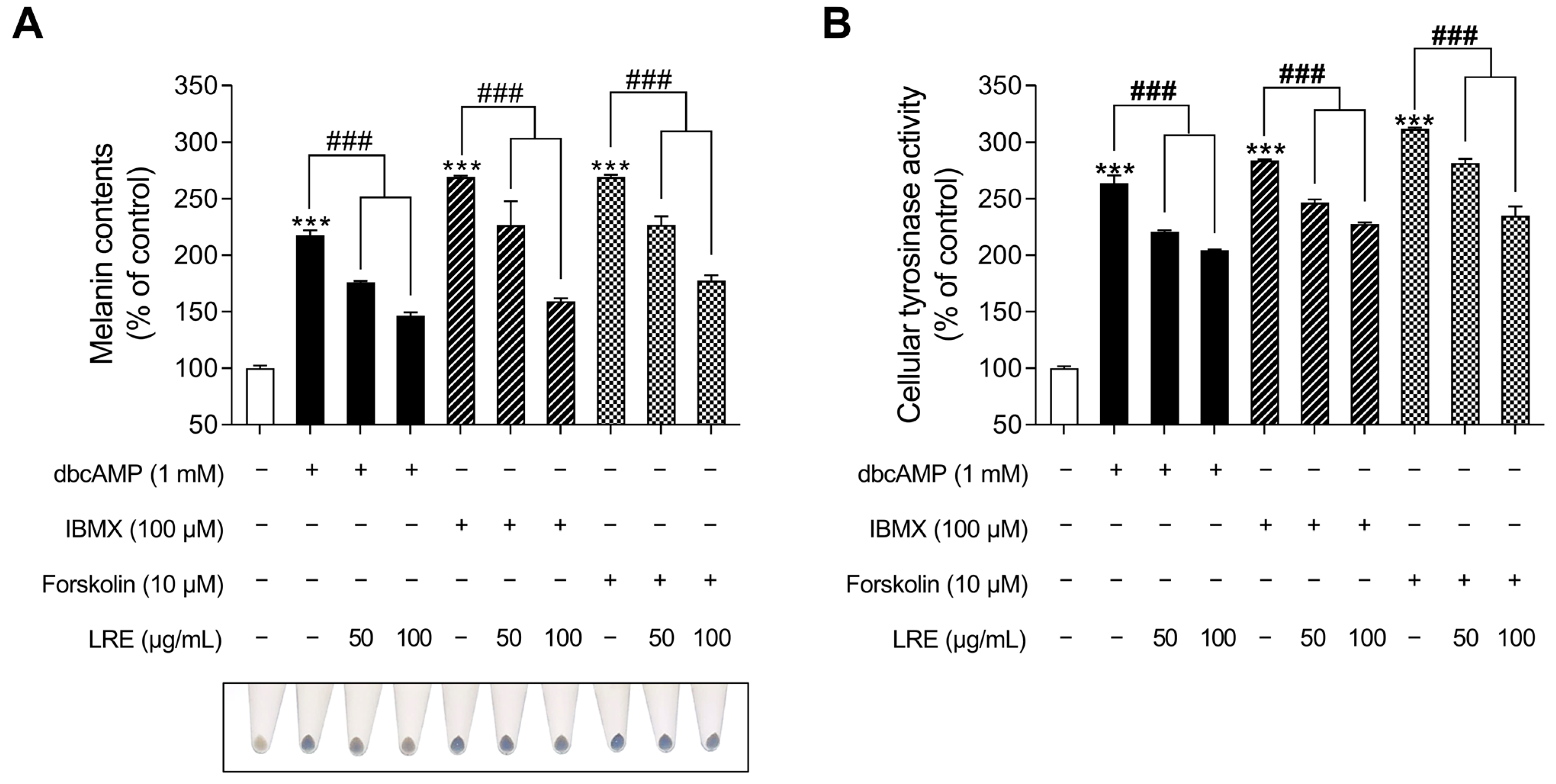
2.5. Inhibitory Effects of LRE on cAMP-Mediated Melanogenesis in B16F10 Cells
2.6. Characterization of LRE via High-Performance Liquid Chromatography-High-Resolution Mass Spectrometry Analysis
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Preparation of L. lancifolium Extract
4.2. HPLC-HRMS Analysis
4.3. Cell Viability Assay
4.4. Measurement of Intracellular Melanin Content
4.5. Measurement of Tyrosinase Activity Assay
4.6. PCR and Quantitative Real-Time PCR
4.7. Western Blot Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Montagna, W.; Carlisle, K. The architecture of black and white facial skin. J. Am. Acad. Dermatol. 1991, 24, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Swalwell, H.; Latimer, J.; Haywood, R.M.; Birch-Machin, M.A. Investigating the role of melanin in UVA/UVB-and hydrogen peroxide-induced cellular and mitochondrial ROS production and mitochondrial DNA damage in human melanoma cells. Free Radic. Biol. Med. 2012, 52, 626–634. [Google Scholar] [CrossRef]
- Bourhim, T.; Villareal, M.O.; Gadhi, C.; Hafidi, A.; Isoda, H. Depigmenting effect of argan press-cake extract through the down-regulation of Mitf and melanogenic enzymes expression in B16 murine melanoma cells. Cytotechnology 2018, 70, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.B.; Fitzpatrick, T.B. Biochemistry of melanin formation. Physiol. Rev. 1950, 30, 91–126. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K. Quantitative analysis of eumelanin and pheomelanin in humans, mice, and other animals: A comparative review. Pigment Cell Res. 2003, 16, 523–531. [Google Scholar] [CrossRef]
- Thody, A.J.; Higgins, E.M.; Wakamatsu, K.; Ito, S.; Burchill, S.A.; Marks, J.M. Pheomelanin as well as eumelanin is present in human epidermis. J. Investig. Dermatol. 1991, 97, 340–344. [Google Scholar] [CrossRef]
- Zanetti, R.; Prota, G.; Napolitano, A.; Martinez, C.; Sancho-Garnier, H.; Østerlind, A.; Sacerdote, C.; Rosso, S. Development of an integrated method of skin phenotype measurement using the melanins. Melanoma Res. 2001, 11, 551–557. [Google Scholar] [CrossRef]
- Chung, K.W.; Park, Y.J.; Choi, Y.J.; Park, M.H.; Ha, Y.M.; Uehara, Y.; Yoon, J.H.; Chun, P.; Moon, H.R.; Chung, H.Y. Evaluation of in vitro and in vivo anti-melanogenic activity of a newly synthesized strong tyrosinase inhibitor (E)-3-(2, 4 dihydroxybenzylidene) pyrrolidine-2, 5-dione (3-DBP). Biochim. Et. Biophys. Acta BBA-Gen. Subj. 2012, 1820, 962–969. [Google Scholar] [CrossRef]
- Riley, P.A. Melanogenesis and melanoma. Pigment Cell Res. 2003, 16, 548–552. [Google Scholar] [CrossRef]
- Abdel-Daim, M.; Funasaka, Y.; Komoto, M.; Nakagawa, Y.; Yanagita, E.; Nishigori, C. Pharmacogenomics of metabotropic glutamate receptor subtype 1 and in vivo malignant melanoma formation. J. Dermatol. 2010, 37, 635–646. [Google Scholar] [CrossRef]
- Miyamura, Y.; Coelho, S.G.; Wolber, R.; Miller, S.A.; Wakamatsu, K.; Zmudzka, B.Z.; Ito, S.; Smuda, C.; Passeron, T.; Choi, W. Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment Cell Res. 2007, 20, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Briganti, S.; Camera, E.; Picardo, M. Chemical and instrumental approaches to treat hyperpigmentation. Pigment Cell Res. 2003, 16, 101–110. [Google Scholar] [CrossRef]
- Ruiz-Maldonado, R.; Orozco-Covarrubias, M.d.l.L. Postinflammatory hypopigmentation and hyperpigmentation. In Proceedings of the Seminars in Cutaneous Medicine and Surgery; WB Saunders Co.: Philadelphia, PA, USA, 1997; pp. 36–43. [Google Scholar]
- Fistarol, S.K.; Itin, P.H. Disorders of pigmentation. JDDG J. Der Dtsch. Dermatol. Ges. 2010, 8, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Solano, F.; Briganti, S.; Picardo, M.; Ghanem, G. Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006, 19, 550–571. [Google Scholar] [CrossRef] [PubMed]
- Rigopoulos, D.; Gregoriou, S.; Katsambas, A. Hyperpigmentation and melasma. J. Cosmet. Dermatol. 2007, 6, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Behrooz, K. Skin Depigmenting Agents: Where Do We Stand? In Pigmentation Disorders; Shahin, A., Ed.; IntechOpen: Rijeka, Croatia, 2022; p. Ch. 5. [Google Scholar]
- Thody, A.J.; Graham, A. Does α-MSH Have a Role in Regulating Skin Pigmentation in Humans? Pigment Cell Res. 1998, 11, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Nylander, K.; Bourdon, J.C.; Bray, S.E.; Gibbs, N.K.; Kay, R.; Hart, I.; Hall, P.A. Transcriptional activation of tyrosinase and TRP-1 by p53 links UV irradiation to the protective tanning response. J. Pathol. 2000, 190, 39–46. [Google Scholar] [CrossRef]
- Cui, F.; She, X.D.; Li, X.F.; Shen, Y.N.; Lü, G.X.; Liu, W.D. Effects of Malassezia isolates on cytokines production associated with melanogenesis by keratinocytes. Zhongguo Yi Xue Ke Xue Yuan Xue Bao Acta Acad. Med. Sin. 2007, 29, 196–200. [Google Scholar]
- Liyanage, A.; Liyanage, G.; Sirimanna, G.; Schürer, N. Comparative Study on Depigmenting Agents in Skin of Color. J. Clin. Aesthetic Dermatol. 2022, 15, 12–17. [Google Scholar]
- Sarkar, R.; Arora, P.; Garg, K.V. Cosmeceuticals for Hyperpigmentation: What is Available? J. Cutan. Aesthetic Surg. 2013, 6, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Jung, S.-H. Recent development of signaling pathways inhibitors of melanogenesis. Cell. Signal. 2017, 40, 99–115. [Google Scholar] [CrossRef]
- Zheng, Y.; Du, X.; Zhang, L.; Jia, T.; Zhang, H.; Peng, B.; Hao, Y.; Tong, Z.; Che, D.; Geng, S. Hydroquinone-induced skin irritant reaction could be achieved by activating mast cells via mas-related G protein–coupled receptor X2. Exp. Dermatol. 2023, 32, 436–446. [Google Scholar] [CrossRef]
- Nordlund, J.J.; Grimes, P.E.; Ortonne, J.P. The safety of hydroquinone. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Kawai, K.; Kawai, K. Contact allergy to kojic acid in skin care products. Contact Dermat. 1995, 32, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Ebanks, J.P.; Wickett, R.R.; Boissy, R.E. Mechanisms regulating skin pigmentation: The rise and fall of complexion coloration. Int. J. Mol. Sci. 2009, 10, 4066–4087. [Google Scholar] [CrossRef] [PubMed]
- Ishack, S.; Lipner, S.R. Exogenous ochronosis associated with hydroquinone: A systematic review. Int. J. Dermatol. 2022, 61, 675–684. [Google Scholar] [CrossRef]
- Jow, T.; Hantash, B.M. Hydroquinone-Induced Depigmentation: Case Report and Review of the Literature. Dermatitis 2014, 25, e1–e5. [Google Scholar] [CrossRef]
- Ahn, M.J.; Hur, S.J.; Kim, E.H.; Lee, S.H.; Shin, J.S.; Kim, M.K.; Uchizono, J.A.; Whang, W.K.; Kim, D.S. Scopoletin from Cirsium setidens Increases Melanin Synthesis via CREB Phosphorylation in B16F10 Cells. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2014, 18, 307–311. [Google Scholar] [CrossRef]
- Schwartz, C.; Jan, A.; Zito, P.M. Hydroquinone. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Nautiyal, A.; Wairkar, S. Management of hyperpigmentation: Current treatments and emerging therapies. Pigment Cell Melanoma Res. 2021, 34, 1000–1014. [Google Scholar] [CrossRef]
- Draelos, Z.D. Skin lightening preparations and the hydroquinone controversy. Dermatol. Ther. 2007, 20, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Mishima, Y.; Hatta, S.; Ohyama, Y.; Inazu, M. Induction of melanogenesis suppression: Cellular pharmacology and mode of differential action. Pigment Cell Res. 1988, 1, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Kang, M.; Chung, H.S.; Cho, C.; Hong, M.C.; Shin, M.K.; Bae, H. Survey and mechanism of skin depigmenting and lightening agents. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2006, 20, 921–934. [Google Scholar] [CrossRef]
- Maeda, K.; Fukuda, M. Arbutin: Mechanism of its depigmenting action in human melanocyte culture. J. Pharmacol. Exp. Ther. 1996, 276, 765–769. [Google Scholar]
- Halder, R.M.; Nordlund, J.J. Topical treatment of pigmentary disorders. In The Pigmentary System: Physiology and Pathophysiology; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2006; pp. 1163–1174. [Google Scholar]
- Garcia, A.; Fulton, J.E., Jr. The combination of glycolic acid and hydroquinone or kojic acid for the treatment of melasma and related conditions. Dermatol. Surg. 1996, 22, 443–447. [Google Scholar] [CrossRef]
- Cardoso, R.; Valente, R.; Souza da Costa, C.H.; da S. Gonçalves Vianez, J.L., Jr.; Santana da Costa, K.; de Molfetta, F.A.; Nahum Alves, C. Analysis of Kojic Acid Derivatives as Competitive Inhibitors of Tyrosinase: A Molecular Modeling Approach. Molecules 2021, 26, 2875. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C.; Hyun, C.-G. Inhibitory Effects of Pinostilbene Hydrate on Melanogenesis in B16F10 Melanoma Cells via ERK and p38 Signaling Pathways. Int. J. Mol. Sci. 2020, 21, 4732. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.T.E.; Frcpi. Treatment of melasma using kojic acid in a gel containing hydroquinone and glycolic acid. Dermatol. Surg. 1999, 25, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Huang, T.; Fernando, S.; Chung, K. Mutagenicity studies of kojic acid. Toxicol. Lett. 1991, 59, 213–220. [Google Scholar] [CrossRef]
- Qian, W.; Liu, W.; Zhu, D.; Cao, Y.; Tang, A.; Gong, G.; Su, H. Natural skin-whitening compounds for the treatment of melanogenesis. Exp. Ther. Med. 2020, 20, 173–185. [Google Scholar] [CrossRef]
- Chiang, H.-M.; Chien, Y.-C.; Wu, C.-H.; Kuo, Y.-H.; Wu, W.-C.; Pan, Y.-Y.; Su, Y.-H.; Wen, K.-C. Hydroalcoholic extract of Rhodiola rosea L. (Crassulaceae) and its hydrolysate inhibit melanogenesis in B16F0 cells by regulating the CREB/MITF/tyrosinase pathway. Food Chem. Toxicol. 2014, 65, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.-P.; Cheng, K.-W.; Zhu, Q.; Wang, X.-C.; Lin, Z.-X.; Wang, M. Tyrosinase inhibitory constituents from the roots of Morus nigra: A structure—Activity relationship study. J. Agric. Food Chem. 2010, 58, 5368–5373. [Google Scholar] [CrossRef] [PubMed]
- Sirat, H.M.; Rezali, M.F.; Ujang, Z. Isolation and identification of radical scavenging and tyrosinase inhibition of polyphenols from Tibouchina semidecandra L. J. Agric. Food Chem. 2010, 58, 10404–10409. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, T.; Jin, Z.-Y.; Xu, X.-M.; Wang, J.-H.; Zha, X.-Q.; Chen, H.-Q. Structural characterisation, physicochemical properties and antioxidant activity of polysaccharide from Lilium lancifolium Thunb. Food Chem. 2015, 169, 430–438. [Google Scholar] [CrossRef]
- Zhang, T.; Gao, J.; Jin, Z.-Y.; Xu, X.-M.; Chen, H.-Q. Protective effects of polysaccharides from Lilium lancifolium on streptozotocin-induced diabetic mice. Int. J. Biol. Macromol. 2014, 65, 436–440. [Google Scholar] [CrossRef]
- Wang, P.; Li, J.; Attia, F.A.K.; Kang, W.; Wei, J.; Liu, Z.; Li, C. A critical review on chemical constituents and pharmacological effects of Lilium. Food Sci. Hum. Wellness 2019, 8, 330–336. [Google Scholar] [CrossRef]
- Kwon, O.-K.; Lee, M.-Y.; Yuk, J.-E.; Oh, S.-R.; Chin, Y.-W.; Lee, H.-K.; Ahn, K.-S. Anti-inflammatory effects of methanol extracts of the root of Lilium lancifolium on LPS-stimulated Raw264. 7 cells. J. Ethnopharmacol. 2010, 130, 28–34. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Xie, C.; Liu, K.; Gu, Z. Isolation of non-starch polysaccharides from bulb of tiger lily (Lilium lancifolium Thunb.) with fermentation of Saccharomyces cerevisiae. Carbohydr. Polym. 2010, 81, 35–40. [Google Scholar] [CrossRef]
- Xue, C.C.; Shergis, J.L.; Zhang, A.L.; Worsnop, C.; Fong, H.; Story, D.; Da Costa, C.; Thien, F.C. Panax ginseng CA Meyer root extract for moderate chronic obstructive pulmonary disease (COPD): Study protocol for a randomised controlled trial. Trials 2011, 12, 164. [Google Scholar] [CrossRef]
- Sun, X.; Gao, R.-L.; Xiong, Y.-K.; Huang, Q.-C.; Xu, M. Antitumor and immunomodulatory effects of a water-soluble polysaccharide from Lilii Bulbus in mice. Carbohydr. Polym. 2014, 102, 543–549. [Google Scholar] [CrossRef]
- Cheng, A.; Wan, F.; Wang, J.; Jin, Z.; Xu, X. Macrophage immunomodulatory activity of polysaccharides isolated from Glycyrrhiza uralensis fish. Int. Immunopharmacol. 2008, 8, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Xie, Z.; Huang, S.; Tai, Y.; Cai, Q.; Jiang, W.; Sun, J.; Yuan, Y. Immune-enhancing effects of polysaccharides extracted from Lilium lancifolium Thunb. Int. Immunopharmacol. 2017, 52, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, H.; Wang, B.; Fu, L.; Yuan, M.; Liu, J.; Zhou, L.; Ding, C. Characterization and antioxidant activities of polysaccharides from the leaves of Lilium lancifolium Thunb. Int. J. Biol. Macromol. 2016, 92, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Sim, W.S.; Choi, S.I.; Jung, T.D.; Cho, B.Y.; Choi, S.H.; Park, S.M.; Lee, O.H. Antioxidant and anti-inflammatory effects of Lilium lancifolium bulbs extract. J. Food Biochem. 2020, 44, e13176. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Yun, N.; Jang, Y.P.; Kim, J. Lilium lancifolium Thunb. extract attenuates pulmonary inflammation and air space enlargement in a cigarette smoke-exposed mouse model. J. Ethnopharmacol. 2013, 149, 148–156. [Google Scholar] [CrossRef]
- Park, T.; Seo, K.-S.; Choi, S.; Yun, K.W. Chemical Constituents of Bulb of Lilium lancifolium Thunberg and Lilium tsingtauense Gilg. Korean J. Plant Resour. 2014, 27, 125–132. [Google Scholar] [CrossRef][Green Version]
- Zhou, J.; An, R.; Huang, X. Genus Lilium: A review on traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2021, 270, 113852. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, Y.; Luo, K.; Xu, L.; Yang, P.; Ming, J. Potential Applications of Lilium Plants in Cosmetics: A Comprehensive Review Based on Research Papers and Patents. Antioxidants 2022, 11, 1458. [Google Scholar] [CrossRef]
- Ferreira, A.M.; de Souza, A.A.; Koga, R.C.R.; Sena, I.D.S.; Matos, M.J.S.; Tomazi, R.; Ferreira, I.M.; Carvalho, J.C.T. Anti-Melanogenic Potential of Natural and Synthetic Substances: Application in Zebrafish Model. Molecules 2023, 28, 1053. [Google Scholar] [CrossRef]
- Wang, R.; Tang, P.; Wang, P.; Boissy, R.E.; Zheng, H. Regulation of tyrosinase trafficking and processing by presenilins: Partial loss of function by familial Alzheimer’s disease mutation. Proc. Natl. Acad. Sci. USA 2006, 103, 353–358. [Google Scholar] [CrossRef]
- del Marmol, V.; Beermann, F. Tyrosinase and related proteins in mammalian pigmentation. FEBS Lett. 1996, 381, 165–168. [Google Scholar] [CrossRef]
- Sun, M.; Xie, H.-F.; Tang, Y.; Lin, S.-Q.; Li, J.-M.; Sun, S.-N.; Hu, X.-L.; Huang, Y.-X.; Shi, W.; Jian, D. G protein-coupled estrogen receptor enhances melanogenesis via cAMP-protein kinase (PKA) by upregulating microphthalmia-related transcription factor-tyrosinase in melanoma. J. Steroid Biochem. Mol. Biol. 2017, 165, 236–246. [Google Scholar] [CrossRef]
- Bentley, N.; Eisen, T.; Goding, C. Melanocyte-specific expression of the human tyrosinase promoter: Activation by the microphthalmia gene product and role of the initiator. Mol. Cell. Biol. 1994, 14, 7996–8006. [Google Scholar] [PubMed]
- Vachtenheim, J.; Borovanský, J. “Transcription physiology” of pigment formation in melanocytes: Central role of MITF. Exp. Dermatol. 2010, 19, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Kim, M.J.; Choi, Y.H.; Kim, B.K.; Kim, K.S.; Park, K.J.; Park, S.M.; Lee, N.H.; Hyun, C.G. Down-regulation of tyrosinase, TRP-1, TRP-2 and MITF expressions by citrus press-cakes in murine B16 F10 melanoma. Asian Pac. J. Trop. Biomed. 2013, 3, 617–622, discussion 621–612. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.-F.; Huang, C.-C.; Lee, M.-Y.; Lin, Y.-S. Fermented broth in tyrosinase-and melanogenesis inhibition. Molecules 2014, 19, 13122–13135. [Google Scholar] [CrossRef]
- Kim, A.; Yim, N.-H.; Im, M.; Jung, Y.P.; Liang, C.; Cho, W.-K.; Ma, J.Y. Ssanghwa-tang, an oriental herbal cocktail, exerts anti-melanogenic activity by suppression of the p38 MAPK and PKA signaling pathways in B16F10 cells. BMC Complement. Altern. Med. 2013, 13, 214. [Google Scholar] [CrossRef]
- Kim, Y.M.; Cho, S.E.; Seo, Y.K. The activation of melanogenesis by p-CREB and MITF signaling with extremely low-frequency electromagnetic fields on B16F10 melanoma. Life Sci. 2016, 162, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Hartman, M.L.; Czyz, M. MITF in melanoma: Mechanisms behind its expression and activity. Cell. Mol. Life Sci. 2015, 72, 1249–1260. [Google Scholar] [CrossRef]
- Saha, B.; Singh, S.K.; Sarkar, C.; Bera, R.; Ratha, J.; Tobin, D.J.; Bhadra, R. Activation of the Mitf promoter by lipid-stimulated activation of p38-stress signalling to CREB. Pigment Cell Res. 2006, 19, 595–605. [Google Scholar] [CrossRef]
- Zhang, H.; Kong, Q.; Wang, J.; Jiang, Y.; Hua, H. Complex roles of cAMP–PKA–CREB signaling in cancer. Exp. Hematol. Oncol. 2020, 9, 32. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Lazarovici, P.; Quirion, R.; Zheng, W. cAMP Response Element-Binding Protein (CREB): A Possible Signaling Molecule Link in the Pathophysiology of Schizophrenia. Front. Mol. Neurosci. 2018, 11, 255. [Google Scholar] [CrossRef] [PubMed]
- Ao, Y.; Park, H.Y.; Olaizola-Horn, S.; Gilchrest, B.A. Activation of cAMP-dependent protein kinase is required for optimal alpha-melanocyte-stimulating hormone-induced pigmentation. Exp. Cell Res. 1998, 244, 117–124. [Google Scholar] [CrossRef]
- Roh, E.; Yun, C.-Y.; Young Yun, J.; Park, D.; Doo Kim, N.; Yeon Hwang, B.; Jung, S.-H.; Park, S.K.; Kim, Y.-B.; Han, S.-B.; et al. cAMP-Binding Site of PKA as a Molecular Target of Bisabolangelone against Melanocyte-Specific Hyperpigmented Disorder. J. Investig. Dermatol. 2013, 133, 1072–1079. [Google Scholar] [CrossRef]
- Herraiz, C.; Journé, F.; Abdel-Malek, Z.; Ghanem, G.; Jiménez-Cervantes, C.; García-Borrón, J.C. Signaling from the Human Melanocortin 1 Receptor to ERK1 and ERK2 Mitogen-Activated Protein Kinases Involves Transactivation of cKIT. Mol. Endocrinol. 2011, 25, 138–156. [Google Scholar] [CrossRef]
- Ge, Y.C.; Li, J.N.; Ni, X.T.; Guo, C.M.; Wang, W.S.; Duan, T.; Sun, K. Cross talk between cAMP and p38 MAPK pathways in the induction of leptin by hCG in human placental syncytiotrophoblasts. Reproduction 2011, 142, 369–375. [Google Scholar] [CrossRef]
- Delghandi, M.P.; Johannessen, M.; Moens, U. The cAMP signalling pathway activates CREB through PKA, p38 and MSK1 in NIH 3T3 cells. Cell. Signal. 2005, 17, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.; Zippin, J.H. Cyclic adenosine monophosphate (cAMP) signaling in melanocyte pigmentation and melanomagenesis. Pigment Cell Melanoma Res. 2021, 34, 28–43. [Google Scholar] [CrossRef]
- Raker, V.K.; Becker, C.; Steinbrink, K. The cAMP Pathway as Therapeutic Target in Autoimmune and Inflammatory Diseases. Front. Immunol. 2016, 7, 123. [Google Scholar] [CrossRef] [PubMed]
- Englaro, W.; Rezzonico, R.; Durand-Clément, M.; Lallemand, D.; Ortonne, J.-P.; Ballotti, R. Mitogen-activated Protein Kinase Pathway and AP-1 Are Activated during cAMP-induced Melanogenesis in B-16 Melanoma Cells (*). J. Biol. Chem. 1995, 270, 24315–24320. [Google Scholar] [CrossRef]
- Sassone-Corsi, P. The cyclic AMP pathway. Cold Spring Harb. Perspect. Biol. 2012, 4, a011148. [Google Scholar] [CrossRef]
- Buscà, R.; Ballotti, R. Cyclic AMP a Key Messenger in the Regulation of Skin Pigmentation. Pigment Cell Res. 2000, 13, 60–69. [Google Scholar] [CrossRef]
- Slominski, A.; Zmijewski, M.A.; Pawelek, J. L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012, 25, 14–27. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Li, Z.Y.; Zhao, H.Q.; Gao, C.; Xiao, M.W.; Jiang, X.M.; Zhu, J.P.; Huang, H.Y.; Xu, G.M.; Xie, M.Z. Effects of different drying methods on the chemical constituents of Lilium lancifolium Thunb. based on UHPLC-MS analysis and antidepressant activity of the main chemical component regaloside A. J. Sep. Sci. 2021, 44, 992–1004. [Google Scholar] [CrossRef]
- Farishian, R.A.; Writtaker, J.H. Phenylalanine Lowers Melanin Synthesis in Mammalian Melanocytes by Reducing Tyrosinase Uptake: Implications for Pigment Reduction in Phenylketonuria. J. Investig. Dermatol. 1980, 74, 85–89. [Google Scholar] [CrossRef]
- Kaushik, N.; Kim, J.H.; Nguyen, L.N.; Kaushik, N.K.; Choi, K.A. Characterization of Bioactive Compounds Having Antioxidant and Anti-Inflammatory Effects of Liliaceae Family Flower Petal Extracts. J. Funct. Biomater. 2022, 13, 284. [Google Scholar] [CrossRef]
- Costin, G.E.; Hearing, V.J. Human skin pigmentation: Melanocytes modulate skin color in response to stress. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2007, 21, 976–994. [Google Scholar] [CrossRef]
- Yardman-Frank, J.M.; Fisher, D.E. Skin pigmentation and its control: From ultraviolet radiation to stem cells. Exp. Dermatol. 2021, 30, 560–571. [Google Scholar] [CrossRef]
- Thawabteh, A.M.; Jibreen, A.; Karaman, D.; Thawabteh, A.; Karaman, R. Skin Pigmentation Types, Causes and Treatment-A Review. Molecules 2023, 28, 4839. [Google Scholar] [CrossRef]
- Searle, T.; Al-Niaimi, F.; Ali, F.R. The top 10 cosmeceuticals for facial hyperpigmentation. Dermatol. Ther. 2020, 33, e14095. [Google Scholar] [CrossRef]
- Bertolotto, C.; Buscà, R.; Abbe, P.; Bille, K.; Aberdam, E.; Ortonne, J.P.; Ballotti, R. Different cis-acting elements are involved in the regulation of TRP1 and TRP2 promoter activities by cyclic AMP: Pivotal role of M boxes (GTCATGTGCT) and of microphthalmia. Mol. Cell. Biol. 1998, 18, 694–702. [Google Scholar] [CrossRef]
- Huang, H.C.; Chang, S.J.; Wu, C.Y.; Ke, H.J.; Chang, T.M. [6]-Shogaol inhibits α-MSH-induced melanogenesis through the acceleration of ERK and PI3K/Akt-mediated MITF degradation. BioMed Res. Int. 2014, 2014, 842569. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; An, S.; Bae, S.; Lee, J.H. Diarylpropionitrile inhibits melanogenesis via protein kinase A/cAMP-response element-binding protein/microphthalmia-associated transcription factor signaling pathway in α-MSH-stimulated B16F10 melanoma cells. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2022, 26, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Nam, G.; An, S.K.; Park, I.C.; Bae, S.; Lee, J.H. Daphnetin inhibits α-MSH-induced melanogenesis via PKA and ERK signaling pathways in B16F10 melanoma cells. Biosci. Biotechnol. Biochem. 2022, 86, 596–609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zhao, R.; Wang, Y.; Liu, T.; Tian, H.; Yin, X.; Yang, L.; Sui, X. Natural surfactant used as an additive in the homogenate-ultrasound-synergistic extraction of regaloside A and polysaccharides from Lilium lancifolium bulbs using Doehlert matrix design. Ind. Crops Prod. 2022, 188, 115689. [Google Scholar] [CrossRef]
- Park, S.; Han, N.; Lee, J.-M.; Lee, J.-H.; Bae, S. Effects of Allium hookeri Extracts on Hair-Inductive and Anti-Oxidative Properties in Human Dermal Papilla Cells. Plants 2023, 12, 1919. [Google Scholar] [CrossRef]
- Ullah, S.; Chung, Y.C.; Hyun, C.G. Induction of Melanogenesis by Fosfomycin in B16F10 Cells Through the Upregulation of P-JNK and P-p38 Signaling Pathways. Antibiotics 2020, 9, 172. [Google Scholar] [CrossRef]
- Kim, M.J.; Mohamed, E.A.; Kim, D.S.; Park, M.J.; Ahn, B.J.; Jeung, E.B.; An, B.S. Inhibitory effects and underlying mechanisms of Artemisia capillaris essential oil on melanogenesis in the B16F10 cell line. Mol. Med. Rep. 2022, 25, 113. [Google Scholar] [CrossRef]
- Waku, T.; Nakada, S.; Masuda, H.; Sumi, H.; Wada, A.; Hirose, S.; Aketa, I.; Kobayashi, A. The CNC-family transcription factor Nrf3 coordinates the melanogenesis cascade through macropinocytosis and autophagy regulation. Cell Rep. 2023, 42, 111906. [Google Scholar] [CrossRef]
- Yu, B.Y.; Ngo, H.H.; Choi, W.J.; Keum, Y.S. Dimethyl Itaconate Inhibits Melanogenesis in B16F10 Cells. Antioxidants 2023, 12, 692. [Google Scholar] [CrossRef]
- Joo, I.H.; Choi, J.H.; Kim, D.H.; Chung, M.J.; Lim, M.H. Ligularia fischeri ethanol extract: An inhibitor of alpha-melanocyte-stimulating hormone-stimulated melanogenesis in B16F10 melanoma cells. J. Cosmet. Dermatol. 2023, 22, 637–644. [Google Scholar] [CrossRef]
- Shin, S.; Ko, J.; Kim, M.; Song, N.; Park, K. Morin Induces Melanogenesis via Activation of MAPK Signaling Pathways in B16F10 Mouse Melanoma Cells. Molecules 2021, 26, 2150. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, B.; Jeon, Y.D.; Song, H.W.; Lee, Y.M.; Song, B.J.; Kim, D.K. Inhibitory Effect of Elaeagnus umbellata Fractions on Melanogenesis in α-MSH-Stimulated B16-F10 Melanoma Cells. Molecules 2021, 26, 1308. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Tsatsakis, A.M.; Tzanakakis, G.; Kim, H.S.; Le, B.; Sifaki, M.; Spandidos, D.A.; Tsukamoto, C.; Golokhvast, K.S.; Izotov, B.N.; et al. Soyasaponin Ag inhibits α-MSH-induced melanogenesis in B16F10 melanoma cells via the downregulation of TRP-2. Int. J. Mol. Med. 2017, 40, 631–636. [Google Scholar] [CrossRef]
- Zhou, X.; Oh, J.H.; Karadeniz, F.; Yang, J.; Lee, H.; Seo, Y.; Kong, C.-S. Anti-Melanogenesis Effect of Rosa rugosa on α-MSH-Induced B16F10 Cells via PKA/CREB Pathway Activation. Appl. Sci. 2023, 13, 184. [Google Scholar]
- Lee, K.R.; Lee, J.S.; Lee, S.; Son, Y.K.; Kim, G.R.; Sim, Y.C.; Song, J.E.; Ha, S.J.; Hong, E.K. Polysaccharide isolated from the liquid culture broth of Inonotus obliquus suppresses invasion of B16-F10 melanoma cells via AKT/NF-κB signaling pathway. Mol. Med. Rep. 2016, 14, 4429–4435. [Google Scholar] [CrossRef]
- Han, H.; Hyun, C. Acenocoumarol, an Anticoagulant Drug, Prevents Melanogenesis in B16F10 Melanoma Cells. Pharmaceuticals 2023, 16, 604. [Google Scholar] [CrossRef]
- Kim, H.M.; Hyun, C.G. Miglitol, an Oral Antidiabetic Drug, Downregulates Melanogenesis in B16F10 Melanoma Cells through the PKA, MAPK, and GSK3β/β-Catenin Signaling Pathways. Molecules 2022, 28, 115. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, S.H.; Kim, Y.H.; Kim, S.H.; Oh, G.S.; Bae, J.E.; Kim, J.B.; Park, N.Y.; Park, K.; Yeom, E.; et al. Nalfurafine Hydrochloride, a κ-Opioid Receptor Agonist, Induces Melanophagy via PKA Inhibition in B16F1 Cells. Cells 2022, 12, 146. [Google Scholar] [CrossRef]
- Choi, M.R.; Lee, H.; Kim, H.K.; Han, J.; Seol, J.E.; Vasileva, E.A.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; Ju, W.S.; et al. Echinochrome A Inhibits Melanogenesis in B16F10 Cells by Downregulating CREB Signaling. Mar. Drugs 2022, 20, 555. [Google Scholar] [CrossRef]
- Yao, C.; Jin, C.L.; Oh, J.H.; Oh, I.G.; Park, C.H.; Chung, J.H. Ardisia crenata extract stimulates melanogenesis in B16F10 melanoma cells through inhibiting ERK1/2 and Akt activation. Mol. Med. Rep. 2015, 11, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Lee, J.; Kim, Y.J.; Hoang, D.H.; Choe, W.; Kang, I.; Kim, S.S.; Ha, J. Jeju Magma-Seawater Inhibits α-MSH-Induced Melanogenesis via CaMKKβ-AMPK Signaling Pathways in B16F10 Melanoma Cells. Mar. Drugs 2020, 18, 473. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Lin, H.; Huang, M.C. The lactoferricin B-derived peptide, LfB17-34, induces melanogenesis in B16F10 cells. Int. J. Mol. Med. 2017, 39, 595–602. [Google Scholar] [CrossRef] [PubMed]
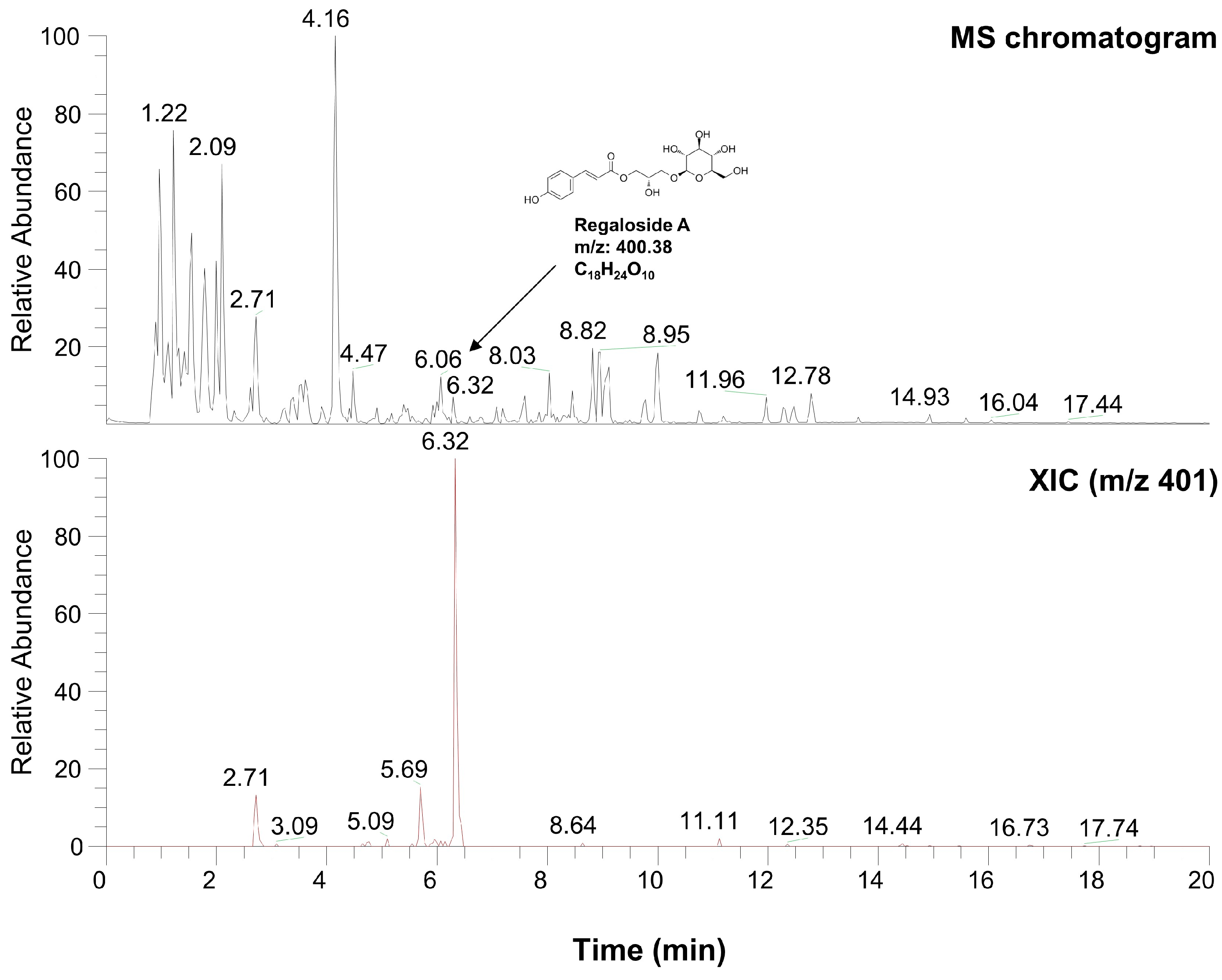
| Number | Name | Formula | M.W. | RT (min) |
|---|---|---|---|---|
| 1 | L-alanyl-L-alpha-aspartyl-L-proline | C12H19O6N3 | 301.296 | 1.22 |
| 2 | Methyl N-acetylhistidinate | C9H13O3N3 | 211.218 | 1.68 |
| 3 | L-phenylalanine | C9H11NO2 | 165.189 | 2.09 |
| 4 | Threonyl-α-glutamylleucine | C15H27O7N3 | 361.391 | 2.71 |
| 5 | Boc-O-methyl-L-threonine | C10H19NO5 | 234.133 | 4.16 |
| 6 | Hopantenic acid glucoside | C16H29O10N | 395.402 | 4.47 |
| 7 | Methyl (4S)-4-[(2-pyridinylcarbonyl)amino]-L-prolinate | C12H15O3N3 | 250.118 | 6.06 |
| 8 | Regaloside A | C18H24O10 | 400.377 | 6.32 |
| 9 | Boc-Lys(Z)-OH | C19H28O6N2 | 380.435 | 8.82 |
| 10 | Z-L-Pro-L-Leu-Gly | C21H29O6N3 | 419.471 | 8.95 |
| 11 | L-Lysyl-L-leucyl-L-valyl-L-leucyl-L-alanyl-L-serine | C29H55O8N7 | 629.789 | 11.96 |
| Target mRNA | Sequences of Primer | Amplicons (bp) | Annealing Temperature (°C) |
|---|---|---|---|
| Gapdh | F: 5′-CATCACTGCCACCCAGAAGACTG-3′ | 153 | 60 |
| R: 5′-ATGCCAGTGAGCTTCCCGTTCAG-3′ | |||
| Mitf | F: 5′-AGAAGCTGGAGCATGCGAACC-3′ | 168 | 60 |
| R: 5′-GTTCCTGGCTGCAGTTCTCAAG-3′ | |||
| Tyrosinase | F: 5′-AGTCGTATCTGGCCATGGCTTCTTG-3′ | 169 | 60 |
| R: 5′-GCAAGCTGTGGTAGTCGTCTTTGTC-3′ | |||
| Tyrp1 | F: 5′-CTGCGATGTCTGCACTGATGACTTG-3′ | 171 | 60 |
| R: 5′-TTTCTCCTGATTGGTCCACCCTCAG-3′ | |||
| Tyrp2 | F: 5′-GCTTGGATGACTACAACCGCCG-3′ | 451 | 60 |
| F: 5′-GGTGGGAAGAAGGGGACCATGT-3′ |
| Antigen | Host | Clonality (Species Reactivity) | Dilution | Manufacturer (Cat. Number) | References |
|---|---|---|---|---|---|
| Mitf | Mouse | Monoclonal (mouse, rat, human) | 1:200 | Santa Cruz (#sc-56725) | [102,103] |
| Tyrosinase | Mouse | Monoclonal (mouse, rat, human) | 1:200 | Santa Cruz (#sc-20035) | [102,104] |
| Tyrp1 | Mouse | Monoclonal (mouse, rat, human) | 1:200 | Santa Cruz (#sc-166857) | [105,106] |
| Tyrp2 | Mouse | Monoclonal (mouse, rat, human) | 1:200 | Santa Cruz (#sc-74439) | [107,108] |
| β-actin | Mouse | Monoclonal (mouse, rat, human) | 1:1000 | Santa Cruz (#sc-47778) | [109,110] |
| PKA | Rabbit | Polyclonal (mouse, rat, human) | 1:1000 | CST (#4782S) | [111,112] |
| Phospho-PKA (Thr198) | Rabbit | Polyclonal (mouse, rat, human) | 1:1000 | CST (#4781S) | [112,113] |
| CREB | Rabbit | Monoclonal (mouse, rat, human···) | 1:1000 | CST(#9197S) | [31,114] |
| Phospho-CREB (Ser133) | Rabbit | Monoclonal (mouse, rat, human···) | 1:1000 | CST (#9198S) | [31,109] |
| ERK | Rabbit | Polyclonal (mouse, rat, human···) | 1:1000 | CST (#9102S) | [106,115] |
| Phospho-ERK (Thr202/Tyr204) | Rabbit | Polyclonal (mouse, rat, human···) | 1:1000 | CST (#9101S) | [106,115] |
| p38 | Rabbit | Polyclonal (mouse, rat, human···) | 1:1000 | CST (#9212S) | [116,117] |
| Phospho-p38 (Thr180/Tyr182) | Rabbit | Polyclonal (mouse, rat, human···) | 1:1000 | CST (#9211S) | [111,116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Han, N.; Lee, J.; Lee, J.-N.; An, S.; Bae, S. Anti-Melanogenic Effects of Lilium lancifolium Root Extract via Downregulation of PKA/CREB and MAPK/CREB Signaling Pathways in B16F10 Cells. Plants 2023, 12, 3666. https://doi.org/10.3390/plants12213666
Park S, Han N, Lee J, Lee J-N, An S, Bae S. Anti-Melanogenic Effects of Lilium lancifolium Root Extract via Downregulation of PKA/CREB and MAPK/CREB Signaling Pathways in B16F10 Cells. Plants. 2023; 12(21):3666. https://doi.org/10.3390/plants12213666
Chicago/Turabian StylePark, Seokmuk, Nayeon Han, Jungmin Lee, Jae-Nam Lee, Sungkwan An, and Seunghee Bae. 2023. "Anti-Melanogenic Effects of Lilium lancifolium Root Extract via Downregulation of PKA/CREB and MAPK/CREB Signaling Pathways in B16F10 Cells" Plants 12, no. 21: 3666. https://doi.org/10.3390/plants12213666
APA StylePark, S., Han, N., Lee, J., Lee, J.-N., An, S., & Bae, S. (2023). Anti-Melanogenic Effects of Lilium lancifolium Root Extract via Downregulation of PKA/CREB and MAPK/CREB Signaling Pathways in B16F10 Cells. Plants, 12(21), 3666. https://doi.org/10.3390/plants12213666






