Changes in Nutrient Components and Digestive Enzymatic Inhibition Activities in Soy Leaves by Ethephon Treatment
Abstract
:1. Introduction
2. Results
2.1. Changes of Proximate Compositions in SLs by Ethephon Treatment
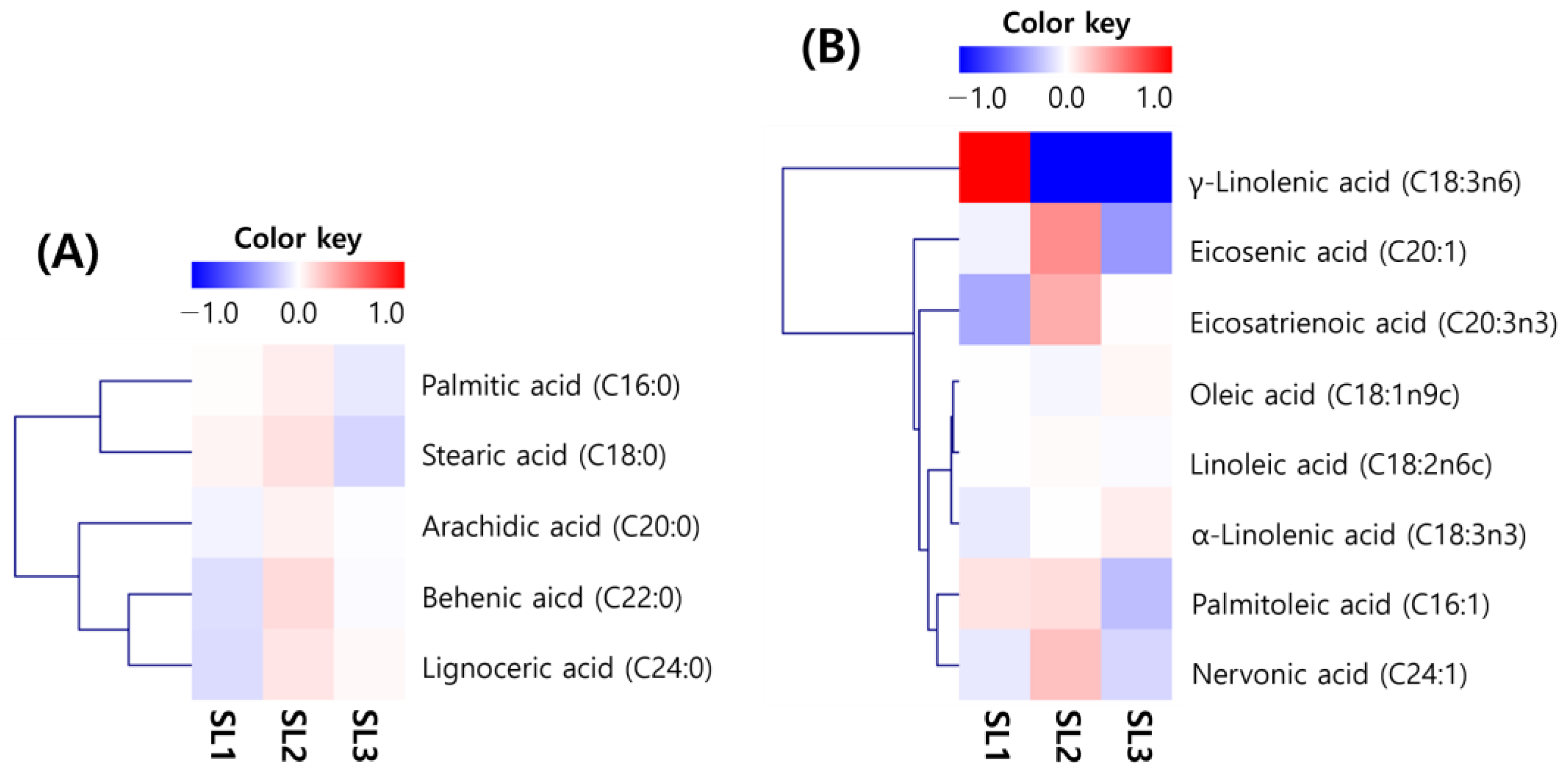
2.2. Changes of Fatty Acid Contents in SLs by Ethephon Treatment
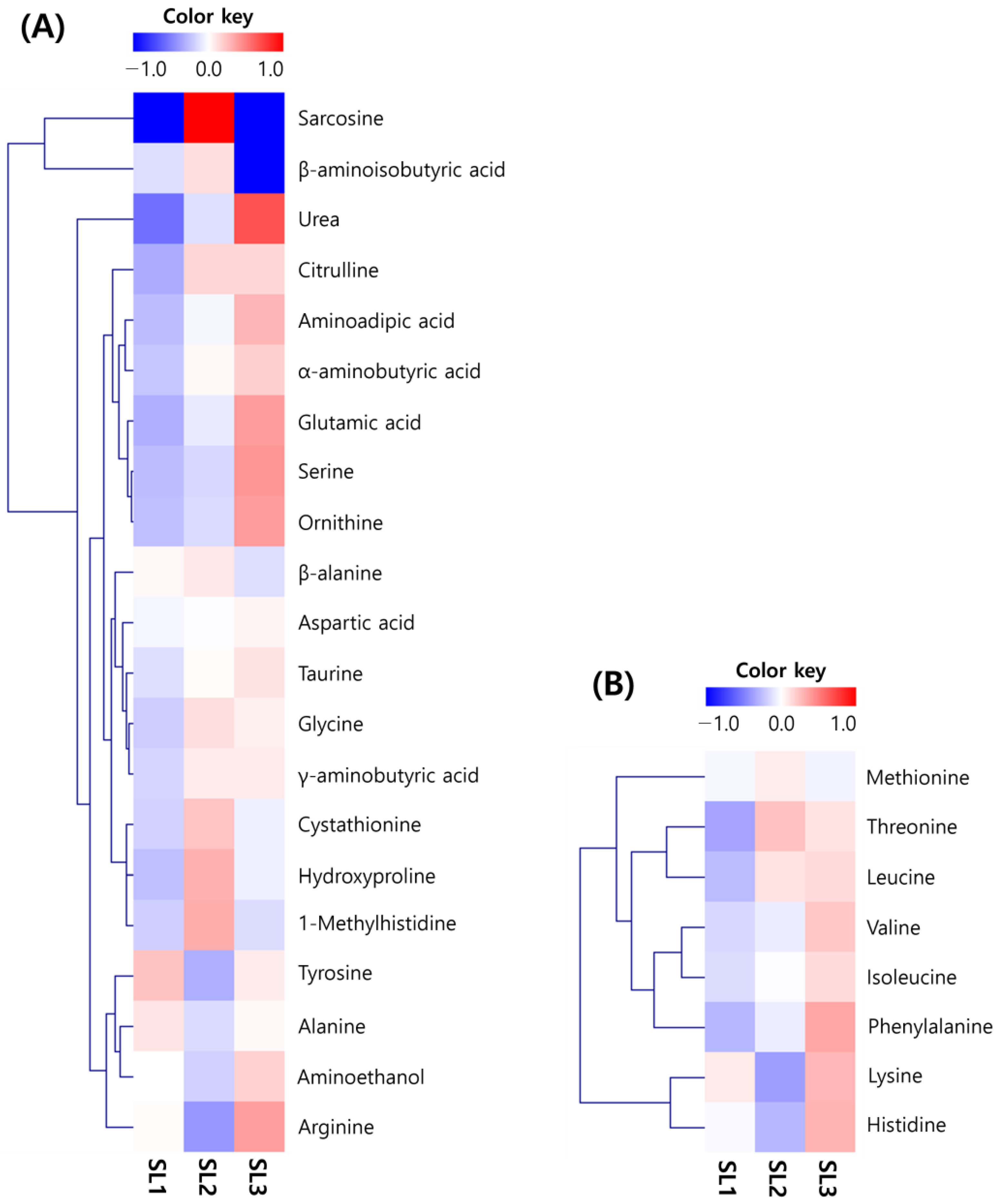
2.3. Changes of Free Amino Acid Contents in SLs by Ethephon Treatment
2.4. Changes of Water-Soluble Vitamin Contents in SLs by Ethephon Treatment
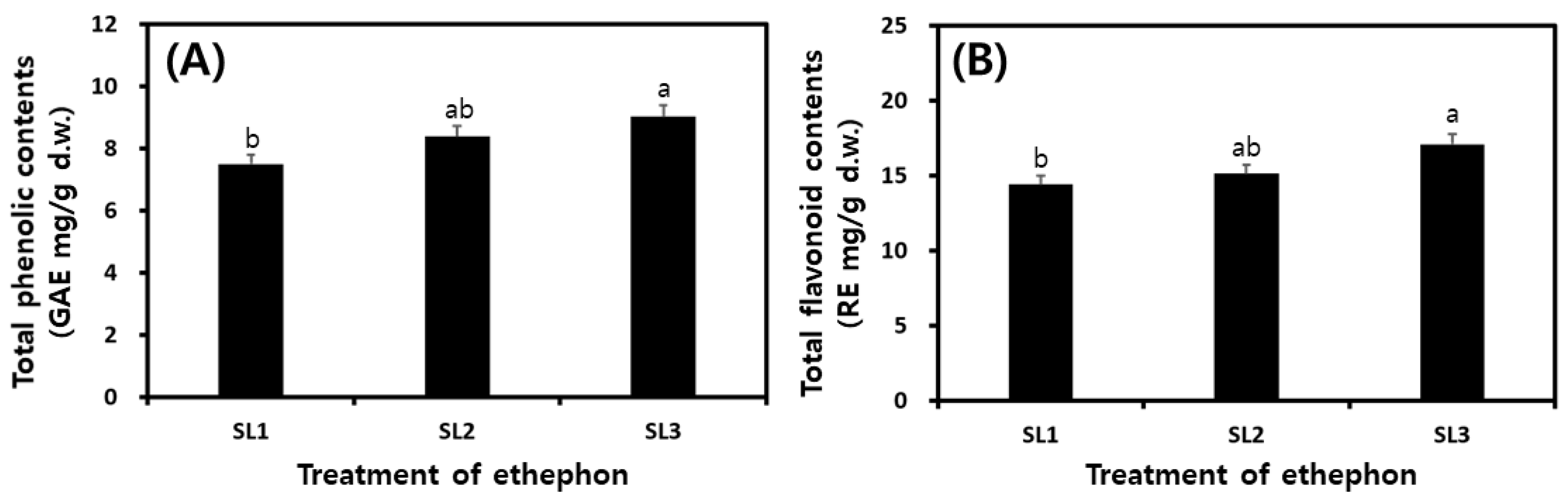
2.5. Changes of TP and TF Contents in SLs by Ethephon Treatment
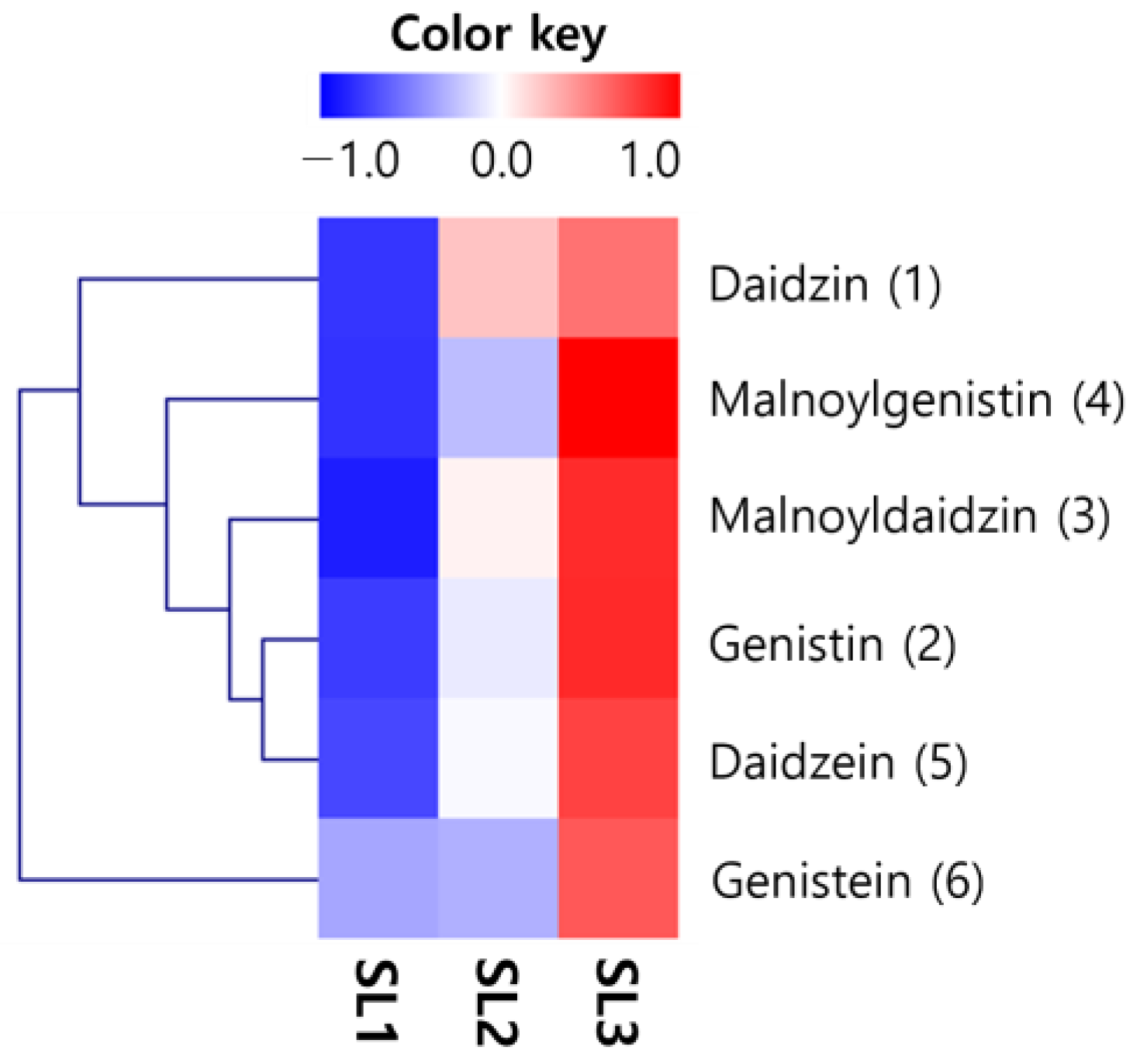
2.6. Changes of Isoflavones Contents in SLs by Ethephon Treatment
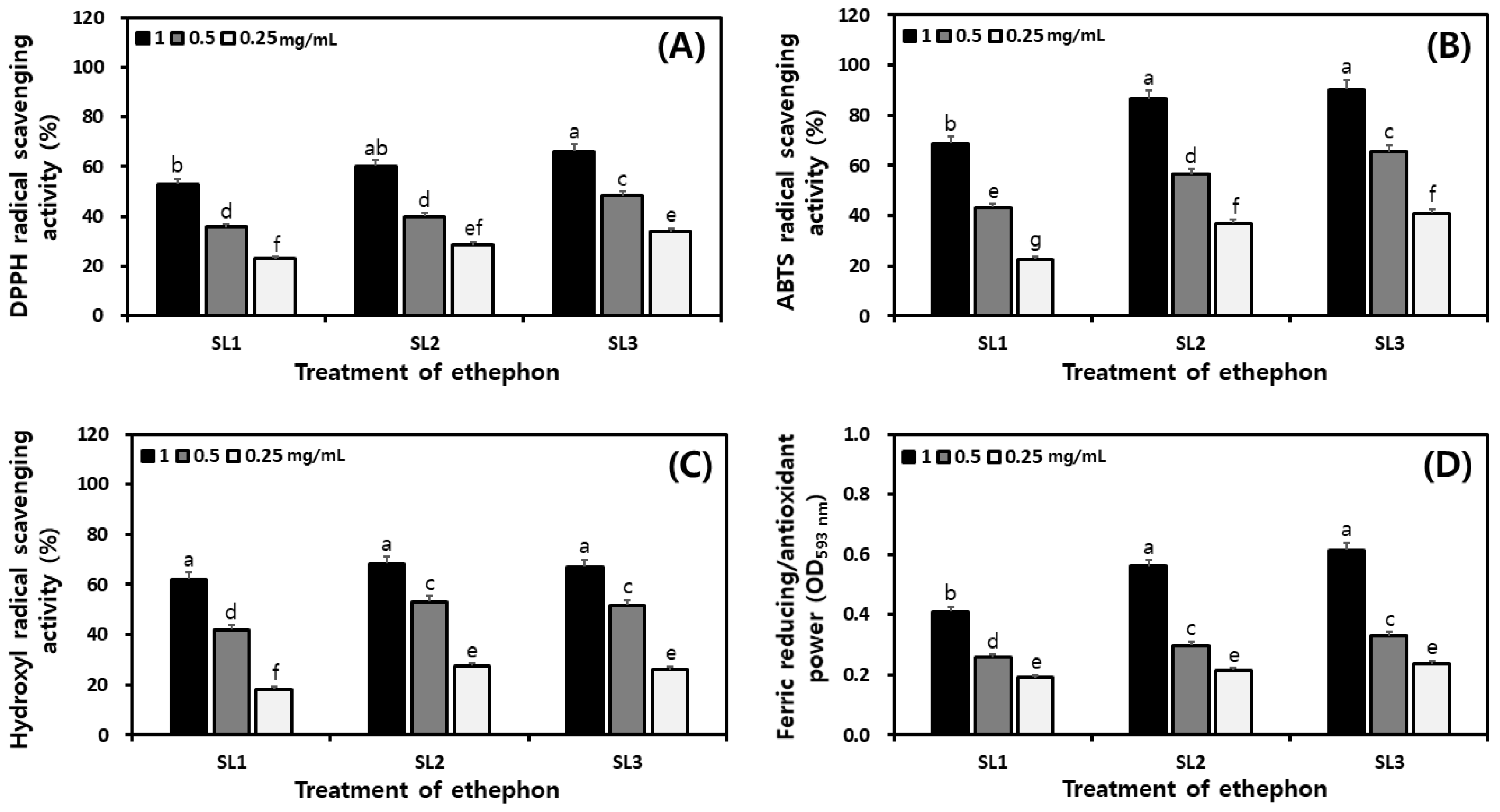
2.7. Changes of Antioxidant Activities in SLs by Ethephon Treatment
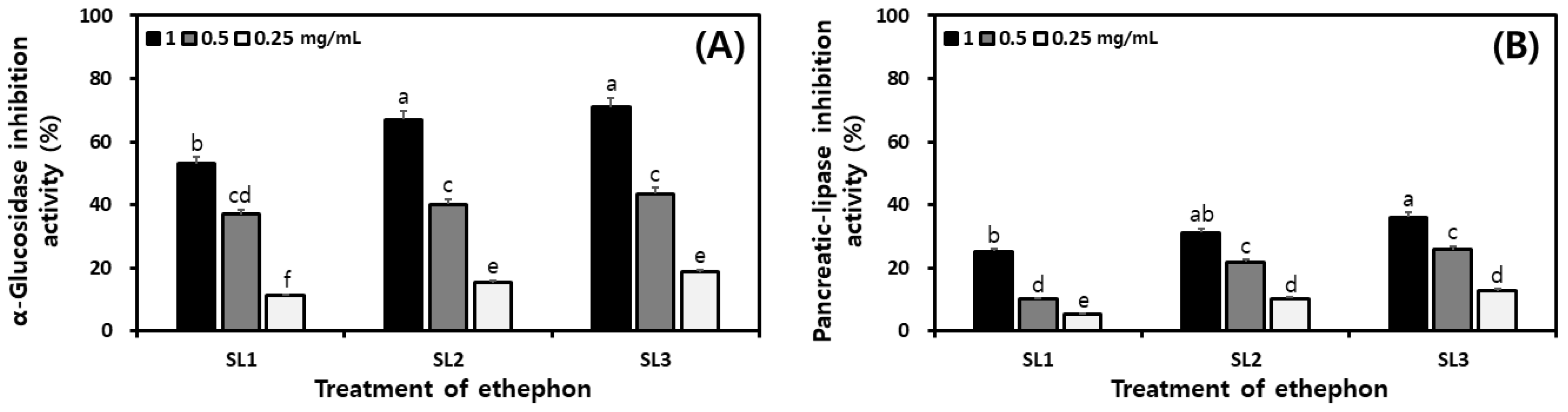
2.8. Changes of Digestive Enzyme Inhibitory Activities in SLs by Ethephon Treatment
3. Discussion
4. Materials and Methods
4.1. Materials, Reagents, and Instruments
4.2. Analysis of Proximate Compositions
4.3. Analysis of Fatty Acids
4.4. Analysis of Free Amino Acids
4.5. Analysis of Water-Soluble Vitamin
4.6. Preparation of Extracts
4.7. Analysis of Total Phenolic and Flavonoid Contents
4.8. Analysis of Isoflavones
4.9. Analysis of Antioxidant Activity
4.10. Analysis of Digestive Enzymes Inhibitory Activity
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hsiao, Y.H.; Ho, C.T.; Pan, M.H. Bioavailability and health benefits of major isoflavone aglycones and their metabolites. J. Funct. Foods 2020, 74, 104164. [Google Scholar] [CrossRef]
- Wang, Q.; Ge, X.; Tian, X.; Zhang, Y.; Zhang, J.; Zhang, P. Soy isoflavone: The multipurpose phytochemical. Biomed. Rep. 2013, 1, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Islam, M.S.; Uddin, M.N.; Hasan, M.M.I.; Akanda, M.R. The potential health benefits of the isoflavone glycoside genistin. Arch. Pharmacal Res. 2020, 43, 395–408. [Google Scholar] [CrossRef]
- Kim, U.H.; Yoon, J.H.; Li, H.; Kang, J.H.; Ji, H.S.; Park, K.H.; Shin, D.H.; Park, H.Y.; Jeong, T.S. Pterocarpan-enriched soy leaf extract ameliorates insulin sensitivity and pancreatic β-cell proliferation in type 2 diabetic mice. Molecules 2014, 19, 18493–18510. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kang, J.H.; Han, J.M.; Cho, M.H.; Chung, Y.J.; Park, K.H.; Shin, D.H.; Park, H.Y.; Choi, M.S.; Jeong, T.S. Anti-obesity effects of soy leaf via regulation of adipogenic transcription factors and fat oxidation in diet-induced obese mice and 3T3-L1 adipocytes. J. Med. Food 2015, 18, 899–908. [Google Scholar] [CrossRef]
- Zang, Y.; Zhang, L.; Igarashi, K.; Yu, C. The anti-obesity and anti-diabetic effects of kaempferol glycosides from unripe soybean leaves in high-fat-diet mice. Food Funct. 2015, 6, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Seo, W.D.; Kang, J.E.; Choi, S.W.; Lee, K.S.; Lee, M.J.; Park, K.D.; Lee, J.H. Comparison of nutritional components (isoflavone, protein, oil, and fatty acid) and antioxidant properties at the growth stage of different parts of soybean [Glycine max (L.) Merrill]. Food Sci. Biotechnol. 2017, 26, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.L.; Kang, S.S.; Cho, K.M.; Park, K.H.; Lee, D.H. Isoflavone-enriched soybean (Glycine max) leaves prevents ovariectomy-induced obesity by enhancing fatty acid oxidation. J. Funct. Foods 2018, 43, 165–172. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, S. Vegetable soybean (Glycine max (L.) Merr.) leaf extracts: Functional components and antioxidant and anti-inflammatory activities. J. Food Sci. 2021, 86, 2468–2480. [Google Scholar] [CrossRef]
- Bakshi, A.; Shemansky, J.M.; Chang, C.; Binder, B.M. History of research on the plant hormone ethylene. J. Plant Growth Regul. 2015, 34, 809–827. [Google Scholar] [CrossRef]
- Fatma, M.; Asgher, M.; Iqbal, N.; Rasheed, F.; Sehar, Z.; Sofo, A.; Khan, N.A. Ethylene signaling under stressful environments: Analyzing collaborative knowledge. Plants 2022, 11, 2211. [Google Scholar] [CrossRef]
- Gautam, H.; Fatma, M.; Sehar, Z.; Iqbal, N.; Albaqami, M.; Khan, N.A. Exogenously-sourced ethylene positively modulates photosynthesis, carbohydrate metabolism, and antioxidant defense to enhance heat tolerance in rice. Int. J. Mol. Sci. 2022, 23, 1031. [Google Scholar] [CrossRef]
- Yuk, H.J.; Song, Y.H.; Curtis-Long, M.J.; Kim, D.W.; Woo, S.G.; Lee, Y.B.; Uddin, Z.; Kim, C.Y.; Park, K.H. Ethylene induced a high accumulation of dietary isoflavones and expression of isoflavonoid biosynthetic genes in soybean (Glycine max) leaves. J. Agric. Food Chem. 2016, 64, 7315–7324. [Google Scholar] [CrossRef]
- Qu, Z.; Tian, Y.; Zhou, X.; Li, X.; Zhou, Q.; Wang, X.; Dong, S. Effects of exogenous sodium nitroprusside spraying on physiological characteristics of soybean leaves at the flowering stage under drought stress. Plants 2023, 12, 1598. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Tian, Y.; Qu, Z.; Wang, J.; Han, D.; Dong, S. Comparing the Salt Tolerance of Different Spring Soybean Varieties at the Germination Stage. Plants 2023, 12, 2789. [Google Scholar] [CrossRef]
- Su, Y.; Yang, H.; Wu, Y.; Gong, W.; Gul, H.; Yan, Y.; Yang, W. Photosynthetic Acclimation of Shade-Grown Soybean Seedlings to a High-Light Environment. Plants 2023, 12, 2324. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Agricultural Sciences. Korean Food Composition Database. Available online: http://koreanfood.rda.go.kr/eng/fctFoodSrchEng/list (accessed on 12 March 2023).
- Sodamade, A.; Bolaji, O.S.; Adeboye, O.O. Proximate analysis, mineral contents and functional properties of Moringa oleifera leaf protein concentrate. IOSR J. Appl. Chem. 2013, 4, 47–51. [Google Scholar]
- Maisarah, A.M.; Asmah, R.; Fauziah, O. Proximate analysis, antioxidant and anti proliferative activities of different parts of Carica papaya. J. Tissue Sci. Eng. 2014, 5, 1. [Google Scholar] [CrossRef]
- Calder, P.C. Functional roles of fatty acids and their effects on human health. J. Parenter. Enter. Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef]
- He, M.; Qin, C.X.; Wang, X.; Ding, N.Z. Plant unsaturated fatty acids: Biosynthesis and regulation. Front. Plant Sci. 2020, 11, 390. [Google Scholar] [CrossRef]
- Kaur, N.; Chugh, V.; Gupta, A.K. Essential fatty acids as functional components of foods-a review. J. Food Sci. Technol. 2014, 51, 2289–2303. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.L.; Liu, M.; Zhao, H.; Meng, J.F.; Fang, Y.L. Effect of exogenous abscisic acid and methyl jasmonate on anthocyanin composition, fatty acids, and volatile compounds of Cabernet Sauvignon (Vitis vinifera L.) grape berries. Molecules 2016, 21, 1354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, C.J. Multifaceted regulations of gateway enzyme phenylalanine ammonia-lyase in the biosynthesis of phenylpropanoids. Mol. Plant 2015, 8, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Heli, Z.; Hongyu, C.; Dapeng, B.; Yee Shin, T.; Yejun, Z.; Xi, Z.; Yingying, W. Recent advances of γ-aminobutyric acid: Physiological and immunity function, enrichment, and metabolic pathway. Front. Nutr. 2022, 9, 1076223. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, W.; Tao, L.; Wei, X.; Gao, L.; Gao, Y.; Suo, J.; Yu, W.; Hu, Y.; Yang, B.; et al. Ethylene treatment promotes umami taste-active amino acids accumulation of Torreya grandis nuts post-harvest by comparative chemical and transcript analyses. Food Chem. 2023, 408, 135214. [Google Scholar] [CrossRef]
- Ban, Y.J.; Song, Y.H.; Kim, J.Y.; Cha, J.Y.; Ali, I.; Baiseitova, A.; Shah, A.B.; Kim, W.Y.; Park, K.H. A significant change in free amino acids of soybean (Glycine max L. Merr) through ethylene application. Molecules 2021, 26, 1128. [Google Scholar] [CrossRef]
- Said, H.M. Water-soluble vitamins. Nutr. Prim. Care Provid. 2015, 111, 30–37. [Google Scholar] [CrossRef]
- Yaman, M.; Çatak, J.; Uğur, H.; Gürbüz, M.; Belli, İ.; Tanyıldız, S.N.; Yıldırım, H.; Cengiz, S.; Yavuz, B.B.; Kişmiroğlu, C.; et al. The bioaccessibility of water-soluble vitamins: A review. Trends Food Sci. Technol. 2021, 109, 552–563. [Google Scholar] [CrossRef]
- Bourgin, M.; Kepp, O.; Kroemer, G. Immunostimulatory effects of vitamin B5 improve anticancer immunotherapy. Oncoimmunology 2022, 11, 2031500. [Google Scholar] [CrossRef]
- Magwaza, L.S.; Mditshwa, A.; Tesfay, S.Z.; Opara, U.L. An overview of preharvest factors affecting vitamin C content of citrus fruit. Sci. Hortic. 2017, 216, 12–21. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, B.; Li, M.; Fu, X.; Zhao, X.; Min, D.; Li, F.; Zhang, X. Ethylene pretreatment induces phenolic biosynthesis of fresh-cut pitaya fruit by regulating ethylene signaling pathway. Postharvest Biol. Technol. 2022, 192, 112028. [Google Scholar] [CrossRef]
- Wairata, J.; Fadlan, A.; Purnomo, A.S.; Taher, M.; Ersam, T. Total phenolic and flavonoid contents, antioxidant, antidiabetic and antiplasmodial activities of Garcinia forbesii King: A correlation study. Arab. J. Chem. 2022, 15, 103541. [Google Scholar] [CrossRef]
- Micronutrient Information Center. Soy Isoflavone. Available online: https://lpi.oregonstate.edu/mic/dietary-factors/phytochemicals/soy-isoflavones (accessed on 20 June 2023).
- Chen, P.; Sun, J.; Liang, Z.; Xu, H.; Du, P.; Li, A.; Meng, Y.; Reshetnik, E.I.; Liu, L.; Li, C. The bioavailability of soy isoflavones in vitro and their effects on gut microbiota in the simulator of the human intestinal microbial ecosystem. Food Res. Int. 2022, 152, 110868. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, J.W.; Martin, B.R.; McCabe, G.P.; McCabe, L.; Jackson, G.S.; Peacock, M.; Barnes, S.; Weaver, C.M. Impact of equol-producing capacity and soy-isoflavone profiles of supplements on bone calcium retention in postmenopausal women: A randomized crossover trial. Am. J. Clin. Nutr. 2015, 102, 695–703. [Google Scholar] [CrossRef]
- Bharati, A.C.; Prasad, B.; Mallick, S.; Masram, D.S.; Kumar, A.; Saxena, G.K. Animal and plant hormone. In Handbook of Biomolecules; Verma, C., Verma, D.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 1, pp. 151–175. [Google Scholar] [CrossRef]
- Choi, H.R.; Baek, M.W.; Cheol, L.H.; Jeong, C.S.; Tilahun, S. Changes in metabolites and antioxidant activities of green ‘Hayward’and gold ‘Haegeum’kiwifruits during ripening with ethylene treatment. Food Chem. 2022, 384, 132490. [Google Scholar] [CrossRef]
- Ban, Y.J.; Song, Y.H.; Kim, J.Y.; Baiseitova, A.; Lee, K.W.; Kim, K.D.; Park, K.H. Comparative investigation on metabolites changes in soybean leaves by ethylene and activation of collagen synthesis. Ind. Crops Prod. 2020, 154, 112743. [Google Scholar] [CrossRef]
- Zhang, M.Q.; Zhang, J.; Zhang, Y.T.; Sun, J.Y.; Prieto, M.A.; Simal-Gandara, J.; Putnik, P.; Li, N.Y.; Liu, C. The link between the phenolic composition and the antioxidant activity in different small berries: A metabolomic approach. LWT 2023, 182, 114853. [Google Scholar] [CrossRef]
- Wang, H.; Chen, J.; Yi, Y.; Wang, L.; Hou, W.; Ai, Y.; Wang, H.; Min, T. Regulation and mechanism of ethylene treatment on storage quality of fresh-cut lotus (Nelumbo nucifera Gaertn.) root slices. Sci. Hortic. 2023, 313, 111900. [Google Scholar] [CrossRef]
- Roheem, F.O.; Ahmed, Q.U.; So’ad, S.M.; Shah, S.A.A.; Latip, J.; Alhassan, A.M.; Mohammad, S.S. Assessment of Free radical scavenging and digestive enzyme inhibitory activities of extract, fractions and isolated compounds from Tetracera macrophylla leaves. J. Herb. Med. 2020, 22, 100351. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, H.; Tian, T.; Hou, Y.; Chen, D.; Zhou, J.; Liu, S.; Yu, Y.; Dai, W.; Zhou, T. Nontargeted and targeted metabolomics analysis for evaluating the effect of “golden flora” amount on the sensory quality, metabolites, and the alpha-amylase and lipase inhibitory activities of Fu brick tea. Food Chem. 2023, 416, 135795. [Google Scholar] [CrossRef]
- Yang, W.T.; Cho, K.M.; Lee, J.H. Comparative analysis of isoflavone aglycones using microwave-assisted acid hydrolysis from soybean organs at different growth times and screening for their digestive enzyme inhibition and antioxidant properties. Food Chem. 2020, 305, 125462. [Google Scholar] [CrossRef]
- Parimelazhagan, T.; Thangaraj, P. Proximate composition analysis. In Pharmacological Assays of Plant-Based Natural Products; Springer: Cham, Switzerland, 2016; pp. 21–31. [Google Scholar] [CrossRef]
- Lee, J.H.; Hwang, C.E.; Son, K.S.; Cho, K.M. Comparisons of nutritional constituents in soybeans during solid state fermentation times and screening for their glucosidase enzymes and antioxidant properties. Food Chem. 2019, 272, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Cho, D.Y.; Jang, K.J.; Lee, J.H.; Jung, J.G.; Kim, M.J.; Jeong, J.B.; Haque, M.A.; Cho, K.M. Changes of γ-Aminobutyric Acid, Phytoestrogens, and Biofunctional Properties of the Isoflavone-Enriched Soybean (Glycine max) Leaves during Solid Lactic Acid Fermentation. Fermentation 2022, 8, 525. [Google Scholar] [CrossRef]
- Datta, S.; Sinha, B.K.; Bhattacharjee, S.; Seal, T. Nutritional composition, mineral content, antioxidant activity and quantitative estimation of water soluble vitamins and phenolics by RP-HPLC in some lesser used wild edible plants. Heliyon 2019, 5, e01431. [Google Scholar] [CrossRef]
- Pungin, A.; Lartseva, L.; Loskutnikova, V.; Shakhov, V.; Popova, E.; Skrypnik, L.; Krol, O. Effect of Salinity Stress on Phenolic Compounds and Antioxidant Activity in Halophytes Spergularia marina (L.) Griseb. and Glaux maritima L. Cultured In Vitro. Plants 2023, 12, 1905. [Google Scholar] [CrossRef]
- Hwang, C.E.; Kim, S.C.; Lee, H.Y.; Suh, H.K.; Cho, K.M.; Lee, J.H. Enhancement of isoflavone aglycone, amino acid, and CLA contents in fermented soybean yogurts using different strains: Screening of antioxidant and digestive enzyme inhibition properties. Food Chem. 2021, 340, 128199. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Sharov, V.; White, J.; Li, J.; Liang, W.; Bhagabati, N.; Braisted, J.; Klapa, M.; Currier, T.; Thiagarajan, M.; et al. TM4: A free, open-source system for microarray data management and analysis. Biotechniques 2003, 34, 374–378. [Google Scholar] [CrossRef] [PubMed]


| Contents 1 (g/100 g) | Treatment Concentration of Ethephon 2 (μg/mL) | ||
|---|---|---|---|
| SL1 | SL2 | SL3 | |
| Moisture | 9.00 ± 0.54 a | 8.90 ± 0.53 a | 8.60 ± 0.52 a |
| Ash | 9.90 ± 0.59 a | 8.80 ± 0.53 b | 10.00 ± 0.61 a |
| Protein | 20.60 ± 1.24 b | 20.90 ± 1.25 b | 25.00 ± 1.50 a |
| Fat | 3.30 ± 0.20 b | 5.30 ± 0.32 a | 3.40 ± 0.19 b |
| Carbohydrate | 57.20 ± 3.43 a | 56.10 ± 3.37 ab | 53.00 ± 3.18 b |
| Contents 1 (mg/100 g) | Treatment Concentration of Ethephon 3 (μg/mL) | ||
|---|---|---|---|
| SL1 | SL2 | SL3 | |
| B2 (Riboflavin) | 0.10 ± 0.01 a | nd 2 | 0.10 ± 0.01 a |
| B3 (Niacin) | 21.90 ± 1.31 a | 8.80 ± 0.53 c | 14.30 ± 0.86 b |
| B5 (Pantothenic acid) | 215.70 ± 12.94 b | 206.00 ± 12.36 b | 431.00 ± 25.86 a |
| B9 (Folic acid) | 8.80 ± 0.53 c | 12.30 ± 0.74 b | 15.40 ± 0.92 a |
| C (Ascorbic acid) | 0.20 ± 0.01 c | 17.60 ± 1.06 b | 40.80 ± 2.45 a |
| Total | 246.70 | 244.70 | 501.60 |
| Contents 1 (μg/g) | Treatment Concentration of Ethephon 2 (μg/mL) | ||
|---|---|---|---|
| SL1 | SL2 | SL3 | |
| Glycosides | |||
| Daidzin (1) | 279.08 ± 18.01 c | 1652.33 ± 99.14 b | 2076.14 ± 124.57 a |
| Genistin (2) | 216.10 ± 12.97 c | 831.35 ± 49.88 b | 1664.39 ± 99.86 a |
| Total | 495.18 | 2483.67 | 3740.54 |
| Malonylglycosides | |||
| Daidzin (3) | 354.12 ± 21.25 c | 3264.16 ± 195.85 b | 5673.07 ± 340.38 a |
| Genistin (4) | 505.07 ± 30.30 c | 1896.63 ± 113.80 b | 5238.65 ± 314.32 a |
| Total | 859.20 | 5160.79 | 10,911.73 |
| Aglycones | |||
| Daidzein (5) | 33.58 ± 2.01 c | 117.23 ± 7.03 b | 208.62 ± 12.52 a |
| Genistein (6) | 42.15 ± 2.53 b | 44.73 ± 2.68 b | 107.11 ± 6.43 a |
| Total | 75.73 | 161.96 | 315.73 |
| Total isoflavones | 1430.11 | 7806.42 | 14,968.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.H.; Cho, D.Y.; Jang, K.J.; Jeong, J.B.; Lee, G.Y.; Jang, M.Y.; Son, K.H.; Lee, J.H.; Lee, H.Y.; Cho, K.M. Changes in Nutrient Components and Digestive Enzymatic Inhibition Activities in Soy Leaves by Ethephon Treatment. Plants 2023, 12, 3640. https://doi.org/10.3390/plants12203640
Lee JH, Cho DY, Jang KJ, Jeong JB, Lee GY, Jang MY, Son KH, Lee JH, Lee HY, Cho KM. Changes in Nutrient Components and Digestive Enzymatic Inhibition Activities in Soy Leaves by Ethephon Treatment. Plants. 2023; 12(20):3640. https://doi.org/10.3390/plants12203640
Chicago/Turabian StyleLee, Ji Ho, Du Yong Cho, Kyeong Jin Jang, Jong Bin Jeong, Ga Young Lee, Mu Yeun Jang, Ki Ho Son, Jin Hwan Lee, Hee Yul Lee, and Kye Man Cho. 2023. "Changes in Nutrient Components and Digestive Enzymatic Inhibition Activities in Soy Leaves by Ethephon Treatment" Plants 12, no. 20: 3640. https://doi.org/10.3390/plants12203640
APA StyleLee, J. H., Cho, D. Y., Jang, K. J., Jeong, J. B., Lee, G. Y., Jang, M. Y., Son, K. H., Lee, J. H., Lee, H. Y., & Cho, K. M. (2023). Changes in Nutrient Components and Digestive Enzymatic Inhibition Activities in Soy Leaves by Ethephon Treatment. Plants, 12(20), 3640. https://doi.org/10.3390/plants12203640