Edible Fruits from the Ecuadorian Amazon: Ethnobotany, Physicochemical Characteristics, and Bioactive Components
Abstract
1. Introduction
2. Results
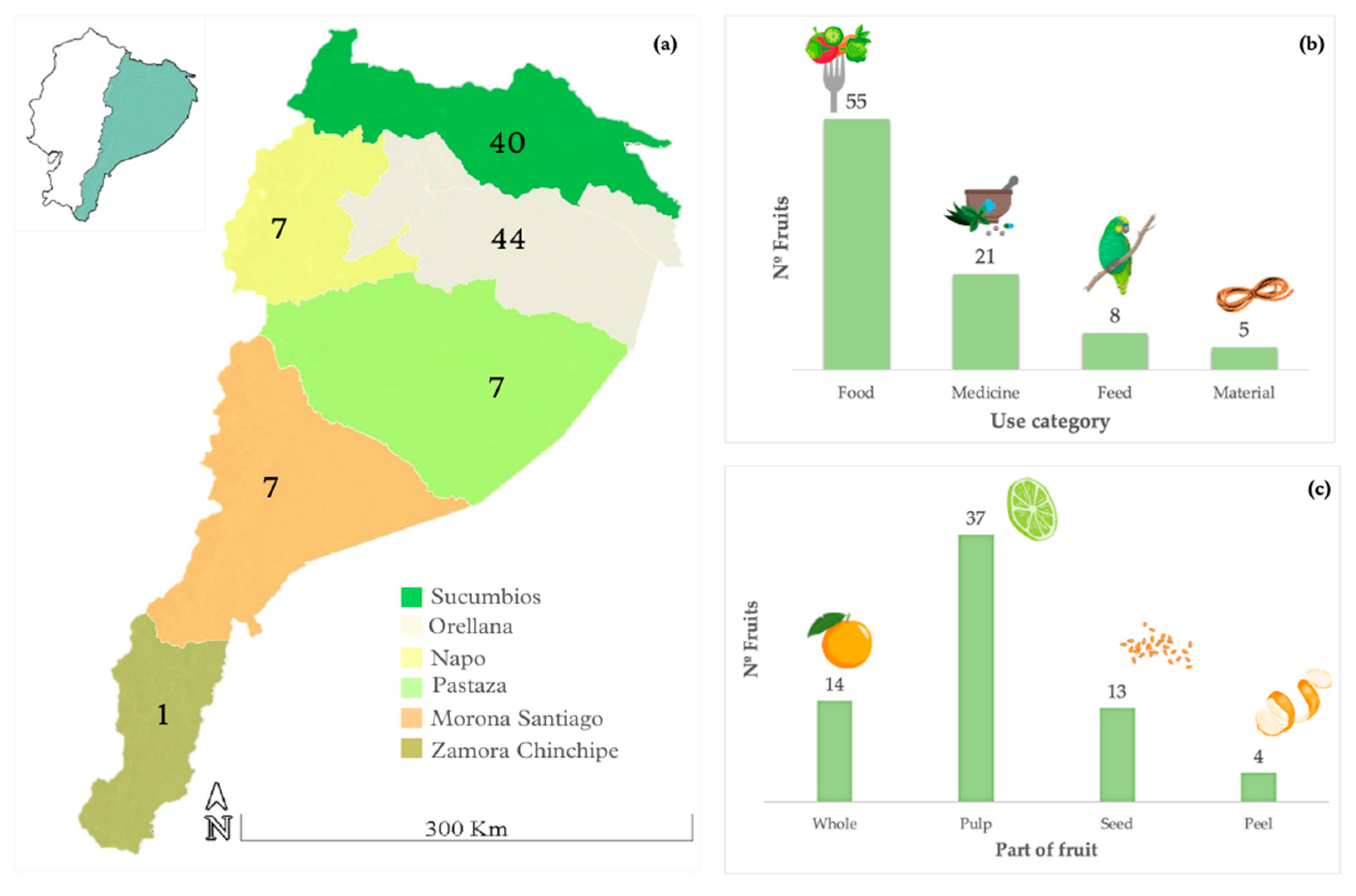
2.1. Ethnobotany
2.2. Physicochemical Characterization
2.3. Bioactive Compounds and Antioxidant Capacity
3. Discussion
3.1. Ethnobotany
3.2. Physicochemical Characterization
3.3. Bioactive Compounds and Antioxidant Capacity
4. Materials and Methods
4.1. Study Area
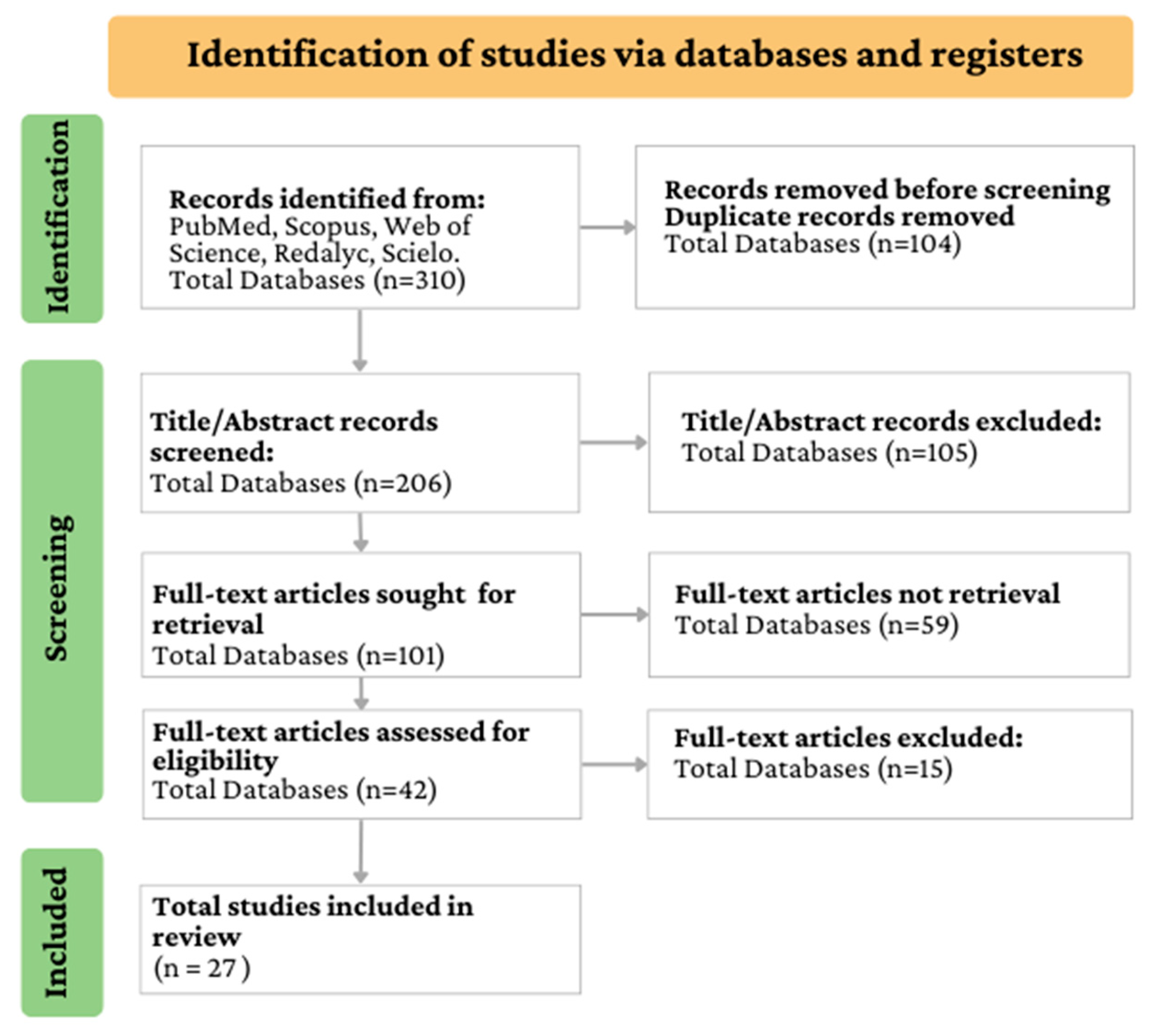
4.2. The Systematic Literature Review
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Charity, S.; Dudley, N.; Oliveira, D.; Stolton, S. Amazonía viva—Informe 2016: Un Enfoque Regional para la Conservación en la Amazonía, 1st ed.; WWF—Fondo Mundial para la Naturaleza: Morges, Switzerland, 2016; Volume 1. [Google Scholar]
- Codeço, C.T.; Dal’Asta, A.P.; Rorato, A.C.; Lana, R.M.; Neves, T.C.; Andreazzi, C.S.; Barbosa, M.; Escada, M.I.S.; Fernandes, D.A.; Rodrigues, D.L.; et al. Epidemiology, biodiversity, and technological trajectories in the Brazilian Amazon: From malaria to COVID-19. Front. Public Health 2021, 9, 647754. [Google Scholar] [CrossRef] [PubMed]
- Organización Del Trabajo de Cooperación Amazónica (OTCA). Available online: http://otca.org/wp-content/uploads/2021/09/biomaz_documento_evaluacion_ES.pdf (accessed on 8 October 2023).
- Marcovitch, J.; Pinsky, V.C. Amazon fund: Financing deforestation avoidance. Rev. Adm. 2014, 49, 280–290. [Google Scholar] [CrossRef]
- Noronha Matos, K.A.; Praia Lima, D.; Pereira Barbosa, A.P.; Zerlotti Mercadante, A.; Campos Chisté, R. Peels of Tucumã (Astrocaryum vulgare) and peach palm (Bactris gasipaes) are by-products classified as very high carotenoid sources. Food Chem. 2019, 272, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nation (FAO). Perspectivas a Mediano Plazo: Perspectivas para la Producción y el Comercio Mundial de Bananos y Frutas Tropicales 2019–2028; FAO: Rome, Italy, 2020. [Google Scholar]
- Vargas-Tierras, Y.B.; Prado-Beltran, J.K.; Nicolalde-Cruz, J.R.; Casanoves, F.; Virginio-Filho, E.D.; Viera-Arroyo, W.F. Characterization and role of Amazonian fruit crops in family farms in the provinces of Sucumbios and Orellana (Ecuador). Corpoica-Cienc. Y Tecnol. Agropecu. 2018, 19, 501–516. [Google Scholar] [CrossRef]
- Machado, C.L.R.; Crespo-Lopez, M.E.; Augusto-Oliveira, M.; de Paula Arrifano, G.; de Matos Macchi, B.; Lopes-Araújo, A.; Santos-Sacramento, L.; Souza-Monteiro, J.R.; Alvarez-Leite, J.I.; de Souza, C.B.A. Eating in the Amazon: Nutritional status of the riverine populations and possible nudge interventions. Foods 2021, 10, 1015. [Google Scholar] [CrossRef]
- Guevara, M.; Tejera, E.; Granda-Albuja, M.G.; Iturralde, G.; Chisaguano-Tonato, M.; Granda-Albuja, S.; Jaramillo-Vivanco, T.; Giampieri, F.; Battino, M.; Alvarez-Suarez, J.M. Chemical composition and antioxidant activity of the main fruits consumed in the western coastal region of Ecuador as a source of health-promoting compounds. Antioxidants 2019, 8, 387. [Google Scholar] [CrossRef]
- Sayago-Ayerdi, S.; García-Martínez, D.L.; Ramírez-Castillo, A.C.; Ramírez-Concepción, H.R.; Viuda-Martos, M. Tropical fruits and their co-products as bioactive compounds and their health effects: A review. Foods 2021, 10, 1952. [Google Scholar] [CrossRef]
- Pereira Barbosa, A.P.; Moraes, A.F.; Chiste, R.C. Physicochemical characterization and quantification of bioactive compounds of Antrocaryon amazonicum fruits cultivated in Brazilian Amazonia. Cyta-J. Food 2020, 18, 616–623. [Google Scholar] [CrossRef]
- Mishra, D.S.; Berwal, M.K.; Singh, A.; Singh, A.K.; Rao, V.V.A.; Yadav, V.; Sharma, B.D. Phenotypic diversity for fruit quality traits and bioactive compounds in red-fleshed Guava: Insights from multivariate analyses and machine learning algorithms. South Afr. J. Bot. 2022, 149, 591–603. [Google Scholar] [CrossRef]
- Costa Lima, P.G.; Coelho–Ferreira, M.; Da Silva Santos, R. Perspectives on medicinal plants in public markets across the Amazon: A review. Econ. Bot 2016, 70, 64–78. [Google Scholar] [CrossRef]
- Stagegaard, J.; Sørensen, M.; Kvist, L.P. Estimations of the importance of plant resources extracted by inhabitants of the Peruvian Amazon flood plains. Perspect. Plant Ecol. Evol. Syst. 2002, 5, 103–122. [Google Scholar] [CrossRef]
- Katz, E.; López, C.L.; Fleury, M.; Miller, R.P.; Payê, V.; Dias, T.; Silva, F.; Oliveira, Z.; Moreira, E. No greens in the forest? note on the limited consumption of greens in the Amazon. Acta Soc. Bot. Pol. 2012, 81, 4. [Google Scholar] [CrossRef]
- Macía, M.J.; Armesilla, P.J.; Cámara-Leret, R.; Paniagua-Zambrana, N.; Villalba, S.; Balslev, H.; Pardo-de-Santayana, M. Palm uses in northwestern South America: A quantitative review. Bot. Rev. 2011, 77, 462–570. [Google Scholar] [CrossRef]
- Viafara, D.; Abreu-Naranjo, R.; Alvarez-Suarez, J.M.; Reyes-Mera, J.J.; Barreno-Ayala, M. Chemical characterisation and antioxidant activity of Aphandra natalia mesocarp and its oil from the Amazon region of Ecuador. J. Food Meas. Charact. 2018, 12, 2835–2843. [Google Scholar] [CrossRef]
- Kronborg, M.; Grández, C.A.; Ferreira, E.; Balslev, H. Aphandra natalia (arecaceae)-a little known source of piassaba fibers from the western Amazon. Rev. Peru. Biol. 2008, 15, 103–113. [Google Scholar] [CrossRef][Green Version]
- Pedersen, H.B. Production and harvest of fibers from Aphandra natalia (palmae) in Ecuador. Ecol. Manag. 1996, 80, 155–161. [Google Scholar] [CrossRef]
- Paniagua-Zambrana, N.; Cámara-Leret, R.; Macía, M.J. Patterns of medicinal use of palms across Northwestern South America. Bot. Rev. 2015, 81, 317–415. [Google Scholar] [CrossRef]
- Jaramillo-Vivanco, T.; Balslev, H.; Montúfar, R.; Cámara, R.M.; Giampieri, F.; Battino, M.; Cámara, M.; Alvarez-Suarez, J.M. Three Amazonian palms as underestimated and little-known sources of nutrients, bioactive compounds and edible insects. Food Chem. 2022, 372, 131273. [Google Scholar] [CrossRef]
- González-Jaramillo, N.; Bailon-Moscoso, N.; Duarte-Casar, R.; Romero-Benavides, J.C. Peach palm (Bactris gasipaes Kunth.): Ancestral tropical staple with future potential. Plants 2022, 11, 3134. [Google Scholar] [CrossRef]
- Innerhofer, S.; Bernhardt, K.-G. Ethnobotanic garden design in the Ecuadorian Amazon. Biodivers. Conserv. 2011, 20, 429–439. [Google Scholar] [CrossRef]
- Arellano-Acuna, E.; Rojas-Zavaleta, I.; Maria Paucar-Menacho, L. Camu-Camu (Myrciaria dubia): Tropical fruit of excellent functional that help to improve the quality of life. Sci. Agropecu. 2016, 7, 433–443. [Google Scholar] [CrossRef]
- Rivera, M.; Ramos, M.; Silva, M.; Briceno, J.; Alvarez, M. Effect of the temperature prior to extraction on the yield and fatty acid profile of Morete oil (Mauritia flexuosa L.F.). Granja-Rev. Cienc. Vida 2022, 35, 98–111. [Google Scholar] [CrossRef]
- Miller, C. Fruit production of the Ungurahua palm (Oenocarpus bataua subsp bataua, Arecaceae) in an indigenous managed reserve. Econ. Bot 2002, 56, 165–176. [Google Scholar] [CrossRef]
- Méndez-Durazno, C.; Cisneros-Perez, P.A.; Loja-Ojeda, B.A.; Monge-Sevilla, R.; Romero-Estévez, D.; Fernández, L.; Espinoza-Montero, P.J. Antioxidant capacity through electrochemical methods and chemical composition of Oenocarpus Bataua and Gustavia Macarenensis from the Ecuadorian Amazon. Antioxidants 2023, 12, 318. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, P. Medicinal plants of the Achuar (Jivaro) of Amazonian Ecuador: Ethnobotanical survey and comparison with other Amazonian pharmacopoeias. J. Ethnopharmacol. 2015, 164, 78–88. [Google Scholar] [CrossRef]
- Ponce-Sanchez, J.; Zurita-Benavides, M.G.; Penuela, M.C. Reproductive ecology of white Cacao (Theobroma bicolor Humb. & Bonpl.) in Ecuador, Western Amazonia: Floral visitors and the impact of fungus and mistletoe on fruit production. Braz. J. Bot. 2021, 44, 479–489. [Google Scholar] [CrossRef]
- Sanchez-Capa, M.; Viteri-Sanchez, S.; Burbano-Cachiguango, A.; Abril-Donoso, M.; Vargas-Tierras, T.; Suarez-Cedillo, S.; Mestanza-Ramón, C. New characteristics in the fermentation process of cocoa (Theobroma cacao L.) “Super Arbol” in La Joya de Los Sachas, Ecuador. Sustainability 2022, 14, 7564. [Google Scholar] [CrossRef]
- Herrera, R.; Vasquez, S.C.; Granja, F.; Molina-Muller, M.; Capa-Morocho, M.; Guaman, A.O. Interaction of N, P and K on soil characteristics, growth and quality of cocoa sprouts and fruits in Ecuadorian Amazon. Bioagro 2022, 34, 277–288. [Google Scholar] [CrossRef]
- Llerena, W.; Samaniego, I.; Angós, I.; Brito, B.; Ortiz, B.; Carrillo, W. Biocompounds content prediction in Ecuadorian fruits using a mathematical model. Foods 2019, 8, 284. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, A.T.; Burgos, J.C.V.; Quintana, Y.G.; Crespo, Y.A. Partial chemical composition, and morphometric and sensory characteristics of organically and conventionally produced fruits of Naranjilla (Solanum quitoense var. Palora) in the Wamani community, Ecuadorian Amazon. Interciencia 2018, 43, 115–119. [Google Scholar]
- Vargas Tierras, Y.; Díaz Martínez, A.; Congo Yépez, C.; Tinoco Jaramillo, L.; Viera Arroyo, W. Comparison of fruit quality traits of Papaya (Carica papaya L.) genotypes from Shushufindi and La Joya de Los Sachas, Ecuador. Corpoica-Cienc. Y Tecnol. Agropecu. 2021, 22, 5–10. [Google Scholar] [CrossRef]
- Valarezo, E.; Ojeda-Riascos, S.; Cartuche, L.; Andrade-Gonzalez, N.; Gonzalez-Sanchez, I.; Meneses, M.A. Extraction and study of the essential oil of Copal (Dacryodes peruviana), an Amazonian Fruit with the highest yield worldwide. Plants 2020, 9, 1658. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Tierras, Y.; Diaz, A.; Caicedo, C.; Macas, J.; Suarez-Tapia, A.; Viera, W. Benefits of legume species in an agroforestry production system of yellow Pitahaya in the Ecuadorian Amazon. Sustainability 2021, 13, 9261. [Google Scholar] [CrossRef]
- Santamaría-Aguilar, D.; Pérez, Á.J.; Liesner, R.L. Mayna yasuniana (Achariaceae), una especie nueva para el Ecuador. Neotrop. Biodivers. 2017, 3, 50–56. [Google Scholar] [CrossRef]
- Weckmüller, H.; Barriocanal, C.; Maneja, R.; Boada, M. Factors affecting traditional medicinal plant knowledge of the Waorani, Ecuador. Sustainability 2019, 11, 4460. [Google Scholar] [CrossRef]
- Kodahl, N. Sacha Inchi (Plukenetia volubilis L.)-from lost crop of the incas to part of the solution to global challenges? Planta 2020, 251, 80. [Google Scholar] [CrossRef]
- Fukalova-Fukalova, T.; Castillo, J.; Parreño, K.; Gaibor, M.; Londoño-Larrea, P. Preliminary studies of performance and lipid profiles of Ecuadorian P. volubilis L. as contribution to agricultural innovation. In Smart Innovation, Systems and Technologies; Springer: Singapore, 2022; Volume 252, pp. 151–163. [Google Scholar]
- Abreu-Naranjo, R.; Paredes-Moreta, J.G.; Granda-Albuja, G.; Iturralde, G.; González-Paramás, A.M.; Alvarez-Suarez, J.M. Bioactive compounds, phenolic profile, antioxidant capacity and effectiveness against lipid peroxidation of cell membranes of Mauritia flexuosa L. fruit extracts from three biomes in the Ecuadorian Amazon. Heliyon 2020, 6, e05211. [Google Scholar] [CrossRef]
- Mera, J.J.R.; Abreu-Naranjo, R.; Alvarez-Suarez, J.M.; Viafara, D. Chemical characterization, fatty acid profile and antioxidant activity of Gustavia macarenensis fruit mesocarp and its oil from the Amazonian region of Ecuador as an unconventional source of vegetable oil. Grasas Y Aceites 2019, 70, e298. [Google Scholar] [CrossRef]
- Llerena, W.; Samaniego, I.; Navarro, M.; Ortíz, J.; Angós, I.; Carrillo, W. Effect of modified atmosphere packaging (MAP) in the antioxidant capacity of Arazá (Eugenia stipitata McVaugh), Naranjilla (Solanum quitoense Lam.), and Tree Tomato (Solanum betaceum Cav.) fruits from Ecuador. J. Food Process. Preserv. 2020, 44, e14757. [Google Scholar] [CrossRef]
- De Araújo, F.F.; Neri-Numa, I.A.; de Paulo Farias, D.; da Cunha, G.R.M.C.; Pastore, G.M. Wild Brazilian species of Eugenia genera (Myrtaceae) as an innovation hotspot for food and pharmacological purposes. Food Res. Int. 2019, 121, 57–72. [Google Scholar] [CrossRef]
- Almeida, R.D.F.; Peters, L.P.; Carvalho, C.M. Nutritional composition and biological activities of Amazonian fruits Araca-Boi, Bacaba, Buriti, Caja and Pataua—A literature review. Rbone-Rev. Bras. Obesidade Nutr. E Emagrecimento 2023, 17, 130–147. [Google Scholar]
- Avila-Sosa, R.; Montero-Rodríguez, A.F.; Aguilar-Alonso, P.; Vera-López, O.; Lazcano-Hernández, M.; Morales-Medina, J.C.; Navarro-Cruz, A.R. Antioxidant properties of Amazonian fruits: A mini review of in vivo and in vitro studies. Oxid. Med. Cell. Longev. 2019, 2019, 8204129. [Google Scholar] [CrossRef] [PubMed]
- Rosan Fortunato Seixas, F.; Kempfer Bassoli, B.; Borghi Virgolin, L.; Chancare Garcia, L.; Soares Janzantti, N. Physicochemical properties and effects of fruit pulps from the Amazon biome on physiological parameters in rats. Nutrients 2021, 13, 1484. [Google Scholar] [CrossRef]
- Toledo Romanienko, D.A. Determinación del Valor Nutritivo y Funcional de Tres Clones Seleccionados de Arazá (Eugenia stipitata) y seis de Borojó (Borojoa patinoi), y Evaluación del Proceso para la Obtención de Pulpas Pasteurizadas y Congeladas. Bachelor Thesis, Escuela Politécnica Nacional, Quito, Ecuador, 2009. [Google Scholar]
- de Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Dias-Audibert, F.L.; Delafiori, J.; de Souza, F.G.; Catharino, R.R.; do Sacramento, C.K.; Pastore, G.M. Chemical characterization of Eugenia stipitata: A native fruit from the Amazon rich in nutrients and source of bioactive compounds. Food Res. Int. 2021, 139, 109904. [Google Scholar] [CrossRef] [PubMed]
- Neri-Numa, I.A.; Carvalho-Silva, L.B.; Morales, J.P.; Malta, L.G.; Muramoto, M.T.; Ferreira, J.E.M.; de Carvalho, J.E.; Ruiz, A.L.T.G.; Maróstica Junior, M.R.; Pastore, G.M. Evaluation of the antioxidant, antiproliferative and antimutagenic potential of Araçá-boi fruit (Eugenia stipitata Mc Vaugh—Myrtaceae) of the Brazilian Amazon Forest. Food Res. Int. 2013, 50, 70–76. [Google Scholar] [CrossRef]
- Gonzalez Collaguazo, J.; Luzuriaga Gutierrez, V. Estudio Botánico y Fitoquímico del Fruto de Gustavia Macarenensis, de la Provincia de Orellana, Ecuador. Bachelor Thesis, Universidad Politecnica Saleciana, Quito, Ecuador, 2020. [Google Scholar]
- Hamacek, F.R.; Della Lucia, C.M.; da Silva, B.P.; Moreira, A.V.B.; Pinheiro-Sant’ana, H.M. Buriti of the Cerrado of Minas Gerais, Brazil: Physical and chemical characterization and content of carotenoids and vitamins. Food Sci. Technol. 2018, 38, 263–269. [Google Scholar] [CrossRef]
- Pereira-Freire, J.A.; de Souza Aquino, J.; Nascimento Campos, A.R.; Freitas Viana, V.G.; da Costa Júnior, J.S.; do Nascimento Silva, J.; Moura, A.K.S.; Citó, A.M.D.G.L.; dos Reis Moreira-Araújo, R.S.; Frota, K.D.M.G.; et al. Nutritional, physicochemical and structural parameters of Mauritia flexuosa fruits and by-products for biotechnological exploration of sustainable goods. Food Technol. Biotechnol. 2022, 60, 155–165. [Google Scholar] [CrossRef]
- Freitas, C.A.B.; Silva, A.S.; Alves, C.N.; Nascimento, W.M.O.; Lopes, A.S.; Lima, M.O.; Muller, R.C.S. Characterization of the fruit pulp of Camu-camu (Myrciaria dubia) of seven different genotypes and their rankings using statistical methods PCA and HCA. J. Braz. Chem. Soc. 2016, 27, 1838–1846. [Google Scholar] [CrossRef]
- Barrios Renteria, J.C.; Espinoza-Espinoza, L.A.; Valdiviezo-Marcelo, J.; Moreno-Quispe, L.A. Sensorially accepted Mangifera indica and Myrciaria dubia yogurts with high ascorbic acid content. Front. Sustain. Food Syst. 2022, 6, 999400. [Google Scholar] [CrossRef]
- Londoño Hernández, L.; Montalvo Rodriguez, C.; Arroyave Sierra, O.J.; Garcia Gonzalez, E. Potential use of Camu-Camu (Myrciaria dubia) in functional food development. Rev. Colomb. Investig. Agroindustriales 2022, 9, 26–41. [Google Scholar] [CrossRef]
- Grigio, M.L.; de Moura, E.A.; Chagas, E.A.; Durigan, M.F.B.; Chagas, P.C.; de Carvalho, G.F.; Zanchetta, J.J. Bioactive compounds in and antioxidant activity of Camu-camu fruits harvested at different maturation stages during postharvest storage. Acta Sci.-Agron. 2021, 43, e50997. [Google Scholar] [CrossRef]
- García-Chacón, J.M.; Marín-Loaiza, J.C.; Osorio, C. Camu camu (Myrciaria dubia (Kunth) McVaugh): An Amazonian Fruit with biofunctional properties–A review. ACS Omega 2023, 8, 5169–5183. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.F.G.; Marmesat, S.; Brito, E.S.; Alves, R.E.; Dobarganes, M.C. Major components in oils obtained from Amazonian palm fruits. Grasas Y Aceites 2013, 64, 328–334. [Google Scholar] [CrossRef]
- Hidalgo, P.S.P.; Nunomura, R.D.S.; Nunomura, S.M. Amazon oilseeds: Chemistry and antioxidant activity of Patawa (Oenocarpus bataua Mart.). Rev. Virtual Química 2016, 8, 130–140. [Google Scholar] [CrossRef]
- Carrillo, W.; Quinteros, M.F.; Carpio, C.; Morales, D.; Vásquez, G.; Álvarez, M.; Silva, M. Identification of fatty acids in Sacha Inchi oil (Plukenetia volubilis L.) from Ecuador. Asian J. Pharm. Clin. Res. 2018, 11, 379–381. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, F.; Kakuda, Y. Sacha Inchi (Plukenetia volubilis L.): Nutritional composition, biological activity, and uses. Food Chem. 2018, 265, 316–328. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, Y.K.; Zhang, P.; Na, Z.; Tang, T.; Shi, Y.X. Chemical composition and oxidative evolution of Sacha Inchi (Plukentia volubilis L.) oil from Xishuangbanna (China). Grasas Y Aceites 2014, 65, e012. [Google Scholar] [CrossRef]
- Gutierrez, L.F.; Rosada, L.M.; Jimenez, A. Chemical composition of Sacha Inchi (Plukenetia volubilis L.) seeds and characteristics of their lipid fraction. Grasas Y Aceites 2011, 62, 76–83. [Google Scholar] [CrossRef]
- Rawdkuen, S.; Murdayanti, D.; Ketnawa, S.; Phongthai, S. Chemical properties and nutritional factors of pressed-cake from tea and Sacha Inchi seeds. Food Biosci. 2016, 15, 64–71. [Google Scholar] [CrossRef]
- Canuto, G.A.B.; Xavier, A.A.O.; Leandro, C.N.; de Benassi, M.T. Physical and chemical characterization of fruit pulps from Amazonia and their correlation to free radical scavenger activity. Rev. Bras. Frutic. 2010, 32, 1196–1205. [Google Scholar] [CrossRef]
- Virgolin, L.B.; Seixas, F.R.F.; Janzantti, N.S. Composition, content of bioactive compounds, and antioxidant activity of fruit pulps from the Brazilian Amazon biome. Pesqui. Agropecu. Bras. 2017, 52, 933–941. [Google Scholar] [CrossRef]
- Gancel, A.-L.; Alter, P.; Dhuique-Mayer, C.; Ruales, J.; Vaillant, F. identifying carotenoids and phenolic compounds in Naranjilla (Solanum quitoense Lam. var. Puyo hybrid), an Andean Fruit. J. Agric. Food Chem. 2008, 56, 11890–11899. [Google Scholar] [CrossRef] [PubMed]
- Mertz, C.; Gancel, A.L.; Gunata, Z.; Alter, P.; Dhuique-Mayer, C.; Vaillant, F.; Perez, A.M.; Ruales, J.; Brat, P. Phenolic compounds, carotenoids and antioxidant activity of three tropical fruits. J. Food Compos. Anal. 2009, 22, 381–387. [Google Scholar] [CrossRef]
- Balslev, H.; Knudsen, T.R.; Byg, A.; Kronborg, M.; Grandez, C. Traditional knowledge, use, and management of Aphandra natalia (Arecaceae) in Amazonian Peru. Econ. Bot 2010, 64, 55–67. [Google Scholar] [CrossRef]
- Balslev, H.; Navarrete, H.; De la Torre, L.; Macía, M. Enciclopedia de las plantas útiles del Ecuador. Herb. QCA Herb. AAU Quito Aarhus 2008, 947, 1–949p. [Google Scholar]
- Boll, T.; Svenning, J.-C.; Vormisto, J.; Normand, S.; Grández, C.; Balslev, H. spatial distribution and environmental preferences of the Piassaba palm Aphandra natalia (Arecaceae) along the Pastaza and Urituyacu rivers in Peru. Ecol. Manag. 2005, 213, 175–183. [Google Scholar] [CrossRef]
- Zillo, R.R.; da Silva, P.P.M.; Spoto, M.H.F.; Martin, J.G.P. Camu-Camu harvested with reddish-green peel preserves its physicochemical characteristics and antioxidant compounds during cold storage. Braz. J. Food Technol. 2019, 22, e2017060. [Google Scholar] [CrossRef]
- Van der Hoek, Y.; Álvarez Solas, S.; Peñuela, M.C. The palm Mauritia flexuosa, a keystone plant resource on multiple fronts. Biodivers. Conserv. 2019, 28, 539–551. [Google Scholar] [CrossRef]
- Quintero-Angel, M.; Martínez-Girón, J.; Orjuela-Salazar, S. Agroindustrial valorization of the pulp and peel, seed, flour, and oil of Moriche (Mauritia flexuosa) from the Bita river, Colombia: A potential source of essential fatty acids. Biomass Convers Biorefin 2023, 13, 10789–10797. [Google Scholar] [CrossRef]
- do Nascimento Silva, N.R.R.; Cavalcante, R.B.M.; da Silva, F.A. Nutritional properties of Buriti (Mauritia flexuosa) and health benefits. J. Food Compos. Anal. 2023, 117, 105092. [Google Scholar] [CrossRef]
- Barboza, N.L.; da Cruz, J.M.; Corrêa, R.F.; Lamarão, C.V.; Lima, A.R.; Inada, N.M.; Sanches, E.A.; de Araújo Bezerra, J.; Campelo, P.H. Buriti (Mauritia flexuosa L. f.): An Amazonian fruit with potential health benefits. Food Res. Int. 2022, 159, 111654. [Google Scholar] [CrossRef] [PubMed]
- Isaza, C.; Martorell, C.; Cevallos, D.; Galeano, G.; Valencia, R.; Balslev, H. Demography of Oenocarpus bataua and implications for sustainable harvest of its fruit in Western Amazon. Popul. Ecol. 2016, 58, 463–476. [Google Scholar] [CrossRef]
- Mosquera, T.; Noriega, P.; Tapia, W.; Perez, S.H. Effectiveness evaluation of cosmetic creams from vegetal Amazon olis: Mauritia flexuosa (Morete) Plukenetia volubilis (Sacha Inchi) and Oenocarpus bataua (Ungurahua). Granja-Rev. Cienc. Vida 2012, 16, 14–22. [Google Scholar] [CrossRef]
- Cordero-Clavijo, L.M.; Serna-Saldívar, S.O.; Lazo-Vélez, M.A.; González, J.F.A.-; Panata-Saquicilí, D.; Briones-Garcia, M. Characterization, functional and biological value of protein-enriched defatted meals from Sacha Inchi (Plukenetia volubilis) and Chocho (Lupinus mutabilis). J. Food Meas. Charact. 2021, 15, 5071–5077. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A. Synthesis of silver nanoparticles using Sacha Inchi (Plukenetia volubilis L.) leaf extracts. Saudi J. Biol. Sci. 2014, 21, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Luis Eduardo;, R.H.; Valdiviezo Rogel, C.J.; Bonilla Bermeo, S.M. Caracterización del aceite de la semilla de Sacha Inchi (Plukenetia volubilis) del cantón San Vicente, Manabí, Ecuador, obtenida mediante procesos no térmicos de extrusión. Granja-Rev. Cienc. Vida 2019, 30, 77–87. [Google Scholar] [CrossRef]
- Abreu, M.M.; Nobrega, P.D.A.; Sales, P.F.; De Oliveira, F.R.; Nascimento, A.A. Antimicrobial and antidiarrheal activities of methanolic fruit peel extract of Pouteria caimito. Pharmacogn. J. 2019, 11, 944–950. [Google Scholar] [CrossRef]
- Alsaif, M.A.; Veeramani, C.; El Newehy, A.S.; Aloud, A.A.; Al-Numair, K.S. Pouteria caimito fruit derived nanoparticles inhibited the Apple ring rot disease as well as extended the shelf-life of sliced apples. Saudi J. Biol. Sci. 2023, 30, 103744. [Google Scholar] [CrossRef]
- Lim, T.K. Pouteria caimito. In Edible Medicinal And Non-Medicinal Plants: Volume 6, Fruits; Lim, T.K., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 129–132. ISBN 978-94-007-5628-1. [Google Scholar]
- Susanto, S.; Bin Arif, A.; Widayanti, S.M.; Matra, D.D. Ascorbic acid extends the shelf-life of Abiu (Pouteria caimito) fruit by maintaining quality and delaying browning symptoms. Hort. J. 2023, 92, 216–226. [Google Scholar] [CrossRef]
- Ramírez, F. Notes about Lulo (Solanum quitoense Lam.): An important South American underutilized Plant. Genet. Resour. Crop Evol. 2021, 68, 93–100. [Google Scholar] [CrossRef]
- Cornejo-Franco, J.F.; Alvarez-Quinto, R.A.; Grinstead, S.; Mollov, D.; Karasev, A.V.; Ochoa, J.; Quito-Avila, D.F. A new Tymovirus isolated from Solanum quitoense: Characterization and prevalence in two Solanaceous crops in Ecuador. Plant Dis. 2019, 103, 2246–2251. [Google Scholar] [CrossRef]
- Chacon, W.D.C.; Valencia, G.A.; Rojas, G.M.A.; Henao, A.C.A. Drying and pyrolysis of Lulo peel: Non-isothermal analysis of physicochemical, kinetics, and master plots. Bioenergy Res. 2020, 13, 927–938. [Google Scholar] [CrossRef]
- Vargas, Y.; Viera, W.; Díaz, A.; Tinoco, L.; Macas, J.; Caicedo, C.; Almeida, M.; Vásquez-Castillo, W. Contribution of agroforestry systems in the cultivation of Naranjilla (Solanum quitoense) grown in the Amazon region of Ecuador. Appl. Sci. 2022, 12, 10637. [Google Scholar] [CrossRef]
- Woods, F.M.; Dozier, W.A.; Ebel, R.C.; Thomas, R.H.; Nesbitt, M.; Wilkins, B.S.; Himelrick, D.G. Fruit quality and antioxidant properties in Alabama-grown blackberries during fruit maturation. Int. J. Fruit Sci. 2007, 6, 67–85. [Google Scholar] [CrossRef]
- Schauss, A.G. Chapter 32—Açaí (Euterpe Oleracea Mart.): A macro and nutrient rich palm fruit from the Amazon rain forest with demonstrated bioactivities in vitro and in vivo. In Bioactive Foods in Promoting Health; Watson, R.R., Preedy, V.R., Eds.; Academic Press: San Diego, CA, USA, 2010; pp. 479–490. ISBN 978-0-12-374628-3. [Google Scholar]
- Rudke, A.R.; Andrade, K.S.; Mazzutti, S.; Zielinski, A.A.F.; Alves, V.R.; Vitali, L.; Ferreira, S.R.S. A comparative study of phenolic compounds profile and in vitro antioxidant activity from Buriti (Mauritia flexuosa) by-Products Extracts. LWT 2021, 150, 111941. [Google Scholar] [CrossRef]
- Azevedo, L.; Ribeiro, P.F.D.; Oliveira, J.A.D.; Correia, M.G.; Ramos, F.M.; de Oliveira, E.B.; Barros, F.; Stringheta, P.C. Camu-Camu (Myrciaria dubia) from commercial cultivation has higher levels of bioactive compounds than native cultivation (Amazon forest) and presents antimutagenic effects in vivo. J. Sci. Food Agric. 2019, 99, 624–631. [Google Scholar] [CrossRef]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-X.; Li, C.-X.; Kakar, M.U.; Khan, M.S.; Wu, P.-F.; Amir, R.M.; Dai, D.-F.; Naveed, M.; Li, Q.-Y.; Saeed, M.; et al. Resveratrol (RV): A Pharmacological review and call for further research. Biomed. Pharmacother. 2021, 143, 112164. [Google Scholar] [CrossRef]
- Rey, F.; Rodrigo, M.J.; Zacarias, L. accumulation of tocopherols and transcriptional regulation of their biosynthesis during cold storage of Mandarin fruit. Postharvest. Biol. Technol. 2021, 180, 111594. [Google Scholar] [CrossRef]
- de Morais Sato, P.; Hatzlhoffer Lourenço, B.; do Manco Machado, R.; Augusto Cardoso, M.; Baeza Scagliusi, F. Food Classifications by Brazilian Amazon Mothers: Interactions With Eating Practices. J. Nutr. Educ. Behav. 2021, 53, 880–885. [Google Scholar] [CrossRef]
- Neves, L.C.; Tosin, J.M.; Benedette, R.M.; Cisneros-Zevallos, L. Post-harvest nutraceutical behavior during ripening and senescence of 8 highly perishable fruit species from the Northern Brazilian Amazon region. Food Chem. 2015, 174, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Suwardi, A.B.; Navia, Z.I. Sustainable use and management of wild edible fruit plants: A case study in the Ulu Masen protected forest, West Aceh, Indonesia. J. Sustain. For. 2023, 42, 811–830. [Google Scholar] [CrossRef]
- Köster, E.P.; Mojet, J. 13—Familiarity, monotony or variety: The role of flavor complexity in food intake. In Flavor, 2nd ed.; Guichard, E., Salles, C., Eds.; Woodhead Publishing: Sawston, UK, 2023; pp. 363–378. ISBN 978-0-323-89903-1. [Google Scholar]
- Berlyne, D.E. Conflict, Arousal, and Curiosity; McGraw-Hill Book Company: New York, NY, USA, 1960. [Google Scholar]
- Silva, G.N.d.M.e.; Rodrigues, E.S.B.; de Macêdo, I.Y.L.; Gil, H.P.V.; Campos, H.M.; Ghedini, P.C.; da Silva, L.C.; Batista, E.A.; de Araújo, G.L.; Vaz, B.G.; et al. Blackberry jam fruit (Randia formosa (Jacq.) K. Schum): An Amazon superfruit with in vitro neuroprotective properties. Food Biosci. 2022, 50, 102084. [Google Scholar] [CrossRef]
- Can-Cauich, C.A.; Sauri-Duch, E.; Betancur-Ancona, D.; Chel-Guerrero, L.; González-Aguilar, G.A.; Cuevas-Glory, L.F.; Pérez-Pacheco, E.; Moo-Huchin, V.M. Tropical fruit peel powders as functional ingredients: Evaluation of their bioactive compounds and antioxidant activity. J. Funct. Foods 2017, 37, 501–506. [Google Scholar] [CrossRef]
- Lima, R.S.; de Carvalho, A.P.A.; Conte-Junior, C.A. Health from Brazilian Amazon food wastes: Bioactive compounds, antioxidants, antimicrobials, and potentials against cancer and oral diseases. Crit. Rev. Food Sci. Nutr. 2022, 169–5183. [Google Scholar] [CrossRef]
- Rocha, J.D.L.; Guilherme, F.A.G.; Rocha, D.I.; Pereira, K.D.R.; Coelho, C.P.; de Souza, L.F. Morphometry of fruits and pyrenes in two morphotypes and populations of Butia purpurascens Glassman (Arecaceae). Cienc. Rural 2022, 52, 3–7. [Google Scholar] [CrossRef]
- Shah, A.S.; Bhat, S.V.; Muzaffar, K.; Ibrahim, S.A.; Dar, B.N. Processing technology, chemical composition, microbial quality and health benefits of dried fruits. Curr. Res. Nutr. Food Sci. 2022, 10, 71–84. [Google Scholar] [CrossRef]
- Campoy, J.A.; Le Dantec, L.; Barreneche, T.; Dirlewanger, E.; Quero-Garcia, J. New insights into fruit firmness and weight control in sweet cherry. Plant Mol. Biol. Rep. 2015, 33, 783–796. [Google Scholar] [CrossRef]
- Espín, R.S.; Rivera, M.G.; Rivera, V.G.; Santos, F.C.; Guerrero, I.H.; Zambrano, L.G.; Borja, E.B. Rendimiento y atributos de calidad de Mora (Rubus glaucus Benth) de cuatro zonas productoras de Bolívar. Rev. Investig. Talent. 2020, 7, 33–45. [Google Scholar] [CrossRef]
- Darnet, S.H.; da Silva, L.H.M.; Rodrigues, A.M.D.; Lins, R.T. Nutritional composition, fatty acid and tocopherol contents of Buriti (Mauritia flexuosa) and Patawa (Oenocarpus bataua) fruit pulp from the Amazon region. Cienc. E Tecnol. Aliment. 2011, 31, 488–491. [Google Scholar] [CrossRef]
- Kodahl, N.; Frandsen, H.B.; Lütken, H.; Petersen, I.L.; Paredes Andrade, N.J.; García-Davila, C.; Sørensen, M. Lipid composition of the Amazonian ‘Mountain Sacha Inchis’ including Plukenetia carolis-vegae Bussmann, Paniagua & C.Téllez. Sci. Rep. 2022, 12, 6450. [Google Scholar] [CrossRef] [PubMed]
- Rogez, H.; Buxant, R.; Mignolet, E.; Souza, J.N.S.; Silva, E.M.; Larondelle, Y. Chemical composition of the pulp of three typical Amazonian fruits: Araça-Boi (Eugenia stipitata), Bacuri (Platonia insignis) and Cupuaçu (Theobroma grandiflorum). Eur. Food Res. Technol. 2004, 218, 380–384. [Google Scholar] [CrossRef]
- de Assis, S.A.; Vellosa, J.C.R.; Brunetti, I.L.; Khalil, N.M.; Leite, K.M.d.S.C.; Martins, A.B.G.; Oliveira, O.M.M.d.F. Antioxidant activity, ascorbic acid and total phenol of exotic fruits occurring in Brazil. Int. J. Food Sci. Nutr. 2009, 60, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Netto, A.B. Tropical fruits as natural, exceptionally rich, sources of bioactive compounds. Int. J. Fruit Sci. 2018, 18, 231–242. [Google Scholar] [CrossRef]
- Rufino, M.D.S.M.; Alves, R.E.; de Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive Compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef]
- Cândido, T.L.N.; Silva, M.R.; Agostini-Costa, T.S. Bioactive compounds and antioxidant capacity of Buriti (Mauritia flexuosa L.f.) from the Cerrado and Amazon Biomes. Food Chem. 2015, 177, 313–319. [Google Scholar] [CrossRef]
- Kondakova, V.; Tsvetkov, I.; Batchvarova, R.; Badjakov, I.; Dzhambazova, T.; Slavov, S. Phenol compounds—Qualitative index in small fruits. Biotechnol. Biotechnol. Equip. 2009, 23, 1444–1448. [Google Scholar] [CrossRef]
- Miean, K.H.; Mohamed, S. Flavonoid (Myricetin, Quercetin, Kaempferol, Luteolin, and Apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J.; Mapelli-Brahm, P.; Stinco, C.M. The Colourless carotenoids Phytoene and Phytofluene: From dietary sources to their usefulness for the functional foods and nutricosmetics industries. J. Food Compos. Anal. 2018, 67, 91–103. [Google Scholar] [CrossRef]
- Luan, A.; Zhang, W.; Yang, M.; Zhong, Z.; Wu, J.; He, Y.; He, J. Unveiling the molecular mechanism involving anthocyanins in pineapple peel discoloration during fruit maturation. Food Chem. 2023, 412, 135482. [Google Scholar] [CrossRef]
- Joaquín-Cruz, E.; Dueñas, M.; García-Cruz, L.; Salinas-Moreno, Y.; Santos-Buelga, C.; García-Salinas, C. Anthocyanin and phenolic characterization, chemical composition and antioxidant activity of Chagalapoli (Ardisia compressa K.) fruit: A tropical source of natural pigments. Food Res. Int. 2015, 70, 151–157. [Google Scholar] [CrossRef]
- Chandra Singh, M.; Price, W.E.; Kelso, C.; Arcot, J.; Probst, Y. Measuring the anthocyanin content of the Australian fruit and vegetables for the development of a food composition database. J. Food Compos. Anal. 2022, 112, 104697. [Google Scholar] [CrossRef]
- Tekaya, M.; Mechri, B.; Cheheb, H.; Attia, F.; Chraief, I.; Ayachi, M.; Boujneh, D.; Hammami, M. Changes in the profiles of mineral elements, phenols, tocopherols, and soluble carbohydrates of olive fruit following foliar nutrient fertilization. LWT—Food Sci. Technol. 2014, 59, 1047–1053. [Google Scholar] [CrossRef]
- Pertuzatti, P.B.; Sganzerla, M.; Jacques, A.C.; Barcia, M.T.; Zambiazi, R.C. Carotenoids, tocopherols and ascorbic acid content in Yellow Passion Fruit (Passiflora edulis) grown under different cultivation systems. LWT—Food Sci. Technol. 2015, 64, 259–263. [Google Scholar] [CrossRef]
- Rollo, A.; Ribeiro, M.M.; Costa, R.L.; Santos, C.; Clavo, P.Z.M.; Mandák, B.; Kalousová, M.; Vebrová, H.; Chuqulin, E.; Torres, S.G.; et al. Genetic structure and pod morphology of Inga edulis cultivated vs. wild populations from the Peruvian Amazon. Forests 2020, 11, 655. [Google Scholar] [CrossRef]
- Ramos, S.L.F.; Lopes, M.T.G.; Meneses, C.; Dequigiovanni, G.; de Macêdo, J.L.V.; Lopes, R.; Sebbenn, A.M.; da Silva, R.F.; de Jesus Pinto Fraxe, T.; Veasey, E.A. Natural populations of Astrocaryum aculeatum Meyer in Amazonia: Genetic diversity and conservation. Plants 2022, 11, 2957. [Google Scholar] [CrossRef] [PubMed]
- Secretaría Técnica de la Circunscripción Territorial Especial Amazónica. Plan Integral para la Amazonía 2021–2025; Secretaría Técnica de la Circunscripción Territorial Especial Amazónica: Puyo, Ecuador, 2021. [Google Scholar]
- Instituto Nacional de Estadísticas y Censos (INEC). Available online: https://www.ecuadorencifras.gob.ec/proyecciones-poblacionales/ (accessed on 8 October 2023).
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
| Fruits | References Included |
|---|---|
| Aphandra natalia | [17] |
| Eugenia stipitata | [32,43,44,45,46,47,48,49,50] |
| Gustavia macarenensis | [27,42,51] |
| Mauritia flexuosa | [21,25,41,44,47,52,53] |
| Myrciaria dubia | [46,54,55,56,57,58] |
| Oenocarpus bataua | [26,27,59,60] |
| Plukenetia volubilis | [61,62,63,64,65] |
| Pouteria caimito | [47,66,67] |
| Solanum quitoense | [32,33,43,68,69] |
| Fruit | Description |
|---|---|
 | Aphandra natalia (Balslev and Henderson) Barfod is a species of palm native to the Ecuadorian Amazon; it is characterized by having a hard, scaly rind, slightly sweet, fibrous pulp with high-fat content, and large seeds that are used for making handicrafts. The mesocarp oil of this fruit has a high oleic acid content (71.92%). However, in Peru, Brazil, and Ecuador, the extraction of fibers for the manufacture of brooms and the collection of leaves for roofing are the most frequent uses for this species [70]. It grows on terraces along rivers at an altitude of up to 800 m but can be cultivated at up to 1000 m above sea level. This species is found in a wide range, extending from south of the Napo River in Ecuador to the lowlands of the western Amazon basin, across the northern Peruvian Amazon to the Acre in Brazil. This species can also be found in home gardens and agroforestry systems, where it provides shade for livestock and protects the soil from erosion [71], but its low densities are due to its dispersal rather than environmental limitations [72]. Family: Arecaceae|Common name: Tagua; Vegetable ivory. |
 | Eugenia stipitata McVaugh has an oval-shaped climacteric berry fruit with a sweet and sour taste and a very pleasant characteristic aroma, which turns from green to yellow or orange when ripe, with three harvests per year. The fruits are used for the preparation of homemade juices, although they also have medicinal uses [43]. This species is native to western Amazonia and the Guianas; it grows in tropical and subtropical climates and is cultivated in Bolivia, Brazil, Colombia, Ecuador, and Peru. It is an approximately 3-meter shrub with dense foliage that grows in fertile, well-drained soils. [49]. Family: Myrtaceae|Common name: Araza; Amazonia guava. |
 | Gustavia macarenensis Philipson is a type of fruit that has a soft, orange pulp with a high-fat content. It is known for its pleasant taste when eaten fresh and can also be used as a purgative. The fruit’s shell can be infused and used as a uterine antihemorrhagic, while the fresh seeds can help counteract dysentery and sinusitis. This makes it a promising fruit for applications in the food, cosmetic, and pharmaceutical industries [27]. This species can be found at various altitudes, ranging from 260 to 1200 m above sea level. Its habitat in Ecuador covers all provinces of the Amazon region. The tree can grow up to 25 m tall, with a dense and rounded crown. Its fruits are ripe when they fall off the tree [42]. Family: Lecythidaceae|Common name: Alan paso; Inaco; Inak; Passo; Sachu pasu. |
 | Myrciaria dubia L.f. berry is round and contains 1 to 4 seeds, with a diameter of approximately 2.5 cm. The color of the berry changes from green to violet–red as it ripens, which is due to the presence of phenolic compounds [54]. This fruit contains various bioactive components like anthocyanins, flavonols, ellagitannins, proanthocyanins, and carotenoids [58]. Peru mainly exports this fruit in the form of flour, extract, and dehydrated products due to its economic potential. The species is native to the Amazon rainforest in Brazil and grows naturally on the banks of rivers and lakes during periods of flooding [73]. Family: Myrtaceae|Common name: Camu-camu; Guayabo; Guayabito; Guapuro blanco. |
 | Mauritia flexuosa (Kunth) McVaugh is a palm that constitutes a socioeconomic resource for local indigenous communities in the Ecuadorian Amazon, growing in poorly drained soils and generating resources such as food, nesting sites, and habitat [74]. This fruit is a highly traded item in local markets. Its vibrant orange pulp is commonly used to make sweets, ice cream, juices, jams, and oil. It is also a rich source of bioactive compounds, with a higher β-carotene content than such products as carrots and spinach. As a result, it can be classified as a functional food [21]. This species has a broad distribution in South America, primarily in the Amazon region, spanning across Bolivia, Brazil, Colombia, Ecuador, Guyana, Peru, Trinidad and Tobago, and Venezuela [75]. It can be in various habitats, ranging from lowland tropical forests to open savannah landscapes, floodplains, and springs [76]. The tree is a palm that grows up to 40 m tall, featuring a cylindrical and smooth stem with a diameter ranging from 30 to 60 cm. It has a spherical crown of elongated leaves reaching up to 6 m in length [77] Family: Arecaceae|Common name: Morete; Aguaje; Buriti; Moriche. |
 | Oenocarpus bataua Mart. tree produces fruit with a valuable violet-colored pulp. This pulp is rich in monounsaturated fats and antioxidants that can be extracted for use. Additionally, the fruit is used to make both fermented and non-fermented beverages and desserts [78]. They constitute a key element in the food security of Amazonian peoples and a promising element for the food and cosmetic industry [79]. This is a type of palm that thrives in tropical rainforests and is commonly found in the Amazon biome, including both flooded and mainland forests [60]. It can reach heights between 4 and 26 m and can yield up to eleven tons of fruit per hectare annually [27]. It is one of the most used palms in Amazonia, growing preferably in poorly drained soils; additional uses of it include straw, fiber, and wood [26]. Family: Arecaceae|Common name: Chapil; Patawa; Seje; Ungurahua. |
 | Plukenetia volubilis L. produces fruits whose seeds are appreciated for their high content of unsaturated fatty acids (omegas 3 and 6) and proteins (27–33%), which are rich in essential amino acids and consumed as nuts [80]. The leaves have antioxidant components such as flavonoids, terpenoids, and saponins [81]. This species is a shrubby climbing plant that originates from the Amazon rainforests and can thrive in warm climates, from the high altitudes of the Andean jungle to the lowlands of the Peruvian Amazon [82]. Its seeds contain essential amino acids, making it a promising economic crop in Central and South America, as well as in Southeast Asian countries [80]. Family: Euphorbiaceae|Common name: Sacha inchi; Sacha yuchi; Sacha yuchiqui; Mountain peanuts; Wild peanuts; Inca peanuts; Sacha peanuts. |
 | Pouteria caimito (Ruiz and Pav.) Radlk is a round berry that often has a pointed end with a smooth peel that contains latex. When it ripens, it changes from green to bright yellow and is a climacteric fruit that contains beneficial bioactive compounds [83]. However, it has a short shelf life due to browning and high levels of ethylene production [84]. The fruit’s pulp is translucent, white, juicy, soft, sweet, and mucilaginous. It is usually eaten raw, but it can also be used to make soft drinks, ice creams, sorbets, and other desserts. This tree is a perennial that can grow up to 5–15 m tall and is native to the Amazon region of South America [85]. It is also found in many other places, including Central America, the West Indies, Northern Australia, and Malaysia. It is grown on farms, in orchards and gardens, and in urban forestry in some regions of northern Brazil [86]. Family: Sapotaceae|Common name: Caimito; Cauje; Luma. |
 | Solanum quitoense Lam. is a perennial herbaceous plant native to Ecuador and Colombia; the fruit is edible and resembles a small orange with pubescence, sweet acid flavor, and exotic and very pleasant characteristic aroma [87]. In Ecuador, the crop is grown mainly in the Amazon region at the altitude of between 1500 and 2400 m; the common name is naranjilla; it is consumed in juice, although it is also marketed as pulp and has been used to produce bottled juices and ice cream [88]. This plant, a perennial shrub that can grow up to 2.5 m tall, is originally from the Andes and can be found from Venezuela to Peru [89]. It is typically grown at elevations between 1000 and 1900 m. There are two types of this plant: varietas quitoense, which does not have thorns and is found in Colombia and Ecuador, and varietas septentrionale, which has thorns and can be found in Colombia, Panama, and Costa Rica [68]. In Ecuador, it is primarily grown in the mountainous foothills and Amazonian plains for both local consumption and export to Colombia [90]. Family: Solanaceae|Common name: Naranjilla; Lulo. |
| Fruit | Length (cm) | Transverse Diameter (cm) | Weight (g) | Moisture (%) | Pulp Yield (%) | Total Soluble Solids (°Brix) | Titrable Acidity (%) | Maturity Index | Ash (%) | Crude Fibre (%) | Lipids (%) | Proteins (%) | Carbohydrates (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aphandra natalia | 13.56 ± 4.97 | Not applicable | 522.77 ± 48.01 | 44.52 ± 0.11 (Mesocarp) | NR | NR | 0.04 * | NR | 2.62 ± 0.05 * | 11.30 ± 0.59 * | 57.92 ± 3.05 * | 5.11 ± 0.10 * | 23.0 ± 2.35 * | [17] |
| Eugenia stipitata | 5.54 | 7–12 | 30–105 | 80.30–94.42 | 89.65 (pulp + peel) | 3.83–5.06 | 2.40 ± 0.02 | 1.60 ± 0.45 | 2.1–2.94 * | 6.69–8.24 * | 0.59–3.02 * | 9.92–12.68 * | 60.36–64.54 * | [32,43,44,45,46,47,48] |
| Gustavia macarenensis | 5.73–7.04 | 6.09–8.50 | 153.93–280.18 | 61.74–70.94 | 59.34 | 60.05–69.8 (oil pulp) | NR | NR | 2.34–4.08 | 11.53–25.17 | 31.60–53.57 | 12.39–12.8 | 19.73–26.75 | [42,51] |
| Mauritia flexuosa | 5.47 ± 0.15 | 4.59 ± 0.18 | 48.7–51.83 | 54.8–56.23 (pulp) | 11.0–20.19 | 7.73 ± 0.06 | 7.60 ± 0.23 | 1.07 | 2.27 ± 0.05 | 38.0 ± 0.3 | 26.6 ± 0.3 | 2.47 ± 0.07 | 15.1 ± 0.3 | [21,25] |
| Myrciaria dubia | 1.2–2.5 | 1.0–3.2 | 6.9 | 93.2 | 50–60 | 6.2–9.39 | 2.30–4.97 | 2.14 | 1.8–2.64 | 0.1–1.3 | 0.2–0.3 | 0.4–0.5 | 3.5–4.7 | [46,54,55,56] |
| Oenocarpus bataua | 33.3–35.9 | 23.9–24 | 13.23 | 42.4 | 11.84 | NR | NR | NR | 1.1 | 31.5 | 12.8–22.2 38.3 * | 3.3 | 47.2 | [26,59,60] |
| Plukenetia volubilis | 3–5 (star-shaped capsule) | 1.5–2 (oval seed) | NR | 3.3–8.32 (seed) | NR | NR | NR | NR | 2.9–6.46 | 18.0 ± 0.095 | 33.4–54.3 | 24.2–29.7 | 7.29–30.9 | [61,62,63] |
| Pouteria caimito | NR | NR | NR | 81.87–95.8 | NR | 3.8–17.50 | 0.04–5.9 | NR | 0.32–0.49 | NR | 0.1–0.15 | 4.60–4.97 | NR | [47,66,67] |
| Solanum quitoense | 4.7–5.24 | 5.4–5.89 | 65.7–311.54 | 90.74 ± 0.73 (whole fruit) | 63.01–84.72 | 6.53–9.55 | 1.85–3.78 | 1.83–3.72 | 5.84 ± 0.85 | 9.22 ± 0.22 | 0.69 ± 0.05 11.65 * | 11.2 ± 1.99 7.44 * | NR | [32,33,68] |
| Fruit (Scientific Name) | Antioxidant Capacity | Vitamin C (mg/100 g) | Total Phenolics (mg GAE/100 g) | Total Flavonoids (mg/100 g) | Total Carotenoids (mg β-Carotene/kg) | Total Anthocyanin (mg/100 g) | Reference |
|---|---|---|---|---|---|---|---|
 | 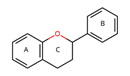 | 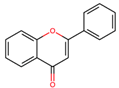 |  |  | |||
| Aphandra natalia | DPPH: 60 ± 1.0 µmol TE/kg (pulp oil) | NR | 8.36 ± 1.84 (pulp oil) | NR | 0.24 ± 0.02 | NR | [17] |
| Eugenia stipitata | DPPH: 392.10–3621.8 * µmol TE/kg (pulp) ABTS: 758.22 ± 5.01 * µmoL TE/g (pulp) DPPH: 84.4 μmol TE/100 (pulp) FRAP: 63 μmol FeSO4/g (pulp) | 5.60–8.3 | 14,243 (seed) 154.20 (pulp) | 125–600.72 * (pulp) 4373 ± 0.23 (seed) | 31.00–33.39 (pulp) | ND | [32,43,44,46,47,49,50] |
| Gustavia macarenensis | DPPH: 688.40 ± 2.28 * µmol AA/100 g (pulp) DPPH: 1146.41 ± 1.12 * µmol AA/100 g (peel) DPPH: 523.82 ± 14.09 * µmol AA/100 g (seed) DPPH: 170 ± 2.86 μmol TE/Kg (pulp oil) | 5.48 ± 0.24 * (pulp) 15.85 ± 0.06 * (peel) 2.20 ± 0.11 * (seed) | 634.30 ± 5.01 * (pulp) 1165.87 ± 17.66 * (peel) 153.16 ± 3.45 * (seed) 156.49 ± 2.62 (pulp oil) | 25.57 ± 0.60 * (pulp) 383.59 ± 8.136 * (peel) 111.39 ± 0.79 * (seed) | 2.62 ± 0.16 (pulp) | 25.57 ± 0.60 * (pulp) 9.13 ± 0.11 * (peel) 5.79 ± 0.12 * (seed) | [27,42] |
| Mauritia flexuosa | DPPH: 14.7–17.98 * µmoL TE/g (pulp) FRAP 11.38–15.68 * µmoL TE/g (Pulp) | 3.28 ± 0.78 33.4 ± 0.48 * (pulp) 59.93 | 725.0–743.2 * (pulp) 29.026–174.36 (shell) | 55.8–196 * (pulp) 20.27–185.58 (shell) | 239.4–216.4 (pulp) | 3.10 mg cyanidin 3-O-glucoside 100 g−1 | [25,41,52,93] |
| Myrciaria dubia | DPPH: 1.93 μmol TE/g (ripe fresh pulp) DPPH: 1328.50 ± 14.40 μmol TE/g (ripe peel) | 960–4752.23 (pulp) 1109.62 ± 56.5 (ripe peel) | 12,798.80 (pulp) 13,348.97 ± 99.94 (ripe peel) | 55.1–211.64 (pulp) | 1 (pulp) 6199.8 (ripe peel) | 170.00 (pulp) 223.14 ± 6.89 (ripe peel) | [57,58,94] |
| Oenocarpus bataua | DPPH: 478.94 ± 4.85 * µmol AA/100 g (pulp) DPPH: 654.56 ± 0.94 * µmol AA/100 g (peel) DPPH: 589.44 ± 5.29 * µmol AA/100 g (seed) | 0.12 ± 0.00 (pulp) | 622.97 ± 4.84 * (pulp) 1009.38 ± 1.80 * (peel) 758.25 ± 3.221 * (seed) | 55.34 ± 0.29 * (pulp) 57.17 ± 0.42 * (peel) 47.15 ± 0.06 * (seed) | NR | 14.82 ± 0.20 * (pulp) 46.48 ± 0.31 * (peel) 14.71 ± 0.02 * (seed) | [27] |
| Plukenetia volubilis (seed) | DPPH: 32.43 ± 1.63% (Oil pressed-cake) FRAP: 732.67 ± 35.29 µmol FeSO4/L (Oil pressed-cake) ORAC: 6.5–9.8 μmol TE/g | NR | 6.58 ± 0.27 (oil seed) 51 (Oil pressed-cake) | NR | NR | NR | [65] |
| Pouteria caimito | DPPH: 734.98 ± 0.26 µmol TE/100 (pulp) FRAP: 1211.03 ± 1.12 µmol TE/100 (pulp) | 2.0–3.11 (pulp) | 172.75 ± 0.77 (pulp) 60.0 ± 0.015 (pulp) | NR | 0.25 (pulp) | NR | [47,66,67] |
| Solanum quitoense | DPPH: 21.26 ± 1.35 * µmol TE/g (pulp) ABTS 76.40 ± 1.33 * µmol TE/g (pulp) ORAC: 0.15 ± 0.02 mM/g (Pulp) | 0.1 * (whole fruit) 4.16 ± 1.49 (pulp) | 775.31–897.58 * | 991.57 ± 20.24 | 34.08 ± 2.42–57.93 * | ND | [32,43,69] |
| Subject | Search Parameters |
|---|---|
| Fruits | “fruit” AND “Amazon” AND “Ecuador” |
| Ethnobotany | “ethnobotany” AND “Ecuador” AND “Amazon” |
| Bioactive compounds | “bioactive” AND “Ecuador” AND “Amazon” |
| Fruit description | “scientific name of fruit” AND “physicochemical” “scientific name of fruit” AND “bioactive” |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Capa, M.; Corell González, M.; Mestanza-Ramón, C. Edible Fruits from the Ecuadorian Amazon: Ethnobotany, Physicochemical Characteristics, and Bioactive Components. Plants 2023, 12, 3635. https://doi.org/10.3390/plants12203635
Sánchez-Capa M, Corell González M, Mestanza-Ramón C. Edible Fruits from the Ecuadorian Amazon: Ethnobotany, Physicochemical Characteristics, and Bioactive Components. Plants. 2023; 12(20):3635. https://doi.org/10.3390/plants12203635
Chicago/Turabian StyleSánchez-Capa, Maritza, Mireia Corell González, and Carlos Mestanza-Ramón. 2023. "Edible Fruits from the Ecuadorian Amazon: Ethnobotany, Physicochemical Characteristics, and Bioactive Components" Plants 12, no. 20: 3635. https://doi.org/10.3390/plants12203635
APA StyleSánchez-Capa, M., Corell González, M., & Mestanza-Ramón, C. (2023). Edible Fruits from the Ecuadorian Amazon: Ethnobotany, Physicochemical Characteristics, and Bioactive Components. Plants, 12(20), 3635. https://doi.org/10.3390/plants12203635










