Isolation and Characterization of Extracellular Vesicles from Arabidopsis thaliana Cell Culture and Investigation of the Specificities of Their Biogenesis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Characterization of Arabidopsis EVs
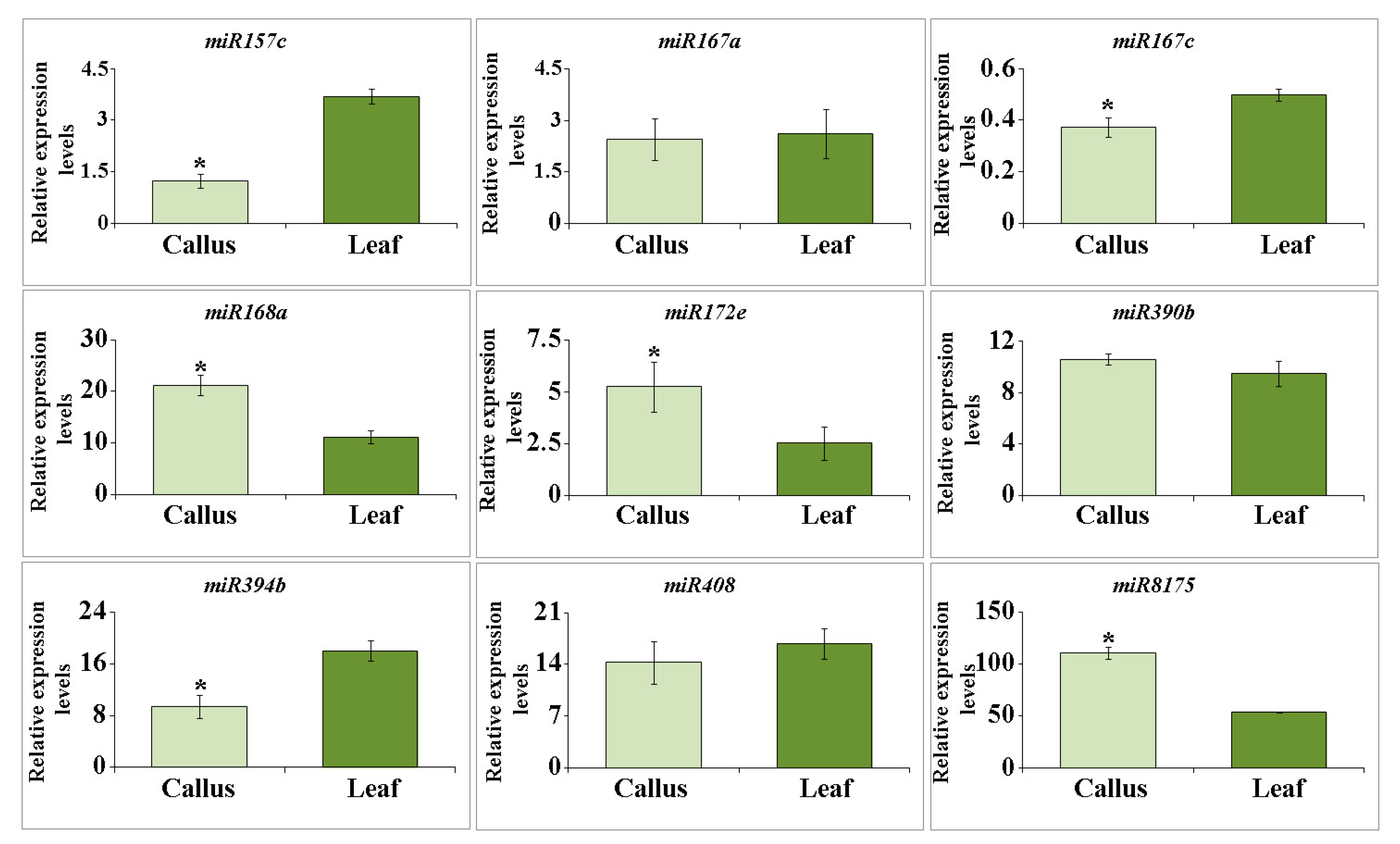
2.2. miRNA Accumulation in Arabidopsis EVs
2.3. Protein Composition of Callus-Derived EVs
2.4. Expression of Genes Participating in EVs Biogenesis
2.5. The Effect of Salicylic Acid on EVs Biogenesis
3. Materials and Methods
3.1. Plant Material and Growth Conditions
3.2. Isolation of EVs
3.3. Scanning Electron Microscopy
3.4. Nanoparticle Tracking Analysis
3.5. Isolation of RNA and miRNA, cDNA Synthesis, and PCR Analysis
3.6. Isolation and Analysis of Protein Cargo
3.6.1. Extraction Procedure
3.6.2. Mass Spectrometry and Protein Identification
3.6.3. Western Blotting
3.6.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patil, S.M.; Sawant, S.S.; Kunda, N.K. Exosomes as drug delivery systems: A brief overview and progress update. Eur. J. Pharm. Biopharm. 2020, 154, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Deng, W.; Klinke, D.J., II. Exosomes: Improved methods to characterize their morphology, RNA content, and surface protein biomarkers. Analyst 2015, 140, 6631–6642. [Google Scholar] [CrossRef] [PubMed]
- Halperin, W.; Jensen, W.A. Ultrastructural changes during growth and embryogenesis in carrot cell cultures. J. Ultrastruct. Res. 1967, 18, 428–443. [Google Scholar] [CrossRef] [PubMed]
- Regente, M.; Pinedo, M.; Elizalde, M.; de la Canal, L. Apoplastic exosome-like vesicles: A new way of protein secretion in plants? Plant Signal. Behav. 2012, 7, 544–546. [Google Scholar] [CrossRef]
- Rutter, B.D.; Innes, R.W. Growing pains: Addressing the pitfalls of plant extracellular vesicle research. New Phytol. 2020, 228, 1505–1510. [Google Scholar] [CrossRef]
- Mu, J.; Zhuang, X.; Wang, Q.; Jiang, H.; Deng, Z.-B.; Wang, B.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D.; et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Food Nutr. Res. 2014, 58, 1561–1573. [Google Scholar] [CrossRef]
- Xiao, J.; Feng, S.; Wang, X.; Long, K.; Luo, Y.; Wang, Y.; Ma, J.; Tang, Q.; Jin, L.; Li, X.; et al. Identification of exosome-like nanoparticle-derived microRNAs from 11 edible fruits and vegetables. PeerJ 2018, 6, e5186. [Google Scholar] [CrossRef]
- Shkryl, Y.; Tsydeneshieva, Z.; Degtyarenko, A.; Yugay, Y.; Balabanova, L.; Rusapetova, T.; Bulgakov, V. Plant exosomal vesicles: Perspective information nanocarriers in biomedicine. Appl. Sci. 2022, 12, 8262. [Google Scholar] [CrossRef]
- Cong, M.; Tan, S.; Li, S.; Gao, L.; Huang, L.; Zhang, H.G.; Qiao, H. Technology insight: Plant-derived vesicles—How far from the clinical biotherapeutics and therapeutic drug carriers? Adv. Drug Deliv. Rev. 2022, 182, 114108. [Google Scholar] [CrossRef]
- Rutter, B.D.; Innes, R.W. Extracellular vesicles as key mediators of plant-microbe interactions. Curr. Opin. Plant Biol. 2018, 44, 16–22. [Google Scholar] [CrossRef]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Karamanidou, T.; Tsouknidas, A. Plant-derived extracellular vesicles as therapeutic nanocarriers. Int. J. Mol. Sci. 2021, 23, 191. [Google Scholar] [CrossRef]
- Gao, C.; Zhuang, X.; Shen, J.; Jiang, L. Plant ESCRT complexes: Moving beyond endosomal sorting. Trends Plant Sci. 2017, 22, 986–998. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Qiao, L.; Wang, M.; He, B.; Lin, F.M.; Palmquist, J.; Huang, S.D.; Jin, H. Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science 2018, 360, 1126–1129. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ding, Y.; Wang, J.; Hillmer, S.; Miao, Y.; Lo, S.W.; Wang, X.; Robinson, D.G.; Jiang, L. EXPO, an exocyst-positive organelle distinct from multivesicular endosomes and autophagosomes, mediates cytosol to cell wall exocytosis in Arabidopsis and tobacco cells. Plant Cell 2010, 22, 4009–4030. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Hamby, R.; Jin, H. Plant extracellular vesicles: Trojan horses of cross-kingdom warfare. FASEB BioAdv. 2021, 3, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Rutter, B.D.; Innes, R.W. Extracellular vesicles isolated from the leaf apoplast carry stress-response proteins. Plant Physiol. 2017, 173, 728–741. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, S.; Koo, Y.; Yang, A.; Dai, Y.; Khant, H.; Osman, S.R.; Chowdhury, M.; Wei, H.; Li, Y.; et al. Characterization of and isolation methods for plant leaf nanovesicles and small extracellular vesicles. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102271. [Google Scholar] [CrossRef]
- Movahed, N.; Cabanillas, D.G.; Wan, J.; Vali, H.; Laliberté, J.F.; Zheng, H. Turnip mosaic virus components are released into the extracellular space by vesicles in infected leaves. Plant Physiol. 2019, 180, 1375–1388. [Google Scholar] [CrossRef]
- Raimondo, S.; Naselli, F.; Fontana, S.; Monteleone, F.; Lo Dico, A.; Saieva, L.; Zito, G.; Flugy, A.; Manno, M.; Di Bella, M.A.; et al. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget 2015, 6, 19514–19527. [Google Scholar] [CrossRef]
- Gui, L.; Dash, B.C.; Luo, J.; Qin, L.; Zhao, L.; Yamamoto, K.; Hashimoto, T.; Wu, H.; Dardik, A.; Tellides, G.; et al. Implantable tissue-engineered blood vessels from human induced pluripotent stem cells. Biomaterials 2016, 102, 120–129. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; van Bel, A.J.; Hückelhoven, R. Do plant cells secrete exosomes derived from multivesicular bodies? Plant Signal. Behav. 2007, 2, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Kwon, C.; Neu, C.; Pajonk, S.; Yun, H.S.; Lipka, U.; Humphry, M.; Bau, S.; Straus, M.; Kwaaitaal, M.; Rampelt, H.; et al. Co-option of a default secretory pathway for plant immune responses. Nature 2008, 451, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Regente, M.; Corti-Monzón, G.; Maldonado, A.M.; Pinedo, M.; Jorrín, J.; de la Canal, L. Vesicular fractions of sunflower apoplastic fluids are associated with potential exosome marker proteins. FEBS Lett. 2009, 583, 3363–3366. [Google Scholar] [CrossRef] [PubMed]
- Woith, E.; Guerriero, G.; Hausman, J.F.; Renaut, J.; Leclercq, C.C.; Weise, C.; Legay, S.; Weng, A.; Melzig, M.F. Plant extracellular vesicles and nanovesicles: Focus on secondary metabolites, proteins and lipids with perspectives on their potential and sources. Int. J. Mol. Sci. 2021, 22, 3719. [Google Scholar] [CrossRef]
- Zhuang, X.; Teng, Y.; Samykutty, A.; Mu, J.; Deng, Z.; Zhang, L.; Cao, P.; Rong, Y.; Yan, J.; Miller, D.; et al. Grapefruit-derived nanovectors delivering therapeutic miR17 through an intranasal route inhibit brain tumor progression. Mol. Ther. 2016, 24, 96–105. [Google Scholar] [CrossRef]
- Raimondo, S.; Nikolic, D.; Conigliaro, A.; Giavaresi, G.; Lo Sasso, B.; Giglio, R.V.; Chianetta, R.; Manno, M.; Raccosta, S.; Corleone, V.; et al. Preliminary results of CitraVes™ effects on low density lipoprotein cholesterol and waist circumference in healthy subjects after 12 weeks: A pilot open-label study. Metabolites 2021, 11, 276. [Google Scholar] [CrossRef]
- Wu, K.; Xing, F.; Wu, S.Y.; Watabe, K. Extracellular vesicles as emerging targets in cancer: Recent development from bench to bedside. Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 538–563. [Google Scholar] [CrossRef]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef]
- Kim, W.S.; Ha, J.H.; Jeong, S.H.; Lee, J.I.; Lee, B.W.; Jeong, Y.J.; Kim, C.Y.; Park, J.Y.; Ryu, Y.B.; Kwon, H.J.; et al. Immunological effects of Aster yomena callus-derived extracellular vesicles as potential therapeutic agents against allergic asthma. Cells 2022, 11, 2805. [Google Scholar] [CrossRef] [PubMed]
- Kocholata, M.; Prusova, M.; Auer Malinska, H.; Maly, J.; Janouskova, O. Comparison of two isolation methods of tobacco-derived extracellular vesicles, their characterization and uptake by plant and rat cells. Sci. Rep. 2022, 12, 19896. [Google Scholar] [CrossRef] [PubMed]
- Sebaihi, N.; De Boeck, B.; Yuana, Y.; Nieuwland, R.; Pétry, J. Dimensional characterization of extracellular vesicles using atomic force microscopy. Meas. Sci. Technol. 2017, 28, 034006. [Google Scholar] [CrossRef]
- Sung, J.; Yang, C.; Viennois, E.; Zhang, M.; Merlin, D. Isolation, purification, and characterization of ginger-derived nanoparticles (GDNPs) from ginger, rhizome of Zingiber officinale. Bio-Protocol 2019, 9, e3390. [Google Scholar] [CrossRef]
- Zhuang, X.; Deng, Z.B.; Mu, J.; Zhang, L.; Yan, J.; Miller, D.; Feng, W.; McClain, C.J.; Zhang, H.G. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J. Extracell. Vesicles 2015, 4, 28713. [Google Scholar] [CrossRef] [PubMed]
- Ekström, K.; Omar, O.; Granéli, C.; Wang, X.; Vazirisani, F.; Thomsen, P. Monocyte exosomes stimulate the osteogenic gene expression of mesenchymal stem cells. PLoS ONE 2013, 8, e75227. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Muto, A.; Van de Velde, J.; Neyt, P.; Himanen, K.; Vandepoele, K.; Van Lijsebettens, M. Functional analysis of the Arabidopsis TETRASPANIN gene family in plant growth and development. Plant Physiol. 2015, 169, 2200–2214. [Google Scholar] [CrossRef]
- Midekessa, G.; Godakumara, K.; Ord, J.; Viil, J.; Lättekivi, F.; Dissanayake, K.; Kopanchuk, S.; Rinken, A.; Andronowska, A.; Bhattacharjee, S.; et al. Zeta potential of extracellular vesicles: Toward understanding the attributes that determine colloidal stability. ACS Omega 2020, 5, 16701–16710. [Google Scholar] [CrossRef]
- Kim, J.; Li, S.; Zhang, S.; Wang, J. Plant-derived exosome-like nanoparticles and their therapeutic activities. Asian J. Pharm. Sci. 2022, 17, 53–69. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, L.; Zhang, Y.; Lu, R. Plant-derived exosomes as a drug-delivery approach for the treatment of inflammatory bowel disease and colitis-associated cancer. Pharmaceutics 2022, 14, 822. [Google Scholar] [CrossRef]
- Li, X.; Lu, C.; Yang, Y.; Yu, C.; Rao, Y. Site-specific targeted drug delivery systems for the treatment of inflammatory bowel disease. Biomed. Pharmacother. 2020, 129, 110486. [Google Scholar] [CrossRef] [PubMed]
- Deregibus, M.C.; Figliolini, F.; D’Antico, S.; Manzini, P.M.; Pasquino, C.; De Lena, M.; Tetta, C.; Brizzi, M.F.; Camussi, G. Charge-based precipitation of extracellular vesicles. Int. J. Mol. Med. 2016, 38, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Garrido, N.; Cordero, C.; Olivo-Martinez, Y.; Badia, J.; Baldomà, L. Cell-to-cell communication by host-released extracellular vesicles in the gut: Implications in health and disease. Int. J. Mol. Sci. 2021, 22, 2213. [Google Scholar] [CrossRef]
- Baldrich, P.; Rutter, B.D.; Karimi, H.Z.; Podicheti, R.; Meyers, B.C.; Innes, R.W. Plant extracellular vesicles contain diverse small RNA species and are enriched in 10- to 17-nucleotide “tiny” RNAs. Plant Cell 2019, 31, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Karimi, Z.H.; Baldrich, P.; Rutter, B.D.; Borniego, L.; Zajt, K.K.; Meyers, B.C.; Innes, R.W. Arabidopsis apoplastic fluid contains sRNA- and circular RNA-protein complexes that are located outside extracellular vesicles. Plant Cell 2022, 34, 1863–1881. [Google Scholar] [CrossRef]
- He, B.; Cai, Q.; Qiao, L.; Huang, C.Y.; Wang, S.; Miao, W.; Ha, T.; Wang, Y.; Jin, H. RNA-binding proteins contribute to small RNA loading in plant extracellular vesicles. Nat. Plants 2021, 7, 539. [Google Scholar] [CrossRef]
- Su, H.G.; Zhang, X.H.; Wang, T.T.; Wei, W.L.; Wang, Y.X.; Chen, J.; Zhou, Y.B.; Chen, M.; Ma, Y.Z.; Xu, Z.S.; et al. Genome-wide identification, evolution, and expression of GDSL-type esterase/lipase gene family in soybean. Front. Plant Sci. 2020, 11, 726. [Google Scholar] [CrossRef]
- Volokita, M.; Rosilio-Brami, T.; Rivkin, N.; Zik, M. Combining comparative sequence and genomic data to ascertain phylogenetic relationships and explore the evolution of the large GDSL-lipase family in land plants. Mol. Biol. Evol. 2011, 28, 551–565. [Google Scholar] [CrossRef]
- Huang, L.M.; Lai, C.P.; Chen, L.O.; Chan, M.T.; Shaw, J.F. Arabidopsis SFAR4 is a novel GDSL-type esterase involved in fatty acid degradation and glucose tolerance. Bot. Stud. 2015, 56, 33. [Google Scholar] [CrossRef]
- Kim, K.J.; Lim, J.H.; Kim, M.J.; Kim, T.; Chung, H.M.; Paek, K.H. GDSL-lipase1 (CaGL1) contributes to wound stress resistance by modulation of CaPR-4 expression in hot pepper. Biochem. Biophys. Res. Commun. 2008, 374, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.K.; Yadav, V.K.; Pant, P.; Singh, S.P.; Maurya, R.; Sable, A.; Sawant, S.V. GhMYB1 regulates SCW stage-specific expression of the GhGDSL promoter in the fibres of Gossypium hirsutum L. Plant Biotechnol. J. 2017, 15, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Burow, M.; Zhang, Z.Y.; Ober, J.A.; Lambrix, V.M.; Wittstock, U.; Gershenzon, J.; Kliebenstein, D.J. ESP and ESM1 mediate indol-3-acetonitrile production from indol-3-ylmethyl glucosinolate in Arabidopsis. Phytochemistry 2008, 69, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ober, J.A.; Kliebenstein, D.J. The gene controlling the quantitative trait locus EPITHIOSPECIFIER MODIFIER1 alters glucosinolate hydrolysis and insect resistance in Arabidopsis. Plant Cell 2006, 18, 1524–1536. [Google Scholar] [CrossRef] [PubMed]
- Galádová, H.; Polozsányi, Z.; Breier, A.; Šimkovič, M. Sulphoraphane affinity-based chromatography for the purification of myrosinase from Lepidium sativum seeds. Biomolecules 2022, 12, 406. [Google Scholar] [CrossRef]
- Liu, Y.; Ahn, J.E.; Datta, S.; Salzman, R.A.; Moon, J.; Huyghues-Despointes, B.; Pittendrigh, B.; Murdock, L.L.; Koiwa, H.; Zhu-Salzman, K. Arabidopsis vegetative storage protein is an anti-insect acid phosphatase. Plant Physiol. 2005, 139, 1545–1556. [Google Scholar] [CrossRef]
- Kongdin, M.; Mahong, B.; Lee, S.K.; Shim, S.H.; Jeon, J.S.; Ketudat Cairns, J.R. Action of multiple rice β-Glucosidases on abscisic acid glucose ester. Int. J. Mol. Sci. 2021, 22, 7593. [Google Scholar] [CrossRef]
- Konishi, T.; Takeda, T.; Miyazaki, Y.; Ohnishi-Kameyama, M.; Hayashi, T.; O’Neill, M.A.; Ishii, T. A plant mutase that interconverts UDP-arabinofuranose and UDP-arabinopyranose. Glycobiology 2007, 17, 345–354. [Google Scholar] [CrossRef]
- Saqib, A.; Scheller, H.V.; Fredslund, F.; Welner, D.H. Molecular characteristics of plant UDP-arabinopyranose mutases. Glycobiology 2019, 29, 839–846. [Google Scholar] [CrossRef]
- Sumiyoshi, M.; Inamura, T.; Nakamura, A.; Aohara, T.; Ishii, T.; Satoh, S.; Iwai, H. UDP-arabinopyranose mutase 3 is required for pollen wall morphogenesis in rice (Oryza sativa). Plant Cell Physiol. 2015, 56, 232–241. [Google Scholar] [CrossRef]
- Zhang, C.; He, M.; Jiang, Z.; Liu, L.; Pu, J.; Zhang, W.; Wang, S.; Xu, F. The xyloglucan endotransglucosylase/hydrolase gene XTH22/TCH4 regulates plant growth by disrupting the cell wall homeostasis in Arabidopsis under boron deficiency. Int. J. Mol. Sci. 2022, 23, 1250. [Google Scholar] [CrossRef] [PubMed]
- Niraula, P.M.; Zhang, X.; Jeremic, D.; Lawrence, K.S.; Klink, V.P. Xyloglucan endotransglycosylase/hydrolase increases tightly-bound xyloglucan and chain number but decreases chain length contributing to the defense response that Glycine max has to Heterodera glycines. PLoS ONE 2021, 16, e0244305. [Google Scholar] [CrossRef] [PubMed]
- Shigeyama, T.; Watanabe, A.; Tokuchi, K.; Toh, S.; Sakurai, N.; Shibuya, N.; Kawakami, N. α-Xylosidase plays essential roles in xyloglucan remodelling, maintenance of cell wall integrity, and seed germination in Arabidopsis thaliana. J. Exp. Bot. 2016, 67, 5615–5629. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.K.; Yokoyama, R.; Nishitani, K. A proteomic approach to apoplastic proteins involved in cell wall regeneration in protoplasts of Arabidopsis suspension-cultured cells. Plant Cell Physiol. 2005, 46, 843–857. [Google Scholar] [CrossRef]
- Pocsfalvi, G.; Turiák, L.; Ambrosone, A.; Del Gaudio, P.; Puska, G.; Fiume, I.; Silvestre, T.; Vékey, K. Physiochemical and protein datasets related to citrus juice sac cells-derived nanovesicles and microvesicles. Data Brief 2018, 22, 251–254. [Google Scholar] [CrossRef]
- Bokka, R.; Ramos, A.P.; Fiume, I.; Manno, M.; Raccosta, S.; Turiák, L.; Sugár, S.; Adamo, G.; Csizmadia, T.; Pocsfalvi, G. Biomanufacturing of tomato-derived nanovesicles. Foods 2020, 9, 1852. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M.D.C.; García-Gomez, P.; Yepes-Molina, L.; Guarnizo, A.L.; Teruel, J.A.; Carvajal, M. Plasma membrane aquaporins mediates vesicle stability in broccoli. PLoS ONE 2018, 13, e0192422. [Google Scholar] [CrossRef]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.B.; Wang, B.; Zhang, L.; et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol. Ther. 2018, 21, 1345–1357. [Google Scholar] [CrossRef]
- Gao, C.; Luo, M.; Zhao, Q.; Yang, R.; Cui, Y.; Zeng, Y.; Xia, J.; Jiang, L. A unique plant ESCRT component, FREE1, regulates multivesicular body protein sorting and plant growth. Curr. Biol. 2014, 24, 2556–2563. [Google Scholar] [CrossRef]
- Shen, J.; Gao, C.; Zhao, Q.; Lin, Y.; Wang, X.; Zhuang, X.; Jiang, L. AtBRO1 functions in ESCRT-I complex to regulate multivesicular body protein sorting. Mol. Plant. 2016, 9, 760–763. [Google Scholar] [CrossRef]
- Caillat, C.; Maity, S.; Miguet, N.; Roos, W.H.; Weissenhorn, W. The role of VPS4 in ESCRT-III polymer remodeling. Biochem. Soc. Trans. 2019, 47, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Korbei, B.; Moulinier-Anzola, J.; De-Araujo, L.; Lucyshyn, D.; Retzer, K.; Khan, M.A.; Luschnig, C. Arabidopsis TOL proteins act as gatekeepers for vacuolar sorting of PIN2 plasma membrane protein. Curr. Biol. 2013, 23, 2500–2505. [Google Scholar] [CrossRef] [PubMed]
- Waghmare, S.; Lileikyte, E.; Karnik, R.; Goodman, J.K.; Blatt, M.R.; Jones, A.M.E. SNAREs SYP121 and SYP122 mediate the secretion of distinct cargo subsets. Plant Physiol. 2018, 178, 1679–1688. [Google Scholar] [CrossRef]
- Holland, S.; Roth, R. Extracellular vesicles in the arbuscular mycorrhizal symbiosis: Current understanding and future perspectives. Mol. Plant. Microbe Interact. 2023, 36, 235–244. [Google Scholar] [CrossRef]
- Lefevere, H.; Bauters, L.; Gheysen, G. Salicylic acid biosynthesis in plants. Front. Plant Sci. 2020, 11, 338. [Google Scholar] [CrossRef]
- Poór, P.; Czékus, Z.; Tari, I.; Ördög, A. The multifaceted roles of plant hormone salicylic acid in endoplasmic reticulum stress and unfolded protein response. Int. J. Mol. Sci. 2019, 20, 5842. [Google Scholar] [CrossRef]
- Bulgakov, V.P.; Vereshchagina, Y.V.; Bulgakov, D.V.; Veremeichik, G.N.; Shkryl, Y.N. The rolB plant oncogene affects multiple signaling protein modules related to hormone signaling and plant defense. Sci. Rep. 2018, 8, 2285. [Google Scholar] [CrossRef]
- Chen, A.; He, B.; Jin, H. Isolation of extracellular vesicles from Arabidopsis. Curr. Protoc. 2022, 2, e352. [Google Scholar] [CrossRef]
- Yang, L.H.; Wang, S.L.; Tang, L.L.; Liu, B.; Ye, W.L.; Wang, L.L.; Wang, Z.Y.; Zhou, M.T.; Chen, B.C. Universal stem-loop primer method for screening and quantification of microRNA. PLoS ONE 2014, 9, e115293. [Google Scholar] [CrossRef]
- Shkryl, Y.N.; Veremeichik, G.N.; Bulgakov, V.P.; Avramenko, T.V.; Günter, E.A.; Ovodov, Y.S.; Muzarok, T.I.; Zhuravlev, Y.N. The production of class III plant peroxidases in transgenic callus cultures transformed with the rolB gene of Agrobacterium rhizogenes. J. Biotechnol. 2013, 168, 64–70. [Google Scholar] [CrossRef]
- Veremeichik, G.N.; Shkryl, Y.N.; Pinkus, S.A.; Bulgakov, V.P. Expression profiles of calcium-dependent protein kinase genes (CDPK1-14) in Agrobacterium rhizogenes pRiA4-transformed calli of Rubia cordifolia under temperature- and salt-induced stresses. J. Plant Physiol. 2014, 171, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhou, W.; Petricoin, E.F., 3rd; Longo, C. Mass spectrometry-based biomarker discovery. Methods Mol. Biol. 2012, 823, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; García-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef] [PubMed]
- Gorpenchenko, T.Y.; Veremeichik, G.N.; Shkryl, Y.N.; Yugay, Y.A.; Grigorchuk, V.P.; Bulgakov, D.V.; Rusapetova, T.V.; Vereshchagina, Y.V.; Mironova, A.A.; Subbotin, E.P.; et al. Suppression of the HOS1 gene affects the level of ROS depending on light and cold. Life 2023, 13, 524. [Google Scholar] [CrossRef]
- Barzin, M.; Bagheri, A.M.; Ohadi, M.; Abhaji, A.M.; Salarpour, S.; Dehghannoudeh, G. Application of plant-derived exosome-like nanoparticles in drug delivery. Pharm. Dev. Technol. 2023, 28, 383–402. [Google Scholar] [CrossRef]
- Alzahrani, F.A.; Khan, M.I.; Kameli, N.; Alsahafi, E.; Riza, Y.M. Plant-Derived Extracellular Vesicles and Their Exciting Potential as the Future of Next-Generation Drug Delivery. Biomolecules 2023, 13, 839. [Google Scholar] [CrossRef]
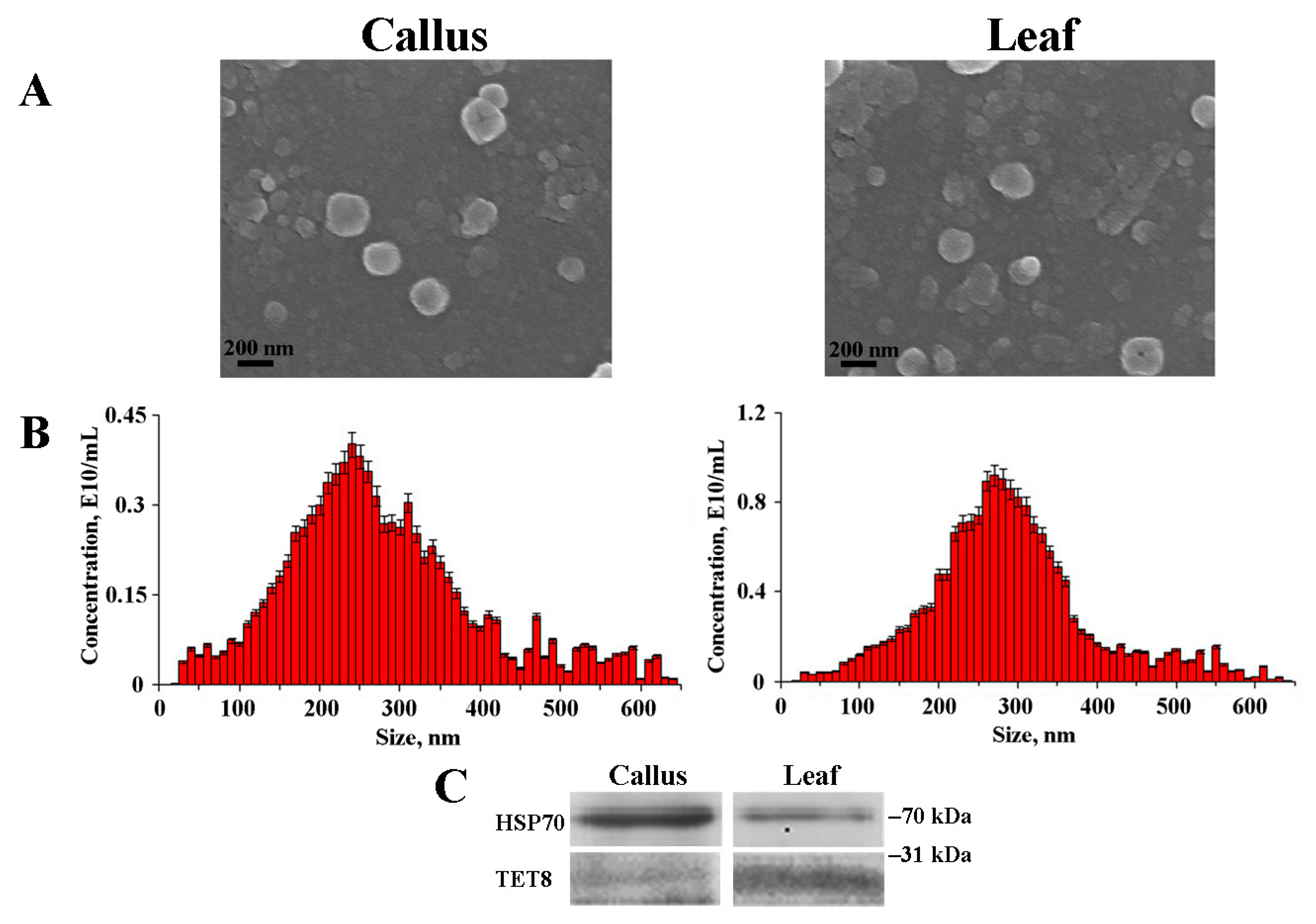


| Samples | Average Size, nm | Ζ-Potential, mV | Concentration, Particles/g FW * |
|---|---|---|---|
| Calli | 222.8 ± 36.5 | −23.8 ± 1.3 | 1.8 × 1010 |
| Leaf apoplastic fluid | 283.6 ± 58.3 | −30.5 ± 2.2 | 2.9 × 1010 |
| Name of Protein | Protein Function | Occurrence in Plant EVs |
|---|---|---|
| Stress responses | ||
| Heat shock 70 kDa protein (HSP70) | Folds de novo synthesized proteins and protect cells from heat stress conditions. | A. thaliana leaf apoplastic fluid [18], C. plantagineum in vitro cultured cells [26], juices from ginger [30], citrus [65], tomato [66], broccoli [67], and grape [68] |
| GDSL esterase/lipase (ESM1) | Regulation of biotic and abiotic stress responses. Possesses antimicrobial and antifungal activities. | A. thaliana leaf apoplastic fluid [18], C. plantagineum in vitro cultured cells [26], and juice from ginger [30] |
| Myrosinase 2 (BGL37) | Involved in the abscisic acid signaling pathway and has redundant functions in glucosinolate breakdown and insect defense. | Juice from ginger [30] |
| Germin-like protein subfamily 3 member 1 (GL31) | Manganese ion binding. May play a role in plant defense. | - |
| Vegetative storage protein 1 (VSP1) | Involved in the jasmonic acid signaling pathway and plays a role in defense against herbivorous insects. | A. thaliana leaf apoplastic fluid [18] |
| Polygalacturonase inhibitor 1 (PGIP1) | Inhibits the pectin-depolymerizing activity of polygalacturonases secreted by microbial pathogens and insects. | C. plantagineum in vitro cultured cells [26], juices from ginger [30] and citrus [65] |
| CCR4-associated factor 1 homolog 4 (CAF1D) | Shows mRNA deadenylation activity and plays a role in plant defense responses to pathogen infections. | - |
| Cell wall remodeling | ||
| UDP-arabinopyranose mutases 1–4 (RGP1, RGP2, RGP3, and RGP4) | Converts UDP-arabinopyranose to UDP-arabinofuranose for cell wall and natural product biosynthesis; also plays a role in the pollen development process. | C. plantagineum in vitro cultured cells [26], juices from citrus [65], tomato [66], and broccoli [67] |
| Xyloglucan endotransglucosylase/hydrolase protein 11 (XTH11) | Participates in cell wall biogenesis, organization, and the xyloglucan metabolic process. | Juices from ginger [30], C. plantagineum in vitro cultured cells [26], and tomato [66] |
| Alpha-xylosidase 1 (XYL1) | Regulation and maintenance of cell wall physical properties. | C. plantagineum in vitro cultured cells [26] and juice from ginger [30] |
| Beta-glucosidases 22 and 23 (BGL22 and BGL23) | Facilitates the hydrolysis of terminal, non-reducing beta-D-glucosyl residues, resulting in the release of beta-D-glucose. It also plays a role in plant protection by releasing scopoletin and other active compounds under both biotic and abiotic stress conditions. | A. thaliana leaf apoplastic fluid [18], C. plantagineum in vitro cultured cells [26], juices from ginger [30], citrus [65], and tomato [66] |
| Various metabolic pathways | ||
| Sucrose synthase 4 (SUS4) | Sucrose-cleaving enzyme that provides UDP-glucose and fructose for various metabolic pathways. | C. plantagineum in vitro cultured cells [26], juices from ginger [30], citrus [65], and tomato [66] |
| Beta carbonic anhydrase 1 (BCA1) | Facilitates the reversible hydration of carbon dioxide, essential for photosynthesis in cotyledons, and is involved in the CO2 signaling pathway. | A. thaliana leaf apoplastic fluid [18], juices from ginger [30], citrus [65], and tomato [66] |
| Phosphoglycerate kinase 3 (PGKY3) | Catalyzes reactions in glycolysis and plays a crucial role in carbohydrate metabolism. | A. thaliana leaf apoplastic fluid [18], C. plantagineum in vitro cultured cells [26], juices from ginger [30], citrus [65], and grape [68] |
| Glyceraldehyde-3-phosphate dehydrogenase GAPC1 (G3PC1) | Participates in the photosynthetic reductive pentose phosphate pathway, crucial for maintaining cellular ATP levels and carbohydrate metabolism. | A. thaliana leaf apoplastic fluid [18], C. plantagineum in vitro cultured cells [26], juices from ginger [30], citrus [65], and tomato [66] |
| Nitrilase 1 and 2 (NRL1 and NRL2) | Plays a key role in the biosynthesis of the plant hormone auxin. It also aids in detoxifying harmful nitriles. | A. thaliana leaf apoplastic fluid [18], juices from ginger [30] and citrus [65] |
| EP1-like glycoprotein 3 (EP1L3) | A protein from the curculin-like lectin family that may be involved in a cell-to-cell programmed cell death (PCD) signaling mechanism. | A. thaliana leaf apoplastic fluid [18], C. plantagineum in vitro cultured cells [26], and juice from ginger [30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yugay, Y.; Tsydeneshieva, Z.; Rusapetova, T.; Grischenko, O.; Mironova, A.; Bulgakov, D.; Silant’ev, V.; Tchernoded, G.; Bulgakov, V.; Shkryl, Y. Isolation and Characterization of Extracellular Vesicles from Arabidopsis thaliana Cell Culture and Investigation of the Specificities of Their Biogenesis. Plants 2023, 12, 3604. https://doi.org/10.3390/plants12203604
Yugay Y, Tsydeneshieva Z, Rusapetova T, Grischenko O, Mironova A, Bulgakov D, Silant’ev V, Tchernoded G, Bulgakov V, Shkryl Y. Isolation and Characterization of Extracellular Vesicles from Arabidopsis thaliana Cell Culture and Investigation of the Specificities of Their Biogenesis. Plants. 2023; 12(20):3604. https://doi.org/10.3390/plants12203604
Chicago/Turabian StyleYugay, Yulia, Zhargalma Tsydeneshieva, Tatiana Rusapetova, Olga Grischenko, Anastasia Mironova, Dmitry Bulgakov, Vladimir Silant’ev, Galina Tchernoded, Victor Bulgakov, and Yury Shkryl. 2023. "Isolation and Characterization of Extracellular Vesicles from Arabidopsis thaliana Cell Culture and Investigation of the Specificities of Their Biogenesis" Plants 12, no. 20: 3604. https://doi.org/10.3390/plants12203604
APA StyleYugay, Y., Tsydeneshieva, Z., Rusapetova, T., Grischenko, O., Mironova, A., Bulgakov, D., Silant’ev, V., Tchernoded, G., Bulgakov, V., & Shkryl, Y. (2023). Isolation and Characterization of Extracellular Vesicles from Arabidopsis thaliana Cell Culture and Investigation of the Specificities of Their Biogenesis. Plants, 12(20), 3604. https://doi.org/10.3390/plants12203604






