Exploring the Phenotypic and Genetic Variabilities in Yield and Yield-Related Traits of the Diallel-Crossed F5 Population of Aus Rice
Abstract
:1. Introduction
2. Results
2.1. Phenotypic Performance of the F5 Aus Rice Population
2.2. Effects of Genetic and Environmental Components on the Agronomical Traits of Aus Rice Population
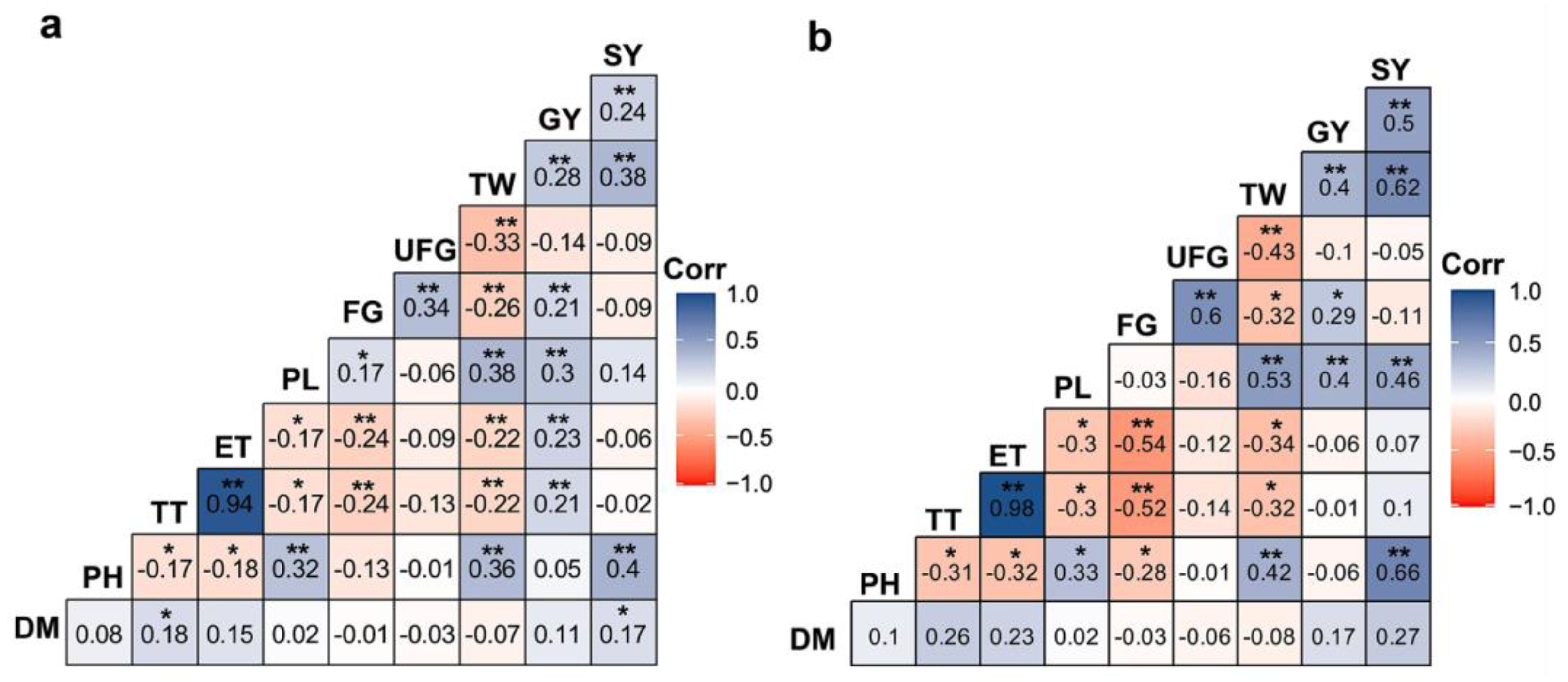
2.3. Phenotype- and Genotype-Based Correlations in the Investigated Agronomical Traits of Aus Rice Population
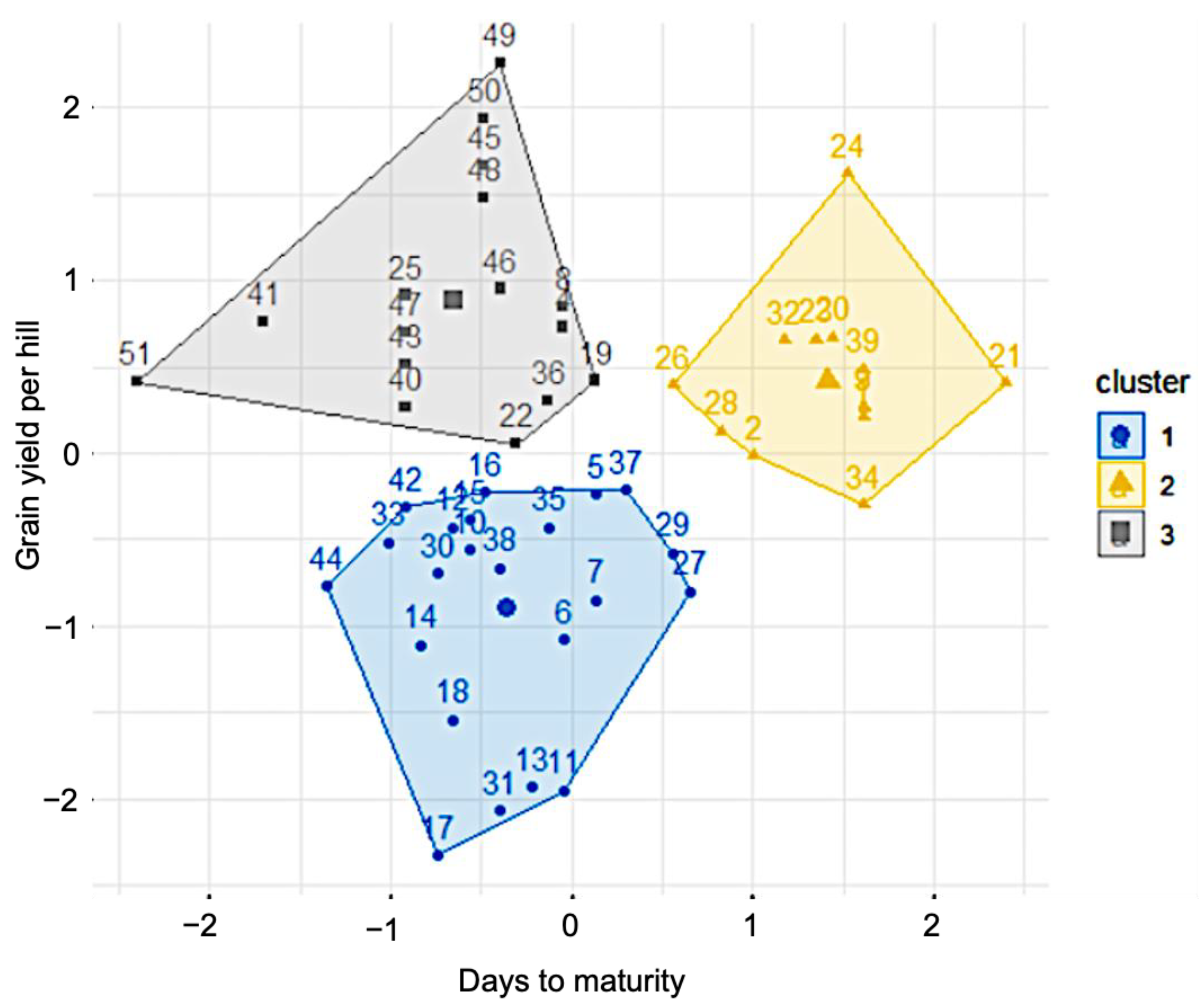
2.4. Trait–Genotype Interactions Using Cluster Analysis
3. Discussion
4. Materials and Methods
4.1. Experimental Details and Materials
4.2. Design of the Experiment and Data Recording
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fukagawa, N.K.; Ziska, L.H. Rice: Importance for global nutrition. J. Nutr. Sci. Vitaminol. 2019, 65, S2–S3. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.S.; Salam, M.U.; Islam, A.; Sarkar, M.A.R.; Mamun, M.A.A.; Rahman, M.C.; Nessa, B.; Kabir, M.J.; Shozib, H.B.; Hossain, M.B. Doubling rice productivity in Bangladesh: A way to achieving SDG 2 and moving forward. Bangladesh Rice J. 2021, 24, 1–47. [Google Scholar] [CrossRef]
- Rahman, M.C.; Rahaman, M.S.; Islam, M.A.; Omar, M.I.; Siddique, M.A.B. Deployment Strategies for Golden Rice in Bangladesh: A Study on Affordability and Varietal Choice with the Target Beneficiaries; Bangladesh Rice Research Institute: Gazipur, Bangladesh, 2021. [Google Scholar] [CrossRef]
- Islam, M.R. Crop calendar mapping of Bangladesh rice paddy field with ALOS-2 ScanSAR data. Adv. Remote Sens. 2021, 10, 115–129. [Google Scholar] [CrossRef]
- Rahman, M.C.; Pede, V.; Balie, J.; Pabuayon, I.M.; Yorobe, J.M.; Mohanty, S. Assessing the market power of millers and wholesalers in the Bangladesh rice sector. J. Agribus. Dev. Emerg. Econ. 2020, 11, 280–295. [Google Scholar] [CrossRef]
- USDA; FAS Foreign Agricultural Service. Russian Federation Grain and Feed Annual. Gain Report number RS1617. Available online: https://apps.fas.usda.gov/newgainapi/api/report/downloadreportbyfilename?filename=Grain%20and%20Feed%20Annual_Moscow_Russian%20Federation_4-18-2016.pdf (accessed on 20 February 2023).
- Shew, A.M.; Durand-Morat, A.; Putman, B.; Nalley, L.L.; Ghosh, A. Rice intensification in Bangladesh improves economic and environmental welfare. Environ. Sci. Policy 2019, 95, 46–57. [Google Scholar] [CrossRef]
- Rahman, N.M.F.; Hasan, M.M.; Hossain, M.I.; Baten, M.A.; Hosen, S.; Ali, M.A.; Kabir, M.S. Forecasting Aus rice area and production in Bangladesh using Box-Jenkins approach. Bangladesh Rice J. 2016, 20, 1–10. [Google Scholar] [CrossRef]
- Shelley, I.J.; Takahashi-Nosaka, M.; Kano-Nakata, M.; Haque, M.S.; Inukai, Y. Rice cultivation in Bangladesh: Present scenario, problems and prospects. J. Int. Coop. Agric. Dev. 2016, 14, 20–29. [Google Scholar] [CrossRef]
- Prasad, B.; Patwary, A.; Biswas, P. Genetic variability and selection criteria in fine rice (Oryza sativa L.). Pak. J. Biol. Sci. 2001, 4, 1188–1190. [Google Scholar]
- Jambhulkar, N.; Bose, L. Genetic variability and association of yield attributing traits with grain yield in upland rice. Genetika 2014, 46, 831–838. [Google Scholar] [CrossRef]
- Picard, C.; Bosco, M. Genotypic and phenotypic diversity in populations of plant-probiotic Pseudomonas spp. colonizing roots. Naturwissenschaften 2007, 95, 1–16. [Google Scholar] [CrossRef]
- Islam, M.N. Chemometrics in nondestructive quality evaluation. In Nondestructive Quality Assessment Techniques for Fresh Fruits and Vegetables; Springer: Berlin/Heidelberg, Germany, 2022; pp. 331–355. [Google Scholar] [CrossRef]
- Anderberg, M.R. Cluster Analysis for Applications: Probability and Mathematical Statistics; A series of Monographs and Textbooks; Academic Press: Cambridge, MA, USA, 2014; Volume 19. [Google Scholar]
- Agahi, K.; Fotokian, M.H.; Farshadfar, E. Correlation and path coefficient analysis for some yield-related traits in rice genotypes (Oryza sativa L.). Asian J. Plant Sci. 2007, 6, 513–517. [Google Scholar] [CrossRef]
- Azam, M.G.; Hossain, M.A.; Sarker, U.; Alam, A.K.M.M.; Nair, R.M.; Roychowdhury, R.; Ercisli, S.; Golokhvast, K.S. Genetic analyses of mungbean [Vigna radiata (L.) Wilczek] breeding traits for selecting superior genotype(s) using multivariate and multi-traits indexing approaches. Plants 2023, 12, 1984. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Nazir, M.F.; Rabnawaz, A.; Mahmood, T.; Safdar, M.E.; Asif, M.; Rehman, A. Estimation of heritability, cortelation and path coefficient analysis in fine grain rice (Oryza sativa L.). J. Anim. Plant Sci. 2011, 21, 660–664. [Google Scholar]
- Demeke, B.; Dejene, T.; Abebe, D. Genetic variability, heritability and genetic advance of morphological, yield related and quality traits in upland rice (Oryza sativa L.) genotypes at pawe, northwestern Ethiopia. Cogent Food Agric. 2023, 9, 2157099. [Google Scholar] [CrossRef]
- Alam, M.A.; Rahman, M.; Ahmed, S.; Jahan, N.; Khan, M.A.A.; Islam, M.R.; Hossain, A. Genetic variation and genotype by environment interaction for agronomic traits in maize (Zea mays L.) hybrids. Plants 2022, 11, 1522. [Google Scholar] [CrossRef]
- Rahman, M.M.; Connor, J.D. The effect of high-yielding variety on rice yield, farm income and household nutrition: Evidence from rural Bangladesh. Agric. Food Secur. 2022, 11, 35. [Google Scholar] [CrossRef]
- Chhogyell, N.; Pradhan, N.; Ghimiray, M.; Bajgai, Y. Evaluation of short duration rice (Oryza sativa L.) varieties as a strategy to cope with climate change. Bhutan Ecol. Soc. 2016, 1, 91–103. [Google Scholar]
- Adhikari, B.N.; Joshi, B.P.; Shrestha, J.; Bhatta, N.R. Genetic variability, heritability, genetic advance and correlation among yield and yield components of rice (Oryza sativa L.). J. Agric. Nat. Resour. 2018, 1, 149–160. [Google Scholar] [CrossRef]
- Idahosa, D.; Alika, J.; Omoregie, A. Genetic variability, heritability and expected genetic advance as indices for yield and yield components selection in cowpea (Vigna unguiculata (L.) Walp. Acad. Arena 2010, 2, 22–26. [Google Scholar]
- Lipi, L.F.; Hasan, M.J.; Akter, A.; Quddus, M.R.; Biswas, P.L.; Ansari, A.; Akter, S. Genetic variation, heritability and genetic advance in some promising rice hybrids. SAARC J. Agric. 2021, 18, 39–49. [Google Scholar] [CrossRef]
- Sivasubramanian, S.S.; Madhava, M.P. Genotypic and phenotypic variability in rice, Madras Agric. J. 1973, 60, 1093–1096. [Google Scholar]
- Johnson, H.W.; Robinson, H.F.; Comstock, R.E. Estimates of genetic and environmental variability in soybeans. Agron. J. 1955, 47, 314–318. [Google Scholar] [CrossRef]
- Khan, M.A.R.; Mahmud, A.; Islam, M.N.; Ghosh, U.K.; Hossain, M.S. Genetic variability and agronomic performances of rice genotypes in different growing seasons in Bangladesh. J. Agric. Food Res. 2023, 14, 100750. [Google Scholar] [CrossRef]
- Tiwari, D.N.; Tripathi, S.R.; Tripathi, M.P.; Khatri, N.; Bastola, B.R. Genetic variability and correlation coefficients of major traits in early maturing rice under rainfed lowland environments of Nepal. Adv. Agric. 2019, 2019, 5975901. [Google Scholar] [CrossRef]
- Seyoum, M.; Alamerew, S.; Bantte, K. Genetic variability, heritability, correlation coefficient and path analysis for yield and yield related traits in upland rice (Oryza sativa L.). J. Plant Sci. 2012, 7, 13–22. [Google Scholar] [CrossRef]
- Sumanth, V.; Suresh, B.; Ram, B.J.; Srujana, G. Estimation of genetic variability, heritability and genetic advance for grain yield components in rice (Oryza sativa L.). J. Pharmacogn. Phytochem. 2017, 6, 1437–1439. [Google Scholar]
- Pratap, A.; Bisen, P.; Loitongbam, B.; Singh, P.K. Assessment of genetic variability for yield and yield components in rice (Oryza sativa L.) germplasms. Int. J. Bio-Resour. Stress Manag. 2018, 9, 87–92. [Google Scholar] [CrossRef]
- Li, X.; Yan, W.; Agrama, H.; Jia, L.; Jackson, A.; Moldenhauer, K.; Yeater, K.; McClung, A.; Wu, D. Unraveling the complex trait of harvest index with association mapping in rice (Oryza sativa L.). PLoS ONE 2012, 7, e29350. [Google Scholar] [CrossRef]
- Srivastava, G.K.; Tripathi, R.S. Response of hybrid and composite rice to number of seedlings and planting geometry. Ann. Agric. Res. 1998, 19, 235–236. [Google Scholar]
- Faysal, A.S.M.; Ali, L.; Azam, M.G.; Sarker, U.; Ercisli, S.; Golokhvast, K.S.; Marc, R.A. Genetic variability, character association and path coefficient analysis in transplant Aman rice genotypes. Plants 2022, 11, 2952. [Google Scholar] [CrossRef]
- Chowhan, S.; Ali, M.K.J.; Nahar, K.; Rahman, M.M.; Ali, M.I.; Islam, M. Yield and morpho-physical characters of some modern Aus rice varieties at Khagrachari. Plant Sci. 2021, 8, 155–160. [Google Scholar] [CrossRef]
- Salam, M. Weed growth and yield of transplant Aus rice (cv. BINAdhan-19) as influenced by spacing of transplanting and level of nitrogen. J. Agric. Food Environ. 2022, 3, 47–53. [Google Scholar] [CrossRef]
- Oladosu, Y.; Rafii, M.Y.; Magaji, U.; Abdullah, N.; Miah, G.; Chukwu, S.C.; Hussin, G.; Ramli, A.; Kareem, I. Genotypic and phenotypic relationship among yield components in rice under tropical conditions. Biomed Res. Int. 2018, 2018, 8936767. [Google Scholar] [CrossRef] [PubMed]
- Brammer, H. Rice Soils of Bangladesh; IRRI: Manila, Philippines, 1978; pp. 35–55. [Google Scholar]
- Azimi, A.M.; Marker, S.; Bhattacharjee, I. Genotypic and phenotypic variability and correlation analysis for yield and its components in late sown wheat (Triticum aestivum L.). J. Pharmacogn. Phytochem. 2017, 6, 167–173. [Google Scholar]
- Burton, G.W. Quantitative inheritance in grasses. In Proceedings of the 6th International Grassland Congress, State College, PA, USA, 17–23 August 1952; pp. 277–283. [Google Scholar]

| Source of Variation | Replication | Genotype | ||
|---|---|---|---|---|
| df (n − 1) | 2 | 50 | ||
| Agronomical Traits | Mean | MD | LSD0.05 | CV (%) |
| Days to maturity (DM) | 106.17 ± 3.85 | 9.35 | 1.20 | 0.70 |
| Plant height (PH) | 114.61 ± 12.83 | 25.75 | 8.12 | 4.38 |
| Number of total tillers per hill (TT) | 13.38 ± 2.65 | 5.19 | 3.17 | 14.63 |
| Number of effective tillers per hill (ET) | 11.72 ± 2.48 | 4.00 | 3.08 | 16.23 |
| Panicle length (PL) | 25.40 ± 2.25 | 3.59 | 2.16 | 5.25 |
| Number of filled grains per panicle (FG) | 122.05 ± 35.86 | 64.39 | 37.31 | 18.87 |
| Number of unfilled grains per panicle (UFG) | 28.73 ± 19.46 | 38.67 | 20.40 | 43.84 |
| 1000-grain weight (TW) | 23.94 ± 3.47 | 3.39 | 1.95 | 5.04 |
| Grain yield per hill (GY) | 23.84 ± 5.57 | 7.67 | 6.37 | 6.50 |
| Straw yield per hill (SY) | 26.06 ± 5.60 | 10.29 | 6.93 | 6.43 |
| Sources of Variation | DM | PH | TT | ET | PL | FG | UFG | TW | GY | SY |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 106.17 | 114.61 | 13.38 | 11.72 | 25.40 | 122.05 | 28.73 | 23.94 | 23.84 | 26.06 |
| σ2e | 0.55 | 25.16 | 3.83 | 3.62 | 1.78 | 530.48 | 158.63 | 1.46 | 15.48 | 18.34 |
| σ2g | 14.41 | 140.99 | 3.06 | 2.35 | 3.35 | 749.17 | 224.77 | 10.75 | 15.64 | 13.02 |
| σ2p | 14.97 | 166.16 | 6.89 | 5.97 | 5.13 | 1279.65 | 383.40 | 12.20 | 31.12 | 31.35 |
| ECV (%) | 0.70 | 4.38 | 14.63 | 16.23 | 5.25 | 18.87 | 43.84 | 5.04 | 16.50 | 16.43 |
| GCV (%) | 3.58 | 10.36 | 13.08 | 13.07 | 7.21 | 22.43 | 52.19 | 13.69 | 16.59 | 13.85 |
| PCV (%) | 3.64 | 11.25 | 19.62 | 20.84 | 8.92 | 29.31 | 68.16 | 14.59 | 23.40 | 21.49 |
| H2 (%) | 96.31 | 84.86 | 44.41 | 39.34 | 65.37 | 58.54 | 58.63 | 88.07 | 50.26 | 41.52 |
| Month | Air Temperature (°C) | Humidity (%) | Rainfall (mm) | Evaporation (mm) | ||
|---|---|---|---|---|---|---|
| Max. | Min. | Ave. | ||||
| March | 34.39 | 21.23 | 27.81 | 81.90 | 4.30 | 121.18 |
| April | 36.05 | 24.13 | 30.09 | 80.57 | 62.01 | 160.30 |
| May | 35.21 | 25.45 | 30.33 | 82.00 | 162.01 | 152.19 |
| June | 32.93 | 26.72 | 29.83 | 87.33 | 490.58 | 151.56 |
| July | 32.82 | 26.98 | 29.90 | 86.45 | 398.21 | 130.91 |
| Mean | 34.28 | 24.90 | 29.59 | 83.65 | 223.42 | 143.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.A.R.; Mahmud, A.; Ghosh, U.K.; Hossain, M.S.; Siddiqui, M.N.; Islam, A.K.M.A.; Anik, T.R.; Rahman, M.M.; Sharma, A.; Abdelrahman, M.; et al. Exploring the Phenotypic and Genetic Variabilities in Yield and Yield-Related Traits of the Diallel-Crossed F5 Population of Aus Rice. Plants 2023, 12, 3601. https://doi.org/10.3390/plants12203601
Khan MAR, Mahmud A, Ghosh UK, Hossain MS, Siddiqui MN, Islam AKMA, Anik TR, Rahman MM, Sharma A, Abdelrahman M, et al. Exploring the Phenotypic and Genetic Variabilities in Yield and Yield-Related Traits of the Diallel-Crossed F5 Population of Aus Rice. Plants. 2023; 12(20):3601. https://doi.org/10.3390/plants12203601
Chicago/Turabian StyleKhan, Md. Arifur Rahman, Apple Mahmud, Uttam Kumar Ghosh, Md. Saddam Hossain, Md. Nurealam Siddiqui, A. K. M. Aminul Islam, Touhidur Rahman Anik, Md. Mezanur Rahman, Anket Sharma, Mostafa Abdelrahman, and et al. 2023. "Exploring the Phenotypic and Genetic Variabilities in Yield and Yield-Related Traits of the Diallel-Crossed F5 Population of Aus Rice" Plants 12, no. 20: 3601. https://doi.org/10.3390/plants12203601
APA StyleKhan, M. A. R., Mahmud, A., Ghosh, U. K., Hossain, M. S., Siddiqui, M. N., Islam, A. K. M. A., Anik, T. R., Rahman, M. M., Sharma, A., Abdelrahman, M., Ha, C. V., Mostofa, M. G., & Tran, L.-S. P. (2023). Exploring the Phenotypic and Genetic Variabilities in Yield and Yield-Related Traits of the Diallel-Crossed F5 Population of Aus Rice. Plants, 12(20), 3601. https://doi.org/10.3390/plants12203601












