Bacterial Strains from Saline Environment Modulate the Expression of Saline Stress-Responsive Genes in Pepper (Capsicum annuum)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacteria Strains
2.2. Evaluation of Bacterial Strains for Growth Promotion in Capsicum annuum
2.3. Stress Salinity and Bacteria Strains Induced Expression Genes
2.3.1. Bacterial Inoculation after Salt Stress
2.3.2. Bacterial Strain Inoculation before Salt Stress
2.4. RNA Extraction
2.5. Relative Gene Expression Levels by qPCR Analysis
2.6. Statistical Analysis
3. Results
3.1. Effect of Bacteria Isolated from Saline Habitats Inoculation on C. annuum Growth
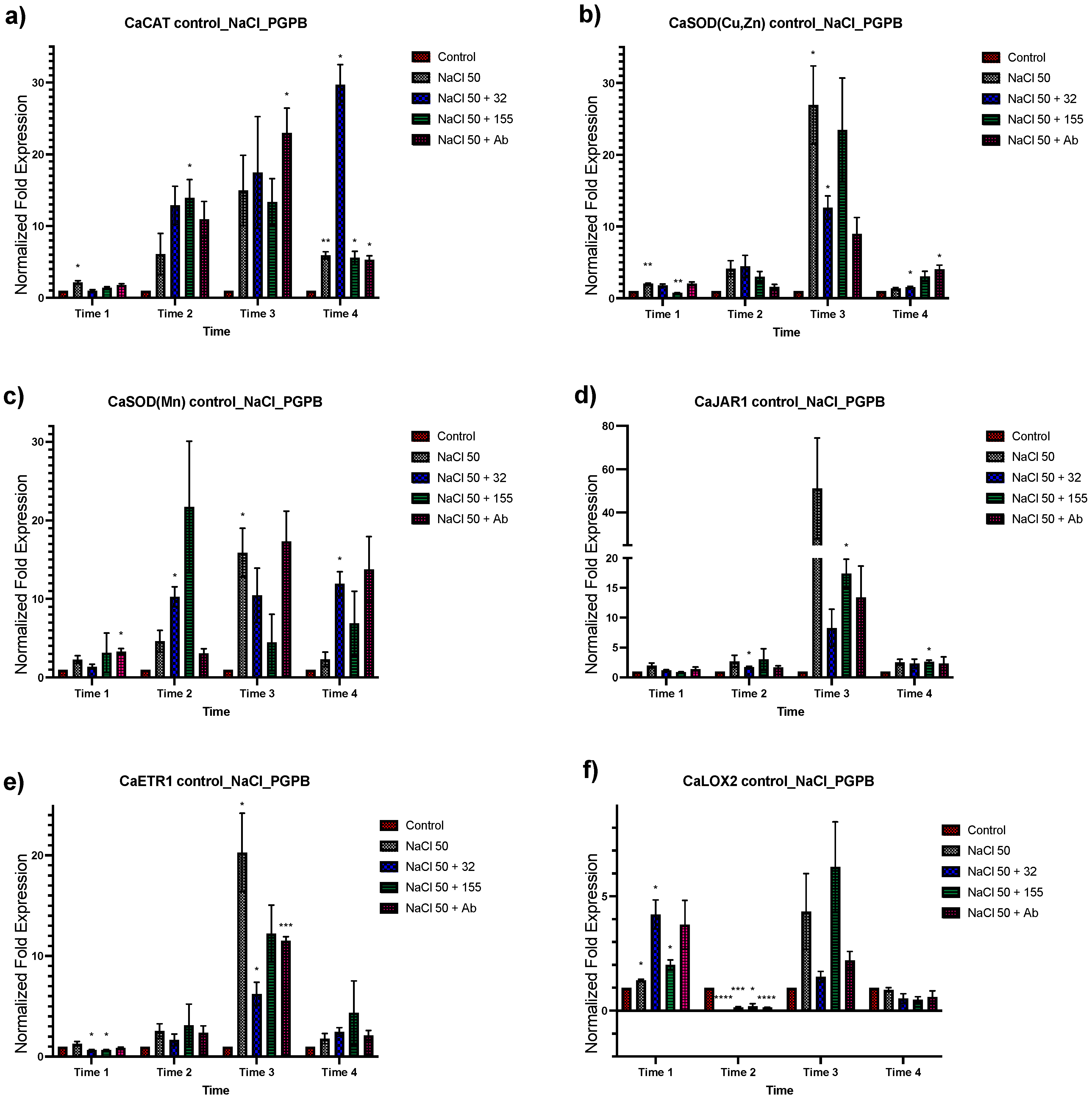
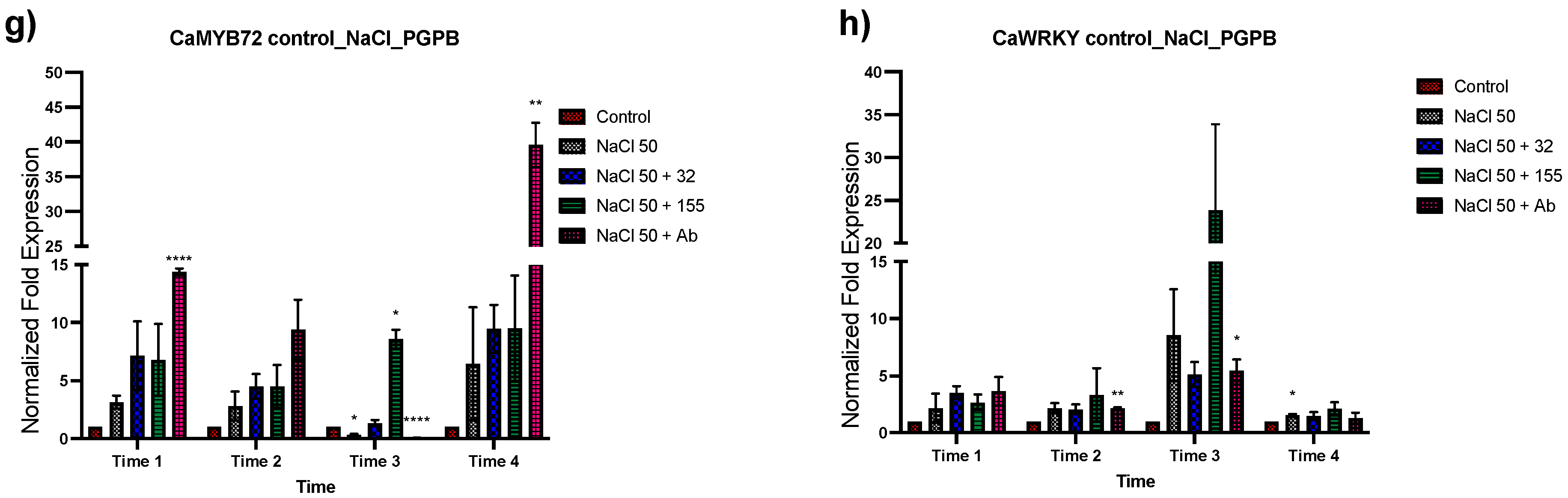
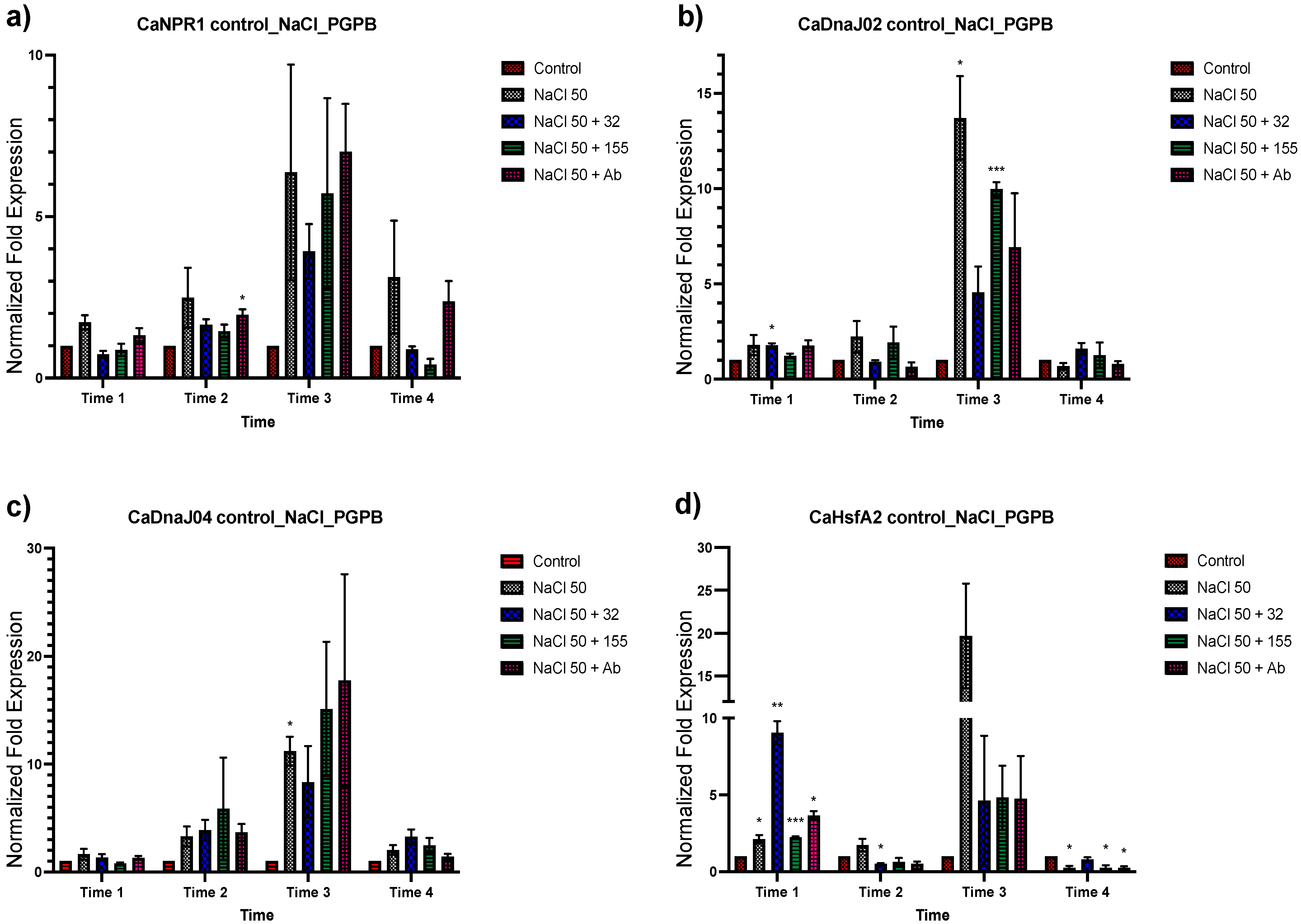
3.2. Expression Analysis of Stress-Related Genes in Plants Inoculated with the Bacterial Strains from Saline Sites
3.3. Effect of Different NaCl Concentrations on Gene Expression of C. annuum
3.4. Expression Analysis of Genes Associated with Salt Tolerance in Plants with Dual Treatment
3.4.1. Bacterial Inoculation after Salt Stress
3.4.2. Bacterial Strain Inoculation before Salt Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.K.; Shabala, S. Mechanisms of plant responses and adaptation to soil salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef] [PubMed]
- Sardar, H.; Khalid, Z.; Ahsan, M.; Naz, S.; Nawaz, A.; Ahmad, R.; Razzaq, K.; Wabaidur, S.M.; Jacquard, C.; Širić, I.; et al. Enhancement of Salinity Stress Tolerance in Lettuce (Lactuca sativa L.) via Foliar Application of Nitric Oxide. Plants 2023, 12, 1115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, G.; Zhao, G.; Xia, C.; Jia, J.; Liu, X.; Kong, X. Characterization of a wheat R2R3-MYB transcription factor gene, TaMYB19, involved in enhanced abiotic stresses in Arabidopsis. Plant Cell Physiol. 2014, 55, 1802–1812. [Google Scholar] [CrossRef] [PubMed]
- Baillo, E.H.; Kimotho, R.N.; Zhang, Z.; Xu, P. Transcription factors associated with abiotic and biotic stress tolerance and their potential for crops improvement. Genes 2019, 10, 771. [Google Scholar] [CrossRef]
- Tang, Y.; Bao, X.; Zhi, Y.; Wu, Q.; Guo, Y.; Yin, X.; Zeng, L.; Li, J.; Zhang, J.; He, W.; et al. Overexpression of a MYB family gene, OsMYB6, increases drought and salinity stress tolerance in transgenic rice. Front. Plant Sci. 2019, 10, 168. [Google Scholar] [CrossRef]
- Ruiz, K.B.; Maldonado, J.; Biondi, S.; Silva, H. RNA-seq analysis of salt-stressed versus non salt-stressed transcriptomes of Chenopodium quinoa Landrace R49. Genes 2019, 10, 1042. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Yang, X.; Cheng, Y.; Kang, Y.; Chai, X. The DnaJ gene family in pepper (Capsicum annuum L.): Comprehensive identification, characterization and expression profiles. Front. Plant Sci. 2017, 8, 689. [Google Scholar] [CrossRef]
- Jacob, P.; Hirt, H.; Bendahmane, A. The heat-shock protein/chaperone network and multiple stress resistance. Plant Biotech. J. 2017, 15, 405–414. [Google Scholar] [CrossRef]
- Pérez-Salamó, I.; Papdi, C.; Rigó, G.; Zsigmond, L.; Vilela, B.; Lumbreras, V.; Nagy, I.; Horváth, B.; Domoki, M.; Darula, Z.; et al. The heat shock factor A4A confers salt tolerance and is regulated by oxidative stress and the mitogen-activated protein kinases MPK3 and MPK6. Plant Physiol. 2014, 165, 319–334. [Google Scholar] [CrossRef]
- Širić, I.; Eid, E.M.; Taher, M.A.; El-Morsy, M.H.E.; Osman, H.E.M.; Kumar, P.; Adelodun, B.; Abou Fayssal, S.; Mioč, B.; Andabaka, Ž.; et al. Combined Use of Spent Mushroom Substrate Biochar and PGPR Improves Growth, Yield, and Biochemical Response of Cauliflower (Brassica oleracea var. botrytis): A Preliminary Study on Greenhouse Cultivation. Horticulturae 2022, 8, 830. [Google Scholar] [CrossRef]
- Barthi, N.; Pandey, S.S.; Barnawal, D.; Patel, V.K.; Kalra, A. Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci. Rep. 2016, 6, 34768. [Google Scholar] [CrossRef]
- Chu, T.N.; Tran, B.T.H.; Bui, L.V.; Hoang, M.T.T. Plant growth-promoting rhizobacterium Pseudomonas PS01 induces salt tolerance in Arabidopsis thaliana. BMC Res. Notes 2019, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kim, M.S.; Sun, Y.; Dowd, S.E.; Shi, H.; Paré, P.W. Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT1. Mol. Plant-Microbe Interact. 2008, 21, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Siddikee, M.D.A.; Glick, B.R.; Chauhan, P.S.; Yim, W.J.; Sa, T. Enhancement of growth and salt tolerance of red pepper seedlings (Capsicum annuum L.) by regulating stress ethylene synthesis with halotolerant bacteria containing 1-aminocyclopropane-1-carboxylic acid deaminase activity. Plant Physiol. Biochem. 2011, 49, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Nabti, E.; Sahnoune, M.; Ghoul, M.; Fischer, D.; Hofmann, A.; Rothballer, M.; Schmid, M.; Hartmann, A. Restoration of growth of durum wheat (Triticum durum var. waha) under saline conditions due to inoculation with the rhizosphere bacterium Azospirillum brasilense NH and extracts of the marine alga Ulva lactuca. J. Plant Growth Regul. 2010, 29, 6–22. [Google Scholar] [CrossRef]
- Barraza, A.; Caamal-Chan, M.G.; Castellanos, T.; Loera-Muro, A. Bacterial community characterization of the rhizobiome of plants belonging to Solanaceae family cultivated in desert soils. Ann. Microbiol. 2020, 70, 34. [Google Scholar] [CrossRef]
- Zacarías-Pérez, Y. Evaluación de Bacterias Halotolerantes de Ambientes Extremos Salinos para Mitigar el Estrés Salino y Promover el Crecimiento en Plantas de Chile (Capsicum annuum L.) var. Caballero. Bachelor’s Thesis, National Technology of Mexico, Technological Institute of La Paz, La Paz, Mexico, 2017. [Google Scholar]
- Castellanos-Cervantes, T.; Díaz De León, J.; Ling, J.; Röder, M. Physiological and genetic analysis of a mapping population responsiveness to plant growth-promoting Azospirillum in wheat. Terra Latinoam. 2023, 41, 1–29. [Google Scholar] [CrossRef]
- Barraza, A.; Estrada-Navarrete, G.; Rodriguez-Alegria, M.E.; Lopez-Munguia, A.; Merino, E.; Quinto, C.; Sanchez, F. Down-regulation of PvTRE1 enhances nodule biomass and bacteroid number in the common bean. New Phytol. 2013, 197, 194–206. [Google Scholar] [CrossRef]
- Wan, H.; Yuan, W.; Ruan, M.; Ye, Q.; Wang, R.; Li, Z.; Zhou, G.; Yao, Z.; Zhao, J.; Liu, S.; et al. Identification of reference genes for reverse transcription quantitative real time PCR normalization in pepper (Capsicum annuum L.). Biochem. Biophys. Res. Comm. 2011, 416, 24–30. [Google Scholar] [CrossRef]
- Guo, M.; Lu, J.P.; Zhai, Y.F.; Chai, W.G.; Gong, Z.H.; Lu, M.H. Genome-wide analysis, expression profile of heat shock factor gene Family (CaHsfs) and characterization of CaHsfA2 in pepper (Capsicum annuum L.). BMC Plant Biol. 2015, 15, 151. [Google Scholar] [CrossRef]
- Huh, S.U.; Lee, G.J.; Jung, J.H.; Kim, Y.; Kim, Y.J.; Paek, K.H. Capsicum annuum transcription factor WRKYa positively regulates defense response upon TMV infection and is a substrate of CaMK1 and CaMK2. Sci. Rep. 2015, 5, 7981. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Pastrana, R.; Arcos-Ortega, F.; Souza-Perera, R.; Sánchez-Borges, C.; Nakazawa-Ueji, Y.; Garcia-Villalobos, F.; Guzmán-Antonio, A.; Zúñiga-Aguilar, J. Ethylene, but not salicylic acid or methyl jasmonate, induces a resistance response against Phytophthora capsici in habanero pepper. Eur. J. Plant Pathol. 2011, 131, 669–683. [Google Scholar] [CrossRef]
- Mejía-Teniente, L.; de Dalia Duran-Flores, F.; Chapa-Oliver, A.M.; Torres-Pacheco, I.; Cruz-Hernández, A.; González-Chavira, M.M.; Ocampo-Velázquez, R.V.; Guevara-González, R.G. Oxidative and molecular responses in Capsicum annuum L. after hydrogen peroxide, salicylic acid and chitosan foliar applications. Int. J. Mol. Sci. 2013, 14, 10178–10196. [Google Scholar] [CrossRef]
- De-Bashan, L.E.; Hernandez, J.P.; Bashan, Y.; Maler, R. Bacillus pumilus ES4: Candidate plant growth-promoting bacterium to enhance establishment of plants in mine tailings. Environ. Exp. Bot. 2010, 69, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Dillon, J.G.; Carlin, M.; Gutierrez, A.; Nguyen, V.; McLain, N. Patterns of microbial diversity along a salinity gradient in the Guerrero Negro solar saltern, Baja CA Sur, Mexico. Front. Microbiol. 2013, 4, 399. [Google Scholar] [CrossRef]
- Ventosa, A.; de la Haba, R.R.; Sánchez-Porro, C.; Papke, R.T. Microbial diversity of hypersaline environments: A metagenomic approach. Curr. Opin. Microbiol. 2015, 25, 80–87. [Google Scholar] [CrossRef]
- Etesami, H.; Beattie, G.A. Mining halophytes for plant growth-promoting halotolerant bacteria to enhance the salinity tolerance of non-halophytic crops. Front. Microbiol. 2018, 9, 148. [Google Scholar] [CrossRef]
- Tanasupawat, S.; Hashimoto, Y.; Ezaki, T.; Kozaki, M.; Komagata, K. Staphylococus piscifermentans sp. nov., from fermented fish in Thailand. Int. J. Syst. Bacteriol. 1992, 42, 577–581. [Google Scholar] [CrossRef]
- Hassan, A.; Mahgoub. Salt inducible-proteins and conjugal gene transfer of halotolerant Staphylococcus isolated from salinity soil. Egypt. J. Genet. Cytol. 2011, 40, 263–280. [Google Scholar] [CrossRef]
- Kim, J.S.; Makama, M.; Petito, J.; Park, N.H.; Cohan, F.M.; Dungan, R.S. Diversity of bacteria and archaea in hypersaline sediment from Death Valley National Park, California. Microbiologyopen 2012, 1, 135–148. [Google Scholar] [CrossRef]
- Ghyselinck, J.; Velivelli, S.L.S.; Heylen, K.; O’Herlihy, E.; Franco, J.; Rojas, M.; De Vos, P.; Prestwich, B.D. Bioprospecting in potato fields in the central Andean highlands: Screening of rhizobacteria for plant growth-promoting properties. Syst. Appl. Microbiol. 2013, 36, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, Z.; He, Y.; Huang, Y.; Li, X.; Ye, B.C. Plant growth promoting and alleviation of salinity stress in Capsicum annuum L. by Bacillus isolated from saline soil in Xinjiang. Ecotoxicol. Environ. Saf. 2018, 64, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Chatterjee, S.; Behera, B.K.; Dangar, T.K.; Das, B.K.; Mohapatra, T. Isolation and characterization of marine bacteria from East Coast of India: Functional screening for salt stress tolerance. Heliyon 2019, 5, e01869. [Google Scholar] [CrossRef] [PubMed]
- Vega, C.; Rodríguez, M.; Llamas, I.; Béjar, V.; Sampedro, I. Silencing of phytopathogen communication by the halotolerant PGPR Staphylococcus equrum strain EN21. Microorganisms 2020, 881, 42. [Google Scholar] [CrossRef]
- Hyder, S.; Gondal, A.S.; Rizvi, Z.F.; Ahmad, R.; Alam, M.M.; Hannan, A.; Ahmed, W.; Fatima, N.; Inam-UI-Haq, M. Characterization of native plant growth promoting rhizobacteria and their antioomycete potential against Phytophthora capsici affecting chilli pepper (Capsicum annum L.). Sci. Rep. 2020, 10, 13859. [Google Scholar] [CrossRef]
- Kang, Y.; Shen, M.; Wang, H.; Zhao, Q. A possible mechanism of action of plant growth-promoting rhizobacteria (PGPR) strain Bacillus pumilus WP8 via regulation of soil bacterial community structure. J. Gen. Appl. Micribiol 2013, 59, 267–277. [Google Scholar] [CrossRef]
- Araujo, N.A.F.; Brandao, R.M.; Barguil, M.B.; Cardoso, M.G.; Pasqual, M.; Rezende, R.A.L.S.; Pereira, M.M.A.; Buttrós, V.H.T.; Dória, J. Plant growth-promoting bacteria improve growth and modify essential oil in Rose (Rosa hybrid L.) cv. Black prince. Front Sustain. Food Syst. 2020, 18, 606827. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Mukherjee, A.; Rastogi, R.P.; Verma, J.P. Salt-tolerant plant growth promoting Bacillus pumilus strain JPVS11 to enhance plant growth attributes of rice and improve soil health under salinity stress. Microbiol. Res. 2021, 242, 126616. [Google Scholar] [CrossRef]
- Masood, S.; Zhao, X.Q.; Shen, R.F. Bacillus pumilus promotes the growth and nitrogen uptake of tomato plants under nitrogen fertilization. Sci. Hortic. 2020, 272, 109581. [Google Scholar] [CrossRef]
- Mora-Ruiz, M.R.; Alejandre-Colomo, C.; Ledger, T.; González, B.; Orfila, A.; Rosselló-Móra, R. Non-halophilic endophytes associated with the euhalophyte Arthrocnemum macrostachyum and their plant growth promoting activity potential. FEMS Microbiol. Lett. 2018, 365, fny208. [Google Scholar] [CrossRef]
- Pinedo, I.; Ledger, T.; Greve, M.; Poupin, M. Burkholderia phytofirmans PsJN induces long-term metabolic and transcriptional changes involved in Arabidopsis thaliana salt tolerance. Front. Plant Sci. 2015, 6, 466. [Google Scholar] [CrossRef]
- Cuong, D.M.; Kwon, S.J.; Nguyen, B.V.; Chun, S.W.; Kim, J.K.; Park, S.U. Effect of salinity stress on phenylpropanoid genes expression and related gene expression in Wheat Sprout. Agronomy 2020, 10, 390. [Google Scholar] [CrossRef]
- Liang, Q.Y.; Wu, Y.H.; Wang, K.W.; Bai, Z.Y.; Liu, Q.L.; Pan, Y.Z.; Zhang, L.; Jiang, B.B. Chrysanthemum WRKY gene DgWRKY5 enhances tolerance to salt stress in transgenic chrysanthemum. Sci. Rep. 2017, 7, 4799. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.K.; Handa, A.K.; Mattoo, A.K. Transcript abundance patterns of 9- and 13-lipoxygenase subfamily genes members in response to abiotic stresses (heat, cold, drought or salt) in tomato (Solanum lycopersicum L.) highlights member-specific dynamics relevant to each stress. Genes 2019, 10, 683. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Jang, Y.; Lee, S.M.; Oh, B.T.; Chae, J.C.; Lee, K.J. Alleviation of salt stress by Enterobacter sp. EJ01 in tomato and Arabidopsis is accompanied by up regulation of conserved salinity responsive factors in plants. Mol. Cell 2014, 28, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Timmermann, T.; González, B.; Ruz, G.A. Reconstruction of a gene regulatory network of the induced systemic resistance defense response in Arabidopsis using Boolean networks. BMC Bioinform. 2020, 21, 142. [Google Scholar] [CrossRef]
- Poupin, M.J.; Timmermann, T.; Vega, A.; Zuñiga, A.; González, B. Effects of the plant growth-promoting bacterium Burkholderia phytofirmans PsJN throughout the life cycle of Arabidopsis thaliana. PLoS ONE 2013, 8, e69435. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopat. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- Sharma, S.; Chen, C.; Navathe, S.; Chand, R.; Pandey, S.P. A halotolerant growth promoting rhizobacteria triggers induced systemic resistance in plants and defends against fungal infection. Sci. Rep. 2019, 9, 4054. [Google Scholar] [CrossRef]
- Andrási, N.; Rigó, G.; Zsigmond, L.; Pérez-Salamó, I.; Papdi, C.; Klement, E.; Pttkó-Szandtner, A.; Baba, A.I.; Ayaydin, F.; Dasari, R.; et al. The mitogen-activated protein kinase 4-phosphorylated heat shock factor A4a regulates responses to combined salt and heat stresses. J. Exp. Bot. 2019, 70, 4903–4917. [Google Scholar] [CrossRef]
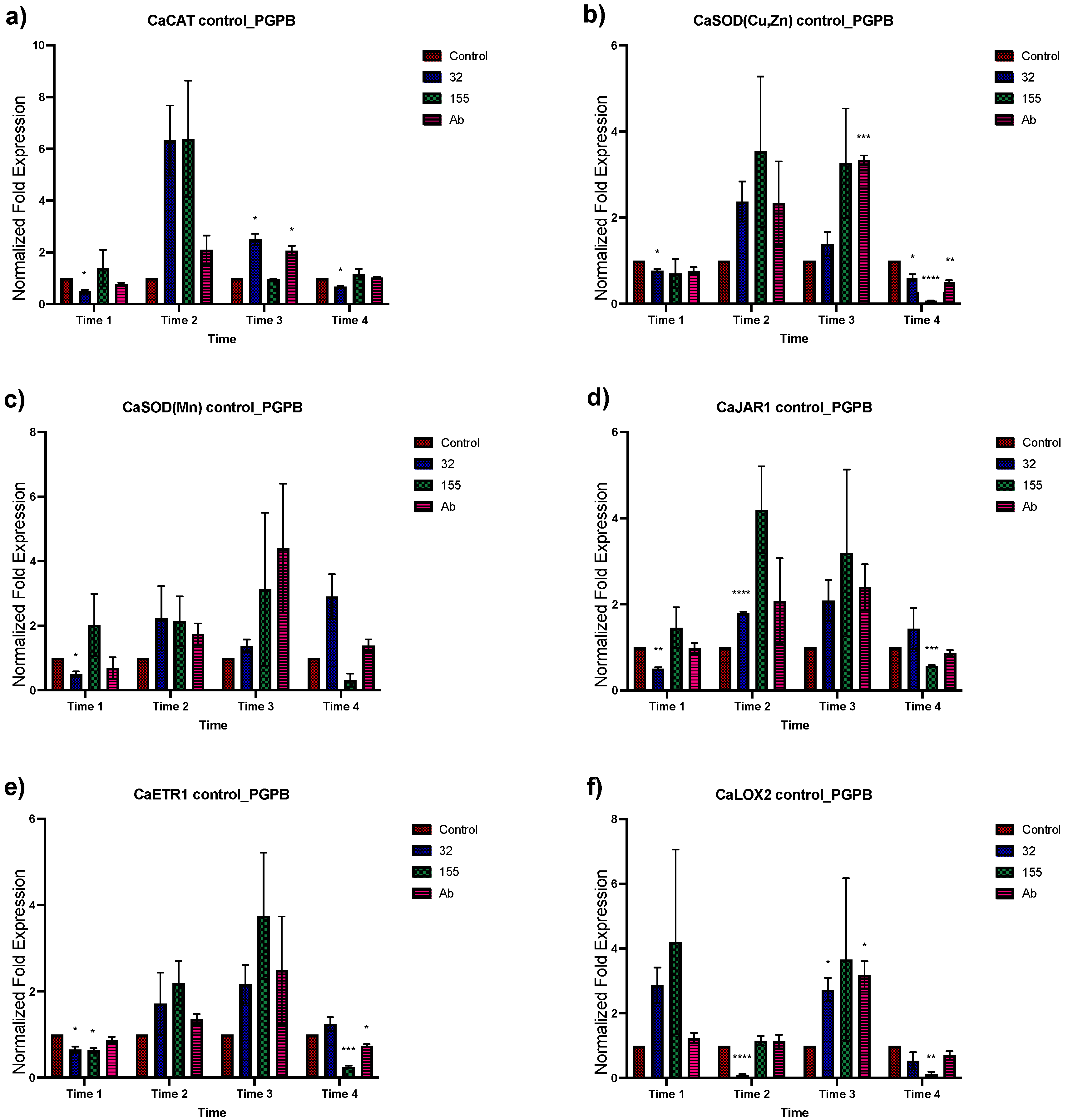

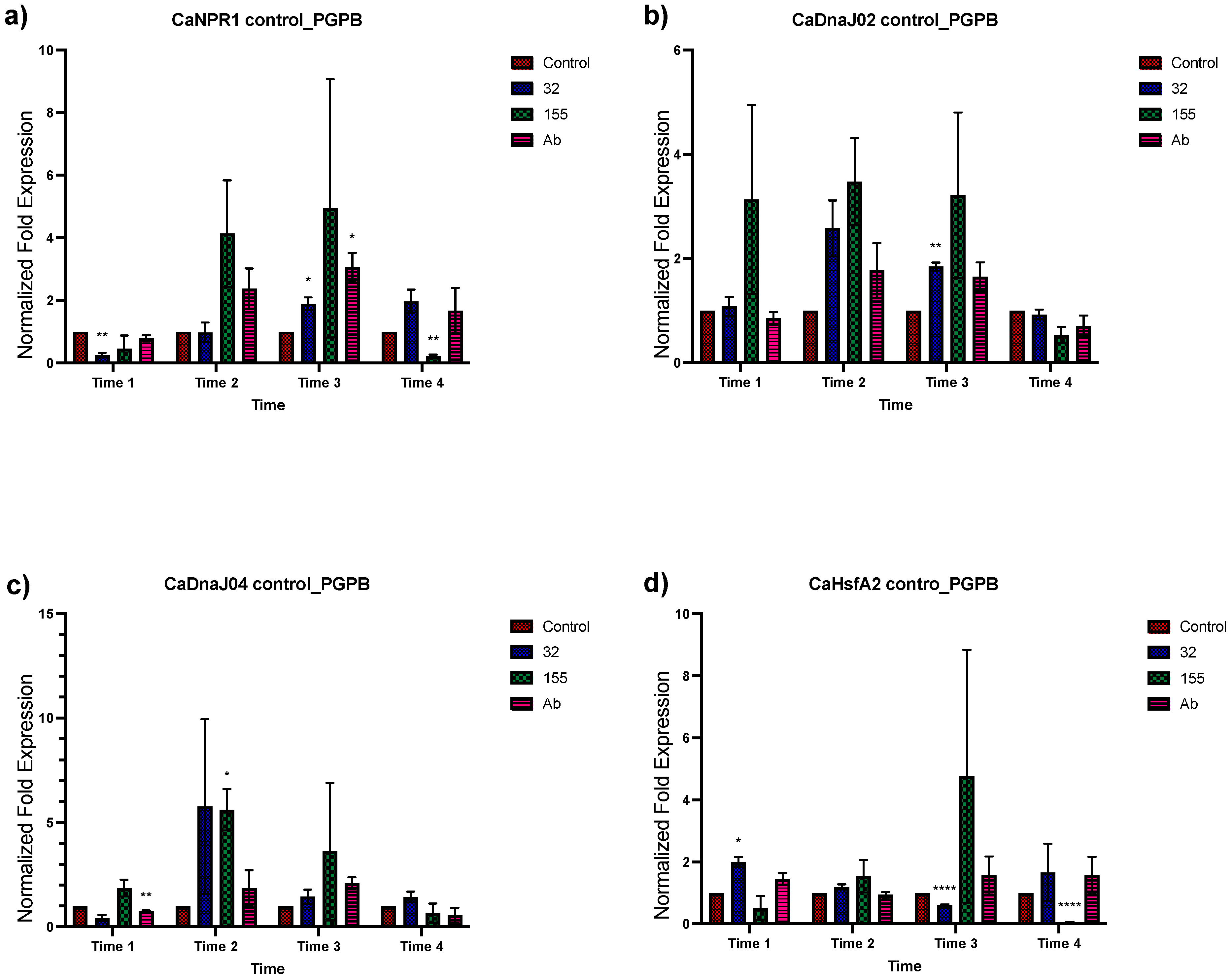

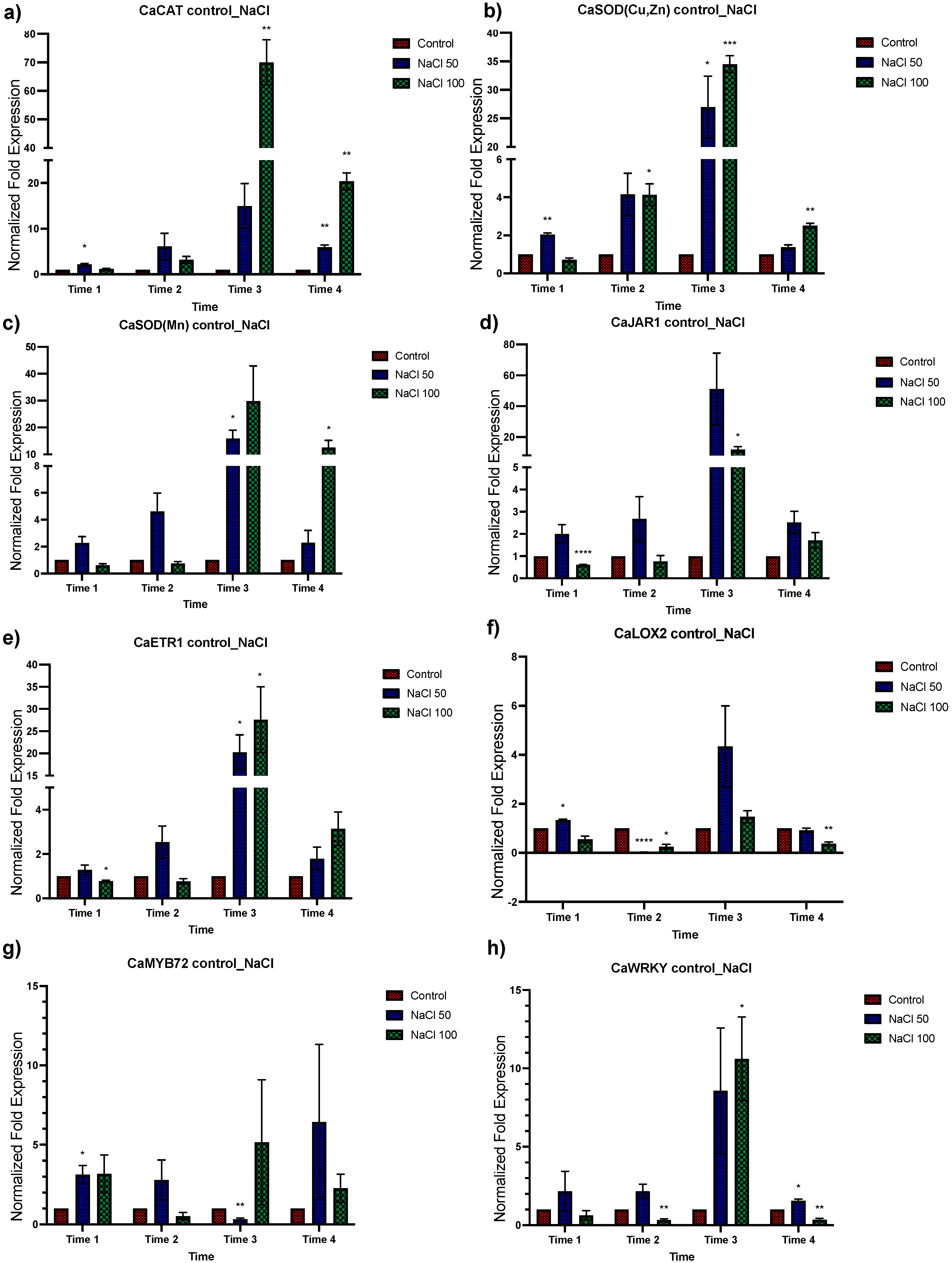
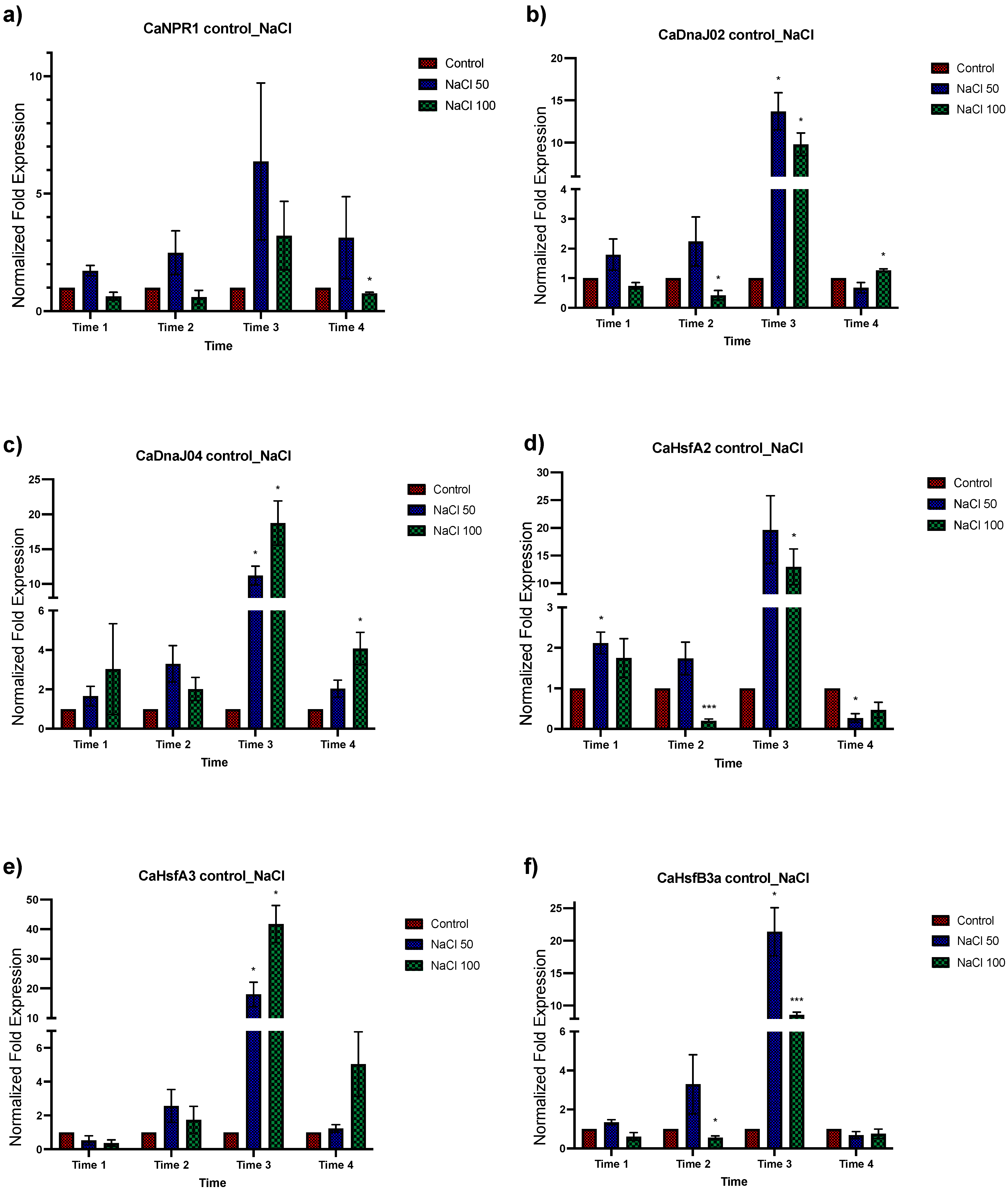

| Name | Primer Sequence | References |
|---|---|---|
| CaDnaJ02-F CaDnaJ02-R | 5′-GCTGGTCATGCATCTGCTGT-3′ 5′-CACTTGGCCGTTTGGACGAA-3′ | [7] |
| CaDnaJ04-F CaDnaJ04-R | 5′-GGCTTGTGTGCCAAAGGCTA-3′ 5′-GGCCGCAGCTACCAGACTTA-3′ | [7] |
| CaHsfA2-F CaHsfA2-R | 5′-GTAGCATCAGTAGCCACAGC-3′ 5′-CAAGCAACTCTTCCCAAATA-3′ | [21] |
| CaHsfA3-F CaHsfA3-R | 5′-CGAAAGTATGATGAAAGAAGAGG-3′ 5′-ATAGTTGCCAAGACCACCC-3′ | [21] |
| CaHsfB3a-F CaHsfB3a-R | 5′-CGACCGACGACATCGTTT C-3′ 5′-TTGTCATTGCTGAACTCCC-3′ | [21] |
| CaUBI-3-F CaUBI-3-R | 5′-TGTCCATCTGCTCTCTGTTG-3′ 5′-CACCCCAAGCACAATAAGAC-3′ | [20] |
| CaMYB72-F CaMYB72-R | 5′-GTCCTCTGGAGTGAGGAAAGGTGCATGGACTGA-3′ 5′-ATCTATTCAGACCAGCTCTAATGGGAACAAGATGCCACTTTCCT-3′ | Designed in this work |
| CaWRKYa-F CaWRKYa-R | 5′-AATTACGAATTCAATTAACAAAGAT-3′ 5′-ATGGAAGAGTATTGGAATTGTTA-3′ | [22] |
| CaLox2-F CaLox2-R | 5′-CGAGCTGTAGTTACGGTAAGGAACAAGAACAAGGAAGATCTG-3′ 5′-GTGTTTGGATCGATGTCGGTGCTGATGAGTTCTAAGGCG-3′ | Designed in this work |
| CaETR1-F CaETR1-R | 5′-CCACATCATTCCTGATTTACTTAGCGTCAAAACTAGGGAG-3′ 5′-CATTCTAACATGTCTACCTGTCTCCTCTTGAGTCCGAATAATACCCA-3′ | Designed in this work |
| CaJAR1-F CaJAR1-R | 5′-CCCTCAGACTTTTAAGGCTTGTGTTCCTCTTGTCACTCA-3′ 5′-CTTCCCTGAGTGGTACCAGAACTTAATGAGATGGTTGTAATG-3′ | Designed in this work |
| CaNPR1-F CaNPR1-R | 5′-GCACAGAGGACAACAGTGGA-3′ 5′-TCAGTGAACGCTTTGGTCAG-3′ | [23] |
| CaCAT-F CaCAT-R | 5′-GTCCATGAGCGTGGAAGCCCCGAAT-3′ 5′-CGCGATGCATGAAGTTCATGGCACC-3′ | [24] |
| CaSOD(Mn)-F CaSOD(Mn)-R | 5′-CTCTGCCATAGACACCAACTT-3′ 5′-CCAAGTTCGGTCCTTTAATAA-3′ | [21] |
| CaSOD(Cu,Zn)-F CaSOD(Cu,Zn)-R | 5′-GTCCTTAGCAGCAGTGAATGTGTTAGTGGCACCATCCTC-3′ 5′-GCCATGAAGTCCAGGTTTTAGGCCAGAGACATTTCCGGTAACTG-3′ | Designed in this work |
| Treat | Length | (cm) | Fresh | Weight | (g Plant−1) | Dry | Weight | (g Plant−1) |
|---|---|---|---|---|---|---|---|---|
| Stem | Root | Stem | Root | Leaf | Stem | Root | Leaf | |
| Not bacteria | 24.30 ± 1.125 | 24.40 ± 0.67 | 2.5 ± 0.22 | 3.8 ± 0.33 | 4.5 ± 0.31 | 0.5 ± 0.0 | 0.6 ± 0.1 | 2.3 ± 0.09 |
| Strain AB | 25.80 ± 0.7348 | 19.6 ± 0.92 ** | 2.8 ± 0.12 | 5.4 ± 0.96 | 4.7 ± 0.2 | 0.5 ± 0.0 | 0.7 ± 0.12 | 2.5 ± 0.09 |
| Strain 32 | 28.50 ± 0.73 * | 21.20 ± 0.73 * | 3.2 ± 0.12 * | 8.7 ± 0.51 *** | 5.4 ± 0.10 * | 0.35 ± 0.07 | 0.98 ± 0.02 ** | 2.5 ± 0.133 |
| Strain 155 | 25.90 ± 0.60 | 19.4 ± 0.24 *** | 2.7 ± 0.2 | 7.4 ± 0.76 ** | 4.8 ± 0.2 | 0.5 ± 0.0.37 | 0.66 ± 0.14 | 2.5 ± 0.133 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caamal-Chan, M.G.; Loera-Muro, A.; Romero-Geraldo, R.D.J.; Ramírez-Serrano, R. Bacterial Strains from Saline Environment Modulate the Expression of Saline Stress-Responsive Genes in Pepper (Capsicum annuum). Plants 2023, 12, 3576. https://doi.org/10.3390/plants12203576
Caamal-Chan MG, Loera-Muro A, Romero-Geraldo RDJ, Ramírez-Serrano R. Bacterial Strains from Saline Environment Modulate the Expression of Saline Stress-Responsive Genes in Pepper (Capsicum annuum). Plants. 2023; 12(20):3576. https://doi.org/10.3390/plants12203576
Chicago/Turabian StyleCaamal-Chan, María Goretty, Abraham Loera-Muro, Reyna De Jesús Romero-Geraldo, and Rogelio Ramírez-Serrano. 2023. "Bacterial Strains from Saline Environment Modulate the Expression of Saline Stress-Responsive Genes in Pepper (Capsicum annuum)" Plants 12, no. 20: 3576. https://doi.org/10.3390/plants12203576
APA StyleCaamal-Chan, M. G., Loera-Muro, A., Romero-Geraldo, R. D. J., & Ramírez-Serrano, R. (2023). Bacterial Strains from Saline Environment Modulate the Expression of Saline Stress-Responsive Genes in Pepper (Capsicum annuum). Plants, 12(20), 3576. https://doi.org/10.3390/plants12203576






