A New Species from the Canary Islands Increases the Diversity of the Red Algal Genus Pterocladiella in the Northeastern Atlantic
Abstract
1. Introduction
2. Results
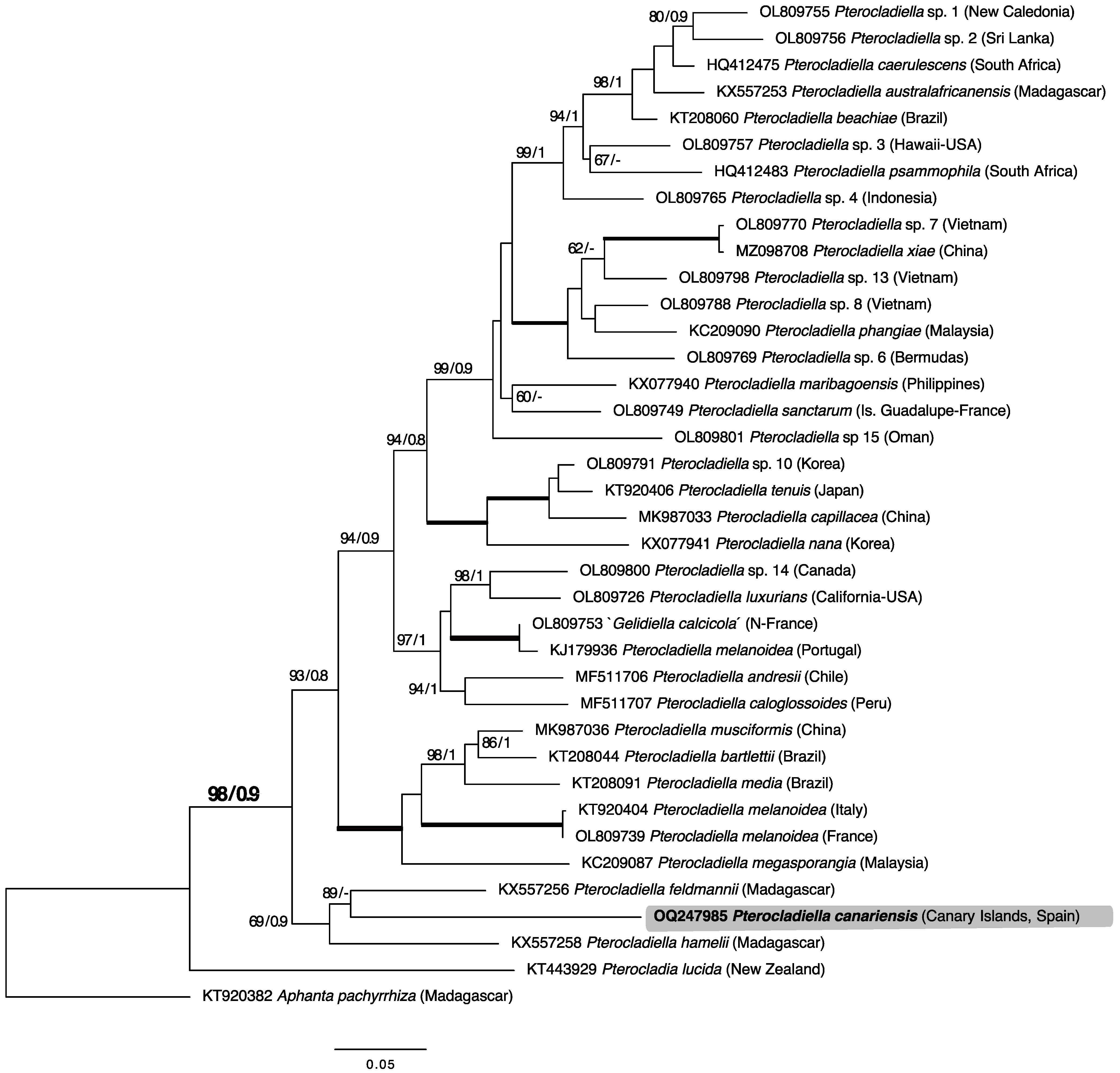
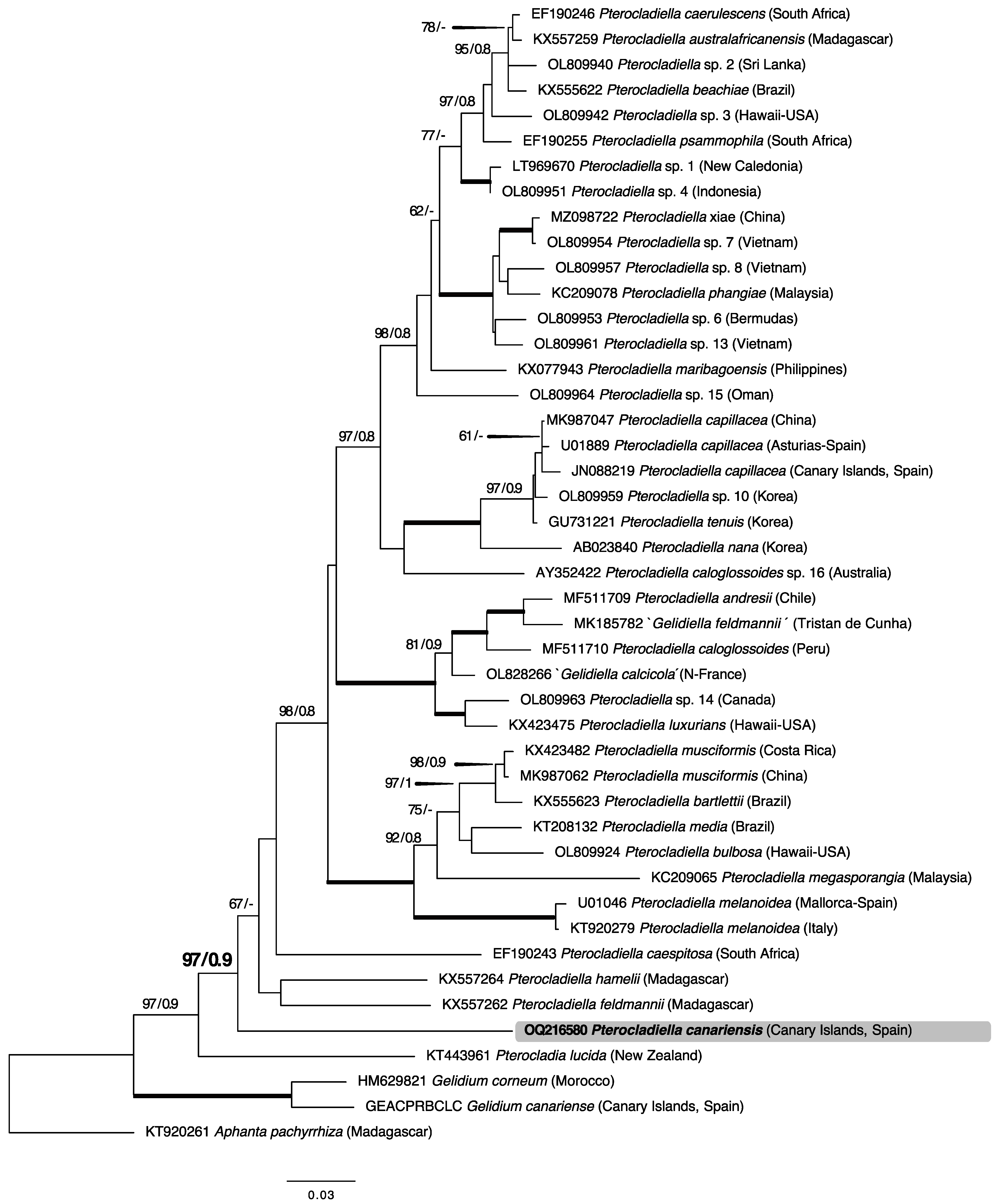
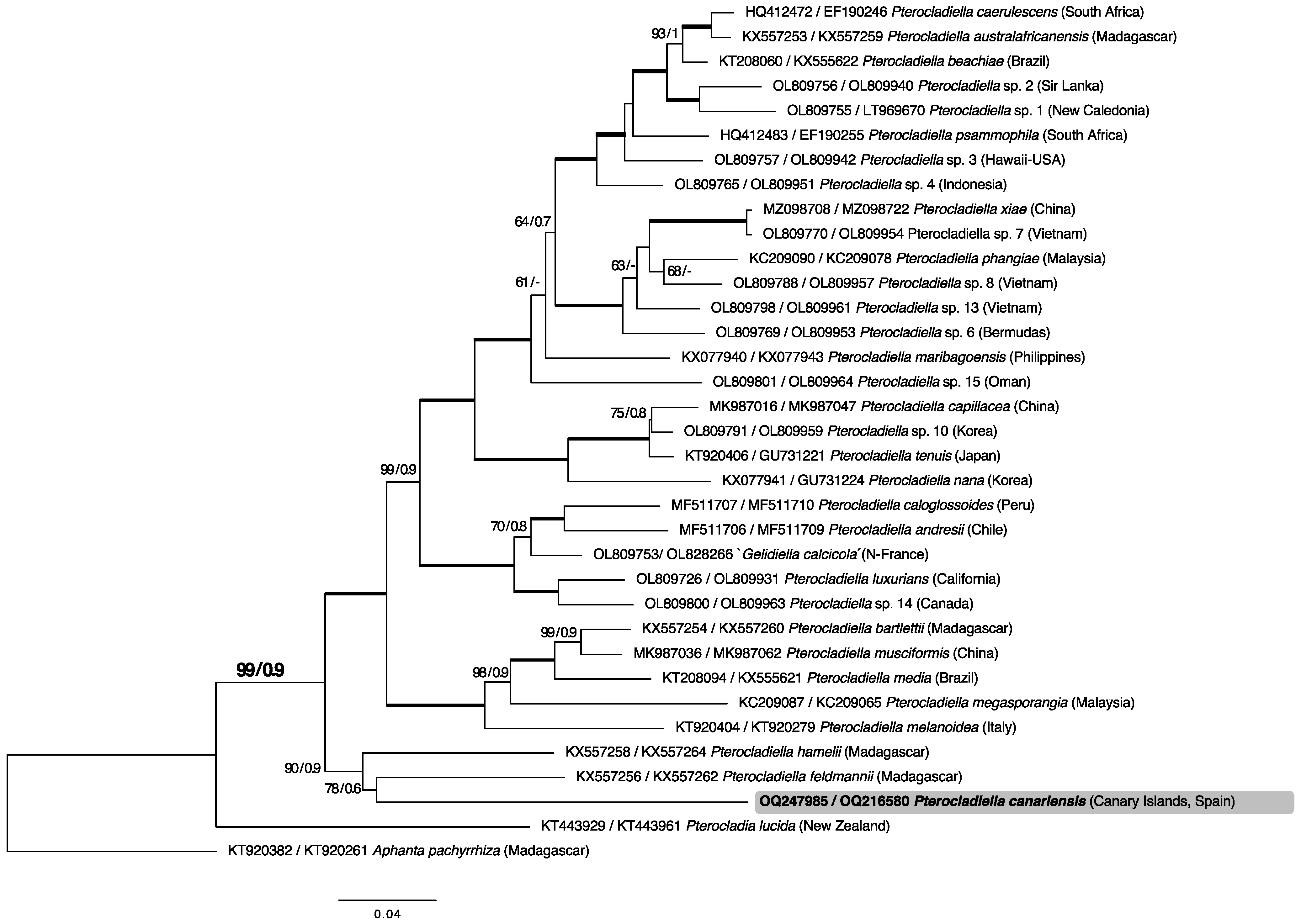
2.1. Phylogenetic Analyses
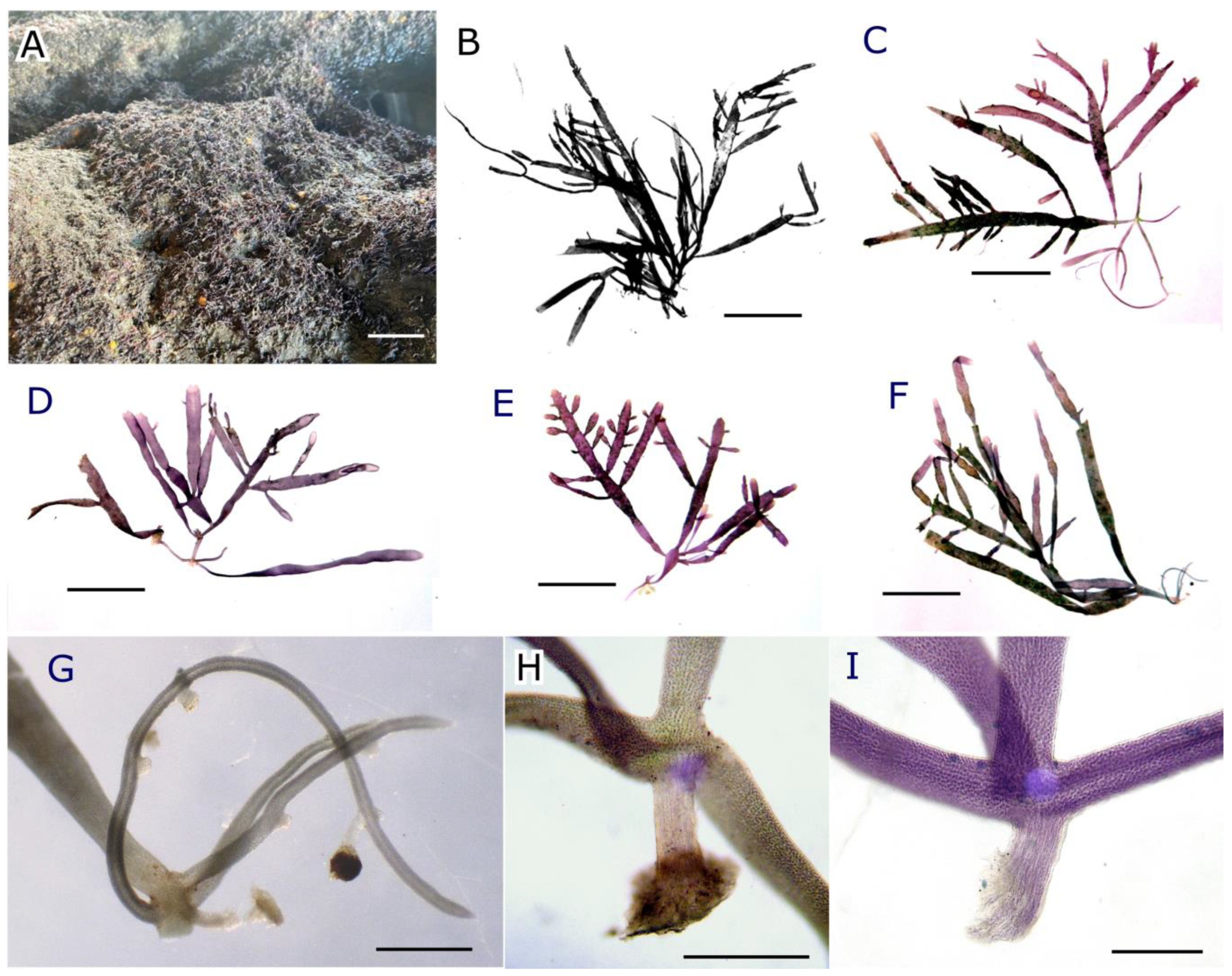
2.2. Morphological Analysis
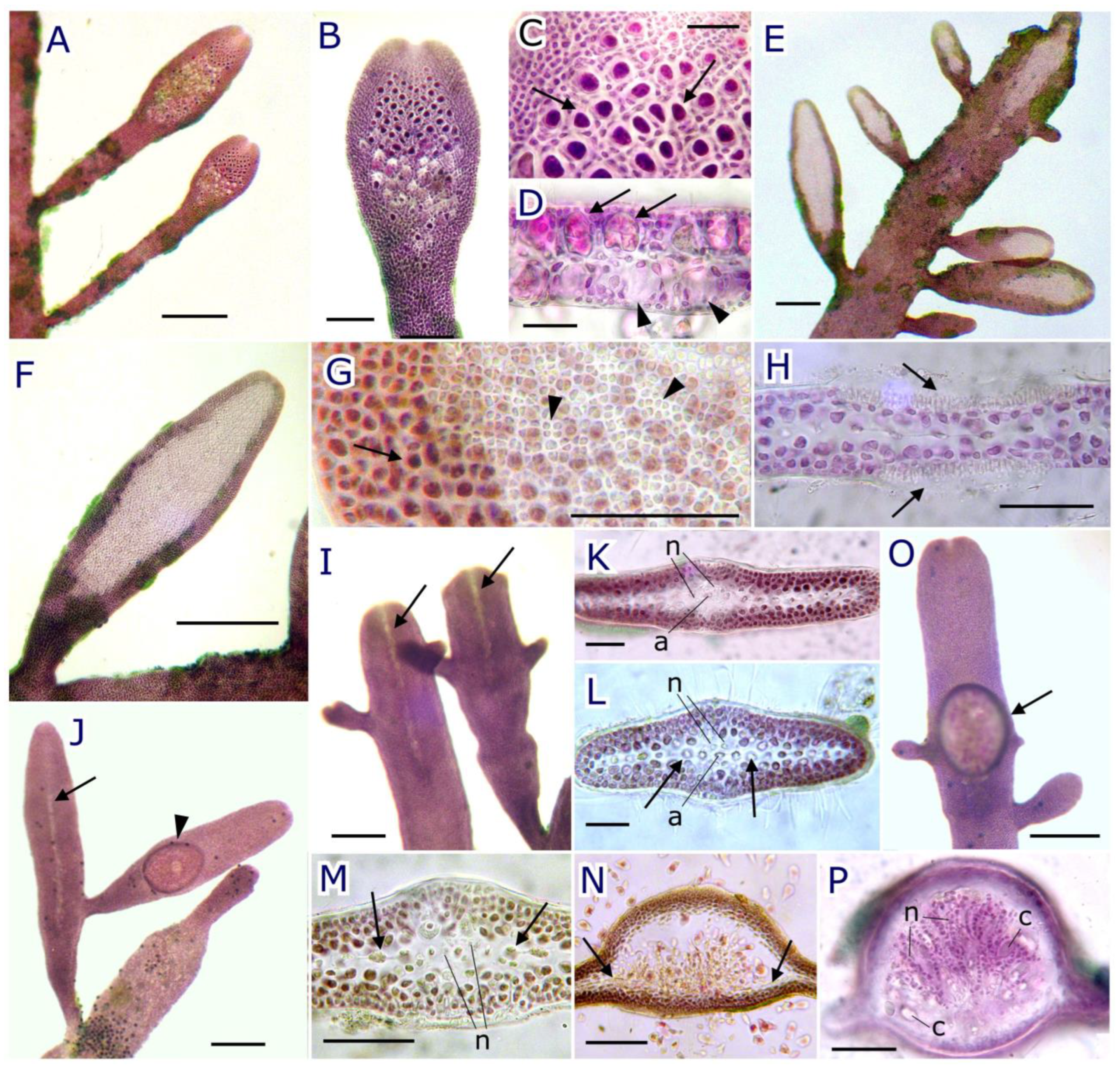
2.2.1. Habit and Vegetative Morphology
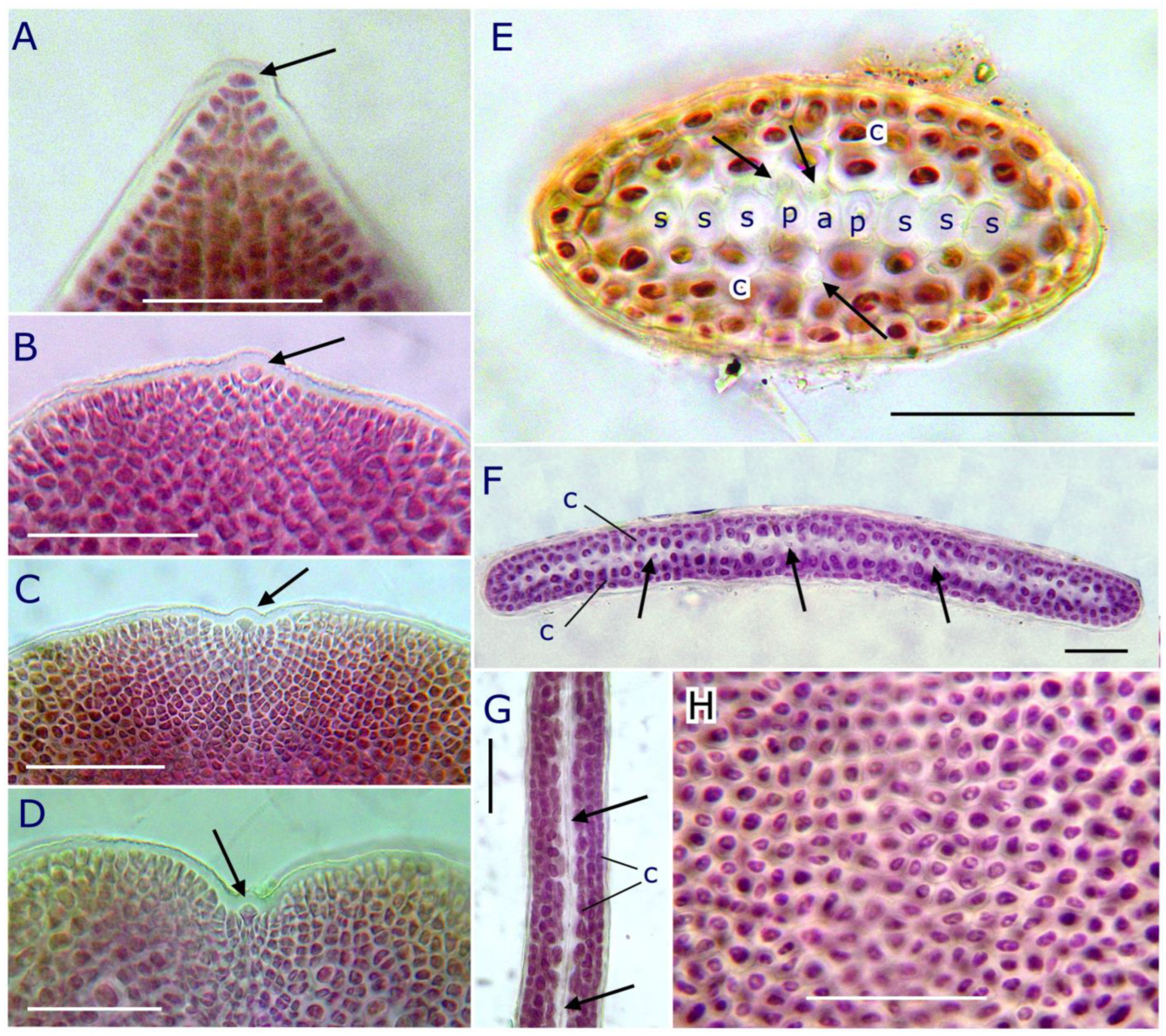
2.2.2. Reproductive Morphology and Seasonality
2.3. Pterocladiella canariensis N.M. Rancel-Rodríguez, J. Afonso-Carrillo, A. Tronholm and M. Sansón sp. nov.
3. Discussion
| Species/ References | Type Locality/ Distribution | Plant Size (mm)/ Branching | Erect Axes Shapes | Cortical Cells: Shape and Size in SV (µm) | Internal Rhizoidal Filaments | Cystocarp: Shape (TS)/ Placental Tissue Position in Cystocarp Cavity | Spermatangia: Sorus Margin/Spermatangial Arrangement | Tetrasporangia: Sorus Margin/Tetrasporangial Arrangement |
|---|---|---|---|---|---|---|---|---|
| Pterocladiella australafricanensis Tronchin and Freshwater [12,13,39] | South Africa/ Western Indo-Pacific and Western Atlantic | ≤30/alternate pinnate up to two orders | lanceolate to ligulate, compressed | circular to elliptical 9.5–17 | many in the outer medulla | elliptical/ central | n.d. | absent/ irregular |
| P. bartlettii (W.R.Taylor) Santelices [39] | Haiti/ Western Atlantic, Central Indo-Pacific, and Western Indo-Pacific | ≤80/opposite to irregular up to four orders | linear, narrow, compressed to cylindrical | circular 7–13 | few in the central medulla | elliptical/ close to the floor | n.d. | absent/ V-shaped rows |
| P. beachiae Freshwater in Thomas and Freshwater [10,39] | Costa Rica/ Western Atlantic | ≤60/pinnate to irregular up to three orders | ligulate, compressed | circular to elliptical 8–15 | many in the central medulla | elliptical/ central | present/ one or both branch surfaces | absent/ irregular |
| P. caespitosa (Kylin) Santelices [57] | South Africa/ South Africa | ≤30/ rare to irregularly alternate up to two orders | thin, slightly compressed to flattened | rounded up to 15 | many in the central medulla | circular/ close to the floor | n.d. | present/ irregular to V-shaped rows |
| P. canariensis N.M. Rancel-Rodríguez, J. Afonso-Carrillo, A. Tronholm and M. Sansón [Present study] | Canary Islands/ Canary Islands | ≤18/sparse and irregularly branched up to two orders | compressed and ribbon-like, attenuate and subcylindrical towards the base | polygonal 3–6 | few in the inner cortex of basal portions | circular/ close to the floor | present/ both branch surfaces | present/ V-shaped rows |
| P. capillacea (S.G.Gmelin) Santelices and Hommersand [3,11,52] | Mediterranean Sea/ Pacific, Atlantic, and Mediterranean Sea | ≤200/pinnate up to four orders | slightly compressed to flattened | circular 4.5–9 | many in the central medulla | triangular and slightly rostrate/ central | present/ both branch surfaces | present/ irregular to horizontal rows |
| P. feldmannii G.H.Boo, L.Le Gall, I.K.Hwang and S.M.Boo [2] | Madagascar/ Madagascar | ≤60/opposite to irregular up to two (three) orders | linear and terete to compressed | polygonal 5–10 × 10–13 | many in inner cortical layers | n.d. | n.d. | absent/ irregular |
| P. hamelii G.H.Boo, L.Le Gall, I.K.Hwang and S.M.Boo [2] | Madagascar/ Madagascar | ≤30/ rare and irregular | thin and compressed | ovoid to rounded 12–13 × 14.3–20 | few in the medulla | n.d. | n.d. | present/ irregular to horizontal rows |
| P. media (E.Y.Dawson) G.H.Boo and K.A.Miller [38,39,49] | California/ Eastern Pacific and Western Atlantic | ≤40/irregular and distal up to three orders | linear and flattened | elliptical 4–7 × 7.5–9.5 | rare in the medulla of basal portion of erect axes | elliptical/ central | n.d. | absent/ irregular to V-shaped rows |
| P. melanoidea (Schousboe ex Bornet) Santelices and Hommersand [45,46,47,48,49] | Morocco/ North-eastern Atlantic and Mediterranean Sea | ≤40/irregular, opposite at right angles to pinnate up to two orders | thin, subcylindrical to flattened | ovoid 5–8 × 6–12 | few in the medulla of basal portions | triangular/ close to the floor | present/ both branch surfaces | present/ V-shaped rows |
| P. sanctarum (Feldman and Hamel) Santelices [9,54] | Guadeloupe, West Indies/ Western Atlantic | ≤20/ few and irregularly branched up to two orders | thin and slightly compressed, ending narrower and cylindrical | square 10–12 | few in the medulla of basal portions | n.d. | n.d. | present/ irregular to horizontal rows |
| ‘Gelidiella calcicola’ Maggs and Guiry [21,22,51] | Ireland/ NE Atlantic | ≤30/ few to densely branched up to two orders | thin, terete to flattened | polygonal 5–18 | few in medulla of stolons | triangular/ close to the floor | n.d. | absent/ V-shaped rows |
| ‘Gelidiella feldmannii’ Baardseth [55,56] | Tristan da Cunha/Tristan da Cunha | ≤15/ irregular, opposite at right angles to pinnate up to two orders | thin, terete, or flattened | n.d. 5–12 | few in the medulla | n.d. | n.d. | present/ V-shaped rows |
4. Materials and Methods
4.1. Taxon Sampling and Morphological Analyses
4.2. DNA Extraction, Sequencing, and Phylogenetic Analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sohrabipour, J.; Lim, P.E.; Maggs, C.A.; Phang, S.M. Two new species and two new records of Pterocladiella (Gelidiales) from Malaysia based on analyses of rbcL and cox1 gene sequences. Phycologia 2013, 52, 517–537. [Google Scholar] [CrossRef]
- Boo, G.H.; Le Gall, L.; Hwang, I.K.; Boo, S.M. Pterocladiella feldmannii sp. nov. and P. hamelii sp. nov. (Gelidiales, Rhodophyta), two new species uncovered in Madagascar during the Atimo Vatae expedition. Cryptogam. Algol. 2016, 37, 179–198. [Google Scholar] [CrossRef]
- Patarra, R.F.; Iha, C.; Pereira, L.; Neto, A.I. Concise review of the species Pterocladiella capillacea (S.G.Gmelin) Santelices & Hommersand. J. Appl. Phycol. 2020, 32, 787–808. [Google Scholar] [CrossRef]
- Santelices, B.; Hommersand, M. Pterocladiella, a new genus in the Gelidiaceae (Gelidiales, Rhodophyta). Phycologia 1997, 36, 114–119. [Google Scholar] [CrossRef]
- Freshwater, D.W.; Fredericq, S.; Hommersand, M.H. A molecular phylogeny of the Gelidiales (Rhodophyta) based on the analysis of plastid rbcL nucleotide sequences. J. Phycol. 1995, 31, 616–632. [Google Scholar] [CrossRef]
- Hommersand, M.H.; Fredericq, S. Vegetative and reproductive development of Pterocladia capillacea (Gelidiales, Rhodophyta) from La Jolla, California. Nova Hedwig. Beih. 1996, 112, 147–160. [Google Scholar]
- Santelices, B. The sexual reproductive development of Pterocladiella bulbosa (Loomis) comb. nov. (Gelidiales, Rhodophyta). Cryptogam. Algol. 1997, 18, 297–307. [Google Scholar]
- Santelices, B. Taxonomic review of the species of Pterocladia (Gelidiales, Rhodophyta). J. Appl. Phycol. 1998, 10, 237–251. [Google Scholar] [CrossRef]
- Santelices, B. Testing the usefulness of attachment structures in the taxonomy of small-sized gelidioids. Phycologia 2007, 46, 293–299. [Google Scholar] [CrossRef]
- Thomas, D.T.; Freshwater, D.W. Studies of Costa Rican Gelidiales (Rhodophyta): Four Caribbean taxa including Pterocladiella beachii sp. nov. Phycologia 2001, 40, 340–350. [Google Scholar] [CrossRef]
- Xia, B.M.; Tseng, C.K.; Wang, Y.Q. Studies on Chinese species of Gelidiella and Pterocladiella (Gelidiales, Rhodophyta). Hydrobiologia 2004, 512, 201–207. [Google Scholar] [CrossRef]
- Tronchin, E.M.; Freshwater, D.W. Four Gelidiales (Rhodophyta) new to Southern Africa, Aphanta pachyrrhiza gen. et sp. nov., Gelidium profundum sp. nov., Pterocladiella caerulescens and P. psammophila sp. nov. Phycologia 2007, 46, 325–348. [Google Scholar] [CrossRef]
- Freshwater, D.W.; Tudor, K.; O’Shaughnessy, K.; Wysor, B. DNA barcoding in the red algal Order Gelidiales: Comparison of COI with rbcL and verification of the “barcoding gap”. Cryptogam. Algol. 2010, 31, 435–449. [Google Scholar]
- Boo, G.H.; Geraldino, P.J.L. Pterocladiella maribagoensis (Gelidiales, Rhodophyta), a new marine alga from Cebu, Philippines. Phytotaxa 2016, 288, 239–248. [Google Scholar] [CrossRef]
- Boo, G.H.; Calderon, M.S.; Boo, S.M. A new marine alga, Pterocladiella andresii sp. nov. (Gelidiales, Rhodophyta) and its relationship to P. caloglossoides from Pacific South America. Phytotaxa 2017, 319, 139–148. [Google Scholar] [CrossRef]
- Wang, X.L.; Yan, S.H.; Wang, Y.Q.; Sun, Z.M.; Xia, B.M.; Wang, G.C. Study of the phylogeny and distribution of Pterocladiella (Pterocladiaceae, Rhodophyta) from China. Phycologia 2020, 59, 165–176. [Google Scholar] [CrossRef]
- Yan, S.; Wang, X.; Wang, G. Pterocladiella xiae sp. nov. (Gelidiales, Rhodophyta), a new species from southern China. Phycologia 2022, 61, 166–174. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. Available online: https://www.algaebase.org (accessed on 27 September 2022).
- Boo, G.H.; Leliaert, F.; Le Gall, L.; Coppejans, E.; De Clerck, O.; Van Nguyen, T.; Payri, C.E.; Miller, K.A.; Yoon, H.S. Ancient Tethyan vicariance and long-distance dispersal drive global diversification and cryptic speciation in the red seaweed Pterocladiella. Front. Plant Sci. 2022, 13, 849476. [Google Scholar] [CrossRef]
- Rodríguez-Prieto, C.; Ballesteros, E.; Boisset, F.; Afonso-Carrillo, J. Guía de Las Macroalgas y Fanerógamas Marinas del Mediterráneo Occidental; Omega: Barcelona, Spain, 2013; pp. 1–656. [Google Scholar]
- Maggs, C.A.; Guiry, M.D. Gelidiella calcicola sp. nov. (Rhodophyta) from the British Isles and northern France. Br. Phycol. J. 1987, 22, 417–434. [Google Scholar] [CrossRef]
- Díaz-Tapia, P.; Bárbara, I. Seaweeds from sand-covered rocks of the Atlantic Iberian Peninsula. Part 2. Palmariales, Ceramiales (excluding Rhodomelaceae), Gelidiales, Gigartinales, Plocamiales, Rhodymeniales and Scytothamniales. Cryptogam. Algol. 2014, 35, 157–199. [Google Scholar] [CrossRef]
- Afonso-Carrillo, J.; Sansón, M. Algas, Hongos y Fanerógamas Marinas de las Islas Canarias. Clave Analítica; Servicio de Publicaciones de la Universidad de La Laguna: La Laguna, Spain, 1999; pp. 1–254. [Google Scholar]
- Gallardo, T.; Bárbara, I.; Afonso-Carrillo, J.; Bermejo, R.; Altamirano, M.; Gómez Garreta, A.; Barceló Martí, M.C.; Rull Lluch, J.; Ballesteros, E.; De la Rosa, J. Nueva lista crítica de las algas bentónicas marinas de España. A new checklist of benthic marine algae of Spain. Algas. Bol. Inf. Soc. Esp. Ficol. 2016, 51, 7–52. [Google Scholar]
- Alfonso, B.; Sangil, C.; Sansón, M. Gelidiales (Rhodophyta) in the Canary Islands: Previous studies and future perspectives. Sci. Insularum 2019, 2, 153–181. [Google Scholar] [CrossRef]
- Elejabeitia, Y.; Reyes, J.; Afonso-Carrillo, J. Algas marinas bentónicas de Punta del Hidalgo, Tenerife (Islas Canarias). Vieraea 1992, 21, 1–28. [Google Scholar]
- Pinedo, S.; Sansón, M.; Afonso-Carrillo, J. Algas marinas bentónicas de Puerto de la Cruz (antes Puerto Orotava), Tenerife (Islas Canarias). Vieraea 1992, 21, 29–60. [Google Scholar]
- Reyes, J.; Sansón, M.; Afonso-Carrillo, J. Notes on some interesting marine algae new from the Canary Islands. Crypt. Bot. 1993, 4, 50–59. [Google Scholar]
- Rancel, N.; Sansón, M.; Afonso-Carrillo, J.; Tronholm, A. Morfología reproductora y vegetativa de Pterocladiella melanoidea (Gelidiales, Rhodophyta) en las Islas Canarias. In Proceedings of the XV Simposio Ibérico de Estudios de Biología Marina (SIEBM), Funchal, Portugal, 9 September 2008. [Google Scholar]
- Rancel, N.; Sansón, M.; Afonso-Carrillo, J. Development of carposporophyte in Pterocladiella melanoidea (Gelidiales, Rhodophyta): An intermediate pattern between Pterocladia and Pterocladiella. Phycologia 2009, 48, 110. [Google Scholar]
- Millar, A.J.K.; Freshwater, D.W. Morphology and molecular phylogeny of the marine algal order Gelidiales (Rhodophyta) from New South Wales, including Lord Howe and Norfolk Island. Aust. Syst. Bot. 2005, 18, 215–263. [Google Scholar] [CrossRef]
- Boo, G.H.; Le Gall, L.; Miller, K.A.; Freshwater, D.W.; Wernberg, T.; Terada, R.; Yoon, K.J.; Boo, S.M. A novel phylogeny of the Gelidiales (Rhodophyta) based on five genes including the nuclear CesA, with descriptions of Orthogonacladia gen. nov. and Orthogonacladiaceae fam. nov. Mol. Phylogenet. Evol. 2016, 101, 359–372. [Google Scholar] [CrossRef]
- Boo, G.H.; Le Gall, L.; Hwang, I.K.; Rousseau, F.; Yoon, H.S. Species Diversity of Gelidium from Southern Madagascar Evaluated by an Integrative Taxonomic Approach. Diversity 2022, 14, 826. [Google Scholar] [CrossRef]
- Boo, G.H.; Nguyen, T.V.; Kim, J.Y.; Le Gall, L.; Rico, J.M.; Bottalico, A.; Boo, S.M. A revised classification of the Gelidiellaceae (Rhodophyta) with descriptions of three new genera: Huismaniella, Millerella and Perronella. Taxon 2016, 65, 965–979. [Google Scholar] [CrossRef]
- Iha, C.; Freshwater, D.W.; Guimarães, S.M.P.B.; Oliveira, M.C. Gelidiorariphycus gen. nov. (Gelidiales, Rhodophyta): A rare genus found in the Americas. Phycologia 2022, 61, 473–483. [Google Scholar] [CrossRef]
- Perrone, C.; Felicini, G.P.; Bottalico, A. The prostrate system of the Gelidiales: Diagnostic and taxonomic importance. Bot. Mar. 2006, 49, 23–33. [Google Scholar] [CrossRef]
- Boo, S.M.; Kim, S.Y.; Hong, I.S.; Hwang, I.K. Reexamination of the genus Pterocladiella (Gelidiaceae, Rhodophyta) in Korea based on morphology and rbcL sequences. Algae 2010, 25, 1–9. [Google Scholar] [CrossRef]
- Boo, G.H.; Hughey, J.R.; Miller, K.A.; Boo, S.M. Mitogenomes from type specimens, a genotyping tool for morphologically simple species: Ten genomes of agar-producing red algae. Sci. Rep. 2016, 6, 35337. [Google Scholar] [CrossRef]
- Iha, C.; Jamas, M.; Guimaraes, S.M.P.B.; Fujii, M.T.; Freshwater, D.W.; Oliviera, M.C. Pterocladiella (Gelidiales, Rhodophyta) species of Brazil including morphological studies of Pterocladiella media and a reassessment of Pterocladiella taylorii. Phycologia 2017, 56, 624–637. [Google Scholar] [CrossRef]
- Pezzolesi, L.; Peña, V.; Le Gall, L.; Gabrielson, P.W.; Kaleb, S.; Hughey, J.R.; Rodondi, G.; Hernández-Kantun, J.J.; Falace, A.; Basso, D.; et al. Mediterranean Lithophyllum stictiforme (Corallinales, Rhodophyta) is a genetically diverse species complex: Implications for species circumscription, biogeography and conservation of coralligenous habitats. J. Phycol. 2019, 55, 473–492. [Google Scholar] [CrossRef]
- Leliaert, F.; Payo, D.A.; Gurgel, C.F.D.; Shils, T.; Draisma, S.G.A.; Saunders, G.W.; Kamiya, M.; Sherwood, A.R.; Lin, S.M.; Huisman, J.M.; et al. Patterns and drivers of species diversity in the Indo-Pacific red seaweed Portieria. J. Biogeogr. 2018, 45, 2299–2313. [Google Scholar] [CrossRef]
- Díaz-Tapia, P.; Ly, M.; Verbruggen, H. Extensive cryptic diversity in the widely distributed Polysiphonia scopulorum (Rhodomelaceae, Rhodophyta): Molecular species delimitation and morphometric analyses. Mol. Phylogenet. Evol. 2020, 152, 106909. [Google Scholar] [CrossRef]
- Gabrielson, P.W.; Hughey, J.R.; Diaz-Pulido, G. Genomics reveals abundant speciation in the coral reef building alga Porolithon onkodes (Corallinales, Rhodophyta). J. Phycol. 2018, 54, 429–434. [Google Scholar] [CrossRef]
- Richards, J.L.; Sauvage, T.; Schmidt, W.E.; Fredericq, S.; Hughey, J.R.; Gabrielson, P.W. The coralline genera Sporolithon and Heydrichia (Sporolithales, Rhodophyta) clarified by sequencing type material of their generitypes and other species. J. Phycol. 2017, 53, 1044–1059. [Google Scholar] [CrossRef]
- Bornet, E.; Schousboe, P.K.A. Les algues de P.-K.-A. Schousboe: Récoltées au Maroc & dans la Méditerranée de 1815 à 1829; Masson, G., Bornet, E., Schousboe, P.K.A., Eds.; Nabu Press: Charleston, SC, USA, 1892; pp. 165–376. [Google Scholar]
- Feldmann, J.; Hamel, G. Floridées de France VII. Gélidiales. Rev. Algol. 1936, 9, 85–140. [Google Scholar]
- Hommersand, M.H.; Fredericq, S. An investigation of cystocarp development in Gelidium pteridifolium with a revised description of the Gelidiales (Rhodophyta). Phycologia 1988, 27, 254–272. [Google Scholar] [CrossRef]
- Huisman, J.M.; Boo, G.H.; Boo, S.M. Gelidiales. In Algae of Australia. Marine Benthic Algae of North-Western Australia. 2. Red algae; Huisman, J.M., Ed.; ABRS and CSIRO Publishing: Canberra, Australia, 2018; pp. 245–264. [Google Scholar]
- De Gregorio, S.; Dibari, S.; Perrone, C. Fertile plants of Pterocladia melanoidea (Rhodophyta, Gelidiales) on the Apulian coasts (Mediterranean Sea). Giorn. Bot. Ital. 1995, 129, 1262–1266. [Google Scholar]
- Fredriksen, S.; Rueness, J. Culture studies on Pterocladia melanoidea (Schousboe ex Bornet) comb. nov. (Gelidiales, Rhodophyta). Phycologia 1990, 29, 182–190. [Google Scholar] [CrossRef]
- Bárbara, I.; Díaz-Tapia, P. New records and additions to the seaweeds of France. Mar. Biodivers. Rec. 2012, 5, 1–7. [Google Scholar] [CrossRef]
- Cormaci, M.; Furnari, G.; Alongi, G. Flora marina bentonica del Mediterraneo: Rhodophyta–Rhodymeniophycidae I. Acrosymphytales, Bonnemaisoniales, Gelidiales, Gigartinales, Gracilariales. Boll. Accad. Gioenia Nat. Sci 2020, 53, 11–346. [Google Scholar] [CrossRef]
- Dawson, E.Y. Notes on Pacific coast marine algae, VII. Bull. So. Cal. Acad. Sci. 1958, 57, 65–80. [Google Scholar]
- Feldmann, J.; Hamel, G. Observations sur quelques Gélidiacées. Rev. Gén. Bot. 1934, 46, 528–549. [Google Scholar]
- Saunders, G.W.; Brooks, C.M.; Scott, S. Preliminary DNA barcode report on the marine red algae (Rhodophyta) from the British Overseas Territory of Tristan da Cunha. Cryptogamie Algol. 2019, 40, 105–117. [Google Scholar] [CrossRef]
- Baardseth, E. The marine algae of Tristan da Cunha. In Results of the Norwegian Scientific Expedition to Tristan Da Cunha; Dybwad, J., Ed.; Det Norske Videnskaps Akademi: Oslo, Sweden, 1937; pp. 1–172. [Google Scholar]
- Norris, R.E. The marine red algae of Natal, South Africa: Order Gelidiales (Rhodophyta). Mem. Bot. Surv. S. Afr. 1992, 61, 1–43. [Google Scholar]
- Martínez, B.; Afonso-Carrillo, J.; Anadón, R.; Araújo, R.; Arenas, F.; Arrontes, J.; Bárbara, I.; Borja, A.; Díez, I.; Duarte, L.; et al. Regresión de las algas marinas en las islas Canarias y en la costa atlántica de la Península Ibérica por efecto del cambio climático. Algas. Bol. Inf. Soc. Esp. Ficol. 2015, 49, 5–12. [Google Scholar]
- Afonso-Carrillo, J. Tiempos de profundas transformaciones en los paisajes submarinos del litoral canario. In Reflexiones Medioambientales en Tiempos de un Coronavirus; Afonso-Carrillo, J., Ed.; Instituto de Estudios Hispánicos de Canarias: Puerto de la Cruz, Spain, 2021; pp. 41–88. [Google Scholar]
- Alfonso, B.; Hernández, J.C.; Sangil, C.; Martín, L.; Expósito, F.J.; Díaz, J.P.; Sansón, M. Fast climatic changes place an endemic Canary Island macroalga at extinction risk. Reg. Environ. Change 2021, 113, 1–16. [Google Scholar] [CrossRef]
- Martín García, L.; Rancel-Rodríguez, N.M.; Sangil, C.; Reyes, J.; Benito, B.M.; Orellana, S.; Sansón, M. Environmental and human factors drive the subtropical marine forests of Gongolaria abies-marina to extinction. Mar. Environ. Res. 2022, 181, 105759. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Prieto, C.; Afonso-Carrillo, J.; De Clerck, O.; Huisman, J.M.; Lin, S.M. Systematic revision of the foliose Halymeniaceae (Halymeniales, Rhodophyta) from Europe, with the description of Halymenia ballesterosii sp. nov. from the Mediterranean Sea and Nesoia hommersandii from the Canary Islands. Eur. J. Phycol. 2020, 55, 454–466. [Google Scholar] [CrossRef]
- Vieira, C.; Henriques, F.; D’hondt, S.; Neto, A.I.; Almada, C.H.; Kaufmann, M.; Sansón, M.; Sangil, C.; De Clerck, O. Lobophora (Dictyotales) species richness, ecology and biogeography across the North-eastern Atlantic archipelagos and description of two new species. J. Phycol. 2020, 56, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Buján, I.; Pimentel, M.; Díaz-Tapia, P. Melanothamnus macaronesicus Rodríguez-Buján & Díaz-Tapia, sp. nov. (Rhodomelaceae, Rhodophyta): A new turf-forming species from the Azores and the Canary Islands. Cryptogam. Algol. 2021, 42, 77–91. [Google Scholar] [CrossRef]
- Thiers, B. Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff. New York Botanical Garden’s Virtual Herbarium. Available online: http://sweetgum.nybg.org/ih/ (accessed on 10 November 2022).
- Freshwater, D.W.; Rueness, J. Phylogenetic relationships of some European Gelidium (Gelidiales, Rhodophyta) species, based on rbcL nucleotide sequence analysis. Phycologia 1994, 33, 187–194. [Google Scholar] [CrossRef]
- Geraldino, P.J.L.; Yang, E.C.; Bu, S.M. Morphology and molecular phylogeny of Hypnea flexicaulis (Gigartinales, Rhodophyta) from Korea. Algae 2006, 21, 417–423. [Google Scholar] [CrossRef]
- Gouy, M.; Guindon, S.; Gascuel, O. SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 2010, 27, 221–224. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.E.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Org. Divers. Evol. 2010, 10, 311–329. [Google Scholar] [CrossRef]
- García-Jiménez, P.; Robaina, R.R. Effects of ethylene on tetrasporogenesis in Pterocladiella capillacea (Rhodophyta). J. Phycol. 2012, 48, 710–715. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rancel-Rodríguez, N.M.; Afonso-Carrillo, J.; Tronholm, A.; Sansón, M. A New Species from the Canary Islands Increases the Diversity of the Red Algal Genus Pterocladiella in the Northeastern Atlantic. Plants 2023, 12, 416. https://doi.org/10.3390/plants12020416
Rancel-Rodríguez NM, Afonso-Carrillo J, Tronholm A, Sansón M. A New Species from the Canary Islands Increases the Diversity of the Red Algal Genus Pterocladiella in the Northeastern Atlantic. Plants. 2023; 12(2):416. https://doi.org/10.3390/plants12020416
Chicago/Turabian StyleRancel-Rodríguez, Nereida M., Julio Afonso-Carrillo, Ana Tronholm, and Marta Sansón. 2023. "A New Species from the Canary Islands Increases the Diversity of the Red Algal Genus Pterocladiella in the Northeastern Atlantic" Plants 12, no. 2: 416. https://doi.org/10.3390/plants12020416
APA StyleRancel-Rodríguez, N. M., Afonso-Carrillo, J., Tronholm, A., & Sansón, M. (2023). A New Species from the Canary Islands Increases the Diversity of the Red Algal Genus Pterocladiella in the Northeastern Atlantic. Plants, 12(2), 416. https://doi.org/10.3390/plants12020416








