Association between the Classification of the Genus of Batrachospermaceae (Rhodophyta) and the Environmental Factors Based on Machine Learning
Abstract
1. Introduction
2. Results
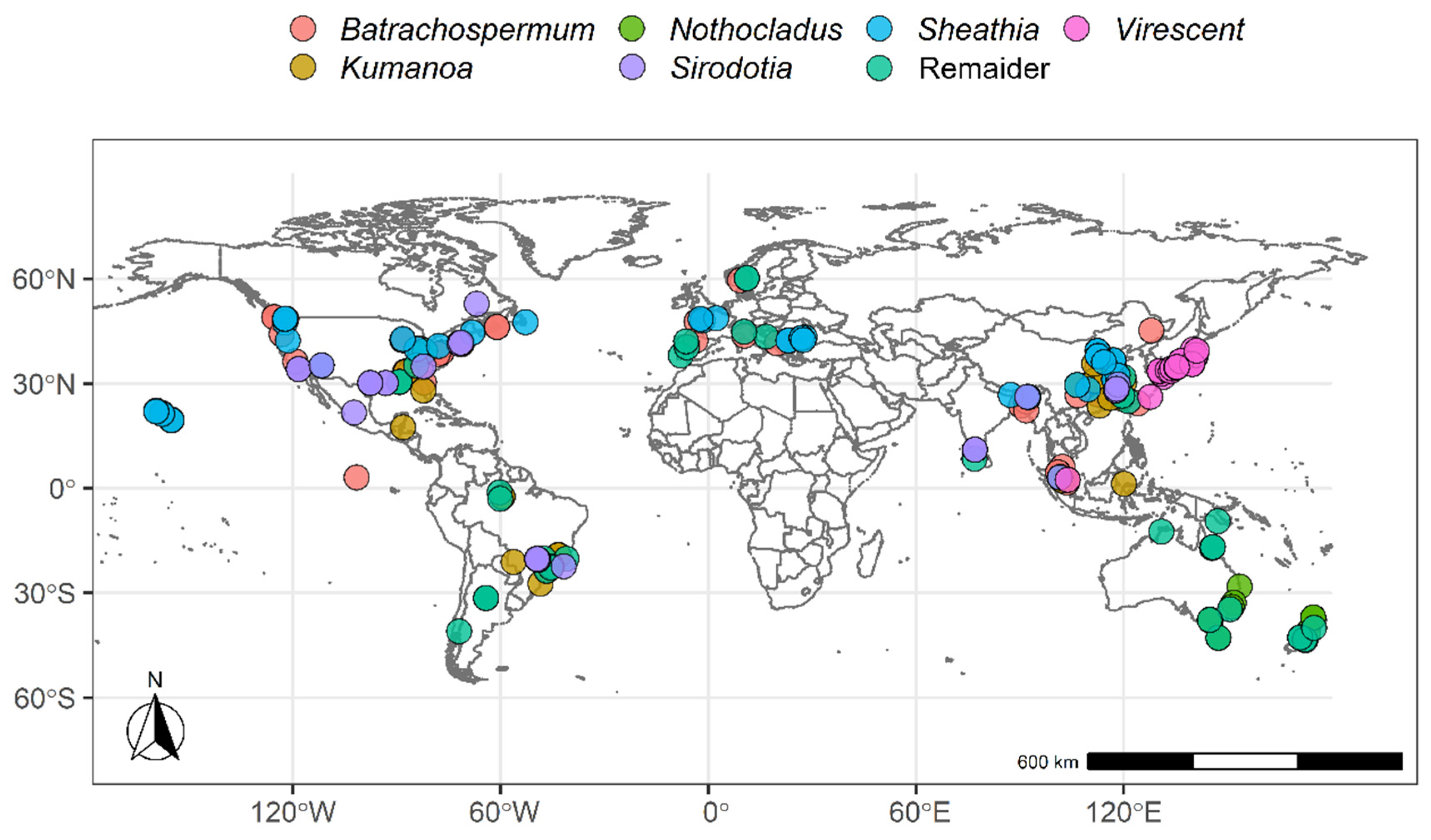
2.1. Descriptive Statistical Analysis
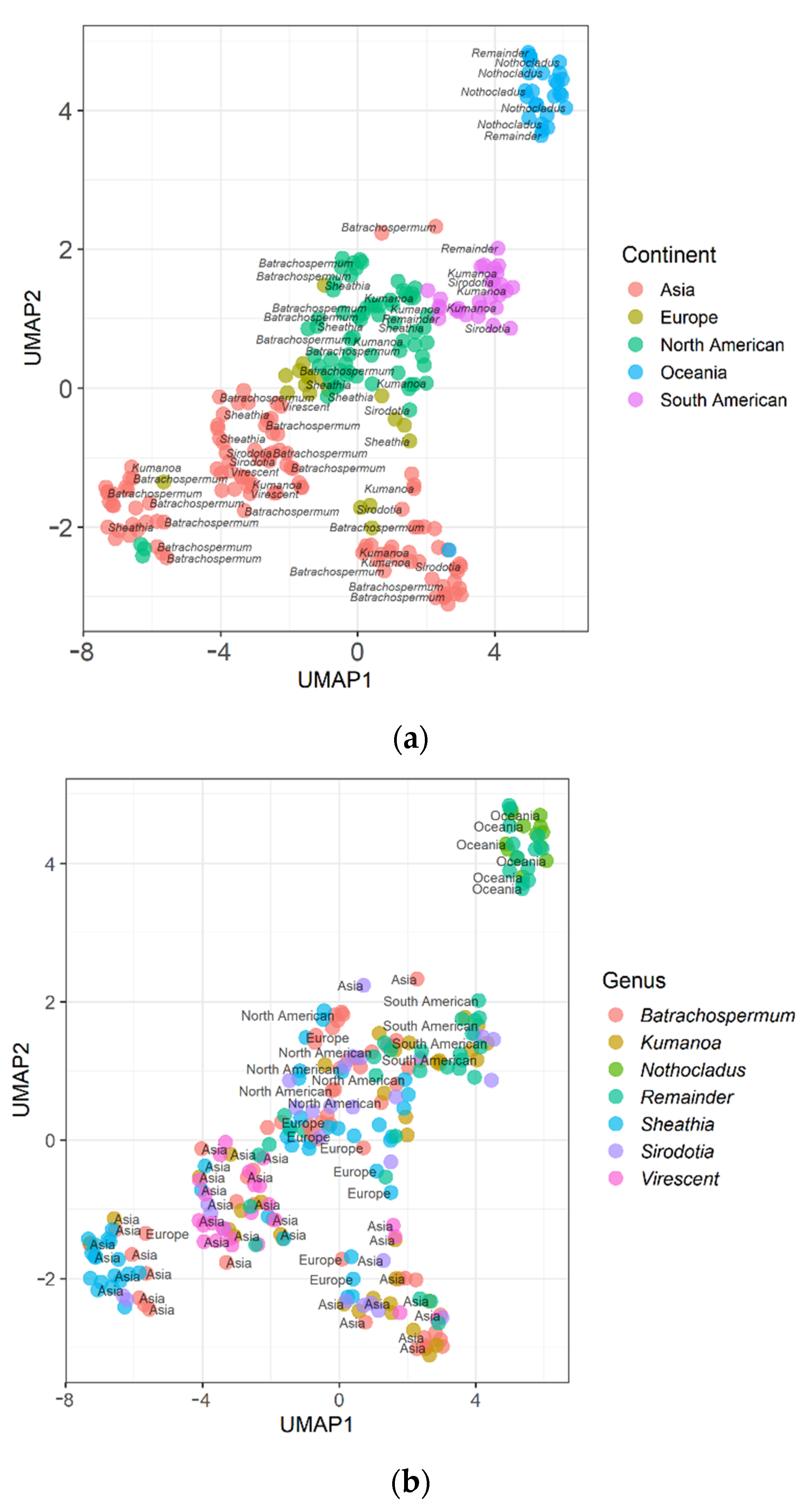
2.2. Results of UMAP
2.3. Results of Machine Learning Methods
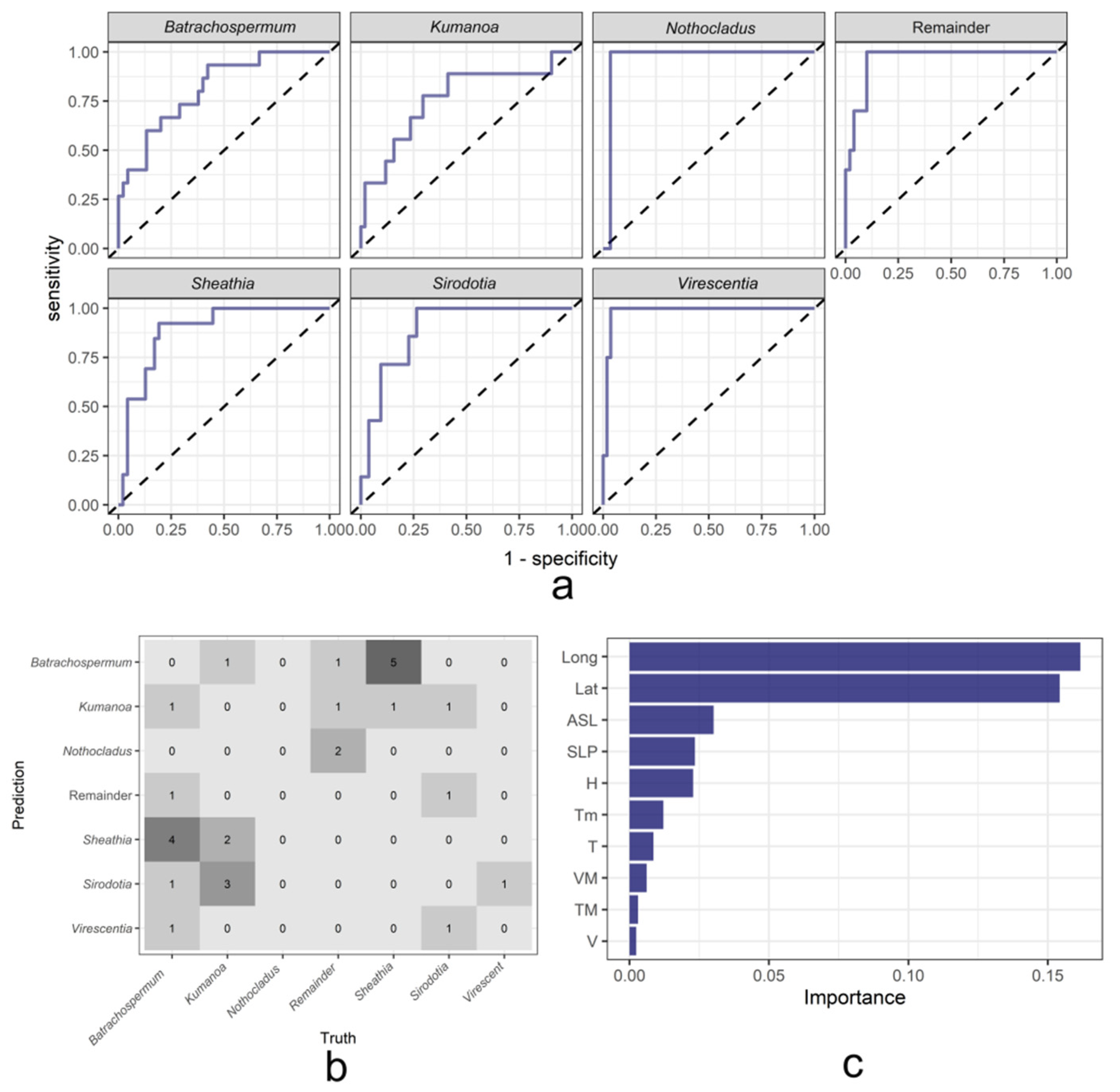
2.3.1. Results of the Random Forest
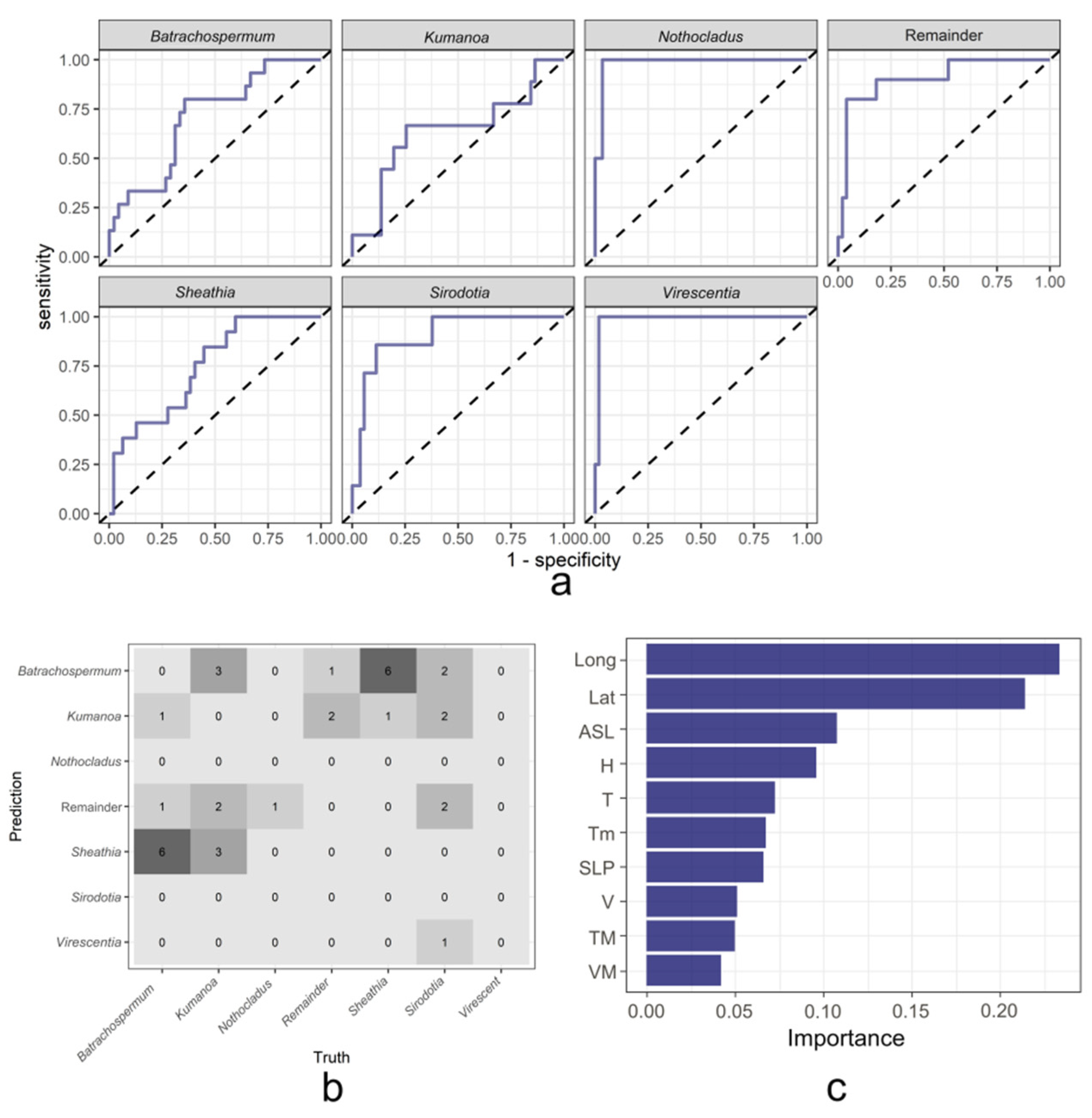
2.3.2. Results of the XGBoost
3. Discussion
4. Materials and Methods
4.1. Data Description
4.2. Methods
4.2.1. UMAP
4.2.2. Random Forest
- (1)
- Use the bootstrap method to resample the original data sample set X, and randomly generate K training sample sets X1, X2, …, XK;
- (2)
- Use each generated training set, generate the corresponding decision tree T1, T2, …, Tk, and select “mtry” attributes (split attributes randomly selected from M attribute sets) on each intermediate node (non-leaf node). The attribute of the best splitting method in the set is used as the splitting attribute of the current node to split on this node;
- (3)
- Each decision tree grows completely without pruning;
- (4)
- Test and classify each decision tree on the original data sample set X;
- (5)
- By voting, the category with the most output from the K decision trees is taken as the category to which the original data sample set X belongs.
4.2.3. XGBoost
- (1)
- The model starts the initial iteration, and a sub-prediction model is constructed in each iteration.
- (2)
- Before each iteration, calculate the first-order and second-order gradients of the loss function under each training sample value.
- (3)
- In each iteration, with the goal of minimizing Equation (4), a decision tree is generated as a sub-prediction model, and the corresponding prediction value ω of each leaf node of the decision tree is calculated.
- (4)
- After each iteration, the newly generated model is added to the previous model. After several iterations, the final prediction model can be obtained.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agardh, C.A. Systema Algarum; Literis Berlingianis: Lundae, Sweden, 1824. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. 2022. Available online: https://www.algaebase.org (accessed on 10 September 2022).
- Sheath, R.G.; Vis, M.L. Red Algae//Freshwater Algae of North America; Academic Press: Cambridge, MA, USA, 2015; pp. 237–264. [Google Scholar] [CrossRef]
- Abdelahad, N.; Bolpagni, R.; Jona Lasinio, G.; Vis, M.L.; Amadio, C.; Laini, A.; Keil, E.J. Distribution, morphology and ecological niche of Batrachospermum and Sheathia species (Batrachospermales, Rhodophyta) in the fontanili of the Po plain (northern Italy). Eur. J. Phycol. 2015, 50, 318–329. [Google Scholar] [CrossRef]
- Siemińska, J. Red list of threatened algae in Poland. In List of Threatened Plants in Poland, 2nd ed.; Polish Academy of Sciences, W Szafer Institute of Botany: Krakowie, Poland, 1992; pp. 7–19. [Google Scholar]
- Nemeth, J. Red list of algae in Hungary. Acta Bot. Hung. 2005, 47, 379–417. [Google Scholar] [CrossRef]
- Sheath, R.G.; Burkholder, J.M. Characteristics of softwater streams in Rhode Island II. Composition and seasonal dynamics of macroalgal communities. Hydrobiologia 1985, 128, 109–118. [Google Scholar] [CrossRef]
- Biggs, B.J.F.; Price, G.M. A survey of filamentous algal proliferations in New Zealand rivers. N. Z. J. Mar. Freshw. Res. 1987, 21, 175–191. [Google Scholar] [CrossRef]
- Biggs, B.J.F. Periphyton communities and their environments in New Zealand rivers. N. Z. J. Mar. Freshw. Res. 1990, 24, 367–386. [Google Scholar] [CrossRef]
- Branco, C.C.Z.; Krupek, R.A.; Peres, C.K. Distribution of stream macroalgal communities from the mid-western region of Paraná State, southern Brazil: Importance of local scale variation. Braz. Arch. Biol. Technol. 2009, 52, 379–386. [Google Scholar] [CrossRef]
- Krupek, R.A.; Branco, C.C.Z. Ecological distribution of stream macroalgae in different spatial scales using taxonomic and morphological groups. Braz. J. Bot. 2012, 35, 273–280. [Google Scholar] [CrossRef]
- Jimenez, J.C.; Fatjo, G.V. Survey and distribution of Batrachospermaceae (Rhodophyta) in tropical, high-altitude streams from central Mexico. Cryptogam. Algol. 2007, 28, 271–282. [Google Scholar]
- Carmona, J.; Bojorge-García, M.; Beltrán, Y.; Ramírez-Rodríguez, R. Phenology of Sirodotia suecica (Batrachospermaceae, Rhodophyta) in a high-altitude stream in central Mexico. Phycol. Res. 2009, 57, 118–126. [Google Scholar] [CrossRef]
- Xie, S.L. Seasonal dynamics of Batrachospermum arcuatum growth and distribution in Jinci Spring, China. J. Shanxi Univ. (Nat. Sci. Ed.) 2009, 32, 596–600. [Google Scholar]
- Xie, S. Seasonal dynamics of Batrachospermum gelatinosum growth and distribution in Niangziguan spring, China. J. Appl. Ecol. 2004, 15, 1931–1934. [Google Scholar]
- Harvey, W.H. Nereis Boreali-Americana: Contributions to a History of the Marine Algae of North America; Smithsonian Institution: Washington, DC, USA, 1858. [Google Scholar]
- Kylin, H. Studien uber die schwedischen Arten der Gattungen Batrachospermum Roth und Sirodotia nov. gen. Nova Acta Reg. Soc. Sci. Upsal. 1912, 3, 1–40. [Google Scholar]
- Skuja, H. Untersuchungen uber die Rhodophyceen des Suβwassers. VI. Nemalionopsis shawii eine neue gattung und Art der Heominthocladioceen. Beih. Zum Bot. Cent. B 1934, 52, 188–192. [Google Scholar]
- Nan, F.; Zhao, Y.; Feng, J.; Lv, J.; Liu, Q.; Liu, X.; Xie, S. Morphological and Molecular Phylogenetic Analysis of a Lemanea Specimen (Batrachospermales, Rhodophyta) from China. Diversity 2022, 14, 479. [Google Scholar] [CrossRef]
- Han, J.-F.; Nan, F.-R.; Feng, J.; Lv, J.-P.; Liu, Q.; Liu, X.-D.; Xie, S.-L. Sheathia matouensis (Batrachospermales, Rhodophyta), a new freshwater red algal species from North China. Phytotaxa 2019, 415, 255–263. [Google Scholar] [CrossRef]
- Necchi, J.O.; Garcia, F.A.; Paiano, M.O. Revision of Batrachospermum sections Acarposporophytum and Aristata (Batrachospermales, Rhodophyta) with the establishment of the new genera Acarposporophycos and Visia. Phytotaxa 2019, 395, 51–65. [Google Scholar] [CrossRef]
- Olden, J.D.; Lawler, J.J.; Poff, N.L. Machine Learning Methods without Tears: A Primer for Ecologists. Q. Rev. Biol. 2008, 83, 171–193. [Google Scholar] [CrossRef]
- Sun, S.; Wang, C.; Ding, H.; Zou, Q. Machine learning and its applications in plant molecular studies. Brief. Funct. Genom. 2020, 19, 40–48. [Google Scholar] [CrossRef]
- Roscher, R.; Bohn, B.; Duarte, M.F.; Garcke, J. Explainable Machine Learning for Scientific Insights and Discoveries. IEEE Access 2020, 8, 42200–42216. [Google Scholar] [CrossRef]
- Chang, C.-I. An Effective Evaluation Tool for Hyperspectral Target Detection: 3D Receiver Operating Characteristic Curve Analysis. IEEE Trans. Geosci. Remote Sens. 2020, 59, 5131–5153. [Google Scholar] [CrossRef]
- Hoo, Z.H.; Candlish, J.; Teare, D. What is an ROC curve? Emerg. Med. J. 2017, 34, 357–359. [Google Scholar] [CrossRef]
- Hinden, H.; Oertli, B.; Menetrey, N.; Sager, L.; Lachavanne, J.-B. Alpine pond biodiversity: What are the related environmental variables? Aquat. Conserv. 2005, 15, 613–624. [Google Scholar] [CrossRef]
- Fu, B. The effects of topography and elevation on precipitation. Acta Geogr. Sin. 1992, 47, 302–314. [Google Scholar]
- Feng, J.; Wang, X.; Fang, J. Altitudinal pattern of species richness and test of the Rapoport’s rules in the Drung river area, southwest China. Acta Sci. Nat. Univ. Pekin. 2006, 42, 515. [Google Scholar]
- Qian, H.; Ricklefs, R.E. Disentangling the effects of geographic distance and environmental dissimilarity on global patterns of species turnover. Glob. Ecol. Biogeogr. 2012, 21, 341–351. [Google Scholar] [CrossRef]
- Wang, P.; Cheng, Q.P.; Kong, G.C.; Wang, Q. Variation characteristics analysis and forecast of relative humidity over past 53 years in Panlong river basin of Yunnan. J. Meteorol. Res. Appl. 2016, 37, 15–20. [Google Scholar]
- Jin, Y.H.; Lian, S.H.; Zhou, D.W.; Xu, J.B.; Peng, C. Study on change of relative humidity in semiarid region under global climate change. J. Northeast Norm. Univ. (Nat. Sci. Ed.) 2009, 41, 134–138. [Google Scholar]
- Cho, Y.-K.; Lee, K.-S.; Park, K.-Y. Year-to-year Variability of the Vertical Temperature Structure in the Youngsan Estuary. Ocean Polar Res. 2009, 31, 239–246. [Google Scholar] [CrossRef]
- Jun, X.; Shubo, C.; Xiuping, H.; Rui, X.; Xiaojie, L. Potential impacts and challenges of climate change on water quality and ecosystem: Case studies in representative rivers in China. J. Resour. Ecol. 2010, 1, 31–35. [Google Scholar]
- Branco, C.C.Z.; Bispo, P.C.; Peres, C.K.; Tonetto, A.F.; Branco, L.H.Z. The roles of environmental conditions and spatial factors in controlling stream macroalgal communities. Hydrobiologia 2014, 732, 123–132. [Google Scholar] [CrossRef]
- Akhtar, T.; Gilani, S.O.; Mushtaq, Z.; Arif, S.; Jamil, M.; Ayaz, Y.; Butt, S.I.; Waris, A. Effective Voting Ensemble of Homogenous Ensembling with Multiple Attribute-Selection Approaches for Improved Identification of Thyroid Disorder. Electronics 2021, 10, 3026. [Google Scholar] [CrossRef]
- Vis, M.L.; Sheath, R.G.; Cole, K.M. Distribution and systematics of Batrachospermum (Batrachospermales, Rhodophyta) in North America. 8b. Section Batrachospermum: Previously described species excluding Batrachospermum gelatinosum. Eur. J. Phycol. 1996, 31, 189–199. [Google Scholar] [CrossRef]
- Entwisle, T.J.; Vis, M.L.; Chiasson, W.B.; Necchi, O., Jr.; Sherwood, A.R. Systematics of the batrachospermales (Rhodophyta)—A synthesis. J. Phycol. 2009, 45, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Kwandrans, J.; Eloranta, P. Diversity of freshwater red algae in Europe. Oceanol. Hydrobiol. Stud. 2010, 39, 161–169. [Google Scholar] [CrossRef]
- Branco, C.C.Z.; Necchi, O. Distribution of stream macroalgae in the eastern Atlantic Rainforest of São Paulo State, southeastern Brazil. Hydrobiologia 1996, 333, 139–150. [Google Scholar] [CrossRef]
- Eloranta, P. Freshwater red algae in Finland. Plant Fungal Syst. 2019, 64, 41–51. [Google Scholar] [CrossRef]
- Chen, L.; Feng, J.; Han, X.-J.; Xie, S.-L. Investigation of a freshwater acrochaetioid alga (Rhodophyta) with molecular and morphological methods. Nord. J. Bot. 2014, 32, 529–535. [Google Scholar] [CrossRef]
- Sherwood, A.R.; Vis, M.L.; Sheath, R.G. Phenology and phylogenetic positioning of the Hawaiian endemic freshwater alga, Batrachospermum spermatiophorum (Rhodophyta, Batrachospermales). Phycol. Res. 2004, 52, 193–203. [Google Scholar] [CrossRef]
- Vis, M.L. Biogeography of River Algae. In River Algae; Springer: Berlin/Heidelberg, Germany, 2016; pp. 219–243. [Google Scholar] [CrossRef]
- Rossignolo, N.L.; Necchi, J.O. Revision of section Setacea of the genus Batrachospermum (Batrachospermales, Rhodophyta) with emphasis on specimens from Brazil. Phycologia 2016, 55, 337–346. [Google Scholar] [CrossRef]
- Ji, L.; Xie, S.L.; Feng, J.; Chen, L.; Wang, J. Molecular systematics of four endemic Batrachospermaceae (Rhodophyta) species in China with multilocus data. J. Syst. Evol. 2014, 52, 92–100. [Google Scholar] [CrossRef]
- Shulian, X.; Zhixin, S. Taxonomy of algal genus Sirodotia Kylin (Batrachospermaceae, Rhodophyta) in China. J. Trop. Subtrop. Bot. 2004, 12, 1–6. [Google Scholar]
- Shulian, X.; Zhixin, S. Three new species of Batrachospermum Roth (Batrachospermaceae, Rhodophyta) in China. Chin. J. Oceanol. Limnol. 2005, 23, 204–209. [Google Scholar] [CrossRef]
- Fang, K.-P.; Nan, F.-R.; Feng, J.; Lv, J.-P.; Liu, Q.; Liu, X.-D.; Xie, S.-L. Batrachospermum qujingense (Batrachospermales, Rhodophyta), a new freshwater red algal species from Southwest China. Phytotaxa 2020, 461, 1–11. [Google Scholar] [CrossRef]
- Chankaew, W.; Sakset, A.; Ganesan, E.K.; Jr, O.N.; West, J.A. Diversity of freshwater red algae at Khao Luang National Park, southern Thailand. Algae 2019, 34, 23–33. [Google Scholar] [CrossRef]
- Xie, S.L.; Feng, J. Batrachospermum hongdongense (sect. Batrachospermum, Batrachospermaceae), a new species from Shanxi, China. Bot. Stud. 2007, 48, 459–464. [Google Scholar]
- Han, J.-F.; Nan, F.-R.; Feng, J.; Lv, J.-P.; Liu, Q.; Kociolek, J.P.; Xie, S.-L. Sheathia jinchengensis (Batrachospermales, Rhodophyta), a new freshwater red algal species described from North China. Phytotaxa 2018, 367, 63–70. [Google Scholar] [CrossRef]
- Feng, J.; Chen, L.; Wang, Y.; Xie, S. Molecular Systematics and Biogeography of Thorea (Thoreales, Rhodophyta) from Shanxi, China. Syst. Bot. 2015, 40, 376–385. [Google Scholar] [CrossRef]
- Mcinnes, L.; Healy, J.; Melville, J. UMAP: Uniform manifold approximation and projection for dimension reduction. arXiv 2018, arXiv:1802.03426. [Google Scholar] [CrossRef]
- Becht, E.; McInnes, L.; Healy, J.; Dutertre, C.-A.; Kwok, I.W.H.; Ng, L.G.; Ginhoux, F.; Newell, E.W. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. 2019, 37, 38–44. [Google Scholar] [CrossRef]
- Weijler, L.; Diem, M.; Reiter, M.; Maurer-Granofszky, M. Detecting Rare Cell Populations in Flow Cytometry Data Using UMAP. Presented at the 2020 25th International Conference on Pattern Recognition (ICPR), Milan, Italy, 10–15 January 2021; pp. 4903–4909. [Google Scholar] [CrossRef]
- Rugard, M.; Jaylet, T.; Taboureau, O.; Tromelin, A.; Audouze, K. Smell compounds classification using UMAP to increase knowledge of odors and molecular structures linkages. PLoS ONE 2021, 16, e0252486. [Google Scholar] [CrossRef]
- Joswiak, M.; Peng, Y.; Castillo, I.; Chiang, L.H. Dimensionality reduction for visualizing industrial chemical process data. Control Eng. Pract. 2019, 93, 104189. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Ho, T.K. The random subspace method for constructing decision forests. IEEE Trans. Pattern Anal. Mach. Intell. 1998, 20, 832–844. [Google Scholar]
- Breiman, L. Bagging predictors. Mach. Learn. 1996, 24, 123–140. [Google Scholar] [CrossRef]
- Ying, Z.; Qianqian, G. Water quality evaluation of Chaohu Lake based on random forest method. Chin. J. Environ. Eng. 2016, 10, 992–998. [Google Scholar]
- Biau, G.; Scornet, E. A random forest guided tour. Test 2016, 25, 197–227. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. Xgboost: A scalable tree boosting system. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar]
- Mitchell, R.; Adinets, A.; Rao, T.; Frank, E. Xgboost: Scalable GPU accelerated learning. arXiv 2018, arXiv:1806.11248. [Google Scholar] [CrossRef]
- Li, S.; Zhang, X. Research on orthopedic auxiliary classification and prediction model based on XGBoost algorithm. Neural Comput. Appl. 2019, 32, 1971–1979. [Google Scholar] [CrossRef]



| Genus | Number of Species | Genus | Number of Species |
|---|---|---|---|
| Acarposporophycos | 1 | Psilosiphon | 1 |
| Balliopsis | 2 | Sheathia | 12 |
| Batrachospermum | 24 | Sirodotia | 9 |
| Kumanoa | 25 | Torularia | 3 |
| Lympha | 1 | Tuomeya | 1 |
| Montagnia | 1 | Virescentia | 4 |
| Nothocladus | 12 | Visia | 1 |
| Petrohua | 1 | Volatus | 3 |
| Genus | Number of Samples |
|---|---|
| Batrachospermum | 61 |
| Kumanoa | 38 |
| Sheathia | 53 |
| Sirodotia | 30 |
| Virescentia | 24 |
| Nothocladus | 13 |
| Remainder | 45 |
| Total | 264 |
| Parameters | Illustration |
|---|---|
| mtry | The number of features to randomly take when building each tree |
| trees | The number of decision trees |
| min_n | Minimum number of samples required for internal node re-splitting |
| tree_depth | Maximum depth of decision tree |
| learn_rate | The learning rate in the ensemble, which is also the weight reduction factor for each weak classifier |
| loss_reduction | Minimum loss reduction required to make a further partition on a leaf node of the tree |
| sample_size | Subsample ratio of the training instance |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Nan, F.; Liu, X.; Liu, Q.; Lv, J.; Feng, J.; Wang, F.; Xie, S. Association between the Classification of the Genus of Batrachospermaceae (Rhodophyta) and the Environmental Factors Based on Machine Learning. Plants 2022, 11, 3485. https://doi.org/10.3390/plants11243485
Yang Q, Nan F, Liu X, Liu Q, Lv J, Feng J, Wang F, Xie S. Association between the Classification of the Genus of Batrachospermaceae (Rhodophyta) and the Environmental Factors Based on Machine Learning. Plants. 2022; 11(24):3485. https://doi.org/10.3390/plants11243485
Chicago/Turabian StyleYang, Qiqin, Fangru Nan, Xudong Liu, Qi Liu, Junping Lv, Jia Feng, Fei Wang, and Shulian Xie. 2022. "Association between the Classification of the Genus of Batrachospermaceae (Rhodophyta) and the Environmental Factors Based on Machine Learning" Plants 11, no. 24: 3485. https://doi.org/10.3390/plants11243485
APA StyleYang, Q., Nan, F., Liu, X., Liu, Q., Lv, J., Feng, J., Wang, F., & Xie, S. (2022). Association between the Classification of the Genus of Batrachospermaceae (Rhodophyta) and the Environmental Factors Based on Machine Learning. Plants, 11(24), 3485. https://doi.org/10.3390/plants11243485








