Coordinating Diverse Functions of miRNA and lncRNA in Fleshy Fruit
Abstract
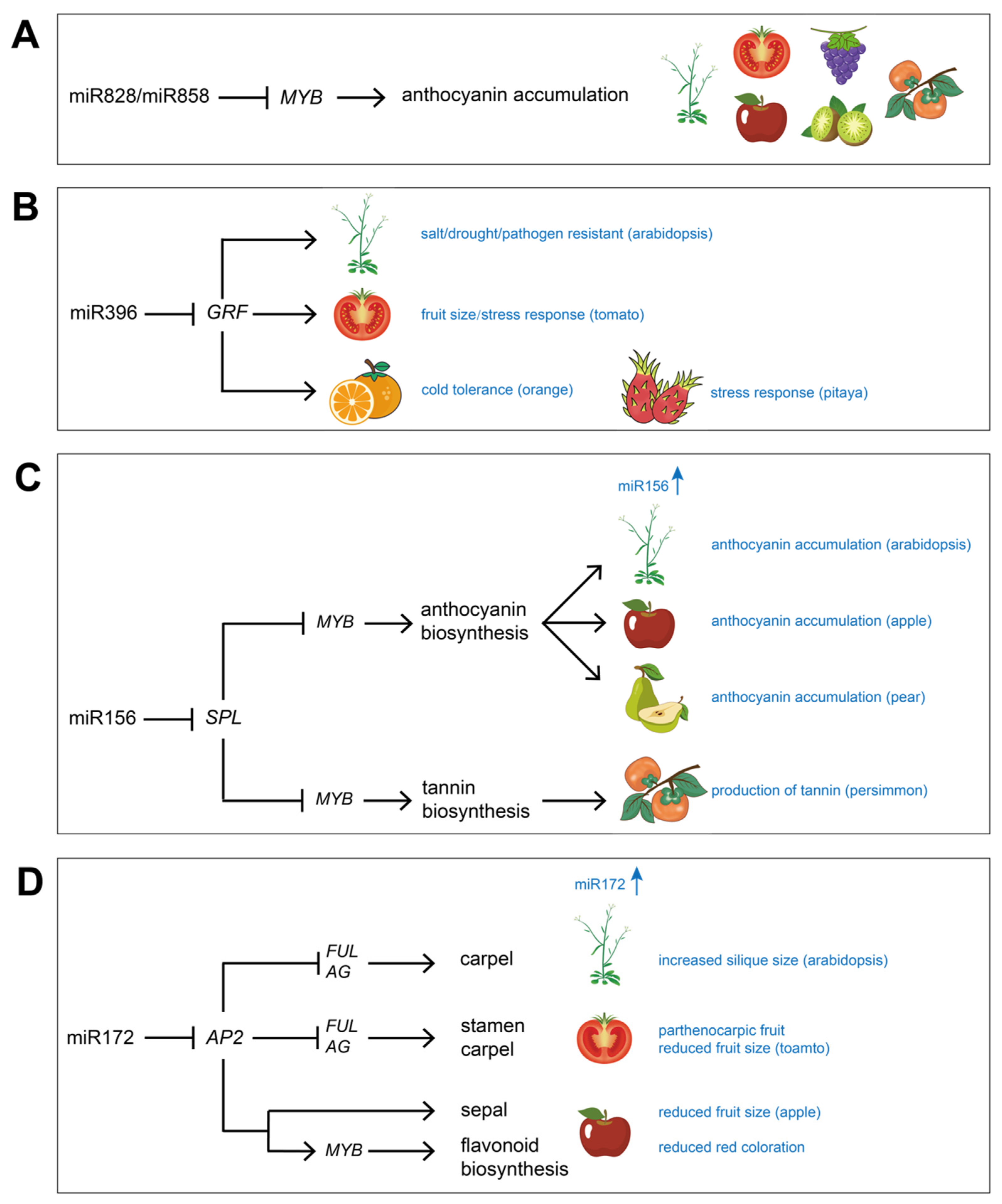
:1. Introduction
2. Functions of miRNAs and lncRNAs in Fruit Development
3. Functions of miRNAs and lncRNAs in Fruit Ripening
4. Functions of miRNAs and lncRNAs in Fruit Responses to Biotic and Abiotic Stress
| Fruit Biology | Classification | Species | Non-Coding RNA | Targets/Downstream | Functionally in Fruit Quality | Research Methods | References |
|---|---|---|---|---|---|---|---|
| Fruit development | Fruit size and number | arabidopsis | miR172C | APETALA2-like | silique fruit expansion | stable (MIR172C::GUS, MIR172CAuxRE::GUS) | [21] |
| miR159a/b | MYB33/MYB65 | altered growth habit, curled leaves, small siliques, and small seeds | T-DNA mutants (mir159ab double mutant) | [40] | |||
| apple | miR172p | AP2 | reduced fruit size, altered floral organ development | stable (MIR172p OE in tomato) | [22,23] | ||
| tomato | miR156 | SPL | fruit growth, ovary and fruit development | stable (AtMIR156b OE) | [15] | ||
| miR159 | SlGAMYB2 (GA biosynthesis gene) | larger fruits | STTM-miR159 | [41] | |||
| miR172d | AP2 | floral organ identity and number | CRISPR/Cas9 (slmir172c-dCR) | [24] | |||
| miR396a/b | GRF | a larger plant, with bigger flowers, leaves, and fruits | STTM-miR396 | [35,36,37] | |||
| miR1917 | CTR4 (altered ethylene response) | fruit size, bigger fruit | STTM-miR1917 | [39] | |||
| miR171a | SlGRAS24 and SlGRAS40 (altered gibberellin and auxin) | cell number and size, smaller tomato fruit | GRAS24 OE | [34] | |||
| miR164a | NAM2/3 | decreased fruit size | CRISPR/Cas9 (slmir164a, slmir164b, slmir164d, slmir164CR) | [38] | |||
| Fruit development | fruit set | tomato | miR159 | SlGAMYB2 (GA biosynthesis gene) | fruit morphology, precocious fruit initiationflattened, fruit with more locules inside | SlMIR159 OE | [11] |
| miR160 | ARF10, ARF16 and ARF17 | sugar accumulation, leaf and flower development, somatic embryo development, pear-shaped fruit | STTM-miR160 | [12,46,47] | |||
| miR166 | SlREV | fruit formation | Overexpression of a microRNA166-resistant version of SlREV (35S::REVRis) | [50] | |||
| miR168 | SlAGO1s | fruit initiation and growth | miR168 loss-of-function (four-point-mutated miR168-resistant 4m-SlAGO1A and 4m-SlAGO1B) | [56] | |||
| pear | PbrmiR397a | LACs | stone cell formation, reduced lignin content and stone cell number | transient (PbrmiR397a OE, pear), stable (PbrmiR397a OE, tobacco) | [51] | ||
| longan | miR160 | ARF10, -16, and -17 | somatic embryo development | target mimics down-regulate miR160 | [52] | ||
| seed development/parthenocarpy | tomato | miR159 | GAMyb-like1 and GAMyb-like2 | parthenocarpy | SlMIR159 OE | [11] | |
| miR166 | SlHB15A | parthenocarpic fruit set | used TILLING to screen for SlHB15A miR166-resistant alleles | [57] | |||
| miR167 | SlARF8 | parthenocarpy | downregulation of miR167 | [11] | |||
| miR168 | SlAGO1s | parthenocarpy | miR168-resistant 4m-SlAGO1A | [56] | |||
| miR172 | AP2 | small parthenocarpic fruit-like organ | CRISPR/Cas9 (slmir172c-dCR) | [24] | |||
| Fruit ripening | fruit color | litchi | miR156a * | LcSPL1/2 | anthocyanin biosynthesis | High-Throughput Sequencing and Degradome Analysis | [67] |
| NEW41 * | CHI | anthocyanin accumulation | |||||
| pear | miR156 * | SPL | Red Peel Coloration, anthocyanin biosynthesis | Degradome Library | [73] | ||
| blueberry | miR156a | VcSPL12 | anthocyanin accumulation | VcMIR156a OE in tomato | [66] | ||
| miR396 * | FtsZs | coloration | Small RNA and Degradome Sequencing | [77] | |||
| miR_n10 * | BAG1 | coloration | |||||
| apple | miR172 | AP2-MYB10 | flavonoidse, reduction in red coloration | miR172 OE | [80] | ||
| MLNC3.2 and MLNC4.6 (lncRNA) | miR156a-SPL2-like and SPL33 | anthocyanin biosynthesis | transient (35S::MLNC3.2, 35S::MLNC4.6, OE-miR156a) | [75] | |||
| miR7125 (light-induced) | MYB16/MYB1-CCRs | promoted anthocyanin synthesis, reduced lignin biosynthesis | transient (miR7125 OE) | [79] | |||
| MdLNC499 (lncRNA) | MdERF109 | fruit coloration | transient (TRV-MdLNC499, TRV-MdERF109, apple fruit), stable (MdLNC499 OE, MdLNC499 RNAi, MdERF109 OE, MdERF109 RNAi, apple calli) | [78] | |||
| mdm-miR828 | TAS4-MdMYB1 | inhibited anthocyanin synthesis | transient (mdm-miR828 OE, apple, stable (mdm-miR828 OE, Arabidopsis) | [82] | |||
| miR858 * | MYB | anthocyanin biosynthesis | small RNA-seq | [26] | |||
| sea buckthorn | LNC1 (lncRNA)-miR156a | SPL9 | anthocyanin accumulation | transient (TRV-LNC1) | [76] | ||
| Fruit ripening | fruit color | grape | miR858 | VvMYB114 | anthocyanin and flavonol accumulation | Degradom, transient/stable (VvMYB114 OE, tobacco) | [69] |
| miR156 | SPL9 | promoted fruit coloration | miR156b/c/d OE in tomato | [74] | |||
| miR3627 * | calcium-transporting ATPase10 | anthocyanin accumulation | sequencing small RNAs, bioinformatics analysis | [101] | |||
| miR828 | VvMYB113/VvMYB114 | anthocyanin and flavonol accumulation | vvi-miR828 OE, Arabidopsis | [69] | |||
| arabidopsis | miR828 | MYB75, MYB90, and MYB113 | anthocyanin accumulation | AtmiR828 OE | [68] | ||
| miR858a | MYB2 | anthocyanin accumulation, anthocyanin biosynthesis | STTM-miR858 | [72] | |||
| miR156 | SPL9 and SPL15 | anthocyanin biosynthesis | MIR156b OE | [65] | |||
| tomato | miR858 | SlMYB7 and SlMYB48 | anthocyanin accumulation | STTM-miR858 | [70] | ||
| kiwifruit | miR858 | AaMYBC1 | anthocyanin biosynthesis | transient (miR858 OE) | [71] | ||
| fruit ripening, fruit softening and fruit quality | persimmon | miR395 * | bHLH | tannin biosynthesis | high-throughput sequencing | [83] | |
| miR156 * | SPL | tannin biosynthesis | |||||
| miR396 * | Flavonoid 3-O-glucosyltransferase (UFGT) | tannin biosynthesis | |||||
| miR858 * | MYB19/20 | reduced the content of proanthocyanidin (PA) | |||||
| miR2991 * | ADH | tannin biosynthesis | |||||
| Fruit ripening | fruit ripening, fruit softening and fruit quality | strawberry | FRILAIR (lncRNA)-miR397 | LAC11a | delayed fruit ripening | transient (miR397 OE, Cas13b-miR397, ocotoploid strawberry) | [100] |
| fan-miR73 | ABI5 | fruit ripening | 5′ -RACE analysis | [85] | |||
| miR399 | PHO2 | flavor, sugar content | miR399a OE (woodland strawberry) | [84] | |||
| tomato | miR157 | SPL-CNR | delayed fruit ripening | miR157 OE | [86,87] | ||
| miR156 | SPL | accelerates tomato fruit softening | VIGS-miR156a | [87] | |||
| miR172 | AP2a | accelerates fruit ripening with enhanced ethylene biosynthesis | miR172 OE | [105] | |||
| miR166 | SlREV | fruit ripening | 35S::REVRis (EIN3, ERFs, AP2, and CTR3 downregulated) | [50] | |||
| miR828 * | EIN2 | ethylene-dependent ripening | high throughput sequencing | [108] | |||
| miR1917 | CTR4 | enhances ethylene response and accelerates fruit ripening | miR1917 OE | [107] | |||
| lncRNA2155 (lncRNA) | RIN, CNR, NOR, ACS4, PSY1 | delayed fruit ripening | CRISPR/Cas9 (lncRNA2155 KO) | [98] | |||
| lncRNA1459 (lncRNA) | PSY1, PDS, ZDS | ripening, ethylene biosynthesis | CRISPR/Cas9 (lncRNA1459 KO) | [10,17] | |||
| lncRNA1840 (lncRNA) | ripening-related genes | ripening, ethylene biosynthesis | TRV-lncRNA1840 | [10] | |||
| kiwifruit | miR164 | NAC6/7 | fruit ripening | miR164 OE (kiwifruit callus) | [88] | ||
| apple | miR7125 | MYB16/MYB1-CCRs | reduced lignin biosynthesis | transient (miR7125 OE, apple fruit) | [79] | ||
| melon | cme-miR393 | CmAFB2 | delayed fruit ripening | cme-miR393-OE | [89] | ||
| Fruit ripening | fruit ripening, fruit softening and fruit quality | grapes | miR479 * | BGA | fruit softing | deep sequencing, bioinformatics analysis | [101] |
| miR399 * | ACO3 | ||||||
| miR397 * | LOX | ||||||
| miR3627 * | Grip22/PAL | ||||||
| miR2950 * | CHS | ||||||
| miR22 * | PE | ||||||
| biotic and abiotic stress in fruit | cold response | arabidopsis | CIL1 (lncRNA) | ROS | enhances cold stress tolerance | T-DNA insertion mutants | [124] |
| orange | miR396b | GRF | cold tolerance | ptr-miR396b OE (transgenic lemon (Citrus limon)) | [119] | ||
| banana | miR393 * | TIR1/AFB | cold stress-specific response | bioinformatics analysis | [121] | ||
| mango | CRlnc26299 * (lncRNA) | RC12B | chilling tolerance | Computational Identification | [120] | ||
| salt tolerance | arabidopsis | miR396 | GRF | salt tolerance | target mimicry (eTM) transgene specific to miR396 | [111] | |
| miR393a/b | TIR1 | salt stress resistance and ABA signaling pathways | mir393ab double mutant | [109,110] | |||
| pitaya | miR396 * | GRF | stress response | bioinformatics analysis | [112] | ||
| heat tolerance | tomato | miR396 * | GRF | drought and heat stress | bioinformatics analysis | [125] | |
| arabidopsis | miR160 | ARF10, ARF16, and ARF17 | heat stress tolerance | eTM-miR160 | [116] | ||
| banana | miR156 * | SPL | heat stress response | bioinformatics analysis | [121] | ||
| mango | MmiR78769 and MmiR101928 (lncRNA) | phospholipase A and phospholipase D | biotic and abiotic stresses | Computational Identification | [120] | ||
| biotic and abiotic stress in fruit | heat tolerance | pear | Novel_188 | Pbr027651.1 | mediate fruit senescence | transient (Novel_188 OE) | [118] |
| LNC_000862 * (lncRNA) | miR390a-Pbr031098.1 | heat tolerance | bioinformatics analysis | [122] | |||
| drought response | arabidopsis | miR396a/b | GRF | drought tolerance | 35S::MIR396a and 35S::MIR396b | [127] | |
| miR159 | MYB101 and MYB33 | drought tolerance | miR159 OE | [128] | |||
| DRIR (lncRNA) | genes involved in ABA signaling | Enhances Drought and Salt Stress Tolerance | DRIR OE | [129] | |||
| tomato | miR169 | NFYA | drought and heat stress | STTM-miR169 | [126] | ||
| miR159 * | MYB | bioinformatics analysis | [125] | ||||
| miR160 * | ARF | ||||||
| miR167 * | ARF | ||||||
| miR393 * | auxin receptor homologous genes | ||||||
| pathogen defense | arabidopsis | miR396 | GRF | pathogen defense | miR396 target mimics lines | [138] | |
| apple | Md-miRLn11 | Md-NBS | pathogen defense | bioinformatics analysis | [132] | ||
| tomato | SlymiR482e-3p | NBS-LRR | enhanced resistance to tomato wilt disease | slymiR482e-3p KO lines | [133] | ||
| miR156 * | SPL | response to ToLCV infections | bioinformatics analysis | [29,135] | |||
| miR159/319 | AP2-like | viral response (tomato leaf curl new delhi virus (tolcndv)) | MicroRNA profiling | [136] | |||
| miR172 | TCP, bHLH | [136] | |||||
| LncRNA4504 (lncRNA) | JA signal pathway genes | pathogen defense (Botrytis cinerea) | TRV-lncRNA4504 | [139] | |||
| pear | pbr-miR156 * | pbRPS6 | viral defense | bioinformatics analysis | [137] | ||
| pbr-miR164 * | pbNAC | ||||||
| pbr-miR399 * | pbTLR | ||||||
| pbr-miR482 * | pbRX-CC |
5. Conclusions and Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Coombe, B.G. The Development of Fleshy Fruits. Annu. Rev. Plant Physiol. 1976, 27, 207–228. [Google Scholar] [CrossRef]
- Seymour, G.B.; Østergaard, L.; Chapman, N.H.; Knapp, S.; Martin, C. Fruit Development and Ripening. Annu. Rev. Plant Biol. 2013, 64, 219–241. [Google Scholar] [CrossRef] [Green Version]
- Seymour, G.B.; Chapman, N.H.; Chew, B.L.; Rose, J.K.C. Regulation of Ripening and Opportunities for Control in Tomato and Other Fruits. Plant Biotechnol. J. 2013, 11, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Alquézar, B.; Peña, L. Fruit Aromas in Mature Fleshy Fruits as Signals of Readiness for Predation and Seed Dispersal. New Phytol. 2013, 197, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Feng, Y.; Yuan, S.; Zhao, X.; Wu, C.; Wang, C.; Xue, Z. Different Regulatory Mechanisms of Plant Hormones in the Ripening of Climacteric and Non-Climacteric Fruits: A Review. Plant Mol. Biol. 2021, 107, 477–497. [Google Scholar] [CrossRef]
- Lin, T.; Zhu, G.; Zhang, J.; Xu, X.; Yu, Q.; Zheng, Z.; Zhang, Z.; Lun, Y.; Li, S.; Wang, X.; et al. Genomic Analyses Provide Insights into the History of Tomato Breeding. Nat. Genet. 2014, 46, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Q.; Shi, J.; Scheben, A.; Zhan, J.; Wang, X.; Liu, G.; Yan, G.; King, G.J.; Edwards, D.; Wang, H. Genetic and Signalling Pathways of Dry Fruit Size: Targets for Genome Editing-based Crop Improvement. Plant Biotechnol. J. 2020, 18, 1124–1140. [Google Scholar] [CrossRef]
- Wang, W.-Q.; Allan, A.C.; Yin, X.-R. Small RNAs With a Big Impact on Horticultural Traits. Crit. Rev. Plant Sci. 2020, 39, 30–43. [Google Scholar] [CrossRef]
- Chen, C.; Zeng, Z.; Liu, Z.; Xia, R. Small RNAs, Emerging Regulators Critical for the Development of Horticultural Traits. Hortic. Res. 2018, 5, 63. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.; Yang, Y.; Li, R.; Fu, D.; Wen, L.; Luo, Y.; Zhu, H. RNA Sequencing and Functional Analysis Implicate the Regulatory Role of Long Non-Coding RNAs in Tomato Fruit Ripening. J. Exp. Bot. 2015, 66, 4483–4495. [Google Scholar] [CrossRef]
- da Silva, E.M.; Silva, G.F.F.E.; Bidoia, D.B.; da Silva Azevedo, M.; de Jesus, F.A.; Pino, L.E.; Peres, L.E.P.; Carrera, E.; López-Díaz, I.; Nogueira, F.T.S. MicroRNA159-targeted SlGAMYB transcription factors are required for fruit set in tomato. Plant J. 2017, 92, 95–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damodharan, S.; Corem, S.; Gupta, S.K.; Arazi, T. Tuning of SlARF10A Dosage by Sly-MiR160a Is Critical for Auxin-Mediated Compound Leaf and Flower Development. Plant J. 2018, 96, 855–868. [Google Scholar] [CrossRef] [Green Version]
- Hendelman, A.; Stav, R.; Zemach, H.; Arazi, T. The Tomato NAC Transcription Factor SlNAM2 Is Involved in Flower-Boundary Morphogenesis. J. Exp. Bot. 2013, 64, 5497–5507. [Google Scholar] [CrossRef] [Green Version]
- Berger, Y.; Harpaz-Saad, S.; Brand, A.; Melnik, H.; Sirding, N.; Alvarez, J.P.; Zinder, M.; Samach, A.; Eshed, Y.; Ori, N. The NAC-Domain Transcription Factor GOBLET Specifies Leaflet Boundaries in Compound Tomato Leaves. Development 2009, 136, 823–832. [Google Scholar] [CrossRef] [Green Version]
- Silva, G.F.F.E.; Silva, E.M.; da Silva Azevedo, M.; Guivin, M.A.C.; Ramiro, D.A.; Figueiredo, C.R.; Carrer, H.; Peres, L.E.P.; Nogueira, F.T.S. MicroRNA156-targeted SPL/SBP box transcription factors regulate tomato ovary and fruit development. Plant J. 2014, 78, 604–618. [Google Scholar] [CrossRef] [PubMed]
- Asefpour Vakilian, K. Machine Learning Improves Our Knowledge about miRNA Functions towards Plant Abiotic Stresses. Sci. Rep. 2020, 10, 3041. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Fu, D.; Zhu, B.; Luo, Y.; Zhu, H. CRISPR/Cas9-Mediated Mutagenesis of LncRNA1459 Alters Tomato Fruit Ripening. Plant J. 2018, 94, 513–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinneny, J.R.; Weigel, D.; Yanofsky, M.F. A Genetic Framework for Fruit Patterning in Arabidopsisthaliana. Development 2005, 132, 4687–4696. [Google Scholar] [CrossRef] [Green Version]
- Sang, Q.; Vayssières, A.; Ó’Maoiléidigh, D.S.; Yang, X.; Vincent, C.; Bertran Garcia de Olalla, E.; Cerise, M.; Franzen, R.; Coupland, G. MicroRNA172 Controls Inflorescence Meristem Size through Regulation of APETALA2 in Arabidopsis. New Phytol. 2022, 235, 356–371. [Google Scholar] [CrossRef]
- Yant, L.; Mathieu, J.; Dinh, T.T.; Ott, F.; Lanz, C.; Wollmann, H.; Chen, X.; Schmid, M. Orchestration of the Floral Transition and Floral Development in Arabidopsis by the Bifunctional Transcription Factor APETALA2. Plant Cell 2010, 22, 2156–2170. [Google Scholar] [CrossRef]
- José Ripoll, J.; Bailey, L.J.; Mai, Q.-A.; Wu, S.L.; Hon, C.T.; Chapman, E.J.; Ditta, G.S.; Estelle, M.; Yanofsky, M.F. MicroRNA Regulation of Fruit Growth. Nat. Plants 2015, 1, 15036. [Google Scholar] [CrossRef]
- Yao, J.-L.; Xu, J.; Cornille, A.; Tomes, S.; Karunairetnam, S.; Luo, Z.; Bassett, H.; Whitworth, C.; Rees-George, J.; Ranatunga, C.; et al. A MicroRNA Allele That Emerged Prior to Apple Domestication May Underlie Fruit Size Evolution. Plant J. 2015, 84, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.-L.; Tomes, S.; Xu, J.; Gleave, A.P. How MicroRNA172 Affects Fruit Growth in Different Species Is Dependent on Fruit Type. Plant Signal. Behav. 2016, 11, e1156833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, W.; Gupta, S.K.; Arazi, T.; Spitzer-Rimon, B. MIR172d Is Required for Floral Organ Identity and Number in Tomato. Int. J. Mol. Sci. 2021, 22, 4659. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, T.C.D.S.; Alves, T.C.; Caneschi, C.M.; Santana, D.D.R.G.; Fernandes-Brum, C.N.; Reis, G.L.D.; Daude, M.M.; Ribeiro, T.H.C.; Gómez, M.M.D.; Lima, A.A.; et al. New Insights into Tomato MicroRNAs. Sci. Rep. 2018, 8, 16069. [Google Scholar] [CrossRef] [Green Version]
- Xia, R.; Zhu, H.; An, Y.; Beers, E.P.; Liu, Z. Apple miRNAs and TasiRNAs with Novel Regulatory Networks. Genome Biol. 2012, 13, R47. [Google Scholar] [CrossRef] [Green Version]
- Ó’Maoiléidigh, D.S.; van Driel, A.D.; Singh, A.; Sang, Q.; Le Bec, N.; Vincent, C.; de Olalla, E.B.G.; Vayssières, A.; Romera Branchat, M.; Severing, E.; et al. Systematic Analyses of the MIR172 Family Members of Arabidopsis Define Their Distinct Roles in Regulation of APETALA2 during Floral Transition. PLoS Biol. 2021, 19, e3001043. [Google Scholar] [CrossRef]
- Xie, J.; Yang, X.; Song, Y.; Du, Q.; Li, Y.; Chen, J.; Zhang, D. Adaptive Evolution and Functional Innovation of Populus-Specific Recently Evolved MicroRNAs. New Phytol. 2017, 213, 206–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, A.; Goswami, K.; Tiwari, M.; Mukherjee, S.K.; Sanan-Mishra, N. Identification and Comparative Analysis of MicroRNAs from Tomato Varieties Showing Contrasting Response to ToLCV Infections. Physiol. Mol. Biol. Plants 2018, 24, 185–202. [Google Scholar] [CrossRef]
- Guo, C.; Xu, Y.; Shi, M.; Lai, Y.; Wu, X.; Wang, H.; Zhu, Z.; Poethig, R.S.; Wu, G. Repression of MiR156 by MiR159 Regulates the Timing of the Juvenile-to-Adult Transition in Arabidopsis. Plant Cell 2017, 29, 1293–1304. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, J.; Zhou, C. The Role of MiR156 in Developmental Transitions in Nicotiana tabacum. Sci. China Life Sci. 2015, 58, 253–260. [Google Scholar] [CrossRef] [Green Version]
- Feng, G.; Xu, L.; Wang, J.; Nie, G.; Bushman, B.S.; Xie, W.; Yan, H.; Yang, Z.; Guan, H.; Huang, L.; et al. Integration of Small RNAs and Transcriptome Sequencing Uncovers a Complex Regulatory Network during Vernalization and Heading Stages of Orchardgrass (Dactylis Glomerata L.). BMC Genom. 2018, 19, 727. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, S.; Jin, J.; Fu, D.; Yang, X.; Weng, X.; Xu, C.; Li, X.; Xiao, J.; Zhang, Q. Coordinated Regulation of Vegetative and Reproductive Branching in Rice. Proc. Natl. Acad. Sci. USA 2015, 112, 15504–15509. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Xian, Z.; Kang, X.; Tang, N.; Li, Z. Genome-Wide Identification, Phylogeny and Expression Analysis of GRAS Gene Family in Tomato. BMC Plant Biol. 2015, 15, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Peng, S.; Xian, Z.; Lin, D.; Hu, G.; Yang, L.; Ren, M.; Li, Z. Overexpression of a Tomato MiR171 Target Gene SlGRAS24 Impacts Multiple Agronomical Traits via Regulating Gibberellin and Auxin Homeostasis. Plant Biotechnol. J. 2017, 15, 472–488. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Li, C.; Li, H.; Zhang, J.; Ye, Z. Silencing GRAS2 Reduces Fruit Weight in Tomato. J. Integr. Plant Biol. 2018, 60, 498–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, T.; Qiao, M.; Liu, H.; Teotia, S.; Zhang, Z.; Zhao, Y.; Wang, B.; Zhao, D.; Shi, L.; Zhang, C.; et al. A Resource for Inactivation of MicroRNAs Using Short Tandem Target Mimic Technology in Model and Crop Plants. Mol. Plant 2018, 11, 1400–1417. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.K.; Vishwakarma, A.; Kenea, H.D.; Galsurker, O.; Cohen, H.; Aharoni, A.; Arazi, T. CRISPR/Cas9 Mutants of Tomato MICRORNA164 Genes Uncover Their Functional Specialization in Development. Plant Physiol. 2021, 187, 1636–1652. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wang, Y.; Liu, H.; Zhang, W.; Chai, M.; Tang, G.; Zhang, Z. MicroRNA1917-CTR1-LIKE PROTEIN KINASE 4 Impacts Fruit Development via Tuning Ethylene Synthesis and Response. Plant Sci. 2020, 291, 110334. [Google Scholar] [CrossRef]
- Allen, R.S.; Li, J.; Stahle, M.I.; Dubroué, A.; Gubler, F.; Millar, A.A. Genetic Analysis Reveals Functional Redundancy and the Major Target Genes of the Arabidopsis MiR159 Family. Proc. Natl. Acad. Sci. USA 2007, 104, 16371–16376. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, F.; Deng, Y.; Zhong, F.; Tian, P.; Lin, D.; Deng, J.; Zhang, Y.; Huang, T. Sly-miR159 Regulates Fruit Morphology by Modulating GA Biosynthesis in Tomato. Plant Biotechnol. J. 2022, 20, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Vivian-Smith, A.; Koltunow, A.M. Genetic Analysis of Growth-Regulator-Induced Parthenocarpy in Arabidopsis. Plant Physiol. 1999, 121, 437–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenn, M.A.; Giovannoni, J.J. Phytohormones in Fruit Development and Maturation. Plant J. 2021, 105, 446–458. [Google Scholar] [CrossRef]
- de Jong, M.; Mariani, C.; Vriezen, W.H. The Role of Auxin and Gibberellin in Tomato Fruit Set. J. Exp. Bot. 2009, 60, 1523–1532. [Google Scholar] [CrossRef] [Green Version]
- Pattison, R.J.; Csukasi, F.; Catalá, C. Mechanisms Regulating Auxin Action during Fruit Development. Physiol. Plant. 2014, 151, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Mei, L.; Wu, M.; Wei, W.; Shan, W.; Gong, Z.; Zhang, Q.; Yang, F.; Yan, F.; Zhang, Q.; et al. SlARF10, an Auxin Response Factor, Is Involved in Chlorophyll and Sugar Accumulation during Tomato Fruit Development. J. Exp. Bot. 2018, 69, 5507–5518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damodharan, S.; Zhao, D.; Arazi, T. A Common miRNA160-Based Mechanism Regulates Ovary Patterning, Floral Organ Abscission and Lamina Outgrowth in Tomato. Plant J. 2016, 86, 458–471. [Google Scholar] [CrossRef] [Green Version]
- Hong, Y.; Jackson, S. Floral Induction and Flower Formation—The Role and Potential Applications of miRNAs. Plant Biotechnol. J. 2015, 13, 282–292. [Google Scholar] [CrossRef]
- Itaya, A.; Bundschuh, R.; Archual, A.; Joung, J.; Fei, Z.; Dai, X.; Zhao, P.; Tang, Y.; Nelson, R.; Ding, B. Small RNAs in Tomato Fruit and Leaf Development. Biochim. Biophys. Acta BBA-Gene Regul. Mech. 2008, 1779, 99–107. [Google Scholar] [CrossRef]
- Hu, G.; Fan, J.; Xian, Z.; Huang, W.; Lin, D.; Li, Z. Overexpression of SlREV Alters the Development of the Flower Pedicel Abscission Zone and Fruit Formation in Tomato. Plant Sci. 2014, 229, 86–95. [Google Scholar] [CrossRef]
- Xue, C.; Yao, J.-L.; Qin, M.-F.; Zhang, M.-Y.; Allan, A.C.; Wang, D.-F.; Wu, J. PbrmiR397a Regulates Lignification during Stone Cell Development in Pear Fruit. Plant Biotechnol. J. 2019, 17, 103–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Lai, Z.; Tian, Q.; Lin, L.; Lai, R.; Yang, M.; Zhang, D.; Chen, Y.; Zhang, Z. Endogenous Target Mimics Down-Regulate MiR160 Mediation of ARF10, -16, and -17 Cleavage during Somatic Embryogenesis in Dimocarpus Longan Lour. Front. Plant Sci. 2015, 6, 956. [Google Scholar] [CrossRef] [Green Version]
- Kang, C.; Liu, Z. Global Identification and Analysis of Long Non-Coding RNAs in Diploid Strawberry Fragaria vesca during Flower and Fruit Development. BMC Genom. 2015, 16, 815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Ren, F.; Wang, X.; Qiu, K.; Sheng, Y.; Xie, Q.; Shi, P.; Zhang, J.; Pan, H. Genome-Wide Identification and Characterization of Long Noncoding RNAs during Peach (Prunus Persica) Fruit Development and Ripening. Sci. Rep. 2022, 12, 11044. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Guo, M.; Huang, K.; Qi, Q.; Li, W.; Yan, J.; He, J.; Guan, Q.; Ma, F.; Xu, J. Genome-Wide Identification and Characterization of Long Noncoding RNAs Involved in Apple Fruit Development and Ripening. Sci. Hortic. 2022, 295, 110898. [Google Scholar] [CrossRef]
- Xian, Z.; Huang, W.; Yang, Y.; Tang, N.; Zhang, C.; Ren, M.; Li, Z. MiR168 Influences Phase Transition, Leaf Epinasty, and Fruit Development via SlAGO1s in Tomato. J. Exp. Bot. 2014, 65, 6655–6666. [Google Scholar] [CrossRef] [Green Version]
- Clepet, C.; Devani, R.S.; Boumlik, R.; Hao, Y.; Morin, H.; Marcel, F.; Verdenaud, M.; Mania, B.; Brisou, G.; Citerne, S.; et al. The MiR166–SlHB15A Regulatory Module Controls Ovule Development and Parthenocarpic Fruit Set under Adverse Temperatures in Tomato. Mol. Plant 2021, 14, 1185–1198. [Google Scholar] [CrossRef]
- Murakami, S. Study of Parthenocarpy in Three Kiwifruit Commercial Cultivars. Int. J. Fruit Sci. 2020, 20, S802–S811. [Google Scholar] [CrossRef]
- Cong, L.; Wu, T.; Liu, H.; Wang, H.; Zhang, H.; Zhao, G.; Wen, Y.; Shi, Q.; Xu, L.; Wang, Z. CPPU May Induce Gibberellin-Independent Parthenocarpy Associated with PbRR9 in ‘Dangshansu’ Pear. Hortic. Res. 2020, 7, 68. [Google Scholar] [CrossRef]
- Shinozaki, Y.; Ezura, H.; Ariizumi, T. The Role of Ethylene in the Regulation of Ovary Senescence and Fruit Set in Tomato (Solanum Lycopersicum). Plant Signal. Behav. 2018, 13, e1146844. [Google Scholar] [CrossRef]
- Serrani, J.C.; Carrera, E.; Ruiz-Rivero, O.; Gallego-Giraldo, L.; Peres, L.E.P.; García-Martínez, J.L. Inhibition of Auxin Transport from the Ovary or from the Apical Shoot Induces Parthenocarpic Fruit-Set in Tomato Mediated by Gibberellins. Plant Physiol. 2010, 153, 851–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mostafa, L.Y.; Kotb, H.R.M. Effect of Brassinosteroids and Gibberellic Acid on Parthenocarpic Fruit Formation and Fruit Quality of Sugar Apple Annona squamosa L. Middle East J. Agric. Res. 2018, 7, 1341–1351. [Google Scholar]
- Lu, L.; Liang, J.; Zhu, X.; Xiao, K.; Li, T.; Hu, J. Auxin- and Cytokinin-Induced Berries Set in Grapevine Partly Rely on Enhanced Gibberellin Biosynthesis. Tree Genet. Genomes 2016, 12, 41. [Google Scholar] [CrossRef]
- Mesejo, C.; Yuste, R.; Reig, C.; Martínez-Fuentes, A.; Iglesias, D.J.; Muñoz-Fambuena, N.; Bermejo, A.; Germanà, M.A.; Primo-Millo, E.; Agustí, M. Gibberellin Reactivates and Maintains Ovary-Wall Cell Division Causing Fruit Set in Parthenocarpic Citrus Species. Plant Sci. 2016, 247, 13–24. [Google Scholar] [CrossRef]
- Gou, J.-Y.; Felippes, F.F.; Liu, C.-J.; Weigel, D.; Wang, J.-W. Negative Regulation of Anthocyanin Biosynthesis in Arabidopsis by a MiR156-Targeted SPL Transcription Factor. Plant Cell 2011, 23, 1512–1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Hou, Y.; Xie, X.; Li, H.; Li, X.; Zhu, Y.; Zhai, L.; Zhang, C.; Bian, S. A Blueberry MIR156a–SPL12 Module Coordinates the Accumulation of Chlorophylls and Anthocyanins during Fruit Ripening. J. Exp. Bot. 2020, 71, 5976–5989. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Lai, B.; Hu, B.; Qin, Y.; Hu, G.; Zhao, J. Identification of MicroRNAs and Their Target Genes Related to the Accumulation of Anthocyanins in Litchi Chinensis by High-Throughput Sequencing and Degradome Analysis. Front. Plant Sci. 2017, 7, 2059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Cai, J.; Yang, Y.; Liu, Z. Overexpression of MicroRNA828 Reduces Anthocyanin Accumulation in Arabidopsis. Plant Cell Tissue Organ Cult. PCTOC 2013, 15, 159–167. [Google Scholar] [CrossRef]
- Tirumalai, V.; Swetha, C.; Nair, A.; Pandit, A.; Shivaprasad, P.V. miR828 and miR858 Regulate VvMYB114 to Promote Anthocyanin and Flavonol Accumulation in Grapes. J. Exp. Bot. 2019, 70, 4775–4792. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Shen, J.; Liu, H.; Li, F.; Ding, N.; Gao, C.; Pattanaik, S.; Patra, B.; Li, R.; Yuan, L. Small Tandem Target Mimic-Mediated Blockage of MicroRNA858 Induces Anthocyanin Accumulation in Tomato. Planta 2015, 242, 283–293. [Google Scholar] [CrossRef]
- Li, Y.; Cui, W.; Qi, X.; Lin, M.; Qiao, C.; Zhong, Y.; Hu, C.; Fang, J. MicroRNA858 Negatively Regulates Anthocyanin Biosynthesis by Repressing AaMYBC1 Expression in Kiwifruit (Actinidia Arguta). Plant Sci. 2020, 296, 110476. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Song, Z.; Zhang, H. Repression of MYBL2 by Both MicroRNA858a and HY5 Leads to the Activation of Anthocyanin Biosynthetic Pathway in Arabidopsis. Mol. Plant 2016, 9, 1395–1405. [Google Scholar] [CrossRef] [Green Version]
- Qian, M.; Ni, J.; Niu, Q.; Bai, S.; Bao, L.; Li, J.; Sun, Y.; Zhang, D.; Teng, Y. Response of MiR156-SPL Module during the Red Peel Coloration of Bagging-Treated Chinese Sand Pear (Pyrus Pyrifolia Nakai). Front. Physiol. 2017, 8, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Z.; Wang, X.; Xuan, X.; Sheng, Z.; Jia, H.; Emal, N.; Liu, Z.; Zheng, T.; Wang, C.; Fang, J. Characterization and Action Mechanism Analysis of VvmiR156b/c/d-VvSPL9 Module Responding to Multiple-Hormone Signals in the Modulation of Grape Berry Color Formation. Foods 2021, 10, 896. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Ma, H.; Zhang, J.; Wu, T.; Song, T.; Tian, J.; Yao, Y. Systematic Identification of Long Noncoding RNAs Expressed during Light-Induced Anthocyanin Accumulation in Apple Fruit. Plant J. 2019, 100, 572–590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Chen, D.; Zhang, T.; Duan, A.; Zhang, J.; He, C. Transcriptomic and Functional Analyses Unveil the Role of Long Non-Coding RNAs in Anthocyanin Biosynthesis during Sea Buckthorn Fruit Ripening. DNA Res. 2018, 25, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Zhai, L.; Li, X.; Xue, Y.; Wang, J.; Yang, P.; Cao, C.; Li, H.; Cui, Y.; Bian, S. Comparative Analysis of Fruit Ripening-Related miRNAs and Their Targets in Blueberry Using Small RNA and Degradome Sequencing. Int. J. Mol. Sci. 2017, 18, 2767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, H.; Yang, T.; Li, Y.; Zhang, J.; Wu, T.; Song, T.; Yao, Y.; Tian, J. The Long Noncoding RNA MdLNC499 Bridges MdWRKY1 and MdERF109 Function to Regulate Early-Stage Light-Induced Anthocyanin Accumulation in Apple Fruit. Plant Cell 2021, 33, 3309–3330. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, H.; Zhang, Y.; Zhang, J.; Niu, S.; Wang, X.; Li, W.; Zhang, J.; Yao, Y. The MdMYB16/MdMYB1-MiR7125-MdCCR Module Regulates the Homeostasis between Anthocyanin and Lignin Biosynthesis during Light Induction in Apple. New Phytol. 2021, 231, 1105–1122. [Google Scholar] [CrossRef]
- Ding, T.; Tomes, S.; Gleave, A.P.; Zhang, H.; Dare, A.P.; Plunkett, B.; Espley, R.V.; Luo, Z.; Zhang, R.; Allan, A.C.; et al. MicroRNA172 Targets APETALA2 to Regulate Flavonoid Biosynthesis in Apple (Malus domestica). Hortic. Res. 2022, 9, uhab007. [Google Scholar] [CrossRef]
- Ramsay, N.A.; Glover, B.J. MYB–BHLH–WD40 Protein Complex and the Evolution of Cellular Diversity. Trends Plant Sci. 2005, 10, 63–70. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, H.-J.; Yang, Y.-Z.; Zhu, Z.-Z.; Li, Y.-N.; Qu, D.; Zhao, Z.-Y. Mdm-MiR828 Participates in the Feedback Loop to Regulate Anthocyanin Accumulation in Apple Peel. Front. Plant Sci. 2020, 11, 608109. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, X.; Luo, Z.; Zhang, Q.; Liu, J. Identification and Characterization of MicroRNAs from Chinese Pollination Constant Non-Astringent Persimmon Using High-Throughput Sequencing. BMC Plant Biol. 2015, 15, 11. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, J.; Cui, W.; Guan, C.; Mao, W.; Zhang, Z. Improvement in Fruit Quality by Overexpressing MiR399a in Woodland Strawberry. J. Agric. Food Chem. 2017, 65, 7361–7370. [Google Scholar] [CrossRef]
- Li, D.; Mou, W.; Luo, Z.; Li, L.; Limwachiranon, J.; Mao, L.; Ying, T. Developmental and Stress Regulation on Expression of a Novel miRNA, Fan-MiR73 and Its Target ABI5 in Strawberry. Sci. Rep. 2016, 6, 28385. [Google Scholar] [CrossRef] [Green Version]
- Karlova, R.; van Haarst, J.C.; Maliepaard, C.; van de Geest, H.; Bovy, A.G.; Lammers, M.; Angenent, G.C.; de Maagd, R.A. Identification of MicroRNA Targets in Tomato Fruit Development Using High-Throughput Sequencing and Degradome Analysis. J. Exp. Bot. 2013, 64, 1863–1878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Kong, J.; Lai, T.; Manning, K.; Wu, C.; Wang, Y.; Qin, C.; Li, B.; Yu, Z.; Zhang, X.; et al. Tuning LeSPL-CNR Expression by SlymiR157 Affects Tomato Fruit Ripening. Sci. Rep. 2015, 5, 7852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Wang, J.; Wu, Y.; Li, D.; Allan, A.C.; Yin, X. Genome-Wide Analysis of Coding and Non-Coding RNA Reveals a Conserved MiR164-NAC Regulatory Pathway for Fruit Ripening. New Phytol. 2020, 225, 1618–1634. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Tian, Y.; Tan, C.; Bai, S.; Hao, J.; Hasi, A. Genome-Wide Identification of MicroRNAs Involved in the Regulation of Fruit Ripening and Climacteric Stages in Melon (Cucumis melo). Hortic. Res. 2020, 7, 106. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, W.; Gao, L.; Zhao, L. Genome-Wide Profiling of Long Non-Coding RNAs from Tomato and a Comparison with MRNAs Associated with the Regulation of Fruit Ripening. BMC Plant Biol. 2018, 18, 75. [Google Scholar] [CrossRef] [Green Version]
- An, N.; Fan, S.; Wang, Y.; Zhang, L.; Gao, C.; Zhang, D.; Han, M. Genome-Wide Identification, Characterization and Expression Analysis of Long Non-Coding RNAs in Different Tissues of Apple. Gene 2018, 666, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, G.; Sharma, S.; Upadhyay, S.K.; Singh, K. Long Non-Coding RNAs Coordinate Developmental Transitions and Other Key Biological Processes in Grapevine. Sci. Rep. 2019, 9, 3552. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Cheng, C.; Feng, X.; Lai, R.; Gao, M.; Chen, W.; Wu, R. Integrated Analysis of LncRNA and MRNA Transcriptomes Reveals the Potential Regulatory Role of LncRNA in Kiwifruit Ripening and Softening. Sci. Rep. 2021, 11, 1671. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Shi, T.; Iqbal, S.; Zhang, Y.; Liu, L.; Gao, Z. Genome-Wide Discovery and Characterization of Flower Development Related Long Non-Coding RNAs in Prunus mume. BMC Plant Biol. 2019, 19, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.; Duan, A.; Zhang, J.; He, C. Genome-Wide Analysis of Long Non-Coding RNAs at the Mature Stage of Sea Buckthorn (Hippophae Rhamnoides Linn) Fruit. Gene 2017, 596, 130–136. [Google Scholar] [CrossRef]
- Tian, Y.; Bai, S.; Dang, Z.; Hao, J.; Zhang, J.; Hasi, A. Genome-Wide Identification and Characterization of Long Non-Coding RNAs Involved in Fruit Ripening and the Climacteric in Cucumis melo. BMC Plant Biol. 2019, 19, 369. [Google Scholar] [CrossRef] [Green Version]
- Bai, L.; Chen, Q.; Jiang, L.; Lin, Y.; Ye, Y.; Liu, P.; Wang, X.; Tang, H. Comparative Transcriptome Analysis Uncovers the Regulatory Functions of Long Noncoding RNAs in Fruit Development and Color Changes of Fragaria pentaphylla. Hortic. Res. 2019, 6, 42. [Google Scholar] [CrossRef] [Green Version]
- Yu, T.; Tzeng, D.T.W.; Li, R.; Chen, J.; Zhong, S.; Fu, D.; Zhu, B.; Luo, Y.; Zhu, H. Genome-Wide Identification of Long Non-Coding RNA Targets of the Tomato MADS Box Transcription Factor RIN and Function Analysis. Ann. Bot. 2019, 123, 469–482. [Google Scholar] [CrossRef] [Green Version]
- Vrebalov, J.; Ruezinsky, D.; Padmanabhan, V.; White, R.; Medrano, D.; Drake, R.; Schuch, W.; Giovannoni, J. A MADS-Box Gene Necessary for Fruit Ripening at the Tomato Ripening-Inhibitor (Rin) Locus. Science 2002, 296, 343–346. [Google Scholar] [CrossRef]
- Tang, Y.; Qu, Z.; Lei, J.; He, R.; Adelson, D.L.; Zhu, Y.; Yang, Z.; Wang, D. The Long Noncoding RNA FRILAIR Regulates Strawberry Fruit Ripening by Functioning as a Noncanonical Target Mimic. PLoS Genet. 2021, 17, e1009461. [Google Scholar] [CrossRef]
- Xue, M.; Yi, H.; Wang, H. Identification of miRNAs Involved in SO2 Preservation in Vitis vinifera L. by Deep Sequencing. Environ. Exp. Bot. 2018, 153, 218–228. [Google Scholar] [CrossRef]
- Kumar, R.; Khurana, A.; Sharma, A.K. Role of Plant Hormones and Their Interplay in Development and Ripening of Fleshy Fruits. J. Exp. Bot. 2013, 65, 4561–4575. [Google Scholar] [CrossRef] [Green Version]
- Alexander, L. Ethylene Biosynthesis and Action in Tomato: A Model for Climacteric Fruit Ripening. J. Exp. Bot. 2002, 53, 2039–2055. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, K.; Grierson, D. A Critical Evaluation of the Role of Ethylene and MADS Transcription Factors in the Network Controlling Fleshy Fruit Ripening. New Phytol. 2019, 221, 1724–1741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, M.-Y.; Nath, U.K.; Vrebalov, J.; Gapper, N.; Lee, J.M.; Lee, D.-J.; Kim, C.K.; Giovannoni, J. Ectopic Expression of miRNA172 in Tomato (Solanum Lycopersicum) Reveals Novel Function in Fruit Development through Regulation of an AP2 Transcription Factor. BMC Plant Biol. 2020, 20, 283. [Google Scholar] [CrossRef]
- Liu, M.; Pirrello, J.; Chervin, C.; Roustan, J.-P.; Bouzayen, M. Ethylene Control of Fruit Ripening: Revisiting the Complex Network of Transcriptional Regulation. Plant Physiol. 2015, 169, 2380–2390. [Google Scholar] [CrossRef] [Green Version]
- Moxon, S.; Jing, R.; Szittya, G.; Schwach, F.; Rusholme Pilcher, R.L.; Moulton, V.; Dalmay, T. Deep Sequencing of Tomato Short RNAs Identifies MicroRNAs Targeting Genes Involved in Fruit Ripening. Genome Res. 2008, 18, 1602–1609. [Google Scholar] [CrossRef] [Green Version]
- Zuo, J.; Zhu, B.; Fu, D.; Zhu, Y.; Ma, Y.; Chi, L.; Ju, Z.; Wang, Y.; Zhai, B.; Luo, Y. Sculpting the Maturation, Softening and Ethylene Pathway: The Influences of MicroRNAs on Tomato Fruits. BMC Genom. 2012, 13, 7. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Hu, L.; Han, N.; Hu, J.; Yang, Y.; Xiang, T.; Zhang, X.; Wang, L. Overexpression of a MiR393-Resistant Form of Transport Inhibitor Response Protein 1 (MTIR1) Enhances Salt Tolerance by Increased Osmoregulation and Na+ Exclusion in Arabidopsis Thaliana. Plant Cell Physiol. 2015, 56, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Denver, J.B.; Ullah, H. MiR393s Regulate Salt Stress Response Pathway in Arabidopsis thaliana through Scaffold Protein RACK1A Mediated ABA Signaling Pathways. Plant Signal. Behav. 2019, 14, 1600394. [Google Scholar] [CrossRef]
- Pegler, J.L.; Nguyen, D.Q.; Oultram, J.M.J.; Grof, C.P.L.; Eamens, A.L. Molecular Manipulation of the MiR396/GRF Expression Module Alters the Salt Stress Response of Arabidopsis thaliana. Agronomy 2021, 11, 1751. [Google Scholar] [CrossRef]
- Cai, X.; Zhang, L.; Xiao, L.; Wen, Z.; Hou, Q.; Yang, K. Genome-Wide Identification of GRF Gene Family and Their Contribution to Abiotic Stress Response in Pitaya (Hylocereus polyrhizus). Int. J. Biol. Macromol. 2022, 223, 618–635. [Google Scholar] [CrossRef] [PubMed]
- Yaish, M.W.; Sunkar, R.; Zheng, Y.; Ji, B.; Al-Yahyai, R.; Farooq, S.A. A Genome-Wide Identification of the miRNAome in Response to Salinity Stress in Date Palm (Phoenix dactylifera L.). Front. Plant Sci. 2015, 6, 946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, R.; Zhang, J.; Ma, Y.; Pan, X.; Dong, C.; Pang, S.; He, S.; Deng, L.; Yi, S.; Zheng, Y.; et al. Combined Analysis of MRNA and miRNA Identifies Dehydration and Salinity Responsive Key Molecular Players in Citrus Roots. Sci. Rep. 2017, 7, 42094. [Google Scholar] [CrossRef] [Green Version]
- Pan, C.; Ye, L.; Zheng, Y.; Wang, Y.; Yang, D.; Liu, X.; Chen, L.; Zhang, Y.; Fei, Z.; Lu, G. Identification and Expression Profiling of MicroRNAs Involved in the Stigma Exsertion under High-Temperature Stress in Tomato. BMC Genom. 2017, 18, 843. [Google Scholar] [CrossRef]
- Lin, J.-S.; Kuo, C.-C.; Yang, I.-C.; Tsai, W.-A.; Shen, Y.-H.; Lin, C.-C.; Liang, Y.-C.; Li, Y.-C.; Kuo, Y.-W.; King, Y.-C.; et al. MicroRNA160 Modulates Plant Development and Heat Shock Protein Gene Expression to Mediate Heat Tolerance in Arabidopsis. Front. Plant Sci. 2018, 9, 68. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Du, G.; Xiang, J.; Hu, C.; Li, X.; Wang, W.; Zhu, H.; Qiao, L.; Zhao, C.; Wang, J.; et al. Genome-Wide Identification of Auxin Response Factor (ARF) Gene Family and the MiR160-ARF18-Mediated Response to Salt Stress in Peanut (Arachis hypogaea L.). Genomics 2022, 114, 171–184. [Google Scholar] [CrossRef]
- Gu, C.; Xu, H.-Y.; Zhou, Y.-H.; Yao, J.-L.; Xie, Z.-H.; Chen, Y.-Y.; Zhang, S.-L. Multiomics Analyses Unveil the Involvement of MicroRNAs in Pear Fruit Senescence under High- or Low-Temperature Conditions. Hortic. Res. 2020, 7, 196. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Wang, M.; Zhang, H.-Y.; Liu, J.-H. The MiR396b of Poncirus Trifoliata Functions in Cold Tolerance by Regulating ACC Oxidase Gene Expression and Modulating Ethylene–Polyamine Homeostasis. Plant Cell Physiol. 2016, 57, 1865–1878. [Google Scholar] [CrossRef] [Green Version]
- Moh, N.M.M.; Zhang, P.; Chen, Y.; Chen, M. Computational Identification of miRNAs and Temperature-Responsive LncRNAs From Mango (Mangifera indica L.). Front. Genet. 2021, 12, 607248. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, Y.; Tang, R.; Qu, H.; Duan, X.; Jiang, Y. Banana SRNAome and Degradome Identify MicroRNAs Functioning in Differential Responses to Temperature Stress. BMC Genom. 2019, 20, 33. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, X.; Qi, K.; Bao, J.; Zhang, S.; Gu, C. Involvement of Long Non-Coding RNAs in Pear Fruit Senescence under High- and Low-Temperature Conditions. Hortic. Plant J. 2022, in press. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, L.; Zhu, B.; Zhu, H.; Luo, Y.; Wang, Q.; Zuo, J. Integrative Analysis of Long Non-Coding RNA Acting as CeRNAs Involved in Chilling Injury in Tomato Fruit. Gene 2018, 667, 25–33. [Google Scholar] [CrossRef]
- Liu, G.; Liu, F.; Wang, Y.; Liu, X. A Novel Long Non-Coding RNA CIL1 Enhances Cold Stress Tolerance in Arabidopsis. Plant Sci. 2022, 323, 111370. [Google Scholar] [CrossRef]
- Zhou, R.; Yu, X.; Ottosen, C.-O.; Zhang, T.; Wu, Z.; Zhao, T. Unique miRNAs and Their Targets in Tomato Leaf Responding to Combined Drought and Heat Stress. BMC Plant Biol. 2020, 20, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, S.; Balyan, S.; Jha, S.; Mathur, S. Novel Insights into Expansion and Functional Diversification of MIR169 Family in Tomato. Planta 2020, 251, 55. [Google Scholar] [CrossRef]
- Liu, D.; Song, Y.; Chen, Z.; Yu, D. Ectopic Expression of MiR396 Suppresses GRF Target Gene Expression and Alters Leaf Growth in Arabidopsis. Physiol. Plant. 2009, 136, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.L.; Chua, N.-H. ABA Induction of MiR159 Controls Transcript Levels of Two MYB Factors during Arabidopsis Seed Germination. Plant J. 2007, 49, 592–606. [Google Scholar] [CrossRef]
- Qin, T.; Zhao, H.; Cui, P.; Albesher, N.; Xiong, L. A Nucleus-Localized Long Non-Coding RNA Enhances Drought and Salt Stress Tolerance. Plant Physiol. 2017, 175, 1321–1336. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Yang, S.; Tang, J.; Song, H. miRNA Expression Profiling Reveals the Regulators of Kiwifruit Response to Botrytis cinerea. Physiol. Mol. Plant Pathol. 2022, 120, 101851. [Google Scholar] [CrossRef]
- Wang, C.; Han, J.; Liu, C.; Kibet, K.N.; Kayesh, E.; Shangguan, L.; Li, X.; Fang, J. Identification of MicroRNAs from Amur Grape (Vitis amurensis Rupr.) by Deep Sequencing and Analysis of MicroRNA Variations with Bioinformatics. BMC Genom. 2012, 13, 122. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Lu, Y.; Bai, S.; Zhang, W.; Duan, X.; Meng, D.; Wang, Z.; Wang, A.; Zhou, Z.; Li, T. Cloning and Characterization of miRNAs and Their Targets, Including a Novel miRNA-Targeted NBS–LRR Protein Class Gene in Apple (Golden Delicious). Mol. Plant 2014, 7, 218–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Li, S.; Zhang, S.; Feng, T.; Zhang, Z.; Luo, S.; Mao, H.; Borkovich, K.A.; Ouyang, S. SlymiR482e-3p Mediates Tomato Wilt Disease by Modulating Ethylene Response Pathway. Plant Biotechnol. J. 2021, 19, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Mohanty, J.N.; Chand, S.K.; Joshi, R.K. Can-MiRn37a Mediated Suppression of Ethylene Response Factors Enhances the Resistance of Chilli against Anthracnose Pathogen Colletotrichum truncatum L. Plant Sci. 2018, 267, 135–147. [Google Scholar] [CrossRef]
- Liu, M.; Yu, H.; Zhao, G.; Huang, Q.; Lu, Y.; Ouyang, B. Identification of Drought-Responsive MicroRNAs in Tomato Using High-Throughput Sequencing. Funct. Integr. Genom. 2018, 18, 67–78. [Google Scholar] [CrossRef]
- Naqvi, A.R.; Haq, Q.M.; Mukherjee, S.K. MicroRNA Profiling of Tomato Leaf Curl New Delhi Virus (Tolcndv) Infected Tomato Leaves Indicates That Deregulation of Mir159/319 and Mir172 Might Be Linked with Leaf Curl Disease. Virol. J. 2010, 7, 281. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Zhang, Y.; Wang, S.; Hao, L.; Wang, S.; Xu, C.; Jiang, F.; Li, T. Characterization of Genome-Wide MicroRNAs and Their Roles in Development and Biotic Stress in Pear. Planta 2019, 249, 693–707. [Google Scholar] [CrossRef]
- Soto-Suárez, M.; Baldrich, P.; Weigel, D.; Rubio-Somoza, I.; San Segundo, B. The Arabidopsis MiR396 Mediates Pathogen-Associated Molecular Pattern-Triggered Immune Responses against Fungal Pathogens. Sci. Rep. 2017, 7, 44898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, X.; Li, J.; Min, D.; Zhao, X.; Liu, J.; Li, F.; Li, X.; Zhang, X. LncRNA4504 Involved in Methyl Jasmonate-Induced Resistance to Botrytis Cinerea in Postharvest Tomato Fruit. Sci. Hortic. 2022, 305, 111381. [Google Scholar] [CrossRef]
- Tang, J.; Chu, C. MicroRNAs in Crop Improvement: Fine-Tuners for Complex Traits. Nat. Plants 2017, 3, 17077. [Google Scholar] [CrossRef]
- Miao, C.; Wang, D.; He, R.; Liu, S.; Zhu, J. Mutations in MIR396e and MIR396f Increase Grain Size and Modulate Shoot Architecture in Rice. Plant Biotechnol. J. 2020, 18, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Zong, Y.; Xue, C.; Wang, S.; Jin, S.; Zhu, Z.; Wang, Y.; Anzalone, A.V.; Raguram, A.; Doman, J.L.; et al. Prime Genome Editing in Rice and Wheat. Nat. Biotechnol. 2020, 38, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Molla, K.A.; Sretenovic, S.; Bansal, K.C.; Qi, Y. Precise Plant Genome Editing Using Base Editors and Prime Editors. Nat. Plants 2021, 7, 1166–1187. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Li, J.; Liu, X.; Shan, T.; Qin, R.; Wei, P. Development of Plant Prime-Editing Systems for Precise Genome Editing. Plant Commun. 2020, 1, 100043. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, R.; Tang, Y.; Wang, D. Coordinating Diverse Functions of miRNA and lncRNA in Fleshy Fruit. Plants 2023, 12, 411. https://doi.org/10.3390/plants12020411
He R, Tang Y, Wang D. Coordinating Diverse Functions of miRNA and lncRNA in Fleshy Fruit. Plants. 2023; 12(2):411. https://doi.org/10.3390/plants12020411
Chicago/Turabian StyleHe, Reqing, Yajun Tang, and Dong Wang. 2023. "Coordinating Diverse Functions of miRNA and lncRNA in Fleshy Fruit" Plants 12, no. 2: 411. https://doi.org/10.3390/plants12020411
APA StyleHe, R., Tang, Y., & Wang, D. (2023). Coordinating Diverse Functions of miRNA and lncRNA in Fleshy Fruit. Plants, 12(2), 411. https://doi.org/10.3390/plants12020411





