LC–HRMS for the Identification of Quercetin and Its Derivatives in Spiraea hypericifolia (Rosaceae) and Anatomical Features of Its Leaves
Abstract
1. Introduction
2. Results and Discussion
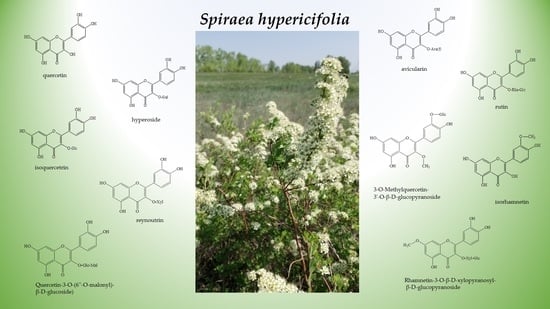
2.1. Flavonoid Assay in an S. hypericifolia Extract by Liquid Chromatography Coupled with High-Resolution Mass Spectrometry (LC–HRMS)
2.2. Anatomical Features of S. hypericifolia Leaves
3. Materials and Methods
3.1. Plant Material and Preparation of the Extract
3.2. Mass Spectrometry Settings and the Spectral Library
3.3. Chemicals
3.4. Anatomic Examination of S. hypericifolia Leaves
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Golovkin, B.N.; Rudenskaya, R.N.; Trofimov, I.A.; Shreter, A.I. Biologically Active Substances of Plant Origin; Nauka: Moscow, Russia, 2001; Volume 1, 350p. (In Russian) [Google Scholar]
- Formica, J.V.; Regelson, W. Review of the biology of quercetin and related bioflavonoids. Food Chem. Toxicol. 1995, 33, 1061–1080. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Milanovic, M.; Procopio, A.C.; Spampinato, G.; Maruca, G.; Perrino, E.V.; Mannino, G.C.; Fagoonee, S.; Luzza, F.; Musarella, C.M. Ancient wheats: Beneficial effects on insulin resistance. Minerva Med. 2020, 12, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Valerio, F.; Mezzapesa, G.N.; Ghannouchi, A.; Mondelli, D.; Logrieco, A.F.; Perrino, E.V. Characterization and antimicrobial properties of essential oils from four wild taxa of Lamiaceae family growing in Apulia. Agronomy 2021, 11, 1431. [Google Scholar] [CrossRef]
- Miean, K.H.; Mohamed, S. Flavonoid (myricetin, quercetin, kaempferol, luteolin and apigenin) content of edible tropical plants. J. Agric. Food. Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef]
- Zhang, M.; Swarts, S.G.; Yin, L.; Liu, C.; Tian, Y.; Cao, Y.; Swatrs, M.; Yang, S.; Zhang, S.B.; Zhang, K.; et al. Antioxidant properties of quercetin. Adv. Exp. Med. Biol. 2011, 701, 283–289. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Func. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Joskova, M.; Franova, S.; Sadlonova, V. Acute bronchodilator effect of quercetin in experimental allergic asthma. Bratisl. Lek. Listy 2011, 112, 9–12. [Google Scholar]
- Rogerio, A.P.; Kanashiro, A.; Fontanari, C.; da Silva, E.V.G.; Lucisano-Valim, Y.M.; Soares, E.G.; Faccioli, L.H. Anti-inflammatory activity of quercetin and isoquercitrin in experimental murine allergic asthma. Inflamm. Res. 2007, 56, 402–408. [Google Scholar] [CrossRef]
- Saeedi-Boroujeni, A.; Mahmoudian-Sani, M.R. Anti-inflammatory potential of quercetin in COVID-19 treatment. J. Inflamm. 2021, 18, 2–9. [Google Scholar] [CrossRef]
- Shoskes, D.A.; Zeitlin, S.I.; Shahed, A.; Rajfer, J. Quercetin in men with category III chronic prostatitis: A preminary prospective, double-blind, placebo-controlled trial. Urology 1999, 54, 960–963. [Google Scholar] [CrossRef]
- Shoskes, D.A.; Albakri, Q.; Thomas, K.; Cook, D. Cytokine polymorphisms in men with chronic prostatis/chronic pelvic pain syndrome: Association with diagnosis and treatment response. J. Urol. 2002, 168, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Sant, G.R. A pilot open label study of Cystoprotek in interstitial cystitis. Int. J. Immunopathol. Pharmacol. 2005, 18, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.L.; Gao, X.; Men, K.; Qiu, J.; Yang, B.; Gou, M.L.; Huang, M.J.; Huang, N.; Qian, Z.Y.; Zhao, X.; et al. Treating acute cystitis with biodegradable micelle-encapsulated quercetin. Int. J. Nanomed. 2012, 7, 2239–2247. [Google Scholar] [CrossRef]
- Patel, R.V.; Mistry, B.M.; Shinde, S.K.; Syed, R.; Singht, V.; Shin, H.S. Therapeutic potential of quercetin as a cardiovascular agent. Eur. J. Med. Chem. 2018, 155, 889–904. [Google Scholar] [CrossRef]
- Javadi, F.; Ahmadzadeh, A.; Eghtesadi, S.; Aryaeian, N.; Zabihiyeganeh, M.; Foroushani, A.R.; Jazayeri, S. The effect of quercetin on inflammatory factors and clinical symptoms in women with rheumatoid arthritis: A double-blind, randomized controlled trial. J. Am. Coll. Nutr. 2017, 36, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, S.D.; de Sousa, L.R.; Burger, M.C.; Lima, M.I.; da Silva, M.F.; Fernandes, J.B. Evaluation of flavonols and derivatives as human cathepsin B inhibitor. Nat. Prod. Res. 2015, 29, 2212–2214. [Google Scholar] [CrossRef]
- Li, L.J.; Li, G.W.; Xie, Y. Regulatory effects of glabridin and quercetin on energy metabolism of breast cancer cells. Zhongguo Zhong Yao Za Zhi 2019, 44, 3786–3791. [Google Scholar] [CrossRef]
- Kim, G.T.; Lee, S.H.; Kim, J.I.; Kim, Y.M. Quercetin regulates the sestrin 2-AMPK-p38 MAPK signaling pathway and induces apoptosis by increasing the generation of intracellular ROS in a p53-independent manner. Int. J. Mol. Med. 2014, 33, 863–869. [Google Scholar] [CrossRef]
- Chang, J.H.; Lai, S.L.; Chen, W.S.; Hung, W.Y.; Chow, J.M.; Hsiao, M.; Lee, W.J.; Chien, M.H. Quercetin suppresses the metastatic ability of lung cancer through inhibiting Snail-dependent Akt activation and Snail-independent ADAM9 expression pathways. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1746–1758. [Google Scholar] [CrossRef]
- Nwaeburu, C.C.; Abukiwan, A.; Zhao, Z.; Herr, I. Quercetin-induced miR-200b-3p regulates the mode of self-renewing divisions in pancreatic cancer. Mol. Cancer 2017, 16, 23. [Google Scholar] [CrossRef] [PubMed]
- Kashchenko, N.I.; Chirikova, N.K.; Olennikov, D.N. Acylated flavonoids of Spiraea genus as α-amylase inhibitors. Russian J. Bioorg. Chem. 2018, 44, 876–886. [Google Scholar] [CrossRef]
- Karpova, E.A.; Lapteva, N.P. Phenolic compounds in taxonomy of the genus Spiraea L. Turczaninowia 2014, 17, 42–56. [Google Scholar] [CrossRef]
- Kostikova, V.A.; Petrova, N.V. Phytoconstituents and bioactivity of plants of the genus Spiraea L. (Rosaceae): A review. Int. J. Mol. Sci. 2021, 22, 11163. [Google Scholar] [CrossRef]
- Kostikova, V.A.; Yu, S.X.; Sharples, M.T. Morphological and biochemical diversity of Spiraea hypericifolia (Rosaceae) growing under natural conditions in Novosibirsk oblast. BOI Web Conf. 2021, 38, 00062. [Google Scholar] [CrossRef]
- Kostikova, V.A.; Zarubaev, V.V.; Esaulkova, I.L.; Sinegubova, E.O.; Kadyrova, R.A.; Shaldaeva, T.M.; Veklich, T.N.; Kuznetsov, A.A. The antiviral, antiradical, and phytochemical potential of dry extracts from Spiraea hypericifolia, S. media, and S. salicifolia (Rosaceae). South Afr. J. Bot. 2022, 147, 215–222. [Google Scholar] [CrossRef]
- Budantsev, A.L. Plant Resources of Russia: Wild Flowering Plants, Their Component Composition and Biological Activity. Families Caprifoliaceae—Lobeliaceae; KMK: Saint Petersburg, Russia; Moscow, Russia, 2011; Volume 4, 630p. (In Russian) [Google Scholar]
- Polozhiy, A.V. Rod Spiraea L.—Tavolga. In Flora Sibiri; Nauka: Novosibirsk, Russia, 1988; Volume 8, pp. 10–20. (In Russian) [Google Scholar]
- Lu, L.T.; Crinan, A. Spiraea Linnaeus. In Flora of China; Wu, Z.Y., Raven, P.H., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MI, USA, 2003; Volume 9, pp. 47–73. [Google Scholar]
- Christodoulakis, N.S.; Mamoucha, S.; Termentzi, A.; Fokialakis, N. Leaf structure and histochemistry of the hardy evergreen Euphorbia characias L. (Mediterranean spurge). Flora 2015, 210, 13–18. [Google Scholar] [CrossRef]
- State Pharmacopoeia of the Russian Federation, 14th ed. Volume 2. Available online: http://www.femb.ru (accessed on 29 April 2022). (In Russian).
- Shinde, V.M.; Dhalwal, K.; Potdar, M.; Mahadik, K.R. Application of quality control principles to herbal drugs. Int. J. Phytomed. 2009, 1, 4–10. [Google Scholar] [CrossRef]
- Sokolova, A.V. Microscopic diagnosis of closely related species of the Spiraea L. section of the Genus Spiraea L. of the Amur region according to the structure of the stem and leaf. Alm. Mod. Sci. Educ. 2016, 9, 98–101. (In Russian) [Google Scholar]
- Bouktaib, M.; Atmani, A.; Rolando, C. Regio- and stereoselective synthesis of the major metabolite of quercetin, quercetin-3-O-β-D-glucuronide. Tetrahedron Lett. 2002, 43, 6263–6266. [Google Scholar] [CrossRef]
- Möhle, B.; Heller, W.; Wellmann, E. UV-induced biosynthesis of quercetin-3-O-β-D-glucuronide in dill cell culture. Phytochemistry 1985, 24, 465–467. [Google Scholar] [CrossRef]
- Murota, K.; Terao, J. Antioxidative flavonoid quercetin: Implications of its intestinal absorption and metabolism. Arch. Biochem. Biophys. 2003, 417, 12–17. [Google Scholar] [CrossRef]
- Kelly, G.S. Quercetin. Monograph. Altern. Med. Rev. 2011, 16, 172–194. [Google Scholar]
- Makino, T.; Shimizu, R.; Kanemaru, M.; Suzuki, Y.; Moriwaki, M.; Mizukami, H. Enzymatically modified isoquercitrin, alpha-oligoglucosyl quercetin 3-O-glucoside, is absorbed more easily than other quercetin glycosides or aglycone after oral administration in rats. Biol. Pharm. Bull. 2009, 32, 2034–2040. [Google Scholar] [CrossRef] [PubMed]
- Materska, M. Quercetin and its derivatives: Chemical structure and bioactivity—A review. Polish J. Food Nutr. Sci. 2008, 58, 407–413. [Google Scholar]
- Janbaz, K.N.; Saeed, S.A.; Gilani, A.H. Protective effect of rutin on paracetamol- and CCl4-induced hepatotoxicity in rodents. Fitoterapia 2002, 73, 557–563. [Google Scholar] [CrossRef]
- Grinberg, L.N.; Rachmilewitz, E.A.; Newmark, H. Protective effects of rutin against hemoglobin oxidation. Biochem. Pharmacol. 1994, 48, 643–649. [Google Scholar] [CrossRef]
- Rotelli, A.E.; Guardia, T.; Juarez, A.O.; de la Rocha, N.E.; Pelzer, L.E. Comparative study of flavonoids in experimental models of inflammation. Pharmacol. Res. 2003, 48, 601–606. [Google Scholar] [CrossRef]
- Obied, H.K.; Allen, M.S.; Bedgood, N.R.; Prenzler, P.D.; Robards, K.; Stockman, R. Bioactivity and analysis of biophenols recovered from olive mill waste. J. Agric. Food Chem. 2005, 53, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, G.; Sun, C.; Peng, F.; Yu, L.; Chen, Y.; Tan, Y.; Cao, X.; Tang, Y.; Xie, X.; et al. Chemistry, pharmacokinetics, pharmacological activities, and toxicity of quercitrin. Phytother. Res. 2022, 36, 1545–1575. [Google Scholar] [CrossRef]
- Valentová, K.; Vrba, J.; Bancířová, M.; Ulrichová, J.; Křen, V. Isoquercitrin: Pharmacology, toxicology, and metabolism. Food Chem. Toxicol. 2014, 68, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Chen, S.; Xia, W.; Sui, H.; Fu, X. Hyperoside: A review of its structure, synthesis, pharmacology, pharmacokinetics and toxicity. Molecules 2022, 27, 3009. [Google Scholar] [CrossRef]
- Yang, W.; Tu, H.; Tang, K.; Huang, H.; Ou, S.; Wu, J. Reynoutrin improves ischemic heart failure in rats via targeting S100A1. Front. Pharmacol. 2021, 12, 703962. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.Y.; Zhang, Z.L.; Rahman, K.; Wang, S.J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Pollini, L.; Tringaniello, C.; Ianni, F.; Blasi, F.; Manes, J.; Cossignani, L. Impact of ultrasound exstraction parameters on the antioxidant properties of Moringa oleifera leaves. Antioxidants 2020, 9, 277. [Google Scholar] [CrossRef]
- Lee, W.J.; Choi, S.W. Quantitative changes of polyphenolic compounds in Mulberry (Morus alba L.) leaves in relation to varieties, harvest period, and heat processing. Prev. Nutr. Food Sci. 2012, 17, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Zhao, Y.Y.; Zeng, H.S.; Zhang, Y.; Lin, R.C.; Sun, W.J. Chemical composition and antioxidant activities of extracts from Apocyni Veneti Folium. Nat. Prod. Res. 2012, 26, 600–608. [Google Scholar] [CrossRef]
- Shi, J.; Li, G.; Wang, H.; Zheng, J.; Suo, Y.; You, J.; Liu, Y. One-step separation of three flavonoids from Poacynum hendersonii by high-speed counter-current chromatography. Phytochem. Anal. 2011, 22, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Kuzieva, S.; Imomova, D.A.; Duschanova, G.M. Structural features of vegetative organs Spiraea hypericifolia L., growing in Uzbekistan. Am. J. Pl. Sci. 2019, 10, 2086–2095. [Google Scholar] [CrossRef]

| ID | Identified Compounds | tR (min) | Mode | Calculated Mass | Measured Mass | Delta Mass (Da) | Delta Mass (ppm) | MzCloud Score |
|---|---|---|---|---|---|---|---|---|
| 1 | Rutin * | 9.22 | Negative | 610.15368 | 610.15338 | 0.00029 | 0.48 | – |
| 2 | Hyperoside * (quercetin-3-galactoside) | 12.39 | Positive | 464.09520 | 464.09548 | −0.00027 | −0.59 | 99.6 |
| 3 | Quercetin * | 12.40 | Positive | 302.04243 | 302.04265 | −0.00022 | −0.73 | 99.9 |
| 4 | Rhamnetin-3-O-β-D-xylopyranosyl-β-D-glucopyranoside | 12.48 | Positive | 610.15339 | 610.15338 | 0.00001 | 0.01 | 96.9 |
| 5 | Isoquercitrin * (quercetin-3-O-β-D-glucopyranoside) | 12.61 | Negative | 464.09548 | 464.09548 | 0.00000 | 0.00 | 99.4 |
| 6 | Quercetin-3-O-(6″-O-malonyl)- β-D-glucoside | 13.58 | Positive | 550.09574 | 550.09587 | −0.00013 | −0.24 | 97.5 |
| 7 | Reynoutrin (quercetin-3-O-β-D-xylopyranoside) | 13.72 | Positive | 434.08471 | 434.08491 | −0.00020 | −0.45 | 98.4 |
| 8 | 3-O-methylquercetin-3′-O-β-D-glucopyranoside | 14.10 | Positive | 478.11095 | 478.11113 | −0.00018 | −0.37 | 97.6 |
| 9 | Isorhamnetin (3′-methylquercetin) | 14.10 | Positive | 316.05826 | 316.05830 | −0.00004 | −0.13 | 99.0 |
| 10 | Avicularin * (quercetin-3-O-α-L-arabinopyranoside) | 14.25 | Negative | 434.08417 | 434.08491 | −0.00074 | −1.71 | – |
| Species | Numbers of Identified Flavonoids by Class | Total | |||||
|---|---|---|---|---|---|---|---|
| Flavones | Flavonols | Flavanones | Isoflavones | Catechins | Anthocyanins | ||
| S. aemiliana | – | 7 | – | – | – | – | 7 |
| S. albiflora | – | 1 | – | – | – | – | 1 |
| S. aquilegifolia | – | 4 | – | – | – | – | 4 |
| S. beauverdiana | – | 7 | – | – | – | – | 7 |
| S. betulifolia | – | 8 | – | – | – | – | 8 |
| S. brahuica | 2 | 1 | – | – | – | – | 3 |
| S. bumalda | – | 4 | – | – | – | – | 4 |
| S. canescens | 1 | 2 | – | – | – | – | 3 |
| S. cantoniensis | – | 3 | – | – | – | – | 3 |
| S. chamaedryfolia | – | 4 | – | – | – | – | 4 |
| S. crenata | – | 6 | – | – | – | – | 6 |
| S. dahurica | – | 2 | – | – | – | – | 2 |
| S. douglasii | – | 2 | – | – | – | – | 2 |
| S. elegans | – | 2 | – | – | – | – | 2 |
| S. flexuosa | – | 3 | – | – | – | – | 3 |
| S. formosana | – | 5 | – | – | – | – | 5 |
| S. humilis | – | 2 | – | – | – | – | 2 |
| S. hypericifolia | 4 | 6 | – | – | 6 | – | 16 |
| S. media | – | 9 | – | – | – | – | 9 |
| S. nipponica | 1 | 1 | – | 1 | 2 | – | 5 |
| S. prunifolia | – | 8 | – | – | 3 | – | 11 |
| S. pubescens | – | 2 | – | – | – | – | 2 |
| S. salicifolia | 4 | 34 | – | – | 3 | – | 41 |
| S. schlothauerae | – | 3 | – | – | – | – | 3 |
| S. sericea | – | 3 | – | – | – | – | 3 |
| S. trilobata | – | 4 | – | – | – | – | 4 |
| S. ussuriensis | – | 3 | – | – | – | – | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrova, N.V.; Chernonosov, A.A.; Koval, V.V.; Andreeva, V.Y.; Erst, A.S.; Kuznetsov, A.A.; Kulikovskiy, M.S.; Wang, W.; Yu, S.-X.; Kostikova, V.A. LC–HRMS for the Identification of Quercetin and Its Derivatives in Spiraea hypericifolia (Rosaceae) and Anatomical Features of Its Leaves. Plants 2023, 12, 381. https://doi.org/10.3390/plants12020381
Petrova NV, Chernonosov AA, Koval VV, Andreeva VY, Erst AS, Kuznetsov AA, Kulikovskiy MS, Wang W, Yu S-X, Kostikova VA. LC–HRMS for the Identification of Quercetin and Its Derivatives in Spiraea hypericifolia (Rosaceae) and Anatomical Features of Its Leaves. Plants. 2023; 12(2):381. https://doi.org/10.3390/plants12020381
Chicago/Turabian StylePetrova, Natalia V., Alexander A. Chernonosov, Vladimir V. Koval, Valeriya Yu. Andreeva, Andrey S. Erst, Alexander A. Kuznetsov, Maxim S. Kulikovskiy, Wei Wang, Sheng-Xiang Yu, and Vera A. Kostikova. 2023. "LC–HRMS for the Identification of Quercetin and Its Derivatives in Spiraea hypericifolia (Rosaceae) and Anatomical Features of Its Leaves" Plants 12, no. 2: 381. https://doi.org/10.3390/plants12020381
APA StylePetrova, N. V., Chernonosov, A. A., Koval, V. V., Andreeva, V. Y., Erst, A. S., Kuznetsov, A. A., Kulikovskiy, M. S., Wang, W., Yu, S.-X., & Kostikova, V. A. (2023). LC–HRMS for the Identification of Quercetin and Its Derivatives in Spiraea hypericifolia (Rosaceae) and Anatomical Features of Its Leaves. Plants, 12(2), 381. https://doi.org/10.3390/plants12020381