1. Introduction
Cultivated potato (
Solanum tuberosum L.) is widely grown and, based on global annual production, is the third most important staple food crop after rice and wheat [
1]. Potatoes are mainly grown for food, with 80–85% processed into frozen products in Europe and the USA [
2]. Global production of processed potato products in more than 70 countries increased from 7.4 million tonnes in 2015 to 8.9 million in 2019 [
3]. These products include French fries, chips and crisps, which are usually processed at high temperatures from potato tubers after cold storage.
During cold storage, potato tubers undergo cold-induced sweetening (CIS), a process in which the stored sucrose is converted to glucose and fructose by plant invertases [
4]. During high-temperature processing of cold-stored tubers, such as frying, the stored hexose sugars react with free amino acids, such as asparagine, through the Maillard reaction resulting in browning of the potato product. The reaction also generally creates organic compounds, which contribute to the aroma and sweet taste of such processed foods [
5]. However, the Maillard reaction can also generate acrylamide, a potential carcinogen and neurotoxin, and various simple carbon compounds associated with the bitter taste and darker colour during the high-temperature processing of potato products [
6]. The level of browning and acrylamide in such processed potato products can be reduced if hexose sugars and non-essential amino acids accumulated in cold-stored tubers are low [
7]. However, because the storage of potato tubers at low temperatures is needed for continuous supply and production of processed potato products, cultivars with a reduced potential to accumulate reducing sugars are desired by potato processors [
8,
9]. This is because management strategies to reduce discolouration and acrylamide formation during high-temperature processing are not always practical [
10]. Despite over 4780 accessions of potatoes held at the International Potato Centre [
11], and other germplasm collections, which provide diverse resources for breeding cultivars with better high-temperature-processing properties, conventional breeding has yet to deliver commercial cultivars with all the necessary traits for high-temperature processing. For example, cv. Atlantic, a popular chipping potato variety in North America, still accumulates reducing sugars during long-term storage [
12,
13]. It is, therefore, essential that new methods for developing good quality potato cultivars for high-temperature processing are sought.
Incorporating traits in potatoes so heat-processed products have low acrylamide-forming potential and reduced browning has been attempted using molecular tools. These have involved strategies to inhibit the activity of the vacuolar invertase (VINV), a key enzyme that hydrolyses sucrose to glucose and fructose in cold-stored tubers, and the two isoforms of asparagine synthetase (AS1 and AS2), the enzymes responsible for the catalysis of asparagine from aspartate and glutamine [
14,
15,
16]. For example, RNA interference (RNAi) of the
AS1 gene has been reported to reduce the production of asparagine, with some potato lines also having up to 70% less acrylamide than wild-type products, whereas RNAi of the
AS2 gene does not appear to decrease asparagine synthetase level and can negatively impact plant growth [
15,
17]. Moreover, switching off the
VInv or
AS1 gene alone does not always guarantee the desirable reduction in acrylamide formation [
15]. Simultaneously reducing or eliminating the expression of the
VInv gene or the activity of the encoded enzyme and the
AS1 gene will likely reduce the accumulation of hexose sugars and possibly slow down the rate of asparagine synthesis leading to the reduced acrylamide-forming potential in cold-stored tubers. This was demonstrated using RNAi to silence the
VInv, AS1 and
AS2 genes, resulting in only one-fifteenth of the acrylamide content in controls in the best RNAi lines [
18]. However, knocking out the
AS2 gene resulted in some RNAi lines being stunted and chlorotic during field trials, confirming earlier observations that the
AS2 may not be a good candidate for genetic manipulation aimed at reducing just the levels of asparagine [
18]. RNAi technology is often associated with incomplete silencing of target genes in various organisms [
17,
18,
19,
20,
21]. As such, gene editing tools that can permanently and completely reduce the activity of target enzymes by modifying genes are a better option than RNAi. An example is the use of the transcription activator-like effector nucleases (TALENs) to knock out the
VInv in the commercial potato cultivar Ranger Russet, resulting in some edited lines having undetectable levels of reducing sugars and the processed chips being light-coloured and containing reduced levels of acrylamide [
22]. In all of the above-reported cases, the lines created are transgenic, as they have foreign DNA, although some of the TALENs-edited lines were suggested to contain no TALEN DNA in the genome [
22]. It is now possible to use another gene editing tool, the Clustered Regularly Interspaced Short Palindromic Repeat (CRISPR) system, to introduce mutations that will permanently eliminate the expression of genes, as with TALENs; but, unlike TALENs, it is simpler, more versatile, more efficient and can achieve similar outcomes with or without foreign DNA [
23].
The CRISPR editing system is a natural antiviral system used by bacteria and has been modified as a tool to edit plant genes [
24,
25]. With this approach, a DNA vector, encoding a plant codon-optimised CRISPR endonuclease (e.g., the Cas9 protein) and a guide RNA (gRNA) complex, is expressed in plant cells where a ribonucleoprotein (RNP) complex formed between the endonuclease and gRNA is directed to cleave target DNA. The gRNA complex comprises a 17–20 bp single guide RNA (sgRNA), complementary to the target DNA sequence, and fused to a gRNA scaffold. The sgRNA replaces the spacer sequences called CRISPR RNA (crRNA), and the gRNA scaffold mimics the trans-activating CRISPR RNA (tracrRNA) found naturally in the bacteria antiviral system. The sgRNA is designed based on knowledge of the protospacer adjacent motif (PAM), the recognition site of a CRISPR endonuclease. Typically, in the RNP complex, the tracrRNA binds to the CRISPR endonuclease, while the sgRNA guides the complex to the target DNA, which cuts at specific sites adjacent to the PAM. For the CRISPR-Cas9 system, which employs the CRISPR-associated protein 9 as the CRISPR endonuclease, the sgRNA directs the RNP to cleave both DNA strands at three nucleotides upstream of the PAM, which is usually NGG. Endogenous DNA repair processes at the cleavage site by non-homologous end-joining (NHEJ) can result in deletions, insertions or substitutions at that site. When this repair results in a frameshift or nonsense mutation, the gene product is impaired or inactivated. Since the first report a decade ago, the CRISPR-Cas9 system has been used to successfully edit genes of many plants, including potatoes [
26]. A recent advance of the technology is the introduction of mRNA of the Cas9 and gRNA complex or an externally assembled RNP to edit plant genes without introducing foreign DNA, making it possible to obtain non-GM (Genetically Modified)-edited plants. These applications make the CRISPR-Cas system a valuable alternative to generating gene-edited transgenic plants, especially in countries where transgenic plants are still regulated or are not favoured by consumers.
The potato genome is amenable to CRISPR-Cas9 editing, and the technology has been used to introduce herbicide and pathogen resistance [
27,
28,
29], remove self-incompatibility [
30,
31], and alter tuber chemical composition and nutritional value [
32,
33,
34]. To increase the efficiency of the CRISPR-Cas9 system, more than one gRNA can be introduced to edit alleles of a gene or multiple genes at similar or different chromosomal locations in a genome, for example, in the tetraploid potato. Successful multiplex gene editing is necessary because a change in polygenic traits may require modifications in more than one gene or all alleles of a gene or genes in a biological or metabolic pathway before a meaningful physiological change is achieved [
32,
35]. Multiplex editing can facilitate the development of multiple traits or improve existing ones in established cultivars. In several recent studies, multiplex gene editing has also been applied to edit target genes in potatoes [
32,
33,
36].
This study is the first report on the application of the CRISPR editing system, specifically multiplex CRISPR-Cas9 editing, to demonstrate that the tool can rapidly generate cultivars with improved quality traits for high-temperature processing of potato products. We used the most common transgenic method involving DNA vectors encoding the Cas9 protein and multiple guide RNAs to mutate the VInv and AS1 genes in two potato cultivars as a proof-of-concept. In addition, we explored the non-transgenic method of inducing mutations, which involved the delivery of RNPs assembled outside of plant cells. We demonstrated that mutations in some events resulted in reduced browning and low acrylamide in crisps made from cold-stored tubers. While the transgenic gene-edited events are valuable, the RNP strategy demonstrates that it is possible to generate edited potato varieties with desirable commercial traits without introduced DNA, and, in jurisdictions which do not regulate them as genetically modified, it will be cheaper to bring them to market, and processed products from them may be readily acceptable to consumers.
3. Discussion
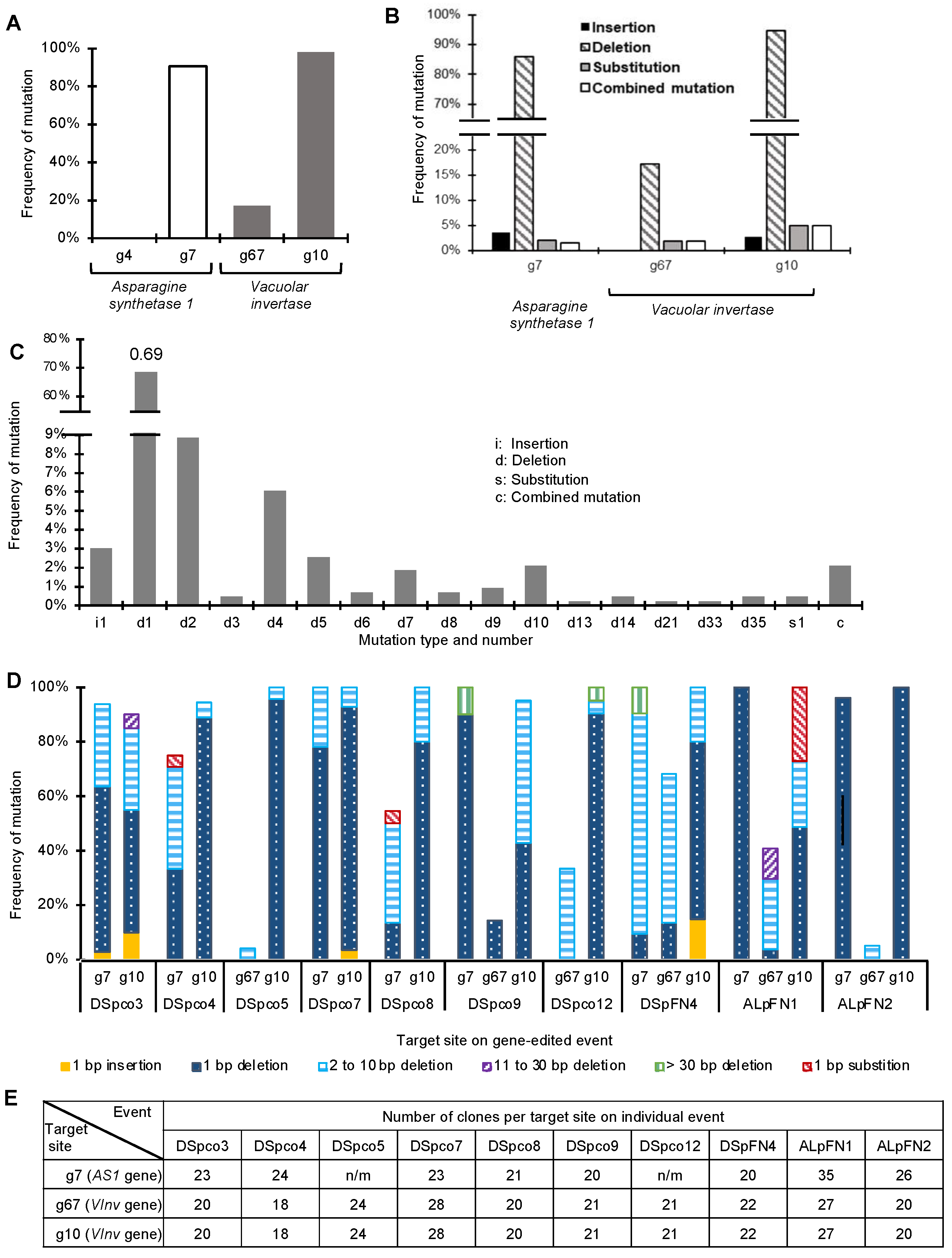
We successfully used the CRISPR-Cas9 system and multiple sgRNAs to edit the
VInv and
AS1 genes in both Atlantic and Desiree potato cultivars, generating events with mutations in either one or both genes. Cold-stored tubers of some of these events had reduced glucose and fructose concentrations. Crisps processed from them were lighter in colour with lower acrylamide concentrations. The significant reduction in browning and acrylamide concentrations in the crisps made from the two Atlantic events is noteworthy, as it demonstrates that CRISPR-Cas9 gene-editing could be used to improve one of the most popular potato chipping varieties, which has a tendency to accumulate reducing sugars [
12]. The acrylamide concentrations in the two events (365.4 ng/g in ALpFN1 and 332.9 ng/g in ALpFN2) were below the only global benchmark for acrylamide in food set by the European Commission Regulation, which is 500 µg/kg (500 ng/g) acrylamide for French fries and 750 µg/kg (750 ng/g) acrylamide for potato crisps or other potato products [
38], further providing evidence that CRISPR-based editing has great potential as a new tool for potato improvement. This is especially important because although potatoes with low browning and acrylamide-forming potential are preferred by the industry for healthy fries and crisps, such cultivars are not readily available. This is because the potato is an outcrossing polyploid species, and cultivars have a high level of heterozygosity, making the breeding and selection of new traits complex and a prolonged process [
39].
For the CRISPR-Cas9 system to be effective as a breeding tool for rapid generation of new or improved potato varieties with desirable traits, it must be possible to modify simultaneously all alleles of a gene or, in the case of a polygenic trait, of more than one gene. To achieve this, it may be necessary to use multiple sgRNAs to achieve relatively high mutation frequencies [
40,
41]. Our study demonstrated that this is possible: in our research, we obtained mutations in both target genes,
VInv and
AS1, in 58% and 100% of generated Desiree and Atlantic transgenic events, respectively. Similarly, Zhao et al. [
33] simultaneously edited the
Sbe1 and
Sbe2 genes of the potato cultivar Desiree with multiple sgRNAs. This resulted in 72% of regenerated lines carrying at least one mutated allele compared to 52% when a single gene was targeted. However, when the
StGBSS1 and
StDMR6-1 genes were targeted for editing with multiple sgRNAs in the potato cultivar Desiree in another study, only the
StDMR6-1 was edited [
36]. The differences in the results suggest that the success of multiplex gene editing may depend on many factors, including the efficiency of the specific sgRNAs used. Multiplexing sgRNAs can be expected to increase the speed and efficiency with which new phenotypes can be developed, and it will be particularly valuable for improving polygenic traits in species such as potatoes [
33,
40].
Biotechnology-based methods to reduce browning and acrylamide formation in high-temperature-processed potato products made from cold-stored tubers have focused on lowering the accumulation of fructose, glucose, and amino acids, particularly asparagine, with varying success. These aims have been achieved to some extent via RNA interference of the
VInv and/or
AS1 and
AS2 genes or via overexpression of invertase inhibitors in some cultivars such as Atlantic, Katahdin, Russet Burbank, Karaka and Ranger Russet [
14,
16,
18,
22,
42,
43]. Inhibition of the activities of VINV or AS1 alone can reduce the acrylamide-forming potential to some extent in crisps of some potato cultivars [
14,
15]. By using the CRISPR-Cas9 system to edit the
VInv and
AS1 at the same time, we achieved a significant reduction (up to 80%) in acrylamide concentration in seven of the eight events with both genes edited and up to 86% in DSpco12, a single
VInv-edited event. The result is in line with Zhu et al. [
18], who achieved up to 93% acrylamide reduction in crisps made from Russet Burbank using RNAi to silence the
VInv,
AS1 and
AS2 genes. Reducing the activity of the VINV with or without a corresponding reduction in the expression of the AS1 and/or AS2 has been shown to result in lighter-coloured crisps and reduced acrylamide-forming potential in some cultivars, indicating that genotypic characteristics of potato lines and cultivars may play a role in the effectiveness of any new breeding technology to improve a cultivar. For example, for any potato cultivar, the role of the three genes in browning and acrylamide-forming potential may have to be established so the one or those with the most critical roles could be targeted. There is a lot to gain in targeting and silencing specific genes that may play vital roles in the expression of traits, as this will reduce cost, effort and potential off-target silencing that may affect other desirable agronomic traits.
It might be expected that a reduction in glucose and fructose concentrations would be balanced by an increased sucrose accumulation in the cold-stored tubers of the edited events, as less sucrose would be hydrolysed because of the disruption to VINV activity. However, this was not the case, except for the event ALpFN1. Reduced VINV activity may not have changed the sucrose concentration substantially in the events, or other enzymes, such as sucrose synthase, may have broken down the sucrose. Sucrose synthase is the predominant enzyme that breaks down sucrose in sink tissues, but when tubers are detached from the plant and stored, its activity declines, and VINV activity becomes predominant [
17,
44,
45]. It is possible that in the events with reduced activity of VINV, other metabolic activities, requirements of the tubers or the re-activation of sucrose synthase induced the hydrolysis of accumulated sucrose. These results suggest that the browning of high-temperature processed potato products may be influenced by factors additional to the level of sucrose in cold-stored tubers.
Our results and many others demonstrate that potato cultivars can be modified with beneficial agronomic traits with health benefits using new breeding technologies, such as the CRISPR-Cas system, RNAi and over-expression of endogenous and exogenous genes [
46,
47,
48]. Most of these techniques involve introducing and integrating foreign DNA in the form of DNA vectors, which contain genes, promoters and selectable marker genes derived from other species, into the genome of plants, making the final products transgenic or GM. Although developing GM crops is much less laborious and more precise than conventional breeding methods, their commercialization is fraught with difficulties, problems or risks, depending on who makes the judgement. It currently costs more and takes longer to bring these products to market because of regulatory hurdles in most countries [
49]. Moreover, more time and effort are needed to educate consumers on the stigma of potential health risks associated with GM products. In most cases, consumer hesitancy in the consumption of GM products is borne out of fear of the unknown and a lack of education on the scientific processes involved in genetic manipulations. Despite these constraints, over 10% of the world’s arable land is cultivated to GM crops, and the increasing marketing of these crops around the globe indicates the negative consumer sentiments towards GM crops are waning, and the crops’ due contribution to the push for global food security is becoming a reality [
50]. Among these are the 51 GM potato cultivars currently recorded on the International Services for the Acquisition of Agri-Tech Applications (ISAAA) databases as commercial GM potatoes. They include the commercialized Innate
® potato, which has stacked RNAi traits, including lowered free asparagine and reducing sugars. These examples strengthen the need for GM potato cultivars such as the ones we generated in this study.
In jurisdictions where GM potatoes with improved traits are not marketable, non-GM potatoes with equivalent agronomic traits, such as those that can be generated with the CRISPR-Cas system using external RNPs, may be valuable alternatives. RNP-particle bombardment is a relatively new strategy for gene-editing plants. It has been applied once in a CRISPR study on potatoes, but no mutation frequency was reported [
51]. In our study, the mutation frequency from RNP-particle bombardment was 0.44%, comparable to the 0.56% reported for wheat [
52]. The primary reason for the lower mutation frequencies for RNP-particle bombardment is that the RNPs are active or functional for a relatively shorter period after introduction into cells or tissues. In contrast, for transgenic plants, DNA vectors encoding the Cas9 and sgRNA are integrated into the potato genome, thereby increasing the chances of continuous production, complexing and activity of RNPs in transgenic cells. The RNP-bombarded cells are, therefore, less-exposed to the editing machinery than transgenic cells, which also undergo selection pressure. Despite the low frequency of editing, the RNP-particle bombardment approach holds real potential for several reasons. It generates non-transgenic events with site-directed nuclease 1 (SDN-1) editing, an outcome of gene editing, which results in a deletion, insertion, or substitution of a base or bases or a combination of the mutations without the introduction of foreign DNA for repair via endogenous non-homologous end-joining. SDN-1 food crops or the process of SDN-1 editing are currently not regulated as GMOs in many jurisdictions (e.g., USA, Canada, Argentina, Japan, and Australia) or are in the process of being deregulated in many other countries, where gene-edited plants and food products will likely make a vital contribution to food security [
53,
54,
55,
56]. Notably, the RNP approach is a relatively cheaper and quicker process of generating new varieties of plants, as it avoids the generation of plant transformation vectors and the associated cumbersome selection processes during the tissue culture of explants. The process does not introduce transgenes into existing cultivars, avoiding some regulatory and financial limitations of developing and bringing transgenic crops to market. Thus, the advantages of using the RNP approach to generate potato cultivars with new or improved agronomic traits include speeding up breeding and commercialization processes, as it avoids the complexities associated with conventional breeding, the potential loss of existing desirable traits in a cultivar and the regulatory and financial barriers associated with GM crops.
With the generation of the SDN-1 events in this study, the Research Stage of the Commercialization Pathway, the journey to bringing laboratory research to the market, which includes the “Gene/Trait Discovery” phase and the proof-of-concept phase of the “Development Stage”, has been achieved successfully [
57]. The other phases of the Development and Commercialization stages will slightly differ for the RNP-generated and GM-edited events. To advance these events to commercialization, it would be of interest to characterize them further to understand the main factor(s) behind the improvement and to distinguish possible chimeras among the events by studying those which will carry the mutations to subsequent generations. For the GM-edited events, obtaining multiple clones will facilitate field trials to obtain data to ensure critical performance indicators such as yield and tolerance to stresses are not affected by the editing. For the RNP approach, the DSRNP217 development was behind the GM events because of the time they were generated. We could not synchronise this event’s clones with those of the GM-edited events in time for the characterization experiments because of COVID-19 restrictions in Western Australia. The first step towards commercialization is to obtain clones and characterize them in detail. Further phenotypic and genotypic characterization is being explored to develop the event for field assessment and market viability. Increasing the efficiency of the RNP-particle bombardment to generate more events with different forms of editing of one or both target genes will dramatically impact the output and commercialization of the event. This could be conducted by optimizing the ratios of Cas9 and gRNA in the RNP complex to deliver the best mutation rates, increasing the penetration of the RNPs into cells by increasing the number of bombardments, or by using cell-penetrating peptides or improving the mutation efficiencies by using protoplasts as explants, as RNPs have been demonstrated to increase mutation rates in some potato cultivars by up to 68% when used to edit genes in protoplasts [
58,
59]. This aspect is worth the attention, as non-GM crops are currently not regulated by gene technology regulators and are accepted by well-informed consumers in many countries. An example is the recent CRISPR-edited wheat with silenced asparagine synthetase gene
TaASN2, which is now being assessed in field trials in the UK [
60,
61]. This trial and the recent announcement of a CRISPR gene-edited tomato for commercial sale in Japan represent a significant advance for the commercialization of gene-edited crops. Hopefully, potato cultivars with low potential for acrylamide formation and CIS will not be far behind.
4. Materials and Methods
4.1. Amplification and Sequencing of Portions of the Vlnv and AS1 Genes Used for sgRNA Design
Preliminary assessment of the reference sequences of the
VInv and AS1 genes at the NCBI databases established the presence of PAM sites appropriate for the design of sgRNAs in the first five exons of both genes. Based on this information, the coding sequence of the
VInv gene (GenBank accession no. HQ110080.1) was used to design the primer pair VInv1-F (5′-CACTGGCTCTACTTGCCTTT-3′) and VInv1-R (5′-GGGTTATCGGGTGTCCATTTAT-3′), which amplified a fragment of the third exon of the gene (593 bp). For the
AS1 gene (GenBank accession no. XM_006343993.2), the primer pair AS1-F (5′-GGCTTTGTTGGGTTGTTCGG-3′) and AS1-R (5′-TAGAGTACAAGTGCCCCGGA-3′) was used to amplify a 530 bp (exons 1 to 5) fragment from cDNA because of the presence of long introns in the selected region of the gene. The fragments were amplified from potato cultivars Atlantic and Desiree, edited in this study. For each cultivar, 100 mg of leaves of in vitro-cultured plants were used for DNA and RNA extraction using the CTAB and TRIzol (Invitrogen, Waltham, MA, USA, catalogue no. 15596026) methods, respectively [
62,
63]. The GoScript™ Reverse Transcriptase (Promega Corporation, Madison, WI, USA, catalogue no. PAA5000) was used to synthesise cDNA from 80 ng of RNA according to the manufacturer’s protocol. The PCRs were conducted with the GoTaq
® Green Master Mix (Promega Corporation, catalogue no. M7122) and used 300 ng of cDNA or 100 ng of DNA for each potato cultivar in separate 20 µL reactions. The PCRs were set up in an Applied Biosystems Veriti Thermal Cycler (Thermo Fisher Scientific, Waltham, MA, USA) with the following profile: initial denaturation at 95 °C for 5 min followed by 30 cycles of 95 °C for 30 s, 55 °C for 30 s, 72 °C for 40 s; and with a final extension at 72 °C for 7 min.
The amplicons were analysed on a 1% agarose gel (Fisher Biotec, Wembley, Australia, catalogue no. AG100) and purified using the Wizard® SV Gel and PCR Clean-Up System (Promega Corporation, catalogue no. A9281). They were ligated to the pGEM-T Easy vector using an insert-to-vector molar ratio of 3:1 and multiplied using Escherichia coli JM109 competent cells (Promega Corporation, catalogue no. A3600 and L2005), following the manufacturer’s protocols. Five (out of 20) microlitres of a suspension of ampicillin-selected white bacterial colonies were further screened by PCR to confirm they contained ligated plasmid DNA using the conditions described above. The Universal M13 primers (M13-F: 5′-GTAAAACGACGGCCAGT-3′ and M13-R: 5′-CAGGAAACAGCTATGAC-3′) were used for the PCR and sequencing of plasmid DNA isolated from overnight cultures of selected bacterial colonies. The selected bacteria colonies were cultured using the media and conditions recommended for the Escherichia coli JM109 competent cells (Promega Corporation). The plasmid DNA was sequenced using the BigDye® Terminator v3.1 Chemistry, the recommended PCR conditions and the ethanol/EDTA precipitation method for the PCR products described in the BigDye® Terminator v3.1 Cycle Sequencing Kit protocol (Applied Biosystems, Waltham, MA, USA). The chromatograms were analysed using the Geneious Prime software (Biomatters Ltd., San Diego, CA, USA).
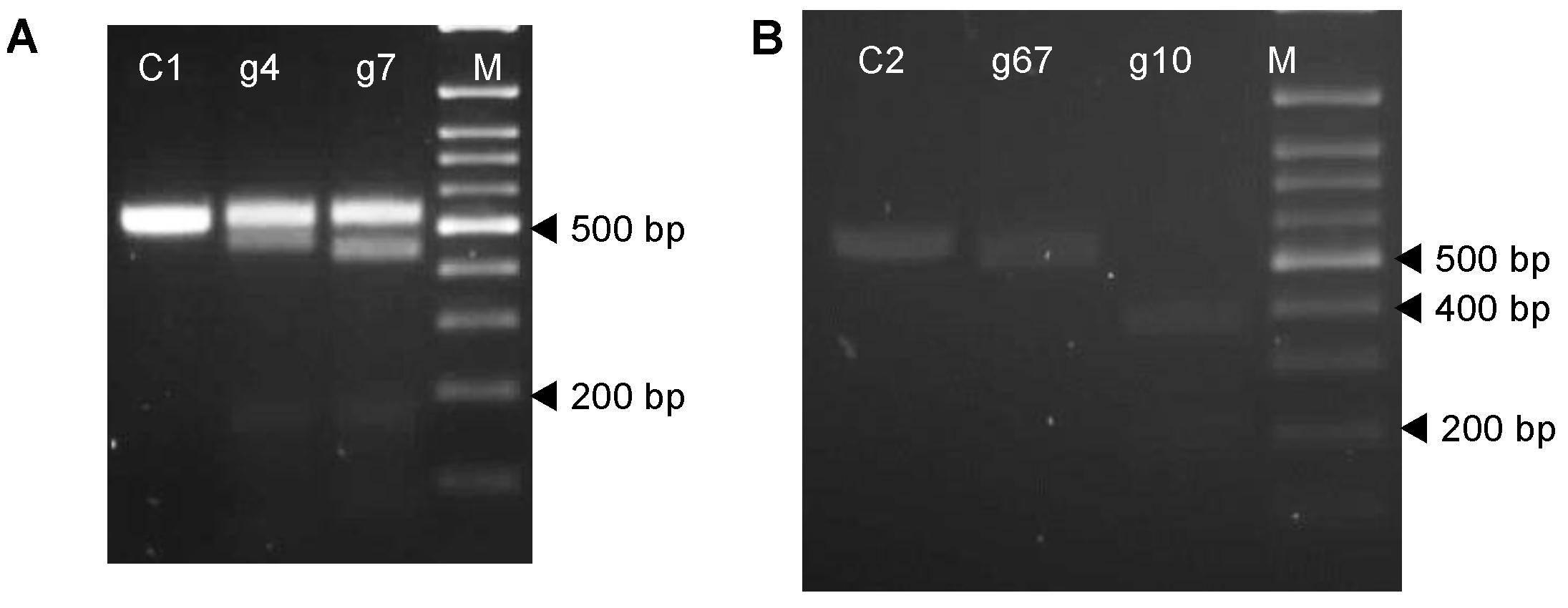
4.2. Design and Testing of the Efficiency of sgRNAs Targeting the Vlnv and AS1 Genes
Sequences of different clones of amplicons of the
Vlnv and
AS1 genes were aligned with the reference sequences for
VInv (accession no. HQ110080.1) and
AS1 (accession no. XM_006343993.2) using the Multalin web tool with standard settings [
64]. Conserved regions without allelic polymorphism were used to select sgRNA target sites using the Cas-Designer [
65]. Two 20-nt target sequences were selected for each gene; g67 and g10 for the
Vlnv gene and g4 and g7 for the
AS1 gene.
To assess the efficiency of the sgRNAs in vitro in Cas9 cleavage assay, each sgRNA with gRNA scaffolds in tandem was first transcribed in vitro from DNA templates. The templates were constructed using overlapping PCRs and the pUC119-gRNA vector (Addgene plasmid #52255; RRID:Addgene_52255;
http://n2t.net/addgene:52255; accessed on 5 June 2019) as described by [
66]. For each sgRNA, the final DNA construct consisted of a T7 promoter sequence, the single guide sequence and the gRNA scaffold. The details of the primers, the overlapping PCR strategy and the PCR conditions were as described previously [
66,
67]. The DNA construct for each sgRNA was transcribed in 20 µL reactions using the HiScribe™ Quick T7 High Yield RNA Synthesis Kit (New England Biolabs, NEB, Ipswich, MA, USA, catalogue no. E2050S). Each reaction consisted of 0.75 X reaction buffer, 7.5 mM each of ATP, GTP, CTP, UTP, 1.5 μL of T7 RNA polymerase mix and 1 μg of template DNA. They were incubated at 37 °C for 14 hr in a thermal cycler. The transcripts were purified using the chloroform extraction and sodium acetate-ethanol precipitation protocol recommended for RNA synthesised with the HiScribe kit. Before the purification, template DNA was removed from the transcripts using TURBO DNase I (Thermo Fisher Scientific, catalogue no. AM2238) following the manufacturer’s protocol.
Cas9 cleavage assays were conducted on amplicons (593 bp and 530 bp, respectively, for the VInv and AS1) generated from the potato cultivars Atlantic and Desiree. The 20 µL reactions consisted of a 10:10:1 molar ratio of Cas9: gRNA: target amplicon. First, a 10 µL reaction containing 0.01 nM of Cas9 (Proteowa Pty Ltd., Murdoch, Australia), 0.01 nM of gRNA and 1X NEBuffer 3.1 (New England Biolabs, NEB, Ipswich, MA, USA, catalogue no. B7203) was incubated at room temperature for 10 min, then 10 µL of 0.001 nM target amplicon was added, and the NEBuffer 3.1 was topped up to 1X final concentration. The reactions were incubated at 37 °C for 15 min and then analysed via electrophoresis using a 2% agarose gel.
4.3. Construction of Gene-Editing Vectors
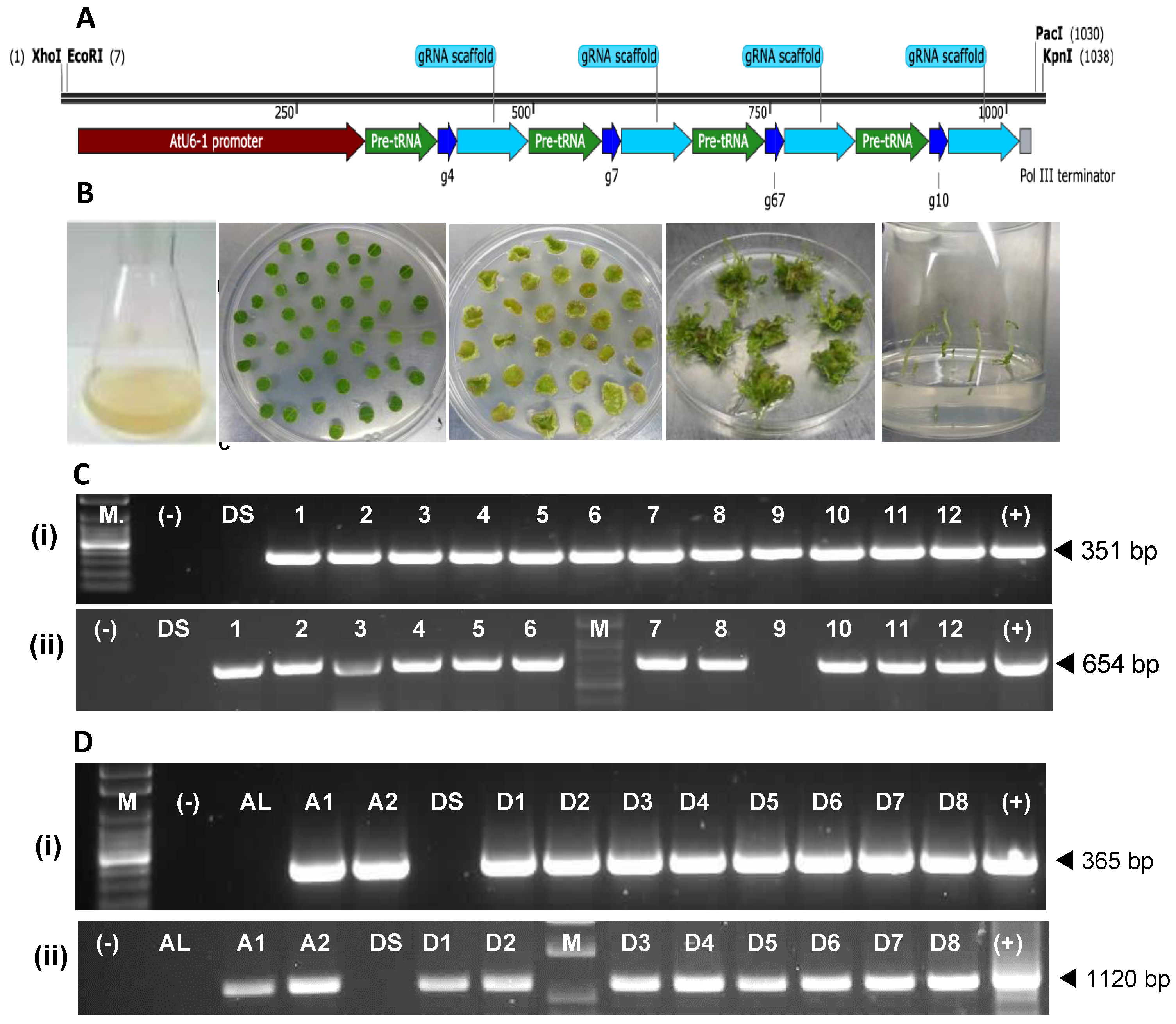
The two Cas9-encoding gene-editing vectors, pFGC-pcoCas9-ASVI and pFN117-Cas9-ASVI, used for transformation, were generated based on the vectors pFGC-pcoCas9 (Addgene plasmid # 52256) and pFN117-Cas9 (donated by Dr. Fatima Naim, Queensland University of Technology), each of which was modified with an ASVI guide RNA cassette. The pFGC-pcoCas9 and pFN117-Cas9 vectors have different regulatory translational and transcriptional elements for the Cas9 sequence, as shown in the vector maps in
Supplementary Figure S1. The ASVI cassette was designed with the
Arabidopsis thaliana U6 promoter (AtU6-1) sequence driving the four sgRNAs with gRNA scaffolds in tandem, each separated by a sequence of the endogenous plant transfer RNA (tRNA) and ending with the Pol III terminator sequence. The
XhoI and
EcoRI restriction sites were added upstream of the AtU6-1 promoter, and the
PacI and
KpnI restriction sites were added downstream of the terminator sequence. The cassette was synthesised commercially (GENEWIZ/Azenta, Burlington, MA, USA) and ligated to pFGC-pcoCas9 using the
EcoRI and
PacI restriction sites to create vector pFGC-pcoCas9-ASVI and to the pFN117-Cas9 using the
XhoI and
KpnI restriction sites to generate vector pFN117-Cas9-ASVI (
Supplementary Figure S1). The ligations were conducted with a 3:1 insert: vector molar ratio using the ligase in the pGEM
®-T Vector System, the plasmid DNA multiplied with the
E. coli JM109 competent cells and purified using the Wizard
® Plus Minipreps DNA Purification System using the recommended protocols provided by Promega Corporation and described in detail above.
The vectors were sequenced to confirm their integrity using the BigDye
® Terminator v3.1 Chemistry, the reactions were cleaned up, and the raw sequence files were edited using the procedures described above. The primers for sequencing were the FGC-F (5′-GAAATTCAGGCCCGGTTGCC-3′) and FGC-R (5′-CTAGGATAAATTATCGCGCGCGGTG-3′) for vector pFGC-pcoCas9-ASVI and, FN117-F (5′-CATAACGTGACTCCCTTAATTCTCC-3′) and FN117-R (5′-CATGTTGACCTCCAAGCTTGAATTC-3′) for vector pFN117-Cas9-ASVI (
Supplementary Figure S1). Competent
A. tumefaciens strain GV3101 cells were modified with the vectors using the freeze–thaw method described by [
67].
4.4. Maintenance of Plants and Agrobacterium-Mediated Transformation
Plantlets of both Atlantic and Desiree, from which leaves were harvested for transformation, were maintained in vitro through regular subculturing of nodal segments on Murashige and Skoog (MS) medium (Sigma, St. Louis, MO, USA, catalogue no. M5519) supplemented with 20 g/L sucrose (pH 5.6) and solidified with 2.8 g/L Gelrite (Sigma-Aldrich, St. Louis, MO, USA, catalogue no G1910). To obtain broader leaves for transformation, two-week-old in vitro plantlets were transferred to a potting mix (40 kg of soil mixed with 20 g of dolomite, 15 g of CaCO3, 40 g of Grower’s blue, 40 g of Osmocote® all-purpose fertiliser) and maintained in a growth chamber at 30 °C, under a 16 h light/ 8 h dark photoperiod for at least four weeks. Fully expanded leaves were harvested, washed under a running tap for 15 min and then surface sterilised by dipping in 70% ethanol for 30 s, followed by incubation for 40 s in 1% sodium hypochlorite solution containing three drops of Tween-20. The leaves were washed four times with sterile water and soaked for two minutes in fresh sterile water with occasional swirling. Leaf discs were prepared by punching the sterile leaves with a sterile cork-borer (0.7 cm in diameter); they were kept in liquid MS medium (pH 5.6) until transformed with modified A. tumefaciens cultures.
The leaf disc transformation was carried out as described by [
68] with some modifications. Overnight bacterial culture (OD
600 = 0.8) was pelleted at 5000×
g for 20 min and was resuspended in liquid MS medium supplemented with 30 g/L sucrose, and 100 µM acetosyringone. The inoculation procedure involved immersing 30 leaf discs in bacterial suspension in a 90 mm Petri dish for 30 min with gentle shaking by placing the dish on a rocking shaker at 30 rpm. After inoculation, the leaf discs were transferred to a callus induction medium (CIM: MS media with Gamborg’s B5 vitamins [MSB5, PhytoTech Labs, catalogue no. M404] + 5 mg/L NAA, 2 mg/L BAP, 16 g/L glucose [pH 5.6], 2.8 g/L Gelrite) and maintained for two days in the dark. Afterwards, the discs were washed once with 200 mg/L Timentin and transferred to CIM supplemented with 200 mg/L Timentin. Five days later, Desiree leaf discs were transferred onto shoot induction medium (SIM) 1 (MSB5 + 0.02 mg/L NAA, 0.15 mg/L GA
3, 2.2 mg/L zeatin riboside, 16 g/L glucose [pH 5.6] 2.8 g/L Gelrite, 200 mg/L Timentin), whereas the Atlantic leaf discs were transferred to SIM 2 (MSB5 + 6 mg/L BAP, 5 mg/L GA
3, 16 g/L glucose [pH 5.6], 2.8 g/L Gelrite, 200 mg/L Timentin). Shoots that developed were individually transferred to a rooting medium made with MS, 20 g/L sucrose and 2.8 g/L Gelrite (pH 5.6). The shooting and rooting media had 0.5 mg/L glufosinate-ammonium for selecting events generated with pFGC-pcoCas9-ASVI or 50 mg/L kanamycin for selecting events generated with pFN117-Cas9-ASVI.
4.5. RNP-Particle Bombardment
Gold particles (0.6 μm in diameter) used for bombardment were freshly prepared as described for the Biolistic
® PDS-1000/He Particle Delivery System (Bio-Rad Lab. Inc., Berkeley, CA, USA) with some modifications. After sterilisation with 70% ethanol, the particles were resuspended in 5 μL of NEB 3.1 buffer and 45 μL of nuclease-free water at a final concentration of 90 mg/mL. The RNP complex for each sgRNA was assembled separately in 25 µL reactions comprising 5 µg of
in vitro-transcribed gRNA-scaffold, 5 µg of Cas9 protein (Proteowa Pty Ltd.) and 1X NEB 3.1 buffer in nuclease-free water. The reactions were incubated for 15 min at 37 °C. For the bombardment, 25 µL of each RNP complex was added to 50 µL of the sterilised gold particles, mixed gently and 15 μL spread on a microcarrier and air-dried in a laminar flow hood before bombardment. Leaf discs to be treated with the RNPs were previously cultured on CIM for five days. Two to four hours before treatment, the discs were transferred onto Petri dishes containing MS solid medium supplemented with 20 g/L sucrose, 0.2 M mannitol (pH 5.6), and 2.8 g/L Gelrite. The discs were bombarded twice with freshly prepared RNP-coated particles at a 9-cm target distance with the PDS-1000/He™ Biolistic system (BioRad Lab. Inc.) using 1100 psi rupture discs. Bombarded leaf discs were transferred and maintained on CIM for two days before transfer to SIM 1 (Desiree) or SIM 2 (Atlantic). Developing shoots were transferred to the rooting medium described in
Section 4.4.
4.6. Screening of Gene-Edited Plants
The transgenic status of regenerated events was assessed using PCRs to establish the integration of Cas9 DNA into the genome by amplifying a portion of the coding sequence. For plants transformed with the pFGC-pcoCas9-ASVI, the primers PFGC9-IF (5′-GAGGAAACTCTCGTTTCGCTTGG-3′) and PFGC9-IR (5′ GCTTTGGAAGAACCTTCTCGT-3′) amplified a 351 bp of the Cas9 DNA, whereas the primers PFN117C9-F (5′-GACGGCACCGAGGAACTG-3′) and PFN117C9-R (5′ TCGTTGGGCAGGTTCTTATC-3′) amplified the 365 bp of the Cas9 coding sequence in the vector pFN117-Cas9-ASVI. In addition, a fragment of the T-DNA integrated into the generated events was amplified to confirm the transgenic status of each event further. For the events transformed with pFGC-pcoCas9-ASVI, the primers BAR-F (5′-CACGGTCAACTTCCGTACC-3′) and BAR-R (5′- CAGATAAAGCCACGCACATTTAGG-3′) amplified a 654 bp fragment made up of part of the BlpR gene and the MAS terminator sequence. Events transformed with vector pFN117-Cas9-ASVI were also screened with primers FN117-F (5′-CATAACGTGACTCCCTTAATTCTCC-3′) and FN117-R (5′-CATGTTGACCTCCAAGCTTGAATTC-3′), which together amplified 1120 bp of the gRNA cassette.
4.7. Detection of Mutation in Gene-Edited Plants by PCR and Sequencing
Mutations in the RNP-treated and transgenic plants were determined by sequencing amplicons of the
VInv and
AS1 genes obtained with the primer pairs 67-F (5′-GGGGAAATATCACATGGGGC-3′) and 10-R (5′-AGTGCAATACCCGTTTTACCAA-3′) for the region containing g67 and g10 target sites; 4-F (5′-GGCTTTGTTGGGTTGTTCGG-3′) and 4-R (5′-AACCCTTCAATGCACAGACAG-3′) for the region containing g4 target site and 7-F (5′-CCTAACGTGGGATAAGAAATCTCT-3′) and 7-R (5′-GATGTGCCAAGTAAAAATCACCA-3′) for the region including the g7 target site. PCRs, done with 100 ng of genomic DNA isolated from 100 mg of leaf tissues following the CTAB method, were conducted using the GoTaq
® Green Master Mix with the temperature profile described under
Section 4.1. The amplicons were sequenced directly and, where required, were cloned using the pGEM-T Easy vector system as described above. Between 18 and 35 clones of each amplicon were sequenced to identify mutation type and frequency.
4.8. Planting, Maintenance of Edited Plants in Soil and Harvesting of Tubers
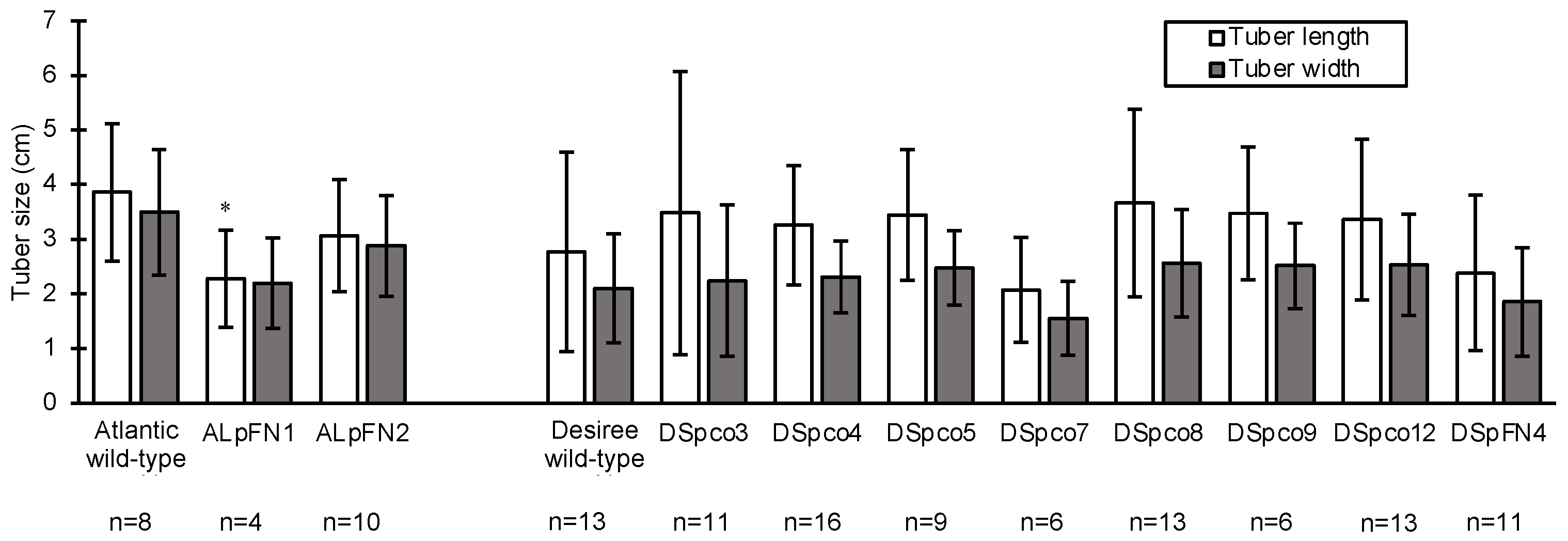
To analyse the effect of editing on the growth and development of the events and to obtain tubers for further characterisation, the events with wild-type Atlantic and Desiree were cloned through subculturing of nodal segments on MS medium (Sigma-Aldrich, St. Louis, MO, USA, catalogue no. M5519) supplemented with 20 g/L sucrose (pH 5.6) and solidified with 2.8 g/L Gelrite (Sigma-Aldrich, St. Louis, MO, USA, catalogue no G1910). Three clones for each of the ten gene-edited events and wild-type were transferred from the rooting media (described above) to pasteurised potting mix (5 L pot for each plant), and the plants were maintained in a Physical Containment Level 2 (PC2) glasshouse at Murdoch University, Western Australia from August to November 2020. The tubers were harvested, washed and air-dried. Tuber width and length were measured with a ruler, after which they were placed in paper bags and stored at 4 °C in the dark for four months before being subjected to sugar content analyses and high-temperature processing.
4.9. Estimation of Sucrose, Glucose, and Fructose Concentrations in Cold-Stored Tubers
Total soluble sugars were extracted from each cold-stored tuber separately using 80% (
v/
v) ethanol, as described previously by McKibbin et al. [
69]. Sugar quantification assay was conducted according to the method of Jones et al. [
70] with modifications made for assessment in a plate reader, as described by McKibbin et al. [
69]. Briefly, soluble sugars were extracted from 300 mg of homogenised tuber tissue by adding 1 mL of 80% ethanol, followed by incubation at 70 °C for an hour with continuous agitation. The supernatant was collected by centrifugation at 10,000×
g for 2 min. The assay was performed in a microtiter plate; each 200 µL reaction contained 100 mM imidazole, 10 mM MgCl
2, 1.1 mM ATP, 0.5 mM NADP, 0.14 U glucose-6-phosphate dehydrogenase (Sigma-Aldrich, St. Louis, MO, USA, catalogue no. G6378) and 20 µL of the tuber extract (previously cooled to room temperature). Absorbance was measured using a Beckman Coulter DTX 880 Multimode Detector microplate reader with an A340 nm absorbance filter and 10 nm bandwidth. Initial absorbance was recorded as the baseline for each reaction. For glucose, fructose and sucrose quantification, 0.12 U hexokinase (Sigma-Aldrich, St. Louis, MO, USA, catalogue no. H4502), 1 U phosphoglucose isomerase (Sigma-Aldrich, St. Louis, MO, USA, catalogue no. P5381) and 4 U invertase (Sigma-Aldrich, St. Louis, MO, USA, catalogue no. I4504) were added accordingly; each was followed by a 10-min incubation at room temperature, and absorbance was recorded. Three readings were recorded for each sample after adding the enzyme. Standard curves were generated for each sugar type using concentrations of 0 to 100 nmol, and sugar concentration in each sample was determined by plotting the absorbance values against the standard curves.
4.10. High-Temperature Processing of Cold-Stored Tubers into Crisps and Acrylamide Assay
The cold-stored tubers of the edited events and wild-type were peeled, precisely cut into 2 mm thick slices using a mandoline slicer and deep-fried in vegetable oil at 191 °C for 60 s. Because there were more events of Desiree, the slices were fried in batches, with each batch including a wild-type. The colour intensity of the crisps was quantified using the FIJI image processing software, which assigns a value of 255 to the brightest (white) colour and zero to the darkest (black) colour [
37]. The acrylamide concentration in the crisps was estimated from 2 g of homogenised crisps using the Acrylamide-ES ELISA kit (Eurofins, Brisbane, AUS, catalogue no. 515680) according to the manufacturer’s instructions.
4.11. Statistical Analyses
The Welch Two Sample t-test was used to determine significant differences in the mean values assigned to the crisp colour intensities, the means of the sucrose, fructose and glucose concentrations in the cold stored tubers, the mean concentrations of acrylamide in the crisps, and the mean tuber lengths and widths between the wild-types and gene-edited events. Statistical analysis was performed using R ver.3.6.3 [
71], and statistical significance was assessed at
p < 0.05. Bar charts were generated using Microsoft Excel.