Patterns of Phenolic Compounds in Betula and Pinus Pollen
Abstract
1. Introduction
2. Results
2.1. Application of Different Methods for the Extraction of Free Phenolic Compounds from Betula pendula and Pinus sylvestris Pollen
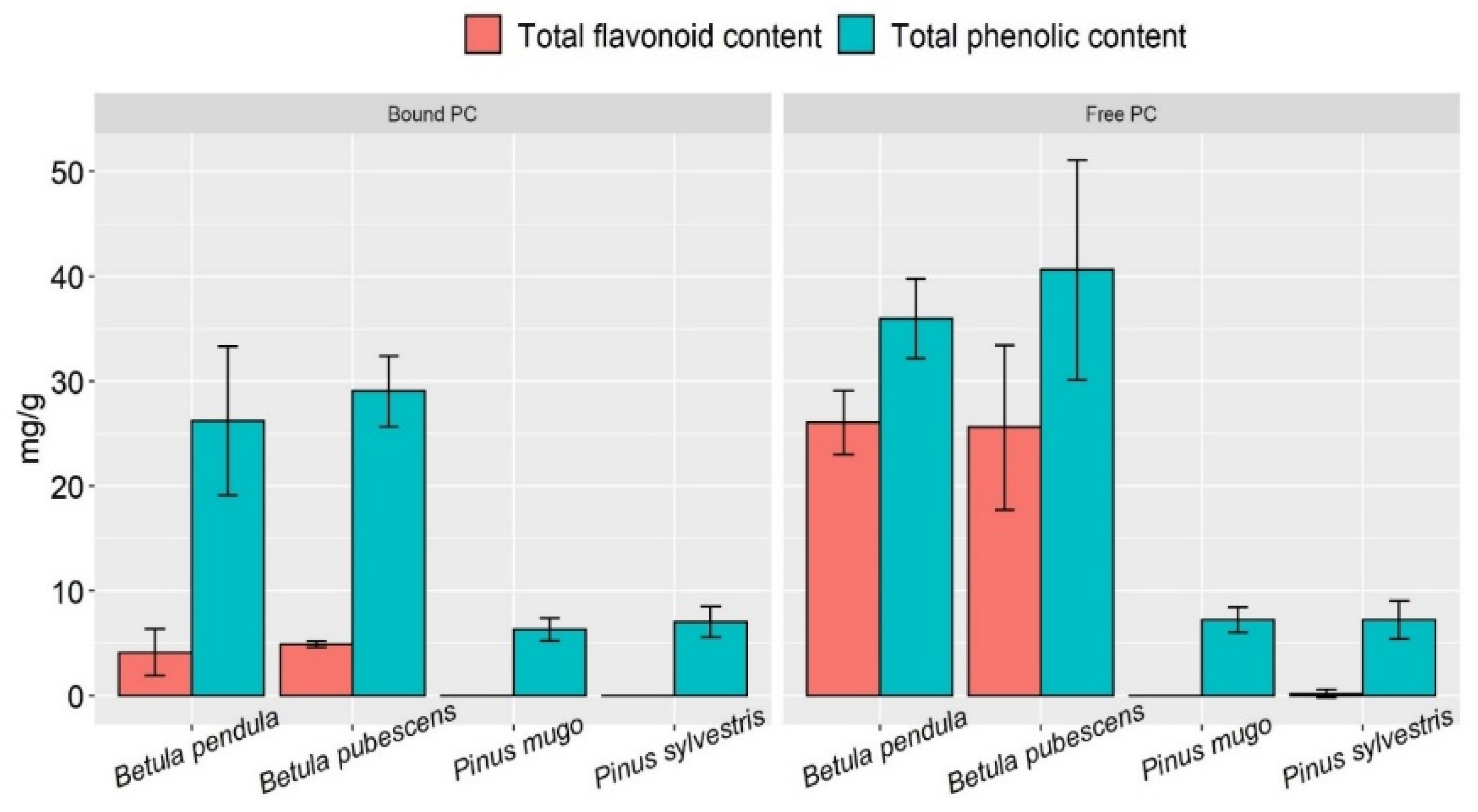
2.2. Free and Cell-Bound Phenolic Compounds, Flavonoid Content in Betula and Pinus Pollen Extracts
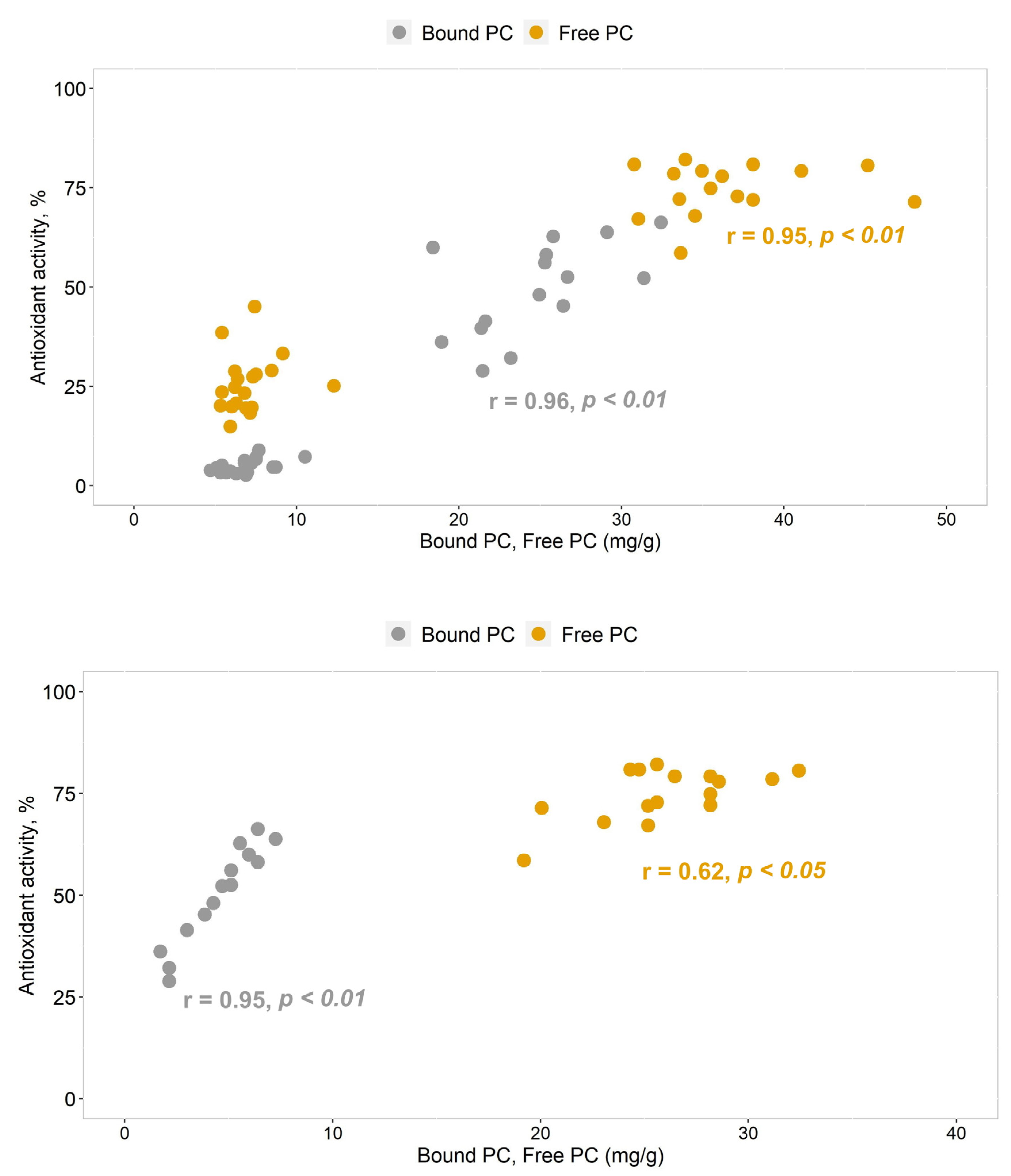
2.3. Bioactivity of Betula and Pinus Pollen
2.4. Chromatography of Betula and Pinus Pollen Phenolic Compounds
2.4.1. High-Performance Thin-Layer Chromatography of Betula and Pinus Pollen Phenolic Compounds
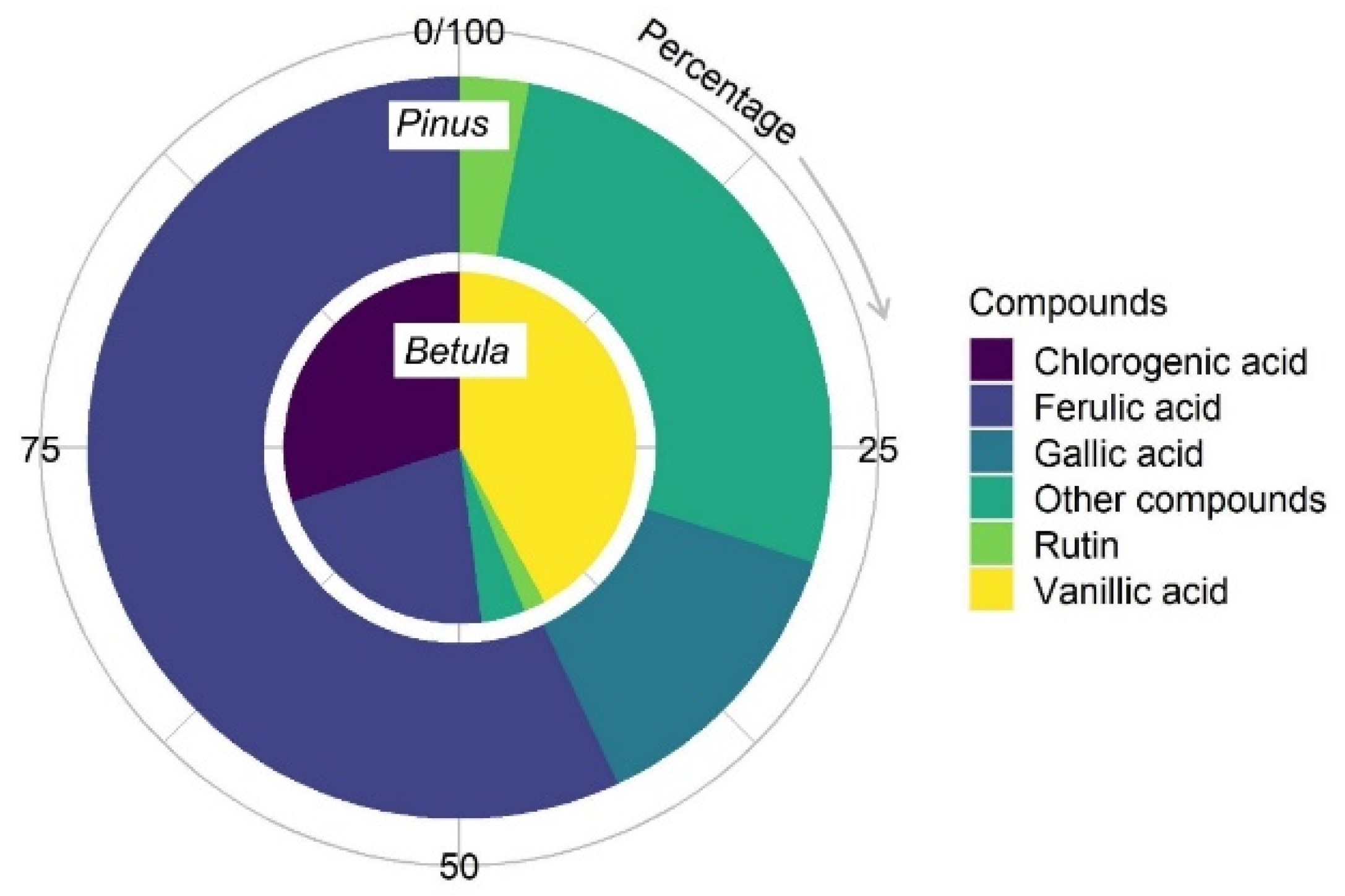
2.4.2. HPLC Analysis of Betula and Pinus Pollen Phenolic Compounds
3. Discussion
4. Materials and Methods
4.1. Chemical Substances
4.2. Collection and Preparation of Betula and Pinus Pollen for Analysis
4.2.1. Collection of Pollen In Situ
4.2.2. Principles of Analysis of Betula and Pinus Pollen Extracts
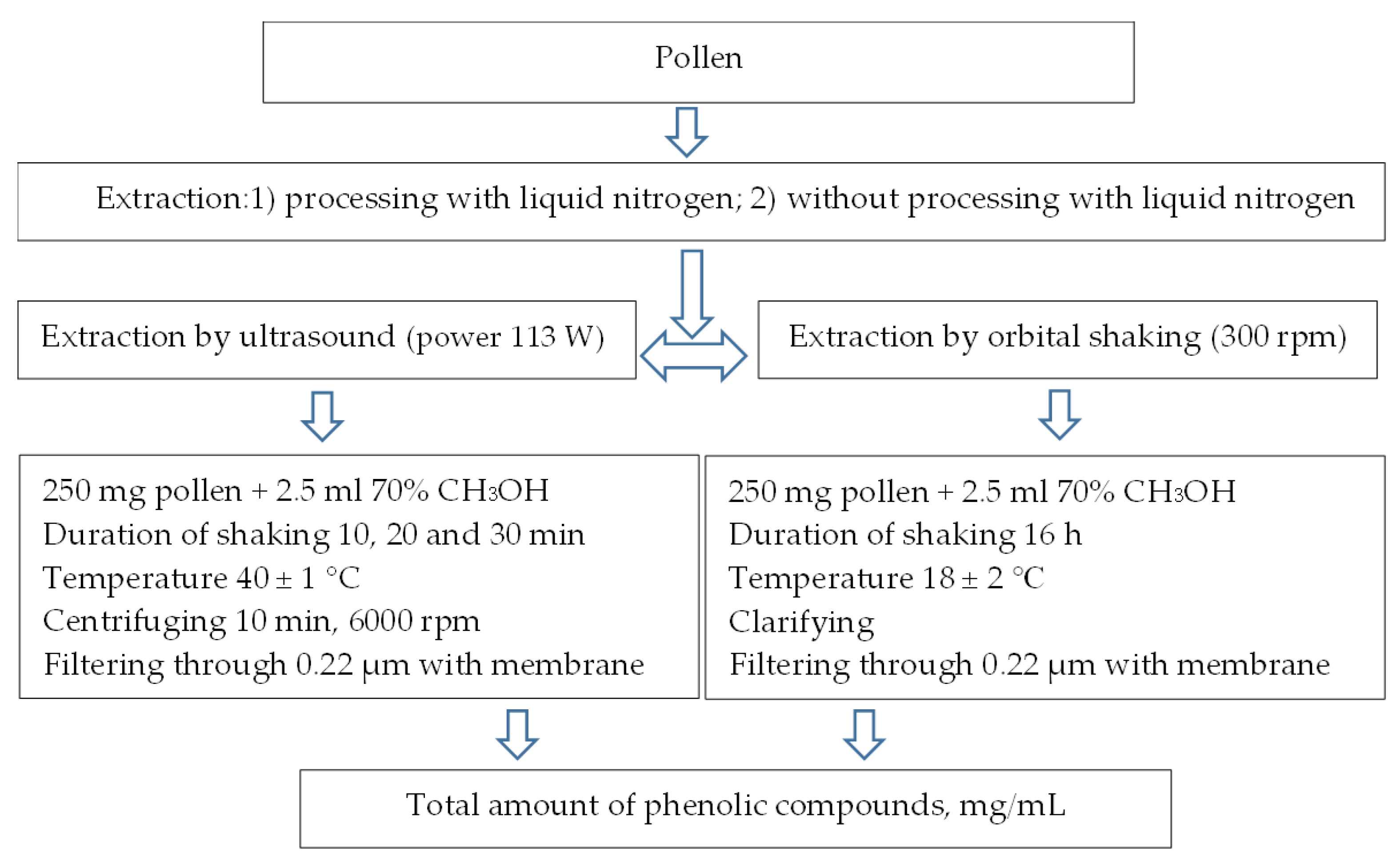
4.3. Extraction of Phenolic Compounds from Betula and Pinus Pollen
4.3.1. Extraction of Free Phenolic Compounds
4.3.2. Extraction of Bound-to-the-Cell-Wall Phenolic Compounds
4.4. Spectrophotometric Analysis of Phenolic Compounds of Betula and Pinus Pollen
4.4.1. The Total Phenolic Compound Content
4.4.2. The Total Content of Flavonoids
4.4.3. Antioxidant Activity Using DPPH• and ABTS• Radical Scavenging Assays
4.5. Chromatographic Analysis of Phenolic Compounds of Betula and Pinus Pollen Extracts
4.5.1. High-Performance Thin Layer Chromatography (HPTLC) Analysis
4.5.2. High-Performance Liquid Chromatography (HPLC-DAD) Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davies, J.M. Pollen Allergens. In Encyclopedia of Environmental Health, 2nd ed.; Nriagu, J., Ed.; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 300–322. ISBN 978-0-444-63951-6. [Google Scholar] [CrossRef]
- Rozema, J.; Broekman, R.A.; Blokker, P.; Meijkamp, B.B.; de Bakker, N.; van de Staaij, J.A.; van Beema, F.; Ariese, F.; Kars, S.M. UV-B absorbance and UV-B absorbing compounds (para-coumaric acid) in pollen and sporopollenin: The perspective to track historic UV-B levels. J. Photochem. Photobiol. B Biol. 2001, 62, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Wiermann, R.; Gubatz, S. Pollen wall and sporopollenin. Int. Rev. Cytol. 1992, 140, 35–72. [Google Scholar] [CrossRef]
- Herminghaus, S.; Arendt, S.; Gubatz, S.; Rittscher, M.; Wiermann, R. Aspects of sporopollenin biosynthesis: Phenols as integrated compounds of the biopolymer. In Sexual Reproduction in Higher Plants; Springer: Berlin/Heidelberg, Germany, 1988; pp. 169–174. [Google Scholar]
- Osthoff, K.S.; Wiermann, R. Phenols as integrated compounds of sporopollenin from Pinus pollen. J. Plant Physiol. 1987, 131, 5–15. [Google Scholar] [CrossRef]
- Shi, J.; Cui, M.; Yang, L.; Kim, Y.J.; Zhang, D. Genetic and biochemical mechanisms of pollen wall development. Trends Plant Sci. 2015, 20, 741–753. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Li, H.; Deng, Z.; Tsao, R. A review on insoluble-bound phenolics in plant-based food matrix and their contribution to human health with future perspectives. Trends Food Sci. Technol. 2020, 105, 347–362. [Google Scholar] [CrossRef]
- Cheng, Y.; Quan, W.; He, Y.; Qu, T.; Wang, Z.; Zeng, M.; Qin, F.; Chen, J.; He, Z. Effects of postharvest irradiation and superfine grinding wall disruption treatment on the bioactive compounds, endogenous enzyme activities, and antioxidant properties of pine (Pinus yunnanensis) pollen during accelerated storage. LWT 2021, 144, 1–9. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Taylor, L.P.; Hepler, P.K. Pollen germination and tube growth. Annu. Rev. Plant Biol. 1997, 48, 461–491. [Google Scholar] [CrossRef]
- Woo, H.H.; Jeong, B.R.; Hawes, M.C. Flavonoids: From cell cycle to biotechnology. Biotechnol. Lett. 2005, 27, 365–374. [Google Scholar] [CrossRef]
- Fraser, W.T.; Lomax, B.H.; Jardine, P.E.; Gosling, W.D.; Sephton, M.A. Pollen and spores as a passive monitor of ultraviolet radiation. Front. Ecol. Evol. 2014, 2, 1–3. [Google Scholar] [CrossRef]
- Shumilovskikh, L.S.; Schlütz, F.; Achterberg, I.; Kvitkina, A.; Bauerochse, A.; Leuschner, H.H. Pollen as nutrient source in Holocene ombrotrophic bogs. Rev. Palaeobot. Palynol. 2015, 221, 171–178. [Google Scholar] [CrossRef]
- Agogué, H.; Casamayor, E.O.; Joux, F.; Obernosterer, I.; Dupuy, C.; Lantoine, F.; Catala, P.; Weinbauer, M.G.; Reinthaler, T.; Herndl, G.J.; et al. Comparison of samplers for the biological characterization of the sea surface microlayer. Limnol. Oceanogr-Meth. 2004, 2, 213–225. [Google Scholar] [CrossRef]
- Graham, M.D.; Vinebrooke, R.D.; Turner, M. Coupling of boreal forests and lakes: Effects of conifer pollen on littoral communities. Limnol. Oceanogr. 2006, 51, 1524–1529. [Google Scholar] [CrossRef]
- Cosmulescu, S.; Trandafir, I.; Nour, V. Chemical composition and antioxidant activity of walnut pollen samples. Not. Bot. Horti Agrobot. Cluj-Napoca. 2015, 43, 361–365. [Google Scholar] [CrossRef]
- Masclaux, H.; Perga, M.E.; Kagami, M.; Desvilettes, C.; Bourdier, G.; Bec, A. How pollen organic matter enters freshwater food webs. Limnol. Oceanogr. 2013, 58, 1185–1195. [Google Scholar] [CrossRef]
- Rösel, S.; Rychła, A.; Wurzbacher, C.; Grossart, H.P. Effects of pollen leaching and microbial degradation on organic carbon and nutrient availability in lake water. Aquat. Sci. 2012, 74, 87–99. [Google Scholar] [CrossRef]
- Filipiak, M. Pollen stoichiometry may influence detrital terrestrial and aquatic food webs. Front. Ecol. Evol. 2016, 4, 1–8. [Google Scholar] [CrossRef]
- Azman, N.A.M.; Skowyra, M.; Muhammad, K.; Gallego, M.G.; Almajano, M.P. Evaluation of the antioxidant activity of Betula pendula leaves extract and its effects on model foods. Pharm. Biol. 2017, 55, 912–919. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Ruočkuvienė, G.; Kaškonas, P.; Akuneca, I.; Maruška, A. Chemometric analysis of bee pollen based on volatile and phenolic compound compositions and antioxidant properties. Food Anal. Methods 2015, 8, 1150–1163. [Google Scholar] [CrossRef]
- Keriene, I.; Mankeviciene, A.; Blazyte, J. The effect of antifungal extracts on the contamination of grain with microfungi. Food Sci. Nutr. 2020, 8, 1375–1382. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, A.J.; Choi, E.M. Antioxidant and anti-inflammatory activity of pine pollen extract in vitro. Phytother. Res. 2009, 23, 41–48. [Google Scholar] [CrossRef]
- Passali, D.; Cingi, C.; Staffa, P.; Passali, F.; Muluk, N.B.; Bellussi, M.L. The international study of the allergic rhinitis survey: Outcomes from 4 geographical regions. Asia Pac. Allergy 2018, 8, e7. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, K. Pollen production in selected species of anemophilous plants. Acta Agrobot. 2008, 61, 41–52. [Google Scholar] [CrossRef]
- Jato, V.; Rodríguez-Rajo, F.J.; Aira, M.J. Use of phenological and pollen-production data for interpreting atmospheric birch pollen curves. Ann. Agric. Environ. Med. 2007, 14, 271–280. [Google Scholar] [PubMed]
- Greenfield, L. Plant pollen production in selected tree species. Canterbury Bot. Soc. J. 1996, 31, 10–13. [Google Scholar]
- Pawlik, M.; Ficek, D. Pine pollen grains in coastal waters of the Baltic Sea. Oceanol. Hydrobiol. Stud. 2016, 45, 35–41. [Google Scholar] [CrossRef]
- Fernando, D.D.; Lazzaro, M.D.; Owens, J.N. Growth and development of conifer pollen tubes. Sex. Plant Reprod. 2005, 18, 149–162. [Google Scholar] [CrossRef]
- Bohne, G.; Woehlecke, H.; Ehwald, R. Water relations of the pine exine. Ann. Bot. 2005, 96, 201–208. [Google Scholar] [CrossRef]
- Augustin, S.; Wex, H.; Niedermeier, D.; Pummer, B.; Grothe, H.; Hartmann, S.; Tomsche, L.; Clauss, T.; Voigtländer, J.; Ignatius, K.; et al. Immersion freezing of birch pollen washing water. Atmos. Chem. Phys. 2013, 13, 10989–11003. [Google Scholar] [CrossRef]
- Pummer, B.G.; Bauer, H.; Bernardi, J.; Bleicher, S.; Grothe, H. Suspendable macromolecules are responsible for ice nucleation activity of birch and conifer pollen. Atmos. Chem. Phys. 2012, 12, 2541–2550. [Google Scholar] [CrossRef]
- Božič, A.; Šiber, A. Mechanics of inactive swelling and bursting of porate pollen grains. Biophys. J. 2022, 121, 782–792. [Google Scholar] [CrossRef]
- Kenđel, A.; Zimmermann, B. Chemical analysis of pollen by FT-Raman and FTIR spectroscopies. Front. Plant Sci. 2020, 11, 352. [Google Scholar] [CrossRef]
- Lou, Y.; Zhu, J.; Yang, Z. (Eds.) Molecular cell biology of pollen walls. In Applied Plant Cell Biology; Springer: Berlin/Heidelberg, Germany, 2014; Volume 22, pp. 179–205. [Google Scholar] [CrossRef]
- Stiebing, C.; Post, N.; Schindler, C.; Göhrig, B.; Lux, H.; Popp, J.; Heutelbeck, A.; Schie, I.W. Revealing the chemical composition of birch pollen grains by Raman spectroscopic imaging. Int. J. Mol. Sci. 2022, 23, 5112. [Google Scholar] [CrossRef] [PubMed]
- Depciuch, J.; Kasprzyk, I.; Drzymała, E.; Parlinska-Wojtan, M. Identification of birch pollen species using FTIR spectroscopy. Aerobiologia 2018, 34, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Bağcıoğlu, M.; Zimmermann, B.; Kohler, A. A multiscale vibrational spectroscopic approach for identification and biochemical characterization of pollen. PLoS ONE 2015, 10, e0137899. [Google Scholar] [CrossRef]
- Burkart, J.; Gratzl, J.; Seifried, T.M.; Bieber, P.; Grothe, H. Isolation of subpollen particles (SPPs) of birch: SPPs are potential carriers of ice nucleatin macromolecules. Biogeosciences 2021, 18, 5751–5765. [Google Scholar] [CrossRef]
- Kanter, U.; Heller, W.; Durner, J.; Winkler, J.B.; Engel, M.; Behrendt, H.; Holzinger, A.; Braun, P.; Hauser, M.; Ferreira, F.; et al. Molecular and immunological characterization of ragweed (Ambrosia artemisiifolia L.) pollen after exposure of the plants to elevated ozone over a whole growing season. PLoS ONE 2013, 8, e61518. [Google Scholar] [CrossRef]
- Atalay, F.E.; Culum, A.A.; Kaya, H.; Gokturk, G.; Yigit, E. Different plant sporopollenin exine capsules and their multifunctional usage. ACS Appl. Bio Mater. 2022, 5, 1348–1360. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J. Insoluble-bound phenolics in food. Molecules 2016, 21, 1216. [Google Scholar] [CrossRef]
- Martins, C.C.; Rodrigues, R.C.; Mercali, G.D.; Rodrigues, E. New insights into non-extractable phenolic compounds analysis. Food Res. Int. 2022, 157, 111487. [Google Scholar] [CrossRef]
- Friedman, M.; Jürgens, H.S. Effect of pH on the stability of plant phenolic compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Routray, W.; Orsat, V. Preparative extraction and separation of phenolic compounds. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2013–2045. [Google Scholar] [CrossRef]
- Feng, W.; Hao, Z.; Li, M. Isolation and structure identification of flavonoids. Flavonoids, from biosynthesis to human health. In Flavonoids—From Biosynthesis to Human Health; Justino, G.C., Ed.; InTech: Rijeka, Croatia, 2017; pp. 17–43. [Google Scholar] [CrossRef]
- Oroian, M.; Ursachi, F.; Dranca, F. Ultrasound-assisted extraction of polyphenols from crude pollen. Antioxidants 2020, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Chiriac, E.R.; Chiţescu, C.L.; Geană, E.I.; Gird, C.E.; Socoteanu, R.P.; Boscencu, R. Advanced analytical approaches for the analysis of polyphenols in plants matrices—A review. Separations 2021, 8, 65. [Google Scholar] [CrossRef]
- Bajkacz, S.; Baranowska, I.; Buszewski, B.; Kowalski, B.; Ligor, M. Determination of flavonoids and phenolic acids in plant materials using SLE-SPE-UHPLC-MS/MS method. Food Anal. Methods 2018, 11, 3563–3575. [Google Scholar] [CrossRef]
- Diego-Taboada, A.; Beckett, S.T.; Atkin, S.L.; Mackenzie, G. Hollow pollen shells to enhance drug delivery. Pharmaceutics 2014, 6, 80–96. [Google Scholar] [CrossRef]
- Quilichini, T.D.; Grienenberger, E.; Douglas, C.J. The biosynthesis, composition and assembly of the outer pollen wall: A tough case to crack. Phytochemistry 2015, 113, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Hemsley, A.R.; Barrie, P.J.; Chaloner, W.G.; Scott, A.C. The composition of sporopollenin and its use in living and fossil plant systematics. Grana 1993, 32, 2–11. [Google Scholar] [CrossRef]
- Prabhakar, A.K.; Lai, H.Y.; Potroz, M.G.; Corliss, M.K.; Park, J.H.; Mundargi, R.C.; Cho, D.; Bang, S.-I.; Cho, N.J. Chemical processing strategies to obtain sporopollenin exine capsules from multi-compartmental pine pollen. J. Ind. Eng. Chem. 2017, 53, 375–385. [Google Scholar] [CrossRef]
- Rowley, J.R. Are the endexines of pteridophytes, gymnosperms and angiosperms structurally equivalent? Rev. Palaeobot. Palynol. 1995, 85, 13–34. [Google Scholar] [CrossRef]
- Mosić, M.; Trifković, J.; Vovk, I.; Gašić, U.; Tešić, Ž.; Šikoparija, B.; Milojković-Opsenica, D. Phenolic composition influences the health-promoting potential of bee-pollen. Biomolecules 2019, 9, 783. [Google Scholar] [CrossRef]
- Taylor, P.E.; Flagan, R.C.; Miguel, A.G.; Valenta, R.; Glovsky, M.M. Birch pollen rupture and the release of aerosols of respirable allergens. Clin. Exp. Allergy 2004, 34, 1591–1596. [Google Scholar] [CrossRef]
- Bohne, G.; Richter, E.; Woehlecke, H.; Ehwald, R. Diffusion barriers of tripartite sporopollenin microcapsules prepared from pine pollen. Ann. Bot. 2003, 9, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Šaulienė, I.; Šukienė, L.; Daunys, G.; Valiulis, G.; Lankauskas, A.; Kokina, I.; Gerbreders, V.; Gavarāne, I. Detection and microscopy of Alnus glutinosa pollen fluorescence peculiarities. Forests 2019, 10, 959. [Google Scholar] [CrossRef]
- Tegelberg, R.; Virjamo, V.; Julkunen-Tiitto, R. Dry-air drying at room temperature—A practical pre-treatment method of tree leaves for quantitative analyses of phenolics? Phytochem. Anal. 2018, 29, 493–499. [Google Scholar] [CrossRef]
- Julkunen-Tiitto, R.; Rousi, M.; Bryant, J.; Sorsa, S.; Keinänen, M.; Sikanen, H. Chemical diversity of several Betulaceae species: Comparison of phenolics and terpenoids in northern birch stems. Trees 1996, 11, 16–22. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Nutr. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Ferreyra, F.M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef]
- Kreft, I.; Vollmannová, A.; Lidiková, J.; Musilová, J.; Germ, M.; Golob, A.; Vombergar, B.; Ačko, D.K.; Luthar, Z. Molecular shield for protection of buckwheat plants from UV-B radiation. Molecules 2022, 27, 5577. [Google Scholar] [CrossRef]
- Raudonė, L.; Raudonis, R.; Janulis, V.; Viškelis, P. Quality evaluation of different preparations of dry extracts of birch (Betula pendula Roth) leaves. J. Nat. Prod. 2014, 28, 1645–1648. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Sachan, A.; Mitra, A. Formation of vanillic acid from ferulic acid by Paecilomyces variotii MTCC 6581. Curr. Sci. 2006, 90, 825–829. Available online: https://www.jstor.org/stable/24089195 (accessed on 10 October 2022).
- Kvasnička, F.; Čopiková, J.; Ševčik, R.; Krátká, J.; Syntytsia, A.; Voldřich, M. Determination of phenolic acids by capillary zone electrophoresis and HPLC. Cent. Eur. J. Chem. 2008, 6, 410–418. [Google Scholar] [CrossRef]
- Fattahi, S.; Zabihi, E.; Abedian, Z.; Pourbagher, R.; Ardekani, A.M.; Mostafazadeh, A.; Akhavan-Niaki, H. Total phenolic and flavonoid contents of aqueous extract of stinging nettle and in vitro antiproliferative effect on hela and BT-474 Cell lines. Int. J. Mol. Cell Med. 2014, 3, 102–107. [Google Scholar] [PubMed]
- Baltrušaitytė, V.; Venskutonis, P.R.; Čeksterytė, V. Radical scavenging activity of different floral origin honey and beebread phenolic extracts. Food Chem. 2007, 101, 502–514. [Google Scholar] [CrossRef]
- Jesionek, W.; Majer-Dziedzic, B.; Choma, I.M. Separation, identification, and investigation of antioxidant ability of plant extract components using TLC, LC–MS, and TLC–DPPH•. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 1147–1153. [Google Scholar] [CrossRef]
- Amarowicz, R.; Weidner, S. Content of phenolic acids in rye caryopses determined using DAD-HPLC method. Czech J. Food Sci. 2001, 19, 201–205. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 10 October 2022).
- R Studio Team. R Studio: Integrated Development for R. RStudio; PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 10 October 2022).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. Available online: https://ggplot2.tidyverse.org (accessed on 10 October 2022).
- Kassambara, A.; Kassambara, M.A. Package ‘ggpubr’; R Foundation for Statistical Computing: Vienna, Austria, 2020; p. 52. [Google Scholar]



| Plant Species | Free PC, mg/mL According to the Extraction Duration | |||||
|---|---|---|---|---|---|---|
| Without Liquid Nitrogen | With Liquid Nitrogen | |||||
| 10 min | 20 min | 30 min | 10 min | 20 min | 30 min | |
| Betulapendula | 1.13 ± 0.03 | 1.07 ± 0.01 | 1.09 ± 0.03 | 1.09 ± 0.01 | 1.08 ± 0.05 | 1.09 ± 0.02 |
| Pinus sylvestris | 0.38 ± 0.01 | 0.40 ± 0.01 | 0.39 ± 0.01 | 0.37 ± 0.01 | 0.38 ± 0.01 | 0.38 ± 0.01 |
| Plant Species | Free PC, mg/mL, When Extraction Duration Is 16 h | |
|---|---|---|
| Without Liquid Nitrogen | With Liquid Nitrogen | |
| Betula pendula | 1.09 ± 0.01 | 1.10 ± 0.01 |
| Pinus sylvestris | 0.46 ± 0.10 | 0.40 ± 0.02 |
| Plant Species | DPPH+, % | ABTS+, % | ||
|---|---|---|---|---|
| Bound PC | Free PC | Bound PC | Free PC | |
| Betula pendula | 49.5 ± 11.1 | 75.5 ± 6.6 | 20.6 ± 4.0 | 37.2 ± 4.7 |
| Betula pubescens | 52.4 ± 11 | 75.2 ± 5.7 | 23.4 ± 6.0 | 31.9 ± 1.1 |
| Pinus sylvestris | >LOD * | 27.0 ± 9.7 | >LOD | >LOD—20.0 |
| Pinus mugo | >LOD | 23.3 ± 5.5 | >LOD | >LOD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerienė, I.; Šaulienė, I.; Šukienė, L.; Judžentienė, A.; Ligor, M.; Buszewski, B. Patterns of Phenolic Compounds in Betula and Pinus Pollen. Plants 2023, 12, 356. https://doi.org/10.3390/plants12020356
Kerienė I, Šaulienė I, Šukienė L, Judžentienė A, Ligor M, Buszewski B. Patterns of Phenolic Compounds in Betula and Pinus Pollen. Plants. 2023; 12(2):356. https://doi.org/10.3390/plants12020356
Chicago/Turabian StyleKerienė, Ilona, Ingrida Šaulienė, Laura Šukienė, Asta Judžentienė, Magdalena Ligor, and Bogusław Buszewski. 2023. "Patterns of Phenolic Compounds in Betula and Pinus Pollen" Plants 12, no. 2: 356. https://doi.org/10.3390/plants12020356
APA StyleKerienė, I., Šaulienė, I., Šukienė, L., Judžentienė, A., Ligor, M., & Buszewski, B. (2023). Patterns of Phenolic Compounds in Betula and Pinus Pollen. Plants, 12(2), 356. https://doi.org/10.3390/plants12020356










