Rhanteriol, a New Rhanterium suaveolens Desf. Lignan with Pharmacological Potential as an Inhibitor of Enzymes Involved in Neurodegeneration and Type 2 Diabetes
Abstract
1. Introduction
2. Results
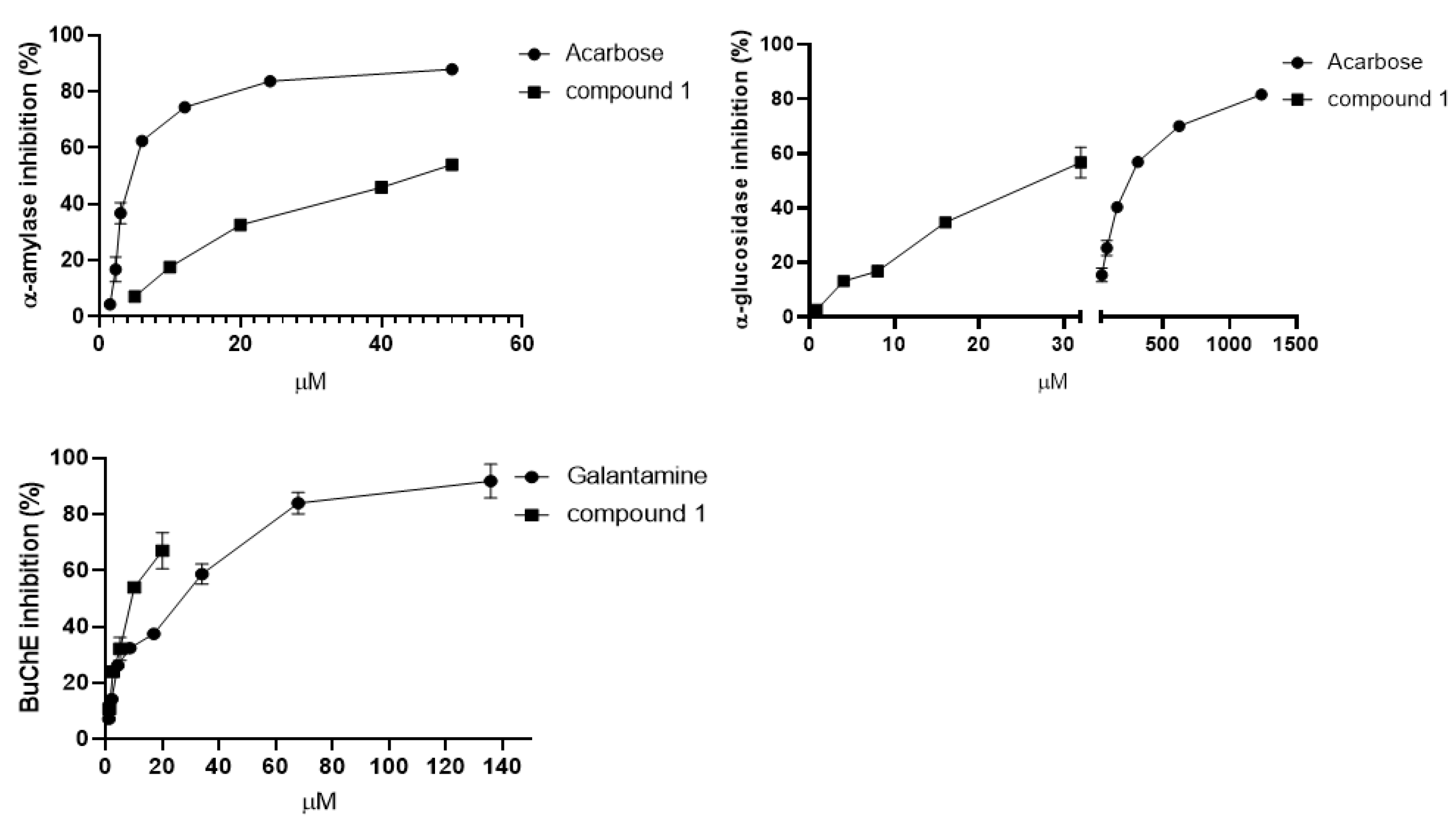
2.1. Isolation, Molecular Elucidation, and Bioactivity
2.2. In Silico Docking of the New Compound from Rhanterium Suaveolens in the Enzymes
3. Discussion
4. Materials and Methods
4.1. Equipment
4.2. Plant Material
4.3. Extraction and Isolation
4.4. α-Amylase Inhibition Assay
4.5. α-Glucosidase Inhibition Assay
4.6. Cholinesterase Inhibition Assay
4.7. Cell Culture
4.8. Cell Viability
4.9. In Silico Molecular Docking
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Lankatillake, C.; Luo, S.; Flavel, M.; Lenon, G.B.; Gill, H.; Huynh, T.; Dias, D.A. Screening natural product extracts for potential enzyme inhibitors: Protocols, and the standardisation of the usage of blanks in α-amylase, α-glucosidase and lipase assays. Plant Methods 2021, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Shafique, Z.; Amjad, S.T.; Bin Asad, M.H.H. Enzymes inhibitors from natural sources with antidiabetic activity: A review. Phytother. Res. 2019, 33, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Bisio, A.; De Mieri, M.; Milella, L.; Schito, A.M.; Parricchi, A.; Russo, D.; Alfei, S.; Lapillo, M.; Tuccinardi, T.; Hamburger, M. Antibacterial and hypoglycemic diterpenoids from Salvia chamaedryoides. J. Nat. Prod. 2017, 80, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Bajgai, J.; Fadriquela, A.; Sharma, S.; Trinh, T.T.; Akter, R.; Jeong, Y.J.; Goh, S.H.; Kim, C.-S.; Lee, K.-J. Therapeutic potential of natural products in treating neurodegenerative disorders and their future prospects and challenges. Molecules 2021, 26, 5327. [Google Scholar] [CrossRef]
- Rallabandi, H.R.; Mekapogu, M.; Natesan, K.; Saindane, M.; Dhupal, M.; Swamy, M.K.; Vasamsetti, B.M.K. Computational Methods Used in Phytocompound-Based Drug Discovery. In Plant-Derived Bioactives; Springer: Berlin/Heidelberg, Germany, 2020; pp. 549–573. [Google Scholar]
- Mocan, A.; Zengin, G.; Crişan, G.; Mollica, A. Enzymatic assays and molecular modeling studies of Schisandra chinensis lignans and phenolics from fruit and leaf extracts. J. Enzyme Inhib. Med. Chem. 2016, 31, 200–210. [Google Scholar] [CrossRef]
- Cao, H.; Chen, X.C. Structures required of flavonoids for inhibiting digestive enzymes. Anti-Cancer Agents Med. Chem. 2012, 12, 929–939. [Google Scholar] [CrossRef]
- Manaharan, T.; Teng, L.L.; Appleton, D.; Ming, C.H.; Masilamani, T.; Palanisamy, U.D. Antioxidant and antiglycemic potential of Peltophorum pterocarpum plant parts. Food Chem. 2011, 129, 1355–1361. [Google Scholar] [CrossRef]
- McCarty, M.F. A chlorogenic acid-induced increase in GLP-1 production may mediate the impact of heavy coffee consumption on diabetes risk. Med. Hypotheses 2005, 64, 848–853. [Google Scholar] [CrossRef]
- Hitana, M.; Najaa, H.; Fattouch, S.; Ghazouani, T.; Sassi, C.B.; Dupas-Farrugia, C.; Oulahal, N.; Neffati, M. Chemical chacracterization and bioactive potential of essential oil isolated from rhanterium suaveolens desf. Species growing in tunisian arid zone. Ital. J. Food Sci. 2020, 32, 983–996. [Google Scholar] [CrossRef]
- Chelly, S.; Chelly, M.; Occhiuto, C.; Cimino, F.; Cristani, M.; Saija, A.; Molonia, M.S.; Ruberto, G.; D’Angelo, V.; Germanò, M.P. Evaluation of Antioxidant, Anti-Inflammatory and Antityrosinase Potential of Extracts from Different Aerial Parts of Rhanterium suaveolens from Tunisia. Chem. Biodivers. 2021, 18, e2100316. [Google Scholar] [CrossRef]
- Hitana, M.; Dupas, C.; Oulahal, N.; Degraeve, P.; Najaa, H.; Bouhamda, T.; Fattouch, S.; Neffati, M. Assessment of antioxidant activities of an endemic species from Tunisia: Rhanterium sueaveolens Desf related to its phenolic composition. Biocatal. Agric. Biotechnol. 2019, 22, 101355. [Google Scholar] [CrossRef]
- Oueslati, M.H.; Jannet, H.B.; Mighri, Z.; Matthew, S.; Abreu, P.M. A new C9 nor-isoprenoid glucoside from Rantherium suaveolens. Nat. Prod. Res. 2007, 21, 884–888. [Google Scholar] [CrossRef]
- Oueslati, M.H.; Mighri, Z.; Jannet, H.B.; Abreu, P.M. New ceramides from Rantherium suaveolens. Lipids 2005, 40, 1075–1079. [Google Scholar] [CrossRef]
- Salah, H.B.; Bouaziz, H.; Allouche, N. Chemical composition of essential oil from Rhanterium suaveolens Desf. and its antimicrobial activity against foodborne spoilage pathogens and mycotoxigenic fungi. J. Essent. Oil-Bear. Plants 2019, 22, 592–603. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Al-Omer, M.S.; Ahmed, A.M.; Hashish, N.E.; Alsaedi, H.M.; Alghazy, S.A.; Abdellatif, A.A.H. Comparative study for the volatile oil constituents and antimicrobial activity of Rhanterium epapposum oliv. Growing in qassim, Saudi arabia. Pharmacogn. J. 2019, 11, 195–199. [Google Scholar] [CrossRef]
- Demirci, B.; Yusufoglu, H.S.; Tabanca, N.; Temel, H.E.; Bernier, U.R.; Agramonte, N.M.; Alqasoumi, S.I.; Al-Rehaily, A.J.; Başer, K.H.C.; Demirci, F. Rhanterium epapposum Oliv. essential oil: Chemical composition and antimicrobial, insect-repellent and anticholinesterase activities. Saudi Pharm. J. 2017, 25, 703–708. [Google Scholar] [CrossRef]
- Lee, C.; Kim, H.; Kho, Y. Agastinol and agastenol, novel lignans from Agastache rugosa and their evaluation in an apoptosis inhibition assay. J. Nat. Prod. 2002, 65, 414–416. [Google Scholar] [CrossRef]
- Saidi, I.; Nimbarte, V.D.; Schwalbe, H.; Waffo-Téguo, P.; Harrath, A.H.; Mansour, L.; Alwasel, S.; Jannet, H.B. Anti-tyrosinase, anti-cholinesterase and cytotoxic activities of extracts and phytochemicals from the Tunisian Citharexylum spinosum L.: Molecular docking and SAR analysis. Bioorg. Chem. 2020, 102, 104093. [Google Scholar] [CrossRef]
- Malik, A.; Anis, I.; Khan, S.B.; Ahmed, E.; Ahmed, Z.; Nawaz, S.A.; Choudhary, M.I. Enzymes inhibiting lignans from Vitex negundo. Chem. Pharm. Bull. 2004, 52, 1269–1272. [Google Scholar] [CrossRef]
- Köse, L.P.; Gulcin, I. Inhibition effects of some lignans on carbonic anhydrase, acetylcholinesterase and butyrylcholinesterase enzymes. Rec. Nat. Prod. 2017, 11, 558–561. [Google Scholar] [CrossRef]
- Koay, Y.-H.; Basiri, A.; Murugaiyah, V.; Chan, K.-L. Isocorilagin, a cholinesterase inhibitor from Phyllanthus niruri. Nat. Prod. Commun. 2014, 9, 515–517. [Google Scholar] [CrossRef] [PubMed]
- Youssef, F.S.; Ashour, M.L.; El-Beshbishy, H.A.; Ahmed Hamza, A.; Singab, A.N.B.; Wink, M. Pinoresinol-4-O-β-D-glucopyranoside: A lignan from prunes (Prunus domestica) attenuates oxidative stress, hyperglycaemia and hepatic toxicity in vitro and in vivo. J. Pharm. Pharmacol. 2020, 72, 1830–1839. [Google Scholar] [CrossRef] [PubMed]
- Amodio, G.; Margarucci, L.; Moltedo, O.; Casapullo, A.; Remondelli, P. Identification of cysteine ubiquitylation sites on the Sec23A protein of the COPII complex required for vesicle formation from the ER. Open Biochem. J. 2017, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Bellone, M.L.; Camero, C.M.; Chini, M.G.; Dal Piaz, F.; Hernandez, V.; Bifulco, G.; De Tommasi, N.; Braca, A. Limonoids from Guarea guidonia and Cedrela odorata: Heat Shock Protein 90 (Hsp90) Modulator Properties of Chisomicine D. J. Nat. Prod. 2021, 84, 724–737. [Google Scholar] [CrossRef]
- Šic Žlabur, J.; Brajer, M.; Voća, S.; Galić, A.; Radman, S.; Rimac-Brnčić, S.; Xia, Q.; Zhu, Z.; Grimi, N.; Barba, F.J. Ultrasound as a promising tool for the green extraction of specialized metabolites from some culinary spices. Molecules 2021, 26, 1866. [Google Scholar] [CrossRef]
- Strzemski, M.; Dresler, S.; Sowa, I.; Czubacka, A.; Agacka-Mołdoch, M.; Płachno, B.J.; Granica, S.; Feldo, M.; Wójciak-Kosior, M. The impact of different cultivation systems on the content of selected secondary metabolites and antioxidant activity of Carlina acaulis plant material. Molecules 2019, 25, 146. [Google Scholar] [CrossRef]
- Chemsa, A.E.; Erol, E.; Öztürk, M.; Zellagui, A.; Özgür, C.; Gherraf, N.; Duru, M.E. Chemical constituents of essential oil of endemic Rhanterium suaveolens Desf. growing in Algerian Sahara with antibiofilm, antioxidant and anticholinesterase activities. Nat. Prod. Res. 2016, 30, 2120–2124. [Google Scholar] [CrossRef]
- De Boer, D.; Nguyen, N.; Mao, J.; Moore, J.; Sorin, E.J. A comprehensive review of cholinesterase modeling and simulation. Biomolecules 2021, 11, 580. [Google Scholar] [CrossRef]
- Walsh, R.; Rockwood, K.; Martin, E.; Darvesh, S. Synergistic inhibition of butyrylcholinesterase by galantamine and citalopram. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2011, 1810, 1230–1235. [Google Scholar] [CrossRef]
- Chatterjee, S.; Mudher, A. Alzheimer’s disease and type 2 diabetes: A critical assessment of the shared pathological traits. Front. Neurosci. 2018, 12, 383. [Google Scholar] [CrossRef]
- Oboh, G.; Agunloye, O.M.; Adefegha, S.A.; Akinyemi, A.J.; Ademiluyi, A.O. Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 diabetes (in vitro): A comparative study. J. Basic. Clin. Physiol. Pharmacol. 2015, 26, 165–170. [Google Scholar] [CrossRef]
- Tamfu, A.N.; Kucukaydin, S.; Yeskaliyeva, B.; Ozturk, M.; Dinica, R.M. Non-alkaloid cholinesterase inhibitory compounds from natural sources. Molecules 2021, 26, 5582. [Google Scholar] [CrossRef]
- De Leo, M.; Iannuzzi, A.M.; Germanò, M.P.; D’Angelo, V.; Camangi, F.; Sevi, F.; Diretto, G.; De Tommasi, N.; Braca, A. Comparative chemical analysis of six ancient italian sweet cherry (Prunus avium L.) varieties showing antiangiogenic activity. Food Chem. 2021, 360, 129999. [Google Scholar] [CrossRef]
- Faraone, I.; Russo, D.; Genovese, S.; Milella, L.; Monné, M.; Epifano, F.; Fiorito, S. Screening of in vitro and in silico α-amylase, α-glucosidase, and lipase inhibitory activity of oxyprenylated natural compounds and semisynthetic derivatives. Phytochemistry 2021, 187, 112781. [Google Scholar] [CrossRef]
- Labanca, F.; Faraone, I.; Nolè, M.R.; Hornedo-Ortega, R.; Russo, D.; García-Parrilla, M.C.; Chiummiento, L.; Bonomo, M.G.; Milella, L. New Insights into the Exploitation of Vitis vinifera L. cv. Aglianico Leaf Extracts for Nutraceutical Purposes. Antioxidants 2020, 9, 708. [Google Scholar] [CrossRef]
- Boudermine, S.; Parisi, V.; Lemoui, R.; Boudiar, T.; Chini, M.G.; Franceschelli, S.; Pecoraro, M.; Pascale, M.; Bifulco, G.; Braca, A. Cytotoxic Sesquiterpenoids from Ammoides atlantica Aerial Parts. J. Nat. Prod. 2022, 85, 647–656. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Li, C.; Begum, A.; Numao, S.; Park, K.H.; Withers, S.G.; Brayer, G.D. Acarbose rearrangement mechanism implied by the kinetic and structural analysis of human pancreatic α-amylase in complex with analogues and their elongated counterparts. Biochemistry 2005, 44, 3347–3357. [Google Scholar] [CrossRef]
- Ren, L.; Qin, X.; Cao, X.; Wang, L.; Bai, F.; Bai, G.; Shen, Y. Structural insight into substrate specificity of human intestinal maltase-glucoamylase. Protein Cell 2011, 2, 827–836. [Google Scholar] [CrossRef]
- Faraone, I.; Russo, D.; Chiummiento, L.; Fernandez, E.; Choudhary, A.; Monné, M.; Milella, L.; Rai, D.K. Phytochemicals of Minthostachys diffusa Epling and their health-promoting bioactivities. Foods 2020, 9, 144. [Google Scholar] [CrossRef] [PubMed]
- Krissinel, E.; Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 2007, 372, 774–797. [Google Scholar] [CrossRef] [PubMed]


| Position | 1 a | HMBC | |
|---|---|---|---|
| δH | δC | ||
| 1 | 133.0 | ||
| 2 | 6.82 d (1.5) | 111.1 | 3, 4, 6, |
| 3 | 149.0 | ||
| 4 | 147.6 | ||
| 5 | 6.74 d (8.0) | 116.3 | 1, 3 |
| 6 | 6.69 dd (8.0, 1.8) | 122.1 | 2, 7 |
| 7a | 2.95 dd (12.5, 5.4) | 34.3 | 1, 2, 6, 8, 8′ |
| 7b | 2.64 dd (12.5, 10.3) | - | 1, 2, 6, 8, 8′ |
| 8 | 2.90 m | 44.3 | |
| 9a | 4.10 dd (8.8, 6.5) | 72.5 | 7, 8, 7′, 8′ |
| 9b | 3.80 ° | 7, 8, 7′, 8′ | |
| -OMe | 3.84 s | 56.3 | 3 |
| 1′ | 134.9 | ||
| 2′ | 6.94 (2.0) | 111.2 | 1′, 4′, 6′, 7′ |
| 3′ | 149.0 | ||
| 4′ | 147.5 | ||
| 5′ | 6.77 d (8.0) | 116.1 | 1′, 3′ |
| 6′ | 6.85 dd (8.0, 2.0) | 120.3 | 2′, 4′, 7′ |
| 7′ | 4.87 ° | 85.4 | 1′, 2′, 6′, 9′ |
| 8′ | 2.78 m | 50.5 | 7′, 8, 9′ |
| 9a’ | 4.67 dd (11.0, 6.2) | 64.6 | 7′, 8, 8′, 7″ |
| 9b’ | 4.47 dd (11.0, 8.0) | 7′, 8, 8′, 7″ | |
| 1″ | 134.9 | ||
| 2″ | 7.82 m | 130.5 | 1″, 6″, 7″ |
| 3″ | 7.44 t (8.0) | 129.5 | 1″, 5″ |
| 4″ | 7.60 br t (8.0) | 134.2 | |
| 5″ | 7.44 t (8.0) | 129.5 | 1″, 5″ |
| 6″ | 7.82 m | 130.5 | 1″, 6″, 7″ |
| 7″ | 167.8 | ||
| -OMe | 3.78 s | 56.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belaabed, S.; Khalfaoui, A.; Parisi, V.; Santoro, V.; Russo, D.; Ponticelli, M.; Monné, M.; Rebbas, K.; Milella, L.; Donadio, G. Rhanteriol, a New Rhanterium suaveolens Desf. Lignan with Pharmacological Potential as an Inhibitor of Enzymes Involved in Neurodegeneration and Type 2 Diabetes. Plants 2023, 12, 301. https://doi.org/10.3390/plants12020301
Belaabed S, Khalfaoui A, Parisi V, Santoro V, Russo D, Ponticelli M, Monné M, Rebbas K, Milella L, Donadio G. Rhanteriol, a New Rhanterium suaveolens Desf. Lignan with Pharmacological Potential as an Inhibitor of Enzymes Involved in Neurodegeneration and Type 2 Diabetes. Plants. 2023; 12(2):301. https://doi.org/10.3390/plants12020301
Chicago/Turabian StyleBelaabed, Soumia, Ayoub Khalfaoui, Valentina Parisi, Valentina Santoro, Daniela Russo, Maria Ponticelli, Magnus Monné, Khellaf Rebbas, Luigi Milella, and Giuliana Donadio. 2023. "Rhanteriol, a New Rhanterium suaveolens Desf. Lignan with Pharmacological Potential as an Inhibitor of Enzymes Involved in Neurodegeneration and Type 2 Diabetes" Plants 12, no. 2: 301. https://doi.org/10.3390/plants12020301
APA StyleBelaabed, S., Khalfaoui, A., Parisi, V., Santoro, V., Russo, D., Ponticelli, M., Monné, M., Rebbas, K., Milella, L., & Donadio, G. (2023). Rhanteriol, a New Rhanterium suaveolens Desf. Lignan with Pharmacological Potential as an Inhibitor of Enzymes Involved in Neurodegeneration and Type 2 Diabetes. Plants, 12(2), 301. https://doi.org/10.3390/plants12020301








