Volatile Metabolome and Aroma Differences of Six Cultivars of Prunus mume Blossoms
Abstract
1. Introduction
2. Results and Discussion
2.1. Analysis of P. mume Blossom Volatile Metabolites by HS-SPME-GC-MS
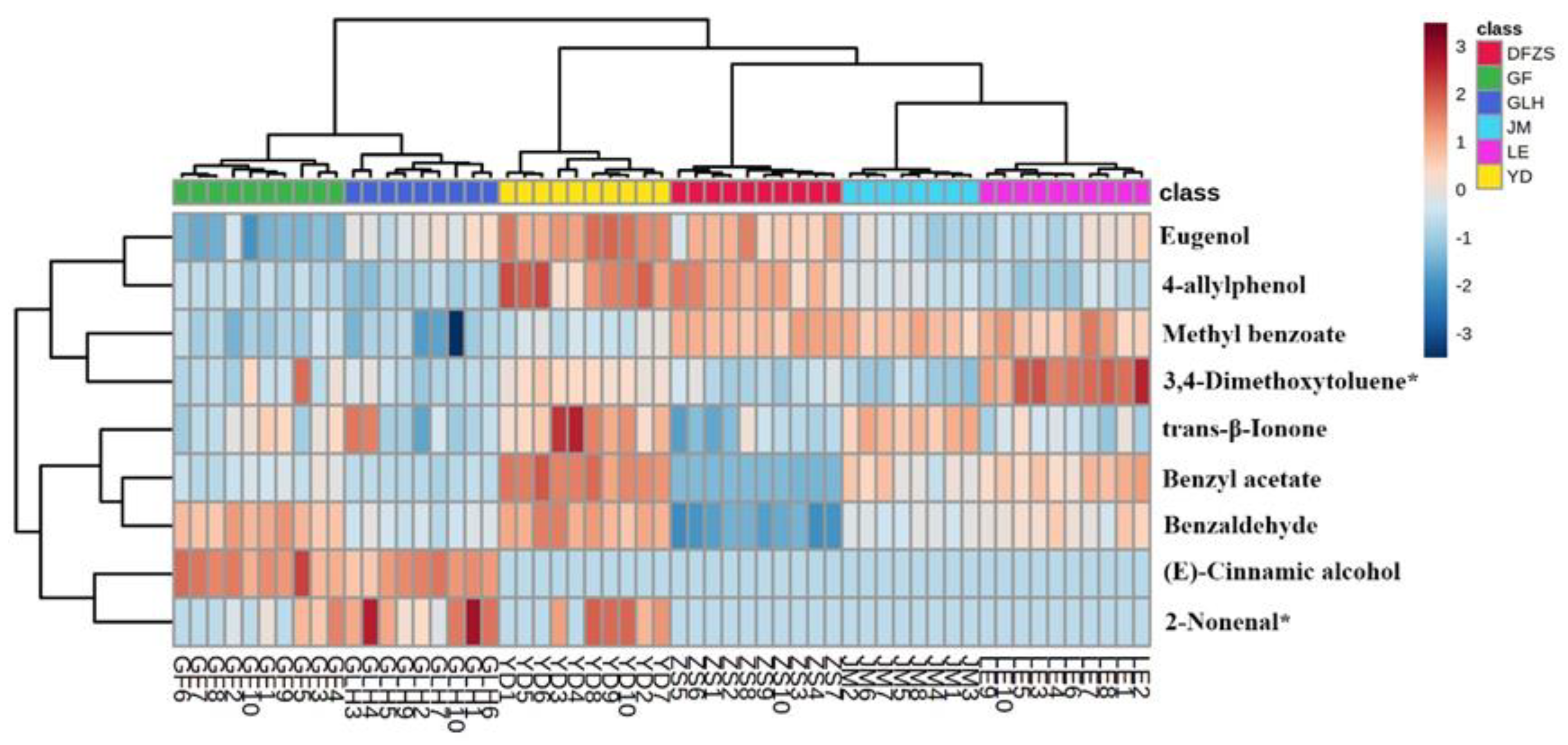
2.2. Analysis of Volatile Metabolite Differences in Six Cultivars of P. mume Blossoms
2.3. Analysis of Aroma Differences of Six Cultivars of P. mume Blossoms
2.4. Correlation between the Differential Compounds and Aroma Characteristics
3. Materials and Methods
3.1. Plant Materials
3.2. Chemicals
3.3. HS-SPME-GC-MS Analysis Method
3.4. Data Processing and Chemometric Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, J. China Mei Flower (Prunus mume) Cultivars in Colour; China Forestry Publishing House: Beijing, China, 2010; pp. 1–8. [Google Scholar]
- Chen, J. The New Revised System of Classification for Chinese Mei Cultivars. J. Beijing For. Univ. 1999, 21, 2–7. [Google Scholar] [CrossRef]
- Lin, X.; Chen, L. Adavances in Genetic Engineering of Floral Scent. Sci. Agric. Sin. 2009, 42, 2076–2084. [Google Scholar] [CrossRef]
- Zhao, Y.; Pan, H.; Zhang, Q.; Sun, M.; Pan, C. Study on the volatile components of different types of Prunus mume varieties. J. Trop. Subtrop. Bot. 2010, 18, 310–315. [Google Scholar]
- Hao, R.-J.; Zhang, Q.; Yang, W.-R.; Wang, J.; Cheng, T.-R.; Pan, H.-T.; Zhang, Q.-X. Emitted and endogenous floral scent compounds of Prunus mume and hybrids. Biochem. Syst. Ecol. 2014, 54, 23–30. [Google Scholar] [CrossRef]
- Zhang, T.; Bao, F.; Hu, L.; Ding, A.; Wang, J.; Cheng, T.; Zhang, Q. A Comparative Analysis of Floral Scent Compounds in Intraspecific Cultivars of Prunus mume with Different Corolla Colours. Molecules 2020, 25, 145. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, Y.; Zhu, H.; Zhang, H.; Xu, J.; Fu, Q.; Bao, M.; Zhang, J. Headspace Volatiles and Endogenous Extracts of Prunus mume Cultivars with Different Aroma Types. Molecules 2021, 26, 7256. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Jia, W.; Wang, Z.; Miao, C. Hangzhou Huading Intellectual Property Agency, Assignee. China Patent CN102660384A, 12 September 2012. [Google Scholar]
- Cao, H.; Li, Z.; Shen, D. Analysis and QSRR study of aroma components of two plum species. J. Anal. Sci. 2009, 25, 130–134. [Google Scholar]
- Zhang, W.; Cao, J.; Li, Q.; Lai, X.; Sun, L.; Chen, R.; Wen, S.; Sun, S.; Lai, Z. HS-SPME and GC/MS volatile component analysis of Yinghong No. 9 dark tea during the pile fermentation process. Food Chem. 2021, 357, 129654. [Google Scholar] [CrossRef] [PubMed]
- NIST Chemistry WebBook. Available online: https://webbook.nist.gov/chemistry/ (accessed on 6 April 2022).
- Klempien, A.; Kaminaga, Y.; Nagegowda, D.A.; Dudareva, N. Contribution of CoA Ligases to Benzenoid Biosynthesis in Petunia Flowers. Plant Cell 2012, 24, 2015–2030. [Google Scholar] [CrossRef] [PubMed]
- Gonda, I.; Davidovich-Rikanati, R.; Bar, E.; Lev, S.; Jhirad, P.; Meshulam, Y.; Wissotsky, G.; Portnoy, V.; Burger, J.; Lewinsohn, E. Differential metabolism of Lephenylalanine in the formation of aromatic volatiles in melon (Cucumis melo L.) fruit. Phytochemistry 2018, 148, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Pichersky, E.; Gershenzon, J. Biochemistry of Plant Volatiles. Plant Physiol. 2004, 135, 1893–1902. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; D’Auria, J.C.; Nam, K.H.; Raguso, R.A.; Pichersky, E. Acetyl-CoA: Benzylalcohol acetyltransferase--an enzyme involved in floral scent production in Clarkia breweri. Plant J. 1998, 14, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Bao, F.; Ding, A.; Zhang, T.; Luo, L.; Wang, J.; Cheng, T.; Zhang, Q. Expansion of PmBEAT genes in the Prunus mume genome induces characteristic floral scent production. Hortic. Res. 2019, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Jayaramaiah, R.H.; Beedkar, S.D.; Singh, P.A.; Joshi, R.S.; Mulani, F.A.; Dholakia, B.B.; Punekar, S.A.; Gade, W.N.; Thulasiram, H.V.; et al. Comparative functional characterization of eugenol synthase from four different Ocimum species: Implications on eugenol accumulation. Biochim. Biophys. Acta 2016, 1864, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Greger, V.; Schieberle, P. Characterization of the key aroma compounds in apricots (Prunus armeniaca) by application of the molecular sensory science concept. J. Agric. Food Chem. 2007, 55, 5221–5228. [Google Scholar] [CrossRef] [PubMed]
- Steinhaus, M.; Sinuco, D.; Polster, J.; Osorio, C.; Schieberle, P. Characterization of the key aroma compounds in pink guava (Psidium guajava L.) by means of aroma re-engineering experiments and omission tests. J. Agric. Food Chem. 2009, 57, 2882–2888. [Google Scholar] [CrossRef] [PubMed]
- Dein, M.; Kerley, T.; Munafo, J.P., Jr. Characterization of Odorants in a 10-Year-Old Riesling Wine. J. Agric. Food Chem. 2021, 69, 11372–11381. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Niu, Y.; Xiao, Z. Characterization of the key aroma compounds in Laoshan green teas by application of odour activity value (OAV), gas chromatography-mass spectrometry-olfactometry (GC-MS-O) and comprehensive two-dimensional gas chromatography mass spectrometry (GC × GC-qMS). Food Chem. 2021, 339, 128136. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-X.; Li, W.-D.; Wang, Y.; Zhong, K.; Zhao, L.; Gao, H.-Y. Characterization of Volatile Compounds in Ten Different Instant Noodle Seasonings by Gas Chromatography–Mass Spectrometry and Odor Activity Values. Chin. J. Anal. Chem. 2021, 49, e21104–e21111. [Google Scholar] [CrossRef]
- Van Gemert, L.J. Compilations of Flavour Threshold Values in Water and Other Media, 2nd ed.; Oliemans Punter & Partners BV: Zeist, The Netherlands, 2011; pp. 215–470. [Google Scholar]
- Ma, L. Study on Aroma Compounds of Zhizhonghe Wujiapi Medicinal Liquor. Master’s Thesis, Shanghai Institute of Technology, Shanghai, China, 27 May 2021. [Google Scholar]
- Paparella, A.; Shaltiel-Harpaza, L.; Ibdah, M. β-Ionone: Its Occurrence and Biological Function and Metabolic Engineering. Plants 2021, 10, 754. [Google Scholar] [CrossRef] [PubMed]
- Perflavory Information System. Available online: www.perflavory.com (accessed on 6 April 2022).
- Zhu, J. Study on the Synergism of Aroma Compounds in Flowers and Fruits. Ph.D. Thesis, Jiangnan University, Wuxi, China, 20 May 2019. [Google Scholar]
- MetaboAnalyst 5.0. Available online: https://www.metaboanalyst.ca/faces/home.xhtml (accessed on 6 April 2022).
- Chen, L.N. Study on Synergistic Effect of Pineapple Aroma. Master’s Thesis, Shanghai Institute of Technology, Shanghai, China, 8 June 2021. [Google Scholar]
- Liu, J.B.; Liu, M.Y.; He, C.C.; Song, H.L.; Wang, Y.; Guo, J. Application of AEDA combined with OAV value calculation for identification of key odor active compounds in cocoa liquor. Food Ferment. Ind. 2013, 39, 180–184. [Google Scholar]
- Chen, J.Y. The Flavour Analysis of Two Natural Products (Fennel Essential Oil and Orange). Master’s Thesis, Shanghai Institute of Technology, Shanghai, China, 25 May 2018. [Google Scholar]
- Zhang, N.; Peng, Y.L.; Chen, X.Y.; Jiao, B.N.; Zhang, Y.H. Analysis on the difference of founctional components of Guanxi pomelo fruits during maturity. J. Fruit Sci. 2022, 39, 1042–1053. [Google Scholar]
- Li, X.R. Changes of Physiologically Active Compounds and Flavor Evolution of Aronia melanocarpa (Michx.) Elliott Wine during Aging. Master’s Thesis, Jiangnan University, Jiangxi, China, 17 June 2021. [Google Scholar]
- Miao, H.L.; Weng, X.C. Preliminary Analysis of Volatile Compounds of Bay Leaves. Nat. Sci. 2009, 15, 326–330. [Google Scholar]
- Liu, H., Q.; Tan, Y.Y.; Huang, J.L.; Tang, X.Y.; Wu, J.W.; Li, Q.T. Aroma Analysis of Star Anise Seeds Oil by Different Extraction Methods. China Condiment. 2022, 47, 146–159. [Google Scholar]
- Jin, Y.L.; Huang, T.; Jiang, R.G.; Huang, F.F.; Liu, Z.H.; Huang, J.N.; Li, Q. Characteristic volatile components of different types of fermented brick tea. Food Ferment. Ind. 2021, 47, 188–196. [Google Scholar]
- Wang, Z.L. Analysis and Formation Mechanism of Flavor Substances in Yeast Products. Master’s Thesis, Shanghai Institute of Technology, Shanghai, China, 27 May 2021. [Google Scholar]
- Wu, J.D.; Niu, J.M.; Zhang, B.; Yang, B.; Zhang, Y.; Wang, Y.J.; Han, S.Y. Extraction process optimization of volatile components and aroma profile analysis in Quercus liaotungensis Koidz and Q. mongolica Fisch. Jiangsu J. Agri. Sci. 2020, 36, 1559–1568. [Google Scholar]
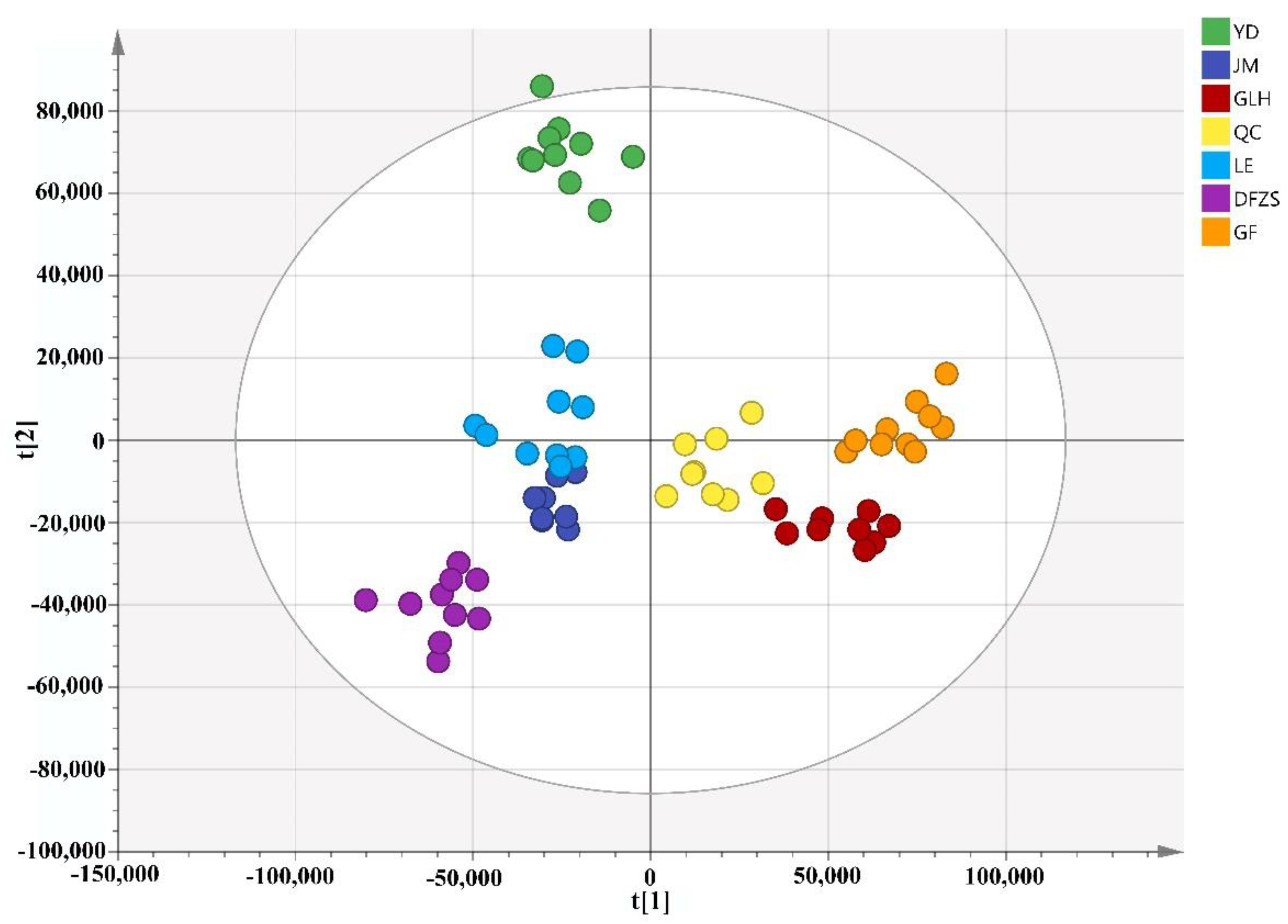
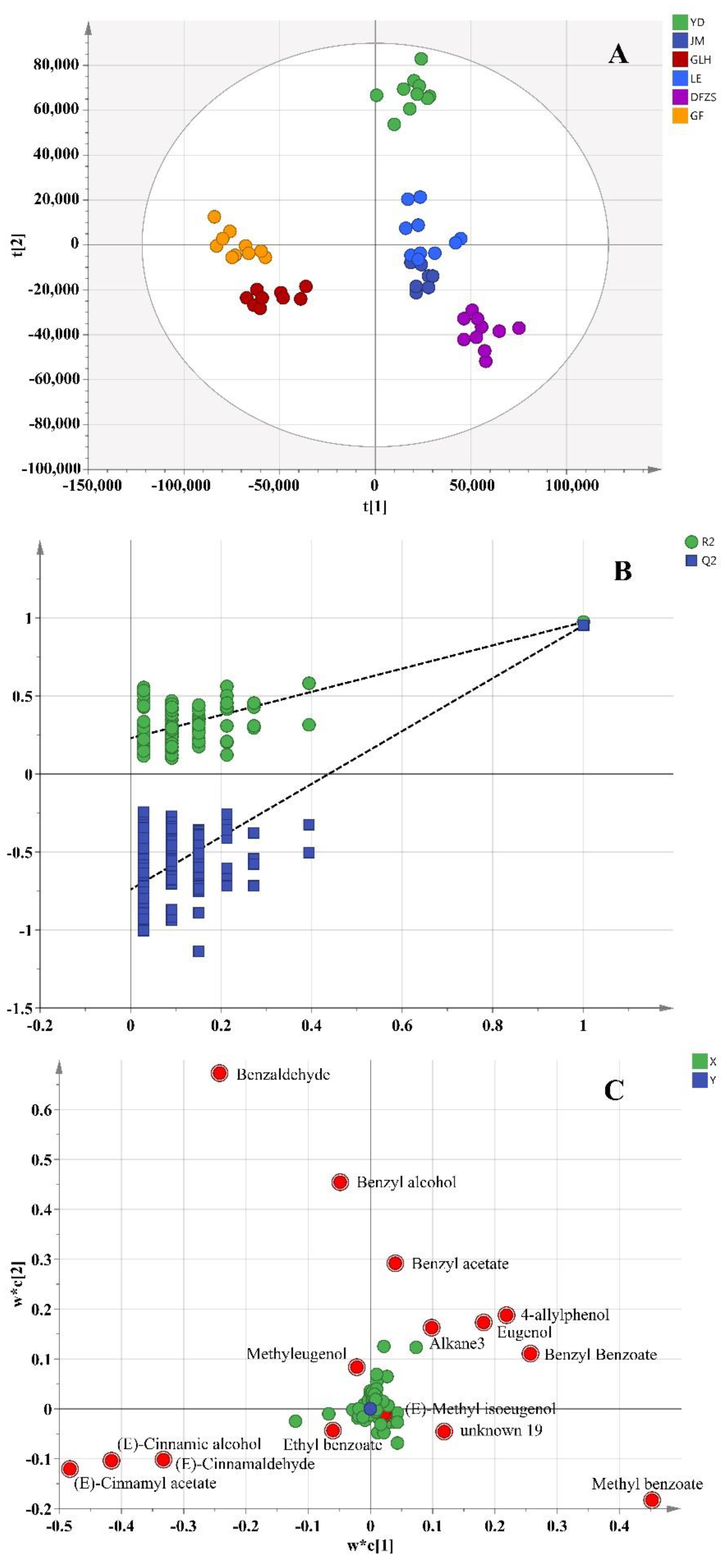
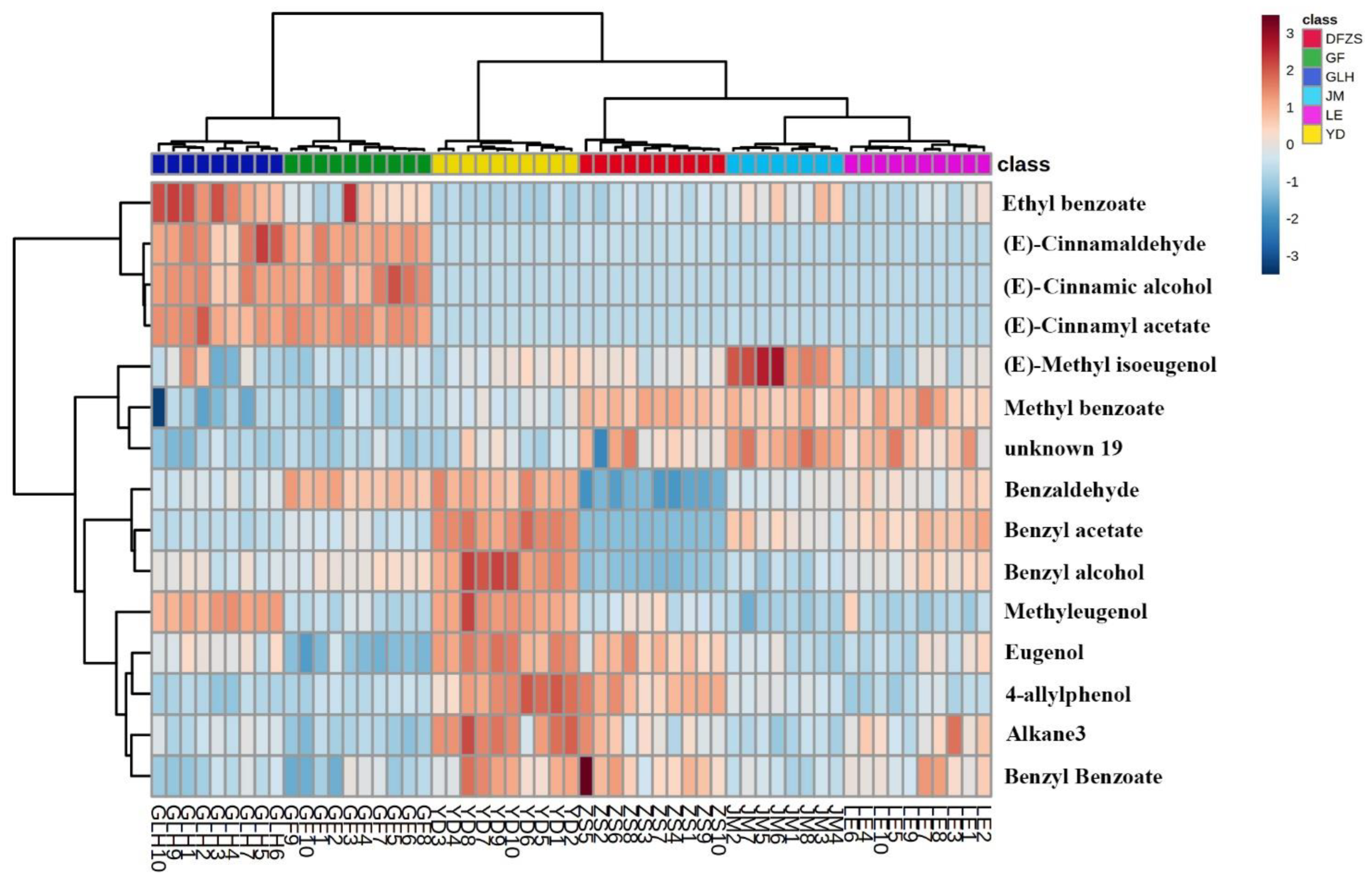
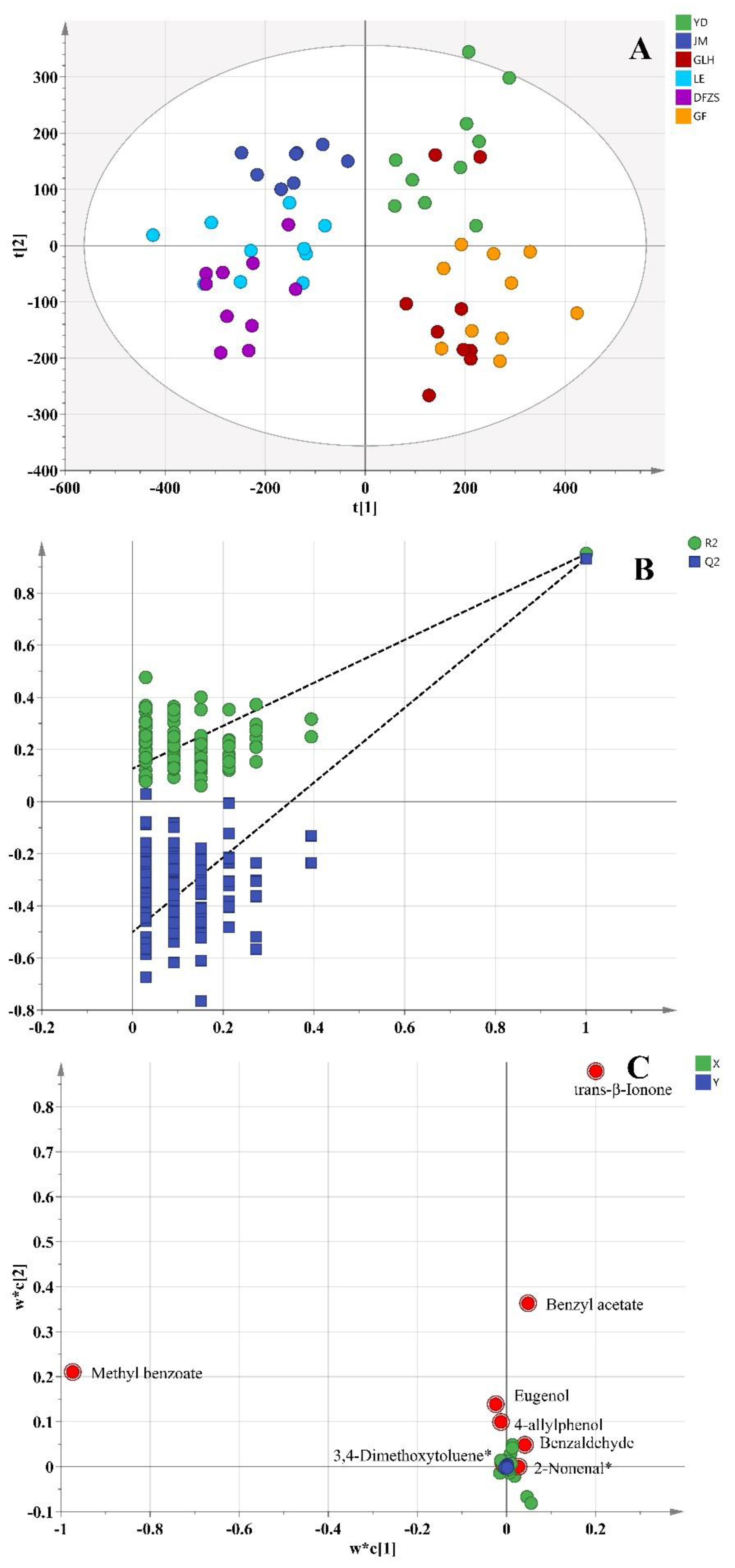

| No. | Compound Name | CAS Number | Measured RI ** | Reference RI | Identification |
|---|---|---|---|---|---|
| Phenylpropanols/Benzenes | |||||
| 1 | o-Xylene | 108-38-3 | 1158.18 ± 0.59 | 1158 | MS, RI |
| 2 | Styrene | 100-42-5 | 1269.78 ± 0.32 | 1263 | MS, RI |
| 3 | Benzaldehyde | 100-52-7 | 1536.47 ± 1.03 | 1529 | MS, RI, Std |
| 4 | Methyl benzoate | 93-58-3 | 1649.52 ± 1.06 | 1641 | MS, RI, Std |
| 5 | Ethyl benzoate | 93-89-0 | 1691.64 ± 0.61 | 1681 | MS, RI, Std |
| 6 | Benzyl formate | 104-57-4 | 1700.61 ± 1.34 | 1705 | MS, RI |
| 7 | 4-Allylanisole | 140-67-0 | 1700.61 ± 1.34 | 1687 | MS, RI |
| 8 | Benzyl acetate | 140-11-4 | 1747.71 ± 0.45 | 1746 | MS, RI, Std |
| 9 | Methyl salicylate | 119-36-8 | 1802.31 ± 0.52 | 1798 | MS, RI |
| 10 | 3,4-Dimethoxytoluene * | 494-99-5 | 1828.76 ± 0.42 | - | MS |
| 11 | Benzyl alcohol | 100-51-6 | 1886.60 ± 0.78 | 1878 | MS, RI, Std |
| 12 | Butyl benzoate | 136-60-7 | 1901.17 ± 1.62 | 1882 | MS, RI |
| 13 | (Z)-Cinnamaldehyde | 57194-69-1 | 1912.02 ± 0.42 | 1884 | MS |
| 14 | Phenylethyl Alcohol | 60-12-8 | 1915.72 ± 0.31 | 1931 | MS, RI |
| 15 | Isoamyl benzoate | 94-46-2 | 1944.08 ± 1.33 | 1937 | MS, RI |
| 16 | Phenylpropyl acetate | 122-72-5 | 1957.25 ± 0.48 | 1965 | MS, RI |
| 17 | Creosol | 93-51-6 | 1957.36 ± 0.89 | 1952 | MS, RI |
| 18 | Pentyl benzoate | 2049-96-9 | 2004.46 ± 0.22 | 2017 | MS, RI |
| 19 | Methyleugenol | 93-15-2 | 2032.82 ± 0.54 | 2030 | MS, RI, Std |
| 20 | (E)-Cinnamaldehyde | 14371-10-9 | 2060.17 ± 0.59 | 2063 | MS, RI, Std |
| 21 | 3-Phenyl-1-propanol | 122-97-4 | 2060.17 ± 0.59 | 2049 | MS, RI |
| 22 | (E)-Methyl cinnamate | 1754-62-7 | 2099.75 ± 0.69 | 2096 | MS, RI |
| 23 | Cinnamyl formate * | 104-65-4 | 2122.36 ± 0.58 | 2094 | MS |
| 24 | Hexyl benzoate * | 6789-88-4 | 2122.36 ± 0.58 | 2096 | MS |
| 25 | 3-Buten-2-one, 4-phenyl- * | 1896-62-4 | 2137.34 ± 1.32 | 2103 | MS |
| 26 | 3-Hexen-1-ol, benzoate, (Z)- | 25152-85-6 | 2166.68 ± 0.67 | 2148 | MS, RI |
| 27 | (E)-Cinnamyl acetate | 21040-45-9 | 2175.61 ± 0.89 | 2182 | MS, RI, Std |
| 28 | Eugenol | 97-53-0 | 2180.34 ± 1.15 | 2186 | MS, RI, Std |
| 29 | (E)-Methyl isoeugenol | 6379-72-2 | 2207.29 ± 0.40 | 2209 | MS, RI, Std |
| 30 | cis-Isoeugenol | 5912-86-7 | 2285.01 ± 0.74 | 2288 | MS, RI |
| 31 | (E)-Cinnamic alcohol | 4407-36-7 | 2312.04 ± 1.41 | 2294 | MS, RI, Std |
| 32 | 4-allylphenol | 501-92-8 | 2371.78 ± 1.24 | 2358 | MS, RI, Std |
| 33 | trans-Isoeugenol | 5932-68-3 | 2394.44 ± 0.99 | 2383 | MS, RI, Std |
| 34 | Benzyl ether * | 103-50-4 | 2455.79 ± 1.57 | 2356 | MS |
| 35 | 5-Indanol * | 1470-94-6 | 2505.96 ± 1.62 | - | MS |
| 36 | 2-Allylphenol * | 1745-81-9 | 2560.28 ± 1.86 | 2132 | MS |
| 37 | Benzyl Benzoate | 120-51-4 | 2642.06 ± 1.70 | 2655 | MS, RI, Std |
| Fatty acid derivatives | |||||
| 38 | Acetic acid, methyl ester | 79-20-9 | 867.67 ± 0.51 | 856 | MS, RI |
| 39 | Hexanal | 66-25-1 | 1092.36 ± 1.21 | 1093 | MS, RI |
| 40 | 2-Hexenal, (E)- | 6728-26-3 | 1226.28 ± 0.32 | 1232 | MS, RI, Std |
| 41 | 1-Hexanol | 111-27-3 | 1358.07 ± 0.64 | 1360 | MS, RI |
| 42 | 3-Hexen-1-ol * | 544-12-7 | 1387.03 ± 0.43 | 1388 | MS, RI |
| 43 | 2-Hexen-1-ol(E) | 928-95-0 | 1409.33 ± 0.39 | 1415 | MS, RI |
| 44 | Nonanal | 124-19-6 | 1424.66 ± 0.50 | 1406 | MS, RI |
| 45 | Acetic acid | 64-19-7 | 1459.71 ± 1.78 | 1461 | MS, RI |
| 46 | 6-Hepten-1-ol, 2-methyl- * | 67133-86-2 | 1478.84 ± 0.66 | 1467 | MS, RI |
| 47 | 2-Nonenal * | 2463-53-8 | 1535.32 ± 0.85 | 1546 | MS, RI |
| 48 | 1-Nonanol | 143-08-8 | 1683.66 ± 1.47 | 1676 | MS, RI |
| 49 | 2,4-Decadienal * | 2363-88-4 | 1852.02 ± 0.73 | 1811 | MS |
| 50 | Alkane1 | 2036.25 ± 0.69 | MS | ||
| 51 | Nonanoic acid | 112-05-0 | 2197.06 ± 0.61 | 2194 | MS, RI |
| 52 | Alkane2 | 2145.80 ± 1.24 | MS | ||
| 53 | Alkane3 | 2265.75 ± 0.74 | MS | ||
| 54 | Alkane4 | 2520.36 ± 1.35 | MS | ||
| Terpenoids | |||||
| 55 | Fenchol * | 1632-73-1 | 1641.38 ± 1.43 | 1591 | MS |
| 56 | Bornyl acetate * | 76-49-3 | 1643.92 ± 0.91 | 1597 | MS |
| 57 | (E)-α-Elemene * | 5951-67-7 | 1675.75 ± 0.63 | 1695 | MS, RI |
| 58 | Borneol * | 507-70-0 | 1721.42 ± 0.52 | 1719 | MS, RI |
| 59 | Carvone * | 99-49-0 | 1769.73 ± 0.86 | 1751 | MS, RI |
| 60 | (Z,E)-α-Farnesene * | 26560-14-5 | 1793.17 ± 1.05 | 1737 | MS |
| 61 | α-Farnesene * | 502-61-4 | 1764.04 ± 0.99 | 1758 | MS, RI |
| 62 | Carveol * | 1197-07-5 | 1858.35 ± 1.28 | 1845 | MS, RI |
| 63 | trans-β-Ionone | 79-77-6 | 1971.82 ± 1.16 | 1958 | MS, RI |
| 64 | Dihydro-β-ionol | 3293-47-8 | 1991.87 ± 0.74 | 1977 | MS, RI |
| others | |||||
| 65 | Dimethyl sulfide | 75-18-3 | - | 753 | MS |
| Number | Compound Name | CAS-Number | VIP | Contents μg/g | |||||
|---|---|---|---|---|---|---|---|---|---|
| YD | JM | GLH | LE | DFZS | GF | ||||
| 1 | Benzaldehyde | 100-52-7 | 3.606 | 157.00 ± 9.21 | 112.00 ± 4.21 | 108.02 ± 6.00 | 126.76 ± 9.83 | 62.61 ± 7.60 | 149.91 ± 8.06 |
| 2 | Methyl benzoate | 93-58-3 | 3.085 | 172.36 ± 7.23 | 207.26 ± 6.85 | 166.90 ± 5.90 | 210.76 ± 11.67 | 212.20 ± 6.35 | 157.84 ± 8.82 |
| 3 | Ethyl benzoate | 93-89-0 | 1.002 | 0.28 ± 0.11 | 1.13 ± 0.38 | 2.38 ± 0.49 | 0.54 ± 0.27 | 0.46 ± 0.14 | 1.21 ± 0.79 |
| 4 | Benzyl acetate | 140-11-4 | 1.762 | 15.99 ± 1.21 | 8.69 ± 2.15 | 4.34 ± 0.38 | 11.29 ± 1.62 | 0.84 ± 0.15 | 5.80 ± 1.16 |
| 5 | Benzyl alcohol | 100-51-6 | 2.202 | 41.41 ± 5.73 | 13.81 ± 2.66 | 19.68 ± 3.19 | 23.67 ± 3.49 | 6.82 ± 1.33 | 22.65 ± 2.73 |
| 6 | Methyleugenol | 93-15-2 | 1.392 | 5.11 ± 0.57 | 1.53 ± 0.38 | 4.83 ± 0.30 | 1.97 ± 0.67 | 2.60 ± 0.58 | 2.06 ± 0.33 |
| 7 | (E)-Cinnamaldehyde | 14371-10-9 | 1.797 | 0.91 ± 0.30 | 0.26 ± 0.09 | 27.52 ± 7.41 | 0.29 ± 0.16 | 1.89 ± 0.31 | 25.09 ± 2.48 |
| 8 | (E)-Cinnamyl acetate | 21040-45-9 | 2.336 | 1.28 ± 0.48 | 1.48 ± 0.84 | 50.96 ± 8.52 | 0.34 ± 0.15 | 0.42 ± 0.08 | 52.22 ± 3.68 |
| 9 | Eugenol | 97-53-0 | 2.580 | 64.87 ± 3.39 | 43.03 ± 3.47 | 48.80 ± 3.25 | 45.79 ± 5.18 | 56.94 ± 5.06 | 34.62 ± 4.05 |
| 10 | (E)-Methyl isoeugenol | 6379-72-2 | 1.244 | 0.73 ± 0.16 | 1.51 ± 0.27 | 0.61 ± 0.37 | 0.56 ± 0.17 | 0.75 ± 0.11 | 0.49 ± 0.10 |
| 11 | Alkane3 | 1.442 | 14.10 ± 2.72 | 4.92 ± 0.86 | 6.30 ± 1.00 | 9.13 ± 2.75 | 8.86 ± 2.65 | 3.97 ± 0.99 | |
| 12 | (E)-Cinnamic alcohol | 4407-36-7 | 2.107 | 0.44 ± 0.08 | 0.18 ± 0.09 | 36.33 ± 6.25 | 0.11 ± 0.08 | 0.30 ± 0.07 | 40.00 ± 7.30 |
| 13 | 4-allylphenol | 501-92-8 | 3.265 | 40.04 ± 9.10 | 15.53 ± 1.98 | 7.52 ± 2.89 | 9.88 ± 3.79 | 34.19 ± 5.29 | 10.92 ± 2.08 |
| 14 | unknown 19 | 1.617 | 7.84 ± 1.82 | 14.20 ± 1.23 | 5.61 ± 1.13 | 11.89 ± 1.97 | 10.22 ± 3.71 | 6.32 ± 0.92 | |
| 15 | Benzyl Benzoate | 120-51-4 | 2.749 | 63.29 ± 11.21 | 40.94 ± 4.13 | 37.39 ± 5.58 | 54.74 ± 9.51 | 64.67 ± 16.78 | 35.52 ± 9.53 |
| No. | Compound Name | Olfactory Threshold/μg/g | VIP | OAV s | |||||
|---|---|---|---|---|---|---|---|---|---|
| YD | JM | GLH | LE | DFZS | GF | ||||
| 1 | Benzaldehyde | 0.2 a | 1.323 | 785.00 ± 46.04 | 560.00 ± 21.04 | 540.10 ± 30.02 | 633.82 ± 49.13 | 313.06 ± 37.98 | 749.56 ± 40.27 |
| 2 | 2-Nonenal * | 0.0005 a | 1.054 | 132.79 ± 112.21 | - | 198.28 ± 97.27 | - | - | 65.09 ± 78.30 |
| 3 | Methyl benzoate | 0.00052 a | 2.804 | 331,469.15 ± 13,902.30 | 398,572.02 ± 13,170.47 | 320,964.28 ± 11,343.53 | 405,298.09 ± 22,440.74 | 408,084.40 ± 12,208.62 | 303,542.79 ± 16,951.69 |
| 4 | Benzyl acetate | 0.002 a | 2.578 | 7993.82 ± 604.97 | 4345.63 ± 1073.17 | 2168.16 ± 191.01 | 5643.68 ± 812.39 | 418.15 ± 74.81 | 2901.00 ± 579.78 |
| 5 | 3,4-Dimethoxytoluene * | 0.053 a | 1.198 | 55.44 ± 5.31 | 15.88 ± 7.43 | 26.54 ± 9.28 | 102.29 ± 13.83 | 25.89 ± 9.85 | 37.98 ± 24.99 |
| 6 | Trans-β-Ionone | 0.000007 a | 2.502 | 64,831.31 ± 16,666.15 | 58,367.53 ± 4829.57 | 33,826.24 ± 22,943.26 | 30,862.43 ± 8876.74 | 21,560.30 ± 11,394.21 | 37,296.25 ± 11,507.64 |
| 7 | Eugenol | 0.007 a | 2.504 | 9267.71 ± 483.68 | 6147.39 ± 496.08 | 6971.31 ± 464.79 | 6540.78 ± 739.34 | 8133.62 ± 722.27 | 4946.27 ± 578.28 |
| 8 | (E)-Cinnamic alcohol | 0.077 a | 1.244 | 5.70 ± 1.06 | 2.34 ± 1.16 | 471.77 ± 81.23 | 1.39 ± 1.00 | 3.89 ± 0.89 | 519.48 ± 94.88 |
| 9 | 4-allylphenol | 0.019 b | 2.356 | 2107.63 ± 479.13 | 817.17 ± 104.23 | 395.70 ± 151.85 | 519.90 ± 199.47 | 1799.25 ± 278.14 | 574.55 ± 109.20 |
| No. | Compound Name | Olfactory Threshold/μg/g | VIP by | Aroma Descriptions c | GLH | GF | YD | DFZS | JM | LE | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Contents | OAVs | C | O | C | O | C | O | C | O | C | O | C | O | ||||
| 1 | Benzaldehyde | 0.2 a | 3.606 | 1.323 | Sharp, sweet, bitter, almond, cherry | ||||||||||||
| 2 | 2-Nonenal * | 0.0005 a | 1.054 | Fatty, green, waxy, cucumber, melon | |||||||||||||
| 3 | Methyl benzoate | 0.00052 a | 3.085 | 2.804 | Phenolic, wintergreen, almond, floral, cananga odorata | ||||||||||||
| 4 | Ethyl benzoate | 0.053 a | 1.002 | Fruit, musty, sweet, wintergreen | |||||||||||||
| 5 | Benzyl acetate | 0.002 a | 1.762 | 2.578 | Sweet, floral, fruit, Jasmine, fresh | ||||||||||||
| 6 | 3,4-Dimethoxytoluene * | 0.053 a | 1.198 | Fruit, musty, sweet, wintergreen | |||||||||||||
| 7 | Benzyl alcohol | 5.5 a | 2.202 | Floral, rose, phenolic, spice | |||||||||||||
| 8 | Trans-β-Ionone | 0.000007 a | 2.502 | Seaweed, violet, flower, raspberry | |||||||||||||
| 9 | Methyleugenol | 0.068 a | 1.392 | Sweet, fresh, warm, spicy, clove, cinnamon | |||||||||||||
| 10 | (E)-Cinnamaldehyde | 6 a | 1.797 | Sweet, spicy, candy, cinnamon, warm | |||||||||||||
| 11 | (E)-Cinnamyl acetate | 0.15 a | 2.336 | Sweet, floral, spicy, spice | |||||||||||||
| 12 | Eugenol | 0.007 a | 2.580 | 2.504 | Sweet, spicy, clove, woody | ||||||||||||
| 13 | (E)-Methyl isoeugenol | n.a. | 1.244 | n.a. | |||||||||||||
| 14 | Alkane3 | n.a. | 1.442 | n.a. | |||||||||||||
| 15 | (E)-Cinnamic alcohol | 0.077 a | 2.107 | 1.244 | Sweet, spice, hyacinth, spicy, cinnamon | ||||||||||||
| 16 | 4-allylphenol | 0.019 b | 3.265 | 2.356 | Phenolic, medicinal, herbal | ||||||||||||
| 17 | unknown 19 | n.a. | 1.617 | n.a. | |||||||||||||
| 18 | Benzyl Benzoate | 0.341 a | 2.749 | Sweet, spice, floral, fruit | |||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Zhao, X.; Cao, X. Volatile Metabolome and Aroma Differences of Six Cultivars of Prunus mume Blossoms. Plants 2023, 12, 308. https://doi.org/10.3390/plants12020308
Li T, Zhao X, Cao X. Volatile Metabolome and Aroma Differences of Six Cultivars of Prunus mume Blossoms. Plants. 2023; 12(2):308. https://doi.org/10.3390/plants12020308
Chicago/Turabian StyleLi, Ting, Xi Zhao, and Xueli Cao. 2023. "Volatile Metabolome and Aroma Differences of Six Cultivars of Prunus mume Blossoms" Plants 12, no. 2: 308. https://doi.org/10.3390/plants12020308
APA StyleLi, T., Zhao, X., & Cao, X. (2023). Volatile Metabolome and Aroma Differences of Six Cultivars of Prunus mume Blossoms. Plants, 12(2), 308. https://doi.org/10.3390/plants12020308








