Extracellular CahB1 from Sodalinema gerasimenkoae IPPAS B-353 Acts as a Functional Carboxysomal β-Carbonic Anhydrase in Synechocystis sp. PCC6803
Abstract
1. Introduction
2. Results
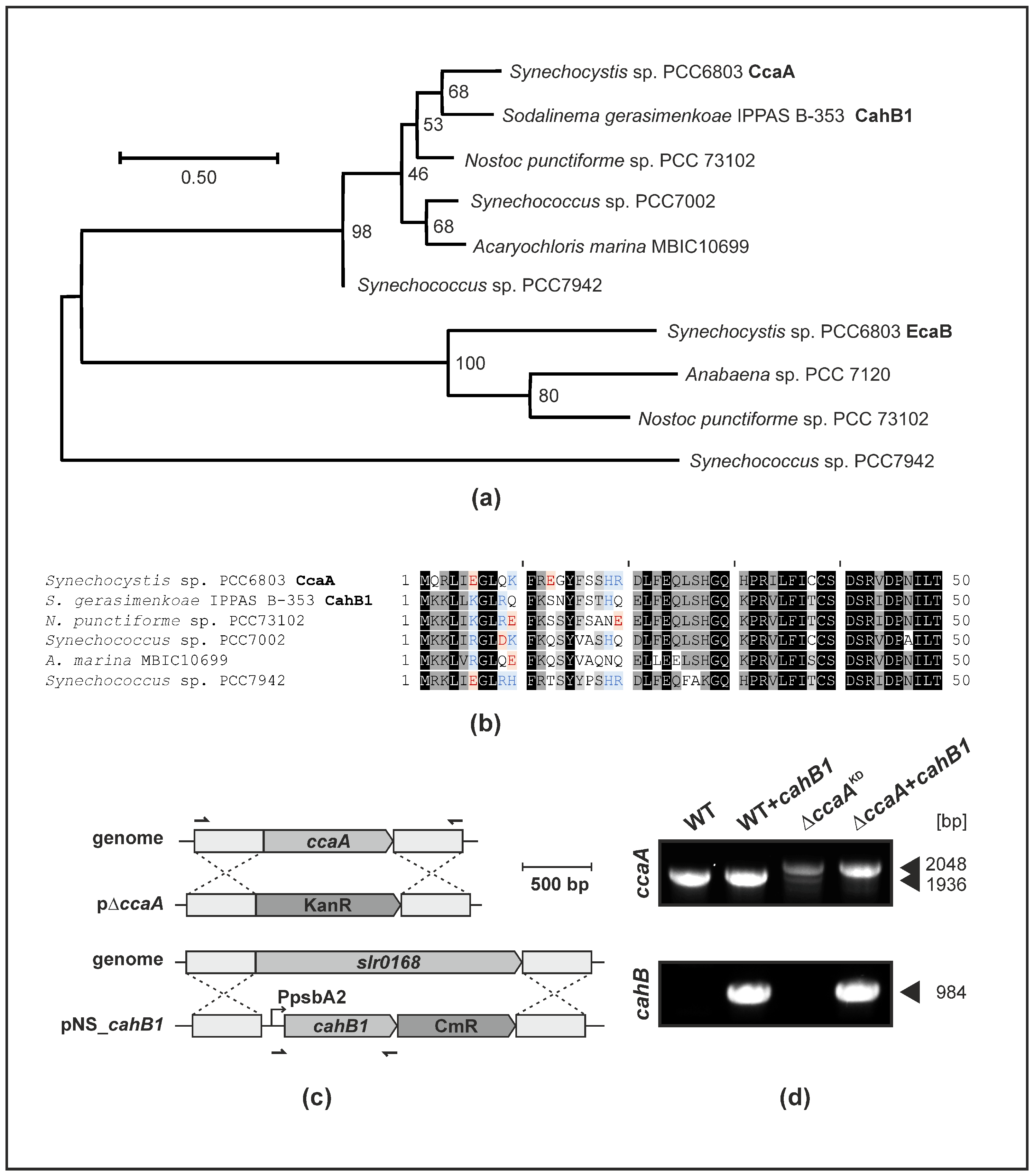
2.1. CahB1 Is Highly Homologous to Synechocystis CcaA
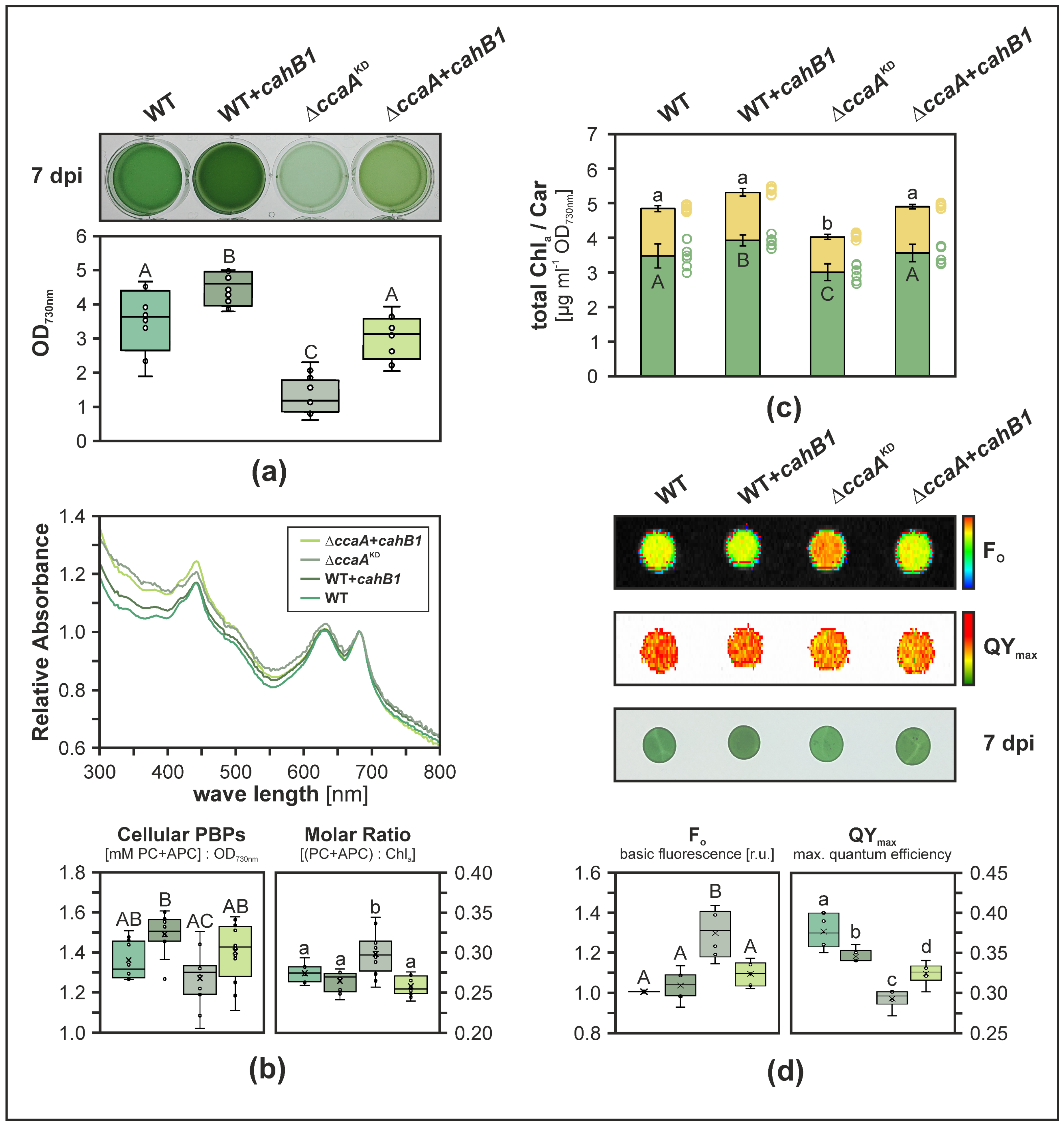
2.2. CahB1 Functionally Complements Loss of Synechocystis CcaA
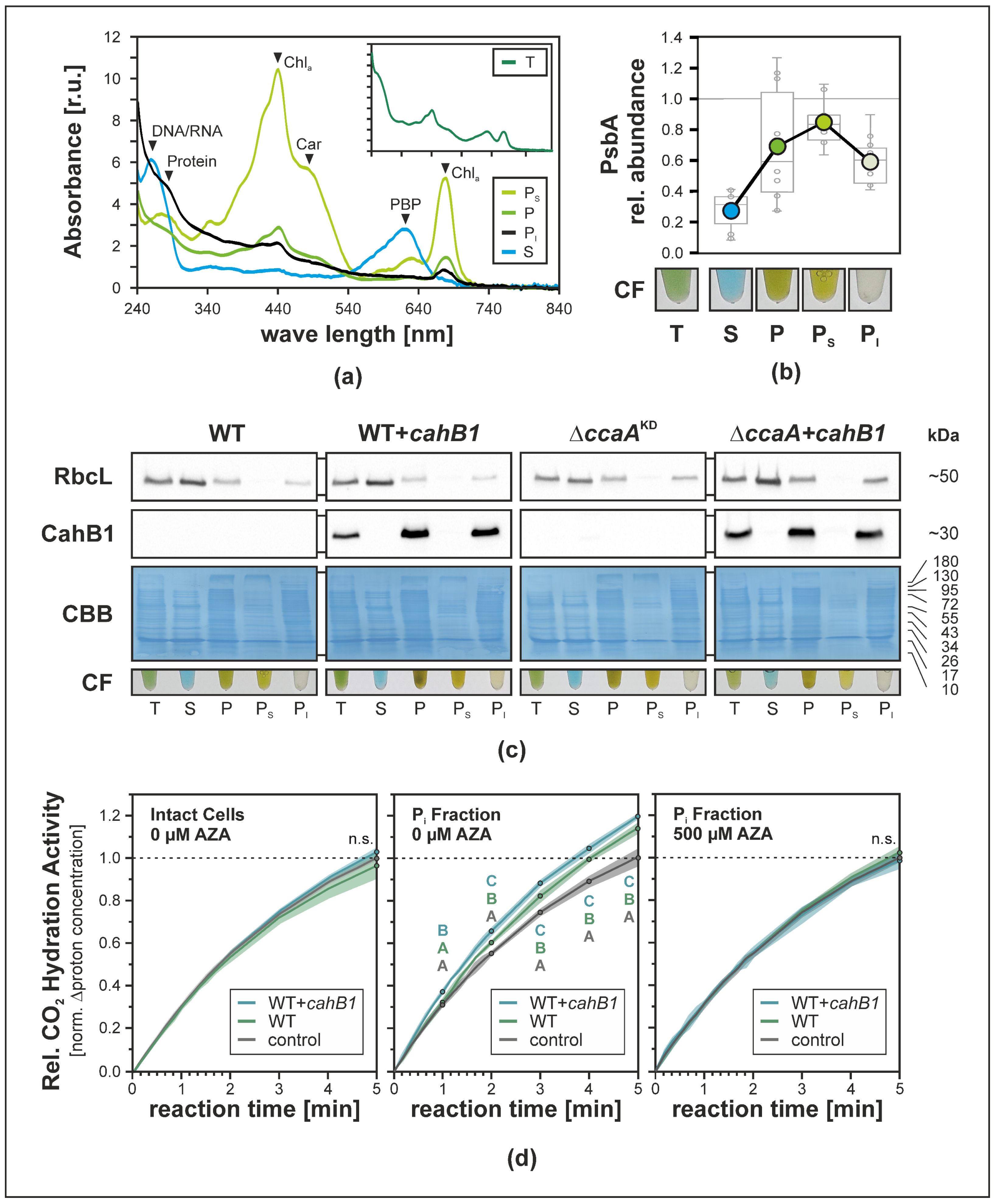
2.3. CahB1 Localizes to the Carboxysome in Synechocystis
2.4. The Sodalinema gerasimenkoae Genome Lacks Genes for Carboxysomal CAs Other Than CahB1
3. Discussion
4. Materials and Methods
- Taxonomy
- Sequence Analysis, Alignment, and Phylogenetic Reconstruction
- Synechocystis cultivation and mutant generation
- Synechocystis photosynthetic measurements
- Synechocystis pigment extraction and quantification
- Synechocystis cell fractionation
- Immunoblot analyses
- CO2 hydration assay
- Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hirakawa, Y.; Senda, M.; Fukuda, K.; Yu, H.Y.; Ishida, M.; Taira, M.; Kinbara, K.; Senda, T. Characterization of a novel type of carbonic anhydrase that acts without metal cofactors. BMC Biol. 2021, 19, 105. [Google Scholar] [CrossRef] [PubMed]
- Badger, M.R.; Price, G.D.; Long, B.M.; Woodger, F.J. The environmental plasticity and ecological genomics of the cyanobacterial CO2 concentrating mechanism. J. Exp. Bot. 2006, 57, 249–265. [Google Scholar] [CrossRef]
- Price, G.D.; Howitt, S.M. The cyanobacterial bicarbonate transporter BicA: Its physiological role and the implications of structural similarities with human SLC26 transporters. Biochem. Cell Biol. 2011, 89, 178–188. [Google Scholar] [CrossRef]
- Kupriyanova, E.; Pronina, N.; Los, D. Carbonic anhydrase—A universal enzyme of the carbon-based life. Photosynthetica 2017, 55, 3–19. [Google Scholar] [CrossRef]
- Wang, Y.; Stessman, D.J.; Spalding, M.H. The CO2 concentrating mechanism and photosynthetic carbon assimilation in limiting CO 2: How Chlamydomonas works against the gradient. Plant J. 2015, 82, 429–448. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.G.; Colman, B. Evidence for HCO3 Transport by the Blue-Green Alga (Cyanobacterium) Coccochloris peniocystis. Plant Physiol. 1980, 65, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Cot, S.S.; So, A.K.; Espie, G.S. A multiprotein bicarbonate dehydration complex essential to carboxysome function in cyanobacteria. J. Bacteriol. 2008, 190, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Pena, K.L.; Castel, S.E.; de Araujo, C.; Espie, G.S.; Kimber, M.S. Structural basis of the oxidative activation of the carboxysomal gamma-carbonic anhydrase, CcmM. Proc. Natl. Acad. Sci. USA 2010, 107, 2455–2460. [Google Scholar] [CrossRef] [PubMed]
- McGurn, L.D.; Moazami-Goudarzi, M.; White, S.A.; Suwal, T.; Brar, B.; Tang, J.Q.; Espie, G.S.; Kimber, M.S. The structure, kinetics and interactions of the β-carboxysomal β-carbonic anhydrase, CcaA. Biochem. J. 2016, 473, 4559–4572. [Google Scholar] [CrossRef]
- So, A.K.; Espie, G.S. Cloning, characterization and expression of carbonic anhydrase from the cyanobacterium Synechocystis PCC6803. Plant Mol. Biol. 1998, 37, 205–215. [Google Scholar] [CrossRef]
- So, A.K.C.; Van Spall, H.G.C.; Coleman, J.R.; Espie, G.S. Catalytic exchange of 18O from 13C18O-labelled CO2 by wild-type cells and ecaA, ecaB, and ccaA mutants of the cyanobacteria Synechococcus PCC7942 and Synechocystis PCC6803. Can. J. Bot. 1998, 76, 1153–1160. [Google Scholar] [CrossRef]
- McGinn, P.J.; Price, G.D.; Maleszka, R.; Badger, M.R. Inorganic carbon limitation and light control the expression of transcripts related to the CO2-concentrating mechanism in the cyanobacterium Synechocystis sp. strain PCC6803. Plant Physiol. 2003, 132, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-L.; Postier, B.L.; Burnap, R.L. Alterations in global patterns of gene expression in Synechocystis sp. PCC 6803 in response to inorganic carbon limitation and the inactivation of ndhR, a LysR family regulator. J. Biol. Chem. 2004, 279, 5739–5751. [Google Scholar] [CrossRef] [PubMed]
- Eisenhut, M.; Von Wobeser, E.A.; Jonas, L.; Schubert, H.; Ibelings, B.W.; Bauwe, H.; Matthijs, H.C.; Hagemann, M. Long-term response toward inorganic carbon limitation in wild type and glycolate turnover mutants of the cyanobacterium Synechocystis sp. strain PCC 6803. Plant Physiol. 2007, 144, 1946–1959. [Google Scholar] [CrossRef] [PubMed]
- Price, G.D.; Badger, M.R.; Woodger, F.J.; Long, B.M. Advances in understanding the cyanobacterial CO2-concentrating-mechanism (CCM): Functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. J. Exp. Bot. 2008, 59, 1441–1461. [Google Scholar] [CrossRef] [PubMed]
- So, A.K.; John-McKay, M.; Espie, G.S. Characterization of a mutant lacking carboxysomal carbonic anhydrase from the cyanobacterium Synechocystis PCC6803. Planta 2002, 214, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Sharp, C.E.; Ataeian, M.; Strous, M.; de Beer, D. Role of Extracellular Carbonic Anhydrase in Dissolved Inorganic Carbon Uptake in Alkaliphilic Phototrophic Biofilm. Front. Microbiol. 2018, 9, 2490. [Google Scholar] [CrossRef] [PubMed]
- Soltes-Rak, E.; Mulligan, M.E.; Coleman, J.R. Identification and characterization of a gene encoding a vertebrate-type carbonic anhydrase in cyanobacteria. J. Bacteriol. 1997, 179, 769–774. [Google Scholar] [CrossRef]
- Kupriyanova, E.V.; Sinetova, M.A.; Bedbenov, V.S.; Pronina, N.A.; Los, D.A. Putative extracellular alpha-class carbonic anhydrase, EcaA, of Synechococcus elongatus PCC 7942 is an active enzyme: A sequel to an old story. Microbiology 2018, 164, 576–586. [Google Scholar] [CrossRef]
- Kupriyanova, E.V.; Sinetova, M.A.; Mironov, K.S.; Novikova, G.V.; Dykman, L.A.; Rodionova, M.V.; Gabrielyan, D.A.; Los, D.A. Highly active extracellular alpha-class carbonic anhydrase of Cyanothece sp. ATCC 51142. Biochimie 2019, 160, 200–209. [Google Scholar] [CrossRef]
- Kupriyanova, E.V.; Sinetova, M.A.; Leusenko, A.V.; Voronkov, A.S.; Los, D.A. A leader peptide of the extracellular cyanobacterial carbonic anhydrase ensures the efficient secretion of recombinant proteins in Escherichia coli. J. Biotechnol. 2022, 344, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Huang, F.; Nilsson, F.; Hagemann, M.; Norling, B. Proteomics of Synechocystis sp. strain PCC 6803. Identification of periplasmic proteins in cells grown at low and high salt concentrations. Eur. J. Biochem. 2000, 267, 5900–5907. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Han, X.; Xu, M.; Kaplan, A.; Espie, G.S.; Mi, H. A thylakoid-located carbonic anhydrase regulates CO2 uptake in the cyanobacterium Synechocystis sp. PCC 6803. New Phytol. 2019, 222, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Kupriyanova, E.V.; Lebedeva, N.V.; Dudoladova, M.V.; Gerasimenko, L.M.; Alekseeva, S.G.; Pronina, N.A.; Zavarzin, G.A. Carbonic Anhydrase Activity of Alkalophilic Cyanobacteria from Soda Lakes. Russ. J. Plant Physiol. 2003, 50, 532–539. [Google Scholar] [CrossRef]
- Kupriyanova, E.V.; Markelova, A.G.; Lebedeva, N.V.; Gerasimenko, L.M.; Zavarzin, G.A.; Pronina, N.A. Carbonic Anhydrase of the Alkaliphilic Cyanobacterium Microcoleus chthonoplastes. Microbiology 2004, 73, 255–259. [Google Scholar] [CrossRef]
- Kupriyanova, E.V.; Sinetova, M.A.; Markelova, A.G.; Allakhverdiev, S.I.; Los, D.A.; Pronina, N.A. Extracellular beta-class carbonic anhydrase of the alkaliphilic cyanobacterium Microcoleus chthonoplastes. J. Photochem. Photobiol. B 2011, 103, 78–86. [Google Scholar] [CrossRef]
- Vullo, D.; Kupriyanova, E.V.; Scozzafava, A.; Capasso, C.; Supuran, C.T. Anion inhibition study of the β-carbonic anhydrase (CahB1) from the cyanobacterium Coleofasciculus chthonoplastes (ex-Microcoleus chthonoplastes). Bioorganic Med. Chem. 2014, 22, 1667–1671. [Google Scholar] [CrossRef]
- Heuer, J.; Kraus, Y.; Vucak, M.; Zeng, A.P. Enhanced sequestration of carbon dioxide into calcium carbonate using pressure and a carbonic anhydrase from alkaliphilic Coleofasciculus chthonoplastes. Eng. Life Sci. 2022, 22, 178–191. [Google Scholar] [CrossRef]
- Hewett-Emmett, D.; Tashian, R.E. Functional diversity, conservation, and convergence in the evolution of the alpha-, beta-, and gamma-carbonic anhydrase gene families. Mol. Phylogenet Evol. 1996, 5, 50–77. [Google Scholar] [CrossRef]
- Samylina, O.S.; Sinetova, M.A.; Kupriyanova, E.V.; Starikov, A.Y.; Sukhacheva, M.V.; Dziuba, M.V.; Tourova, T.P. Ecology and biogeography of the ‘marine Geitlerinema’ cluster and a description of Sodalinema orleanskyi sp. nov., Sodalinema gerasimenkoae sp. nov., Sodalinema stali sp. nov. and Baaleninema simplex gen. et sp. nov. (Oscillatoriales, Cyanobacteria). FEMS Microbiol. Ecol. 2021, 97, fiab104. [Google Scholar] [CrossRef] [PubMed]
- Kupriyanova, E.V.; Cho, S.M.; Park, Y.-I.; Pronina, N.A.; Los, D.A. The complete genome of a cyanobacterium from a soda lake reveals the presence of the components of CO2-concentrating mechanism. Photosynth. Res. 2016, 130, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Fukuzawa, H.; Suzuki, E.; Komukai, Y.; Miyachi, S. A gene homologous to chloroplast carbonic anhydrase (icfA) is essential to photosynthetic carbon dioxide fixation by Synechococcus PCC7942. Proc. Natl. Acad. Sci. USA 1992, 89, 4437–4441. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Chen, M.; Myers, C.; Ludtke, S.J.; Pettitt, B.M.; King, J.A.; Schmid, M.F.; Chiu, W. Visualizing Individual RuBisCO and Its Assembly into Carboxysomes in Marine Cyanobacteria by Cryo-Electron Tomography. J. Mol. Biol. 2018, 430, 4156–4167. [Google Scholar] [CrossRef]
- Kerfeld, C.A.; Melnicki, M.R. Assembly, function and evolution of cyanobacterial carboxysomes. Curr. Opin. Plant Biol. 2016, 31, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Sommer, M.; Cai, F.; Melnicki, M.; Kerfeld, C.A. beta-Carboxysome bioinformatics: Identification and evolution of new bacterial microcompartment protein gene classes and core locus constraints. J. Exp. Bot. 2017, 68, 3841–3855. [Google Scholar] [CrossRef] [PubMed]
- Kautsky, H.; Appel, W.; Amann, H. Chlorophyll fluorescence and carbon assimilation. Part XIII. The fluorescence and the photochemistry of plants. Biochem. Z 1960, 332, 277–292. [Google Scholar] [PubMed]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Brestic, M.; Bussotti, F.; Calatayud, A.; Ferroni, L.; Goltsev, V.; Guidi, L.; Jajoo, A.; Li, P.; et al. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth. Res. 2017, 132, 13–66. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.; Hurry, V.; Clarke, A.K.; Gustafsson, P.; Oquist, G. Chlorophyll fluorescence analysis of cyanobacterial photosynthesis and acclimation. Microbiol. Mol. Biol. Rev. 1998, 62, 667–683. [Google Scholar] [CrossRef] [PubMed]
- Treves, H.; Raanan, H.; Kedem, I.; Murik, O.; Keren, N.; Zer, H.; Berkowicz, S.M.; Giordano, M.; Norici, A.; Shotland, Y. The mechanisms whereby the green alga Chlorella ohadii, isolated from desert soil crust, exhibits unparalleled photodamage resistance. New Phytol. 2016, 210, 1229–1243. [Google Scholar] [CrossRef] [PubMed]
- Long, B.M.; Badger, M.R.; Whitney, S.M.; Price, G.D. Analysis of carboxysomes from Synechococcus PCC7942 reveals multiple Rubisco complexes with carboxysomal proteins CcmM and CcaA. J. Biol. Chem. 2007, 282, 29323–29335. [Google Scholar] [CrossRef] [PubMed]
- Rae, B.D.; Long, B.M.; Badger, M.R.; Price, G.D. Functions, compositions, and evolution of the two types of carboxysomes: Polyhedral microcompartments that facilitate CO2 fixation in cyanobacteria and some proteobacteria. Microbiol. Mol. Biol. Rev. 2013, 77, 357–379. [Google Scholar] [CrossRef] [PubMed]
- Heinhorst, S.; Williams, E.B.; Cai, F.; Murin, C.D.; Shively, J.M.; Cannon, G.C. Characterization of the carboxysomal carbonic anhydrase CsoSCA from Halothiobacillus neapolitanus. J. Bacteriol. 2006, 188, 8087–8094. [Google Scholar] [CrossRef] [PubMed]
- Turmo, A.; Gonzalez-Esquer, C.R.; Kerfeld, C.A. Carboxysomes: Metabolic modules for CO2 fixation. FEMS Microbiol. Lett. 2017, 364, fnx176. [Google Scholar] [CrossRef] [PubMed]
- Bédu, S.; Peltier, G.; Sarrey, F.; Joset, F. Properties of a Mutant from Synechocystis PCC6803 Resistant to Acetazolamide, an Inhibitor of Carbonic Anhydrase. Plant Physiol. 1990, 93, 1312–1315. [Google Scholar] [CrossRef]
- Rae, B.D.; Long, B.M.; Badger, M.R.; Price, G.D. Structural determinants of the outer shell of beta-carboxysomes in Synechococcus elongatus PCC 7942: Roles for CcmK2, K3-K4, CcmO, and CcmL. PLoS ONE 2012, 7, e43871. [Google Scholar] [CrossRef]
- Sommer, M.; Sutter, M.; Gupta, S.; Kirst, H.; Turmo, A.; Lechno-Yossef, S.; Burton, R.L.; Saechao, C.; Sloan, N.B.; Cheng, X.; et al. Heterohexamers Formed by CcmK3 and CcmK4 Increase the Complexity of Beta Carboxysome Shells. Plant Physiol. 2019, 179, 156–167. [Google Scholar] [CrossRef]
- Kimber, M.S. Carboxysomal carbonic anhydrases. Subcell Biochem. 2014, 75, 89–103. [Google Scholar] [CrossRef]
- Gee, C.W.; Niyogi, K.K. The carbonic anhydrase CAH1 is an essential component of the carbon-concentrating mechanism in Nannochloropsis oceanica. Proc. Natl. Acad. Sci. USA 2017, 114, 4537–4542. [Google Scholar] [CrossRef]
- Low, K.O.; Jonet, M.A.; Ismail, N.F.; Illias, R.M. Optimization of a Bacillus sp signal peptide for improved recombinant protein secretion and cell viability in Escherichia coli: Is there an optimal signal peptide design? Bioengineered 2012, 3, 334–338. [Google Scholar] [CrossRef]
- Choo, K.H.; Ranganathan, S. Flanking signal and mature peptide residues influence signal peptide cleavage. BMC Bioinform. 2008, 9, S15. [Google Scholar] [CrossRef] [PubMed]
- Siegesmund, M.A.; Johansen, J.R.; Karsten, U.; Friedl, T. Coleofasciculus gen. nov.(Cyanobacteria): Morphological and Molecular Criteria for Revision of the Genus microcoleus gomont 1. J. Phycol. 2008, 44, 1572–1585. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2016, 44, D67–D72. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Teufel, F.; Almagro Armenteros, J.J.; Johansen, A.R.; Gíslason, M.H.; Pihl, S.I.; Tsirigos, K.D.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 6.0 predicts all five types of signal peptides using protein language models. Nat. Biotechnol. 2022, 40, 1023–1025. [Google Scholar] [CrossRef]
- Hallgren, J.; Tsirigos, K.D.; Pedersen, M.D.; Armenteros, J.J.A.; Marcatili, P.; Nielsen, H.; Krogh, A.; Winther, O. DeepTMHMM predicts alpha and beta transmembrane proteins using deep neural networks. bioRxiv 2022. [Google Scholar] [CrossRef]
- Waterbury, J.B.; Stanier, R.Y. Isolation and growth of cyanobacteria from marine and hypersaline environments. In The Prokaryotes; Springer: Berlin/Heidelberg, Germany, 1981; pp. 221–223. [Google Scholar]
- Viola, S.; Ruhle, T.; Leister, D. A single vector-based strategy for marker-less gene replacement in Synechocystis sp. PCC 6803. Microb. Cell Fact. 2014, 13, 4. [Google Scholar] [CrossRef]
- Gibson, D.G.; Young, L.; Chuang, R.-Y.; Venter, J.C.; Hutchison, C.A.; Smith, H.O. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 2009, 6, 343–345. [Google Scholar] [CrossRef]
- Dann, M.; Ortiz, E.M.; Thomas, M.; Guljamow, A.; Lehmann, M.; Schaefer, H.; Leister, D. Enhancing photosynthesis at high light levels by adaptive laboratory evolution. Nat. Plants 2021, 7, 681–695. [Google Scholar] [CrossRef]
- Zavřel, T.; Sinetova, M.A.; Červený, J. Measurement of Chlorophyll a and Carotenoids Concentration in Cyanobacteria. Bio-protocol 2015, 5, e1467. [Google Scholar] [CrossRef]
- Rakhimberdieva, M.G.; Boichenko, V.A.; Karapetyan, N.V.; Stadnichuk, I.N. Interaction of phycobilisomes with photosystem II dimers and photosystem I monomers and trimers in the cyanobacterium Spirulina platensis. Biochemistry 2001, 40, 15780–15788. [Google Scholar] [CrossRef] [PubMed]
- Luimstra, V.M.; Schuurmans, J.M.; de Carvalho, C.F.M.; Matthijs, H.C.P.; Hellingwerf, K.J.; Huisman, J. Exploring the low photosynthetic efficiency of cyanobacteria in blue light using a mutant lacking phycobilisomes. Photosynth. Res. 2019, 141, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Zlenko, D.V.; Elanskaya, I.V.; Lukashev, E.P.; Bolychevtseva, Y.V.; Suzina, N.E.; Pojidaeva, E.S.; Kononova, I.A.; Loktyushkin, A.V.; Stadnichuk, I.N. Role of the PB-loop in ApcE and phycobilisome core function in cyanobacterium Synechocystis sp. PCC 6803. Biochim. Biophys. Acta (BBA)-Bioenerg. 2019, 1860, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Gandini, C.; Schmidt, S.B.; Husted, S.; Schneider, A.; Leister, D. The transporter Syn PAM 71 is located in the plasma membrane and thylakoids, and mediates manganese tolerance in Synechocystis PCC 6803. New Phytol. 2017, 215, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minagawa, J.; Dann, M. Extracellular CahB1 from Sodalinema gerasimenkoae IPPAS B-353 Acts as a Functional Carboxysomal β-Carbonic Anhydrase in Synechocystis sp. PCC6803. Plants 2023, 12, 265. https://doi.org/10.3390/plants12020265
Minagawa J, Dann M. Extracellular CahB1 from Sodalinema gerasimenkoae IPPAS B-353 Acts as a Functional Carboxysomal β-Carbonic Anhydrase in Synechocystis sp. PCC6803. Plants. 2023; 12(2):265. https://doi.org/10.3390/plants12020265
Chicago/Turabian StyleMinagawa, Jun, and Marcel Dann. 2023. "Extracellular CahB1 from Sodalinema gerasimenkoae IPPAS B-353 Acts as a Functional Carboxysomal β-Carbonic Anhydrase in Synechocystis sp. PCC6803" Plants 12, no. 2: 265. https://doi.org/10.3390/plants12020265
APA StyleMinagawa, J., & Dann, M. (2023). Extracellular CahB1 from Sodalinema gerasimenkoae IPPAS B-353 Acts as a Functional Carboxysomal β-Carbonic Anhydrase in Synechocystis sp. PCC6803. Plants, 12(2), 265. https://doi.org/10.3390/plants12020265





