PEBP Signaling Network in Tubers and Tuberous Root Crops
Abstract
1. Introduction
2. PEBPs in Tuber and Tuberous Root Crops
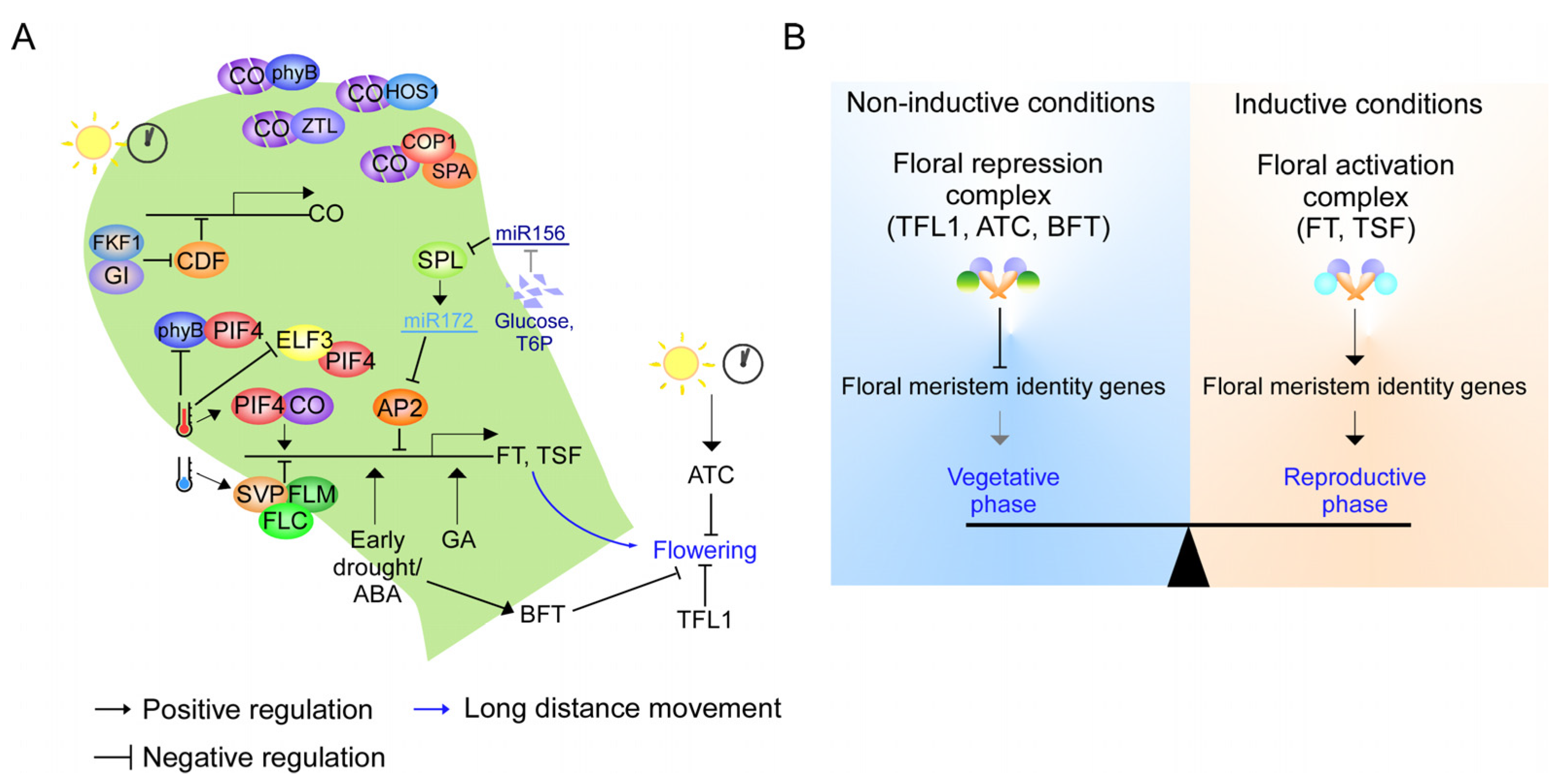
3. PEBP Signaling Network in Flowering Time Regulation
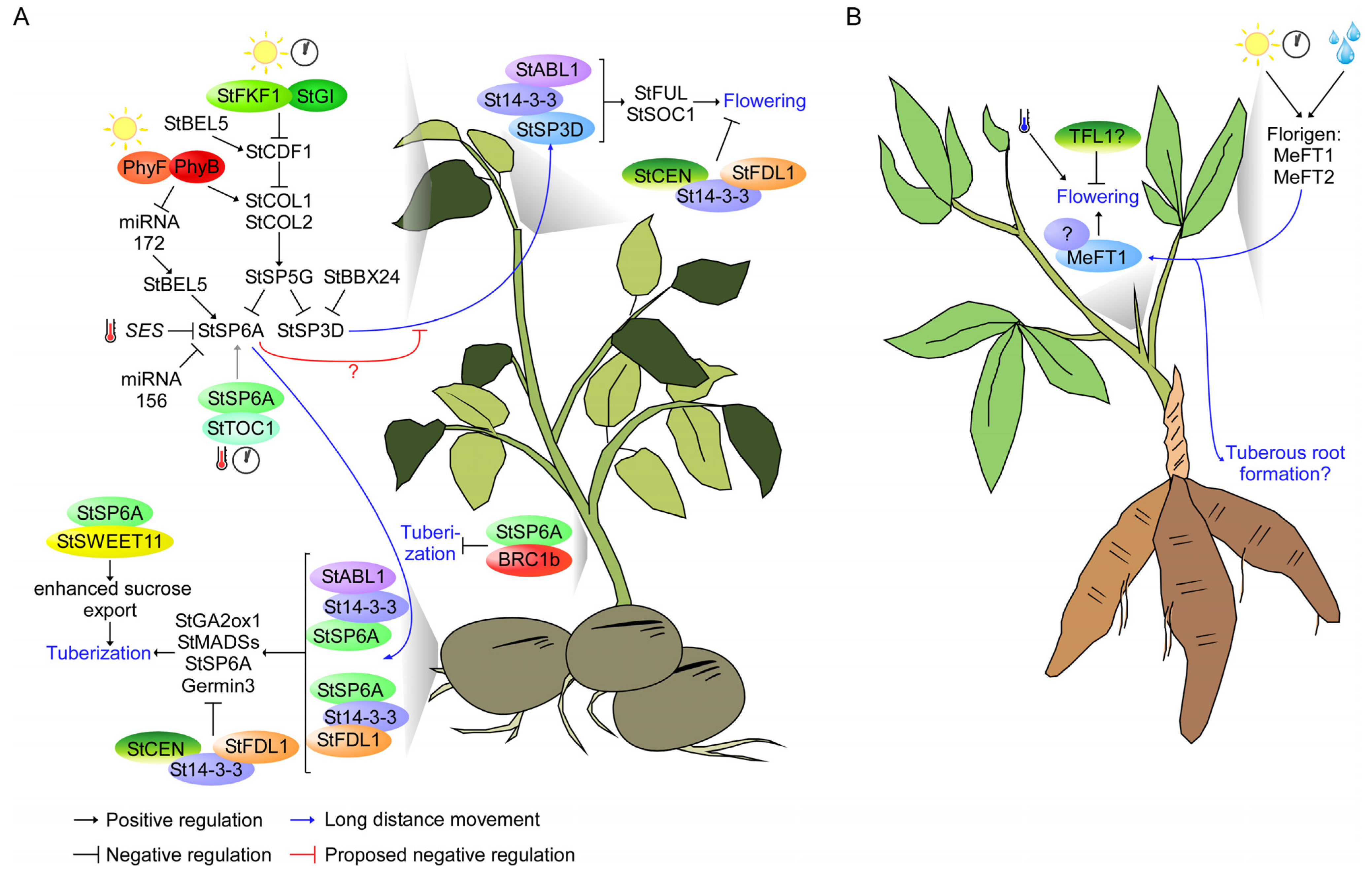
4. PEBP Signaling Network in Underground Storage Organ Formation
5. Conclusions and Future Perspectives
- All Arabidopsis PEBPs are involved in flowering time regulation [15,23], while little is known in other plant species. Furthermore, whether PEBPs also regulate tuberous root formation remains elusive. Therefore, a functional study of the role of PEBP in flowering time and underground storage organ formation is essential to elucidate the PEBP signaling network in each tuber and tuberous root crop;
- Further in-depth studies of the molecular mechanism that controls the function of PEBP are needed. For example, the non-cell-autonomous function of FT requires an interaction with the MCTP transporter and phospholipids [24,46,55,56,133]. However, the mechanism underlying the long-distance transport of FT-like proteins in tubers and tuberous root crops is still elusive. Therefore, further verification of protein transporters and phospholipid interactors of the PEBP family from tubers and tuberous root crops is needed for a complete understanding of the PEBP signaling network in these plants. Furthermore, although StSP5G is a central regulator connecting the photoperiod with floral initiation and tuber formation by regulating StSP3D and StSP6A [21,29,109], it is not clear how StSP5G regulates its downstream targets. StSP5G may interact with other proteins, including St14-3-3s and StTCPs, to modulate floral initiation and tuber formation. Therefore, translating the knowledge from Arabidopsis could accelerate the characterization of the regulatory module of the PEBP-gene family in tubers and tuberous root crops (Figure 2);
- Some plants develop underground storage organs to survive adverse environmental conditions [6]. Whole-genome analysis of the non-tuber-bearing Etuberosum, sister of the Petota (tuber-bearing) section of the Solanum genus, suggests that the non-tuber-bearing phenotype of Etuberosum is caused by the deletion of the fourth exon of SP6A [127]. However, a more in-depth examination is needed to elucidate how PEBP, a flowering time regulator, evolved and diversified its function to regulate underground storage organ formation.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chandrasekara, A.; Josheph Kumar, T. Roots and Tuber Crops as Functional Foods: A Review on Phytochemical Constituents and Their Potential Health Benefits. Int. J. Food Sci. 2016, 2016, 3631647. [Google Scholar] [CrossRef] [PubMed]
- FAO. Crops and Livestock Products; FAO: Roma, Italy, 2020; Available online: https://www.fao.org/faostat/en/#data/QC (accessed on 25 February 2022).
- Kolawole, P.O.; Agbetoye, L.; Ogunlowo, S.A. Sustaining World Food Security with Improved Cassava Processing Technology: The Nigeria Experience. Sustainability 2010, 2, 3681–3694. [Google Scholar] [CrossRef]
- Devaux, A.; Kromann, P.; Ortiz, O. Potatoes for Sustainable Global Food Security. Potato Res. 2014, 57, 185–199. [Google Scholar] [CrossRef]
- Arteca, R.N. Tuberization. In Plant Growth Substances; Springer: New York, NY, USA, 1996; pp. 223–239. [Google Scholar] [CrossRef]
- Zierer, W.; Rüscher, D.; Sonnewald, U.; Sonnewald, S. Tuber and Tuberous Root Development. Annu. Rev. Plant Biol. 2021, 72, 551–580. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, J.F.; Virgós-Soler, A.; Prat, S. Control of photoperiod-regulated tuberization in potato by the Arabidopsis flowering-time gene CONSTANS. Proc. Natl. Acad. Sci. USA 2002, 99, 15211–15216. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Park, S.-J.; Kwon, S.-Y.; Shin, A.-Y.; Moon, K.-B.; Park, J.M.; Cho, H.S.; Park, S.U.; Jeon, J.-H.; Kim, H.-S.; et al. Temporally distinct regulatory pathways coordinate thermo-responsive storage organ formation in potato. Cell Rep. 2022, 38, 110579. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Y.; Zhao, J.; Shi, X.; Ma, Z.; Fan, M. Tuber formation as influenced by the C: N ratio in potato plants. J. Plant Nutr. Soil Sci. 2018, 181, 686–693. [Google Scholar] [CrossRef]
- Kühn, C.; Hajirezaei, M.-R.; Fernie, A.R.; Roessner-Tunali, U.; Czechowski, T.; Hirner, B.; Frommer, W.B. The Sucrose Transporter StSUT1 Localizes to Sieve Elements in Potato Tuber Phloem and Influences Tuber Physiology and Development. Plant Physiol. 2003, 131, 102–113. [Google Scholar] [CrossRef]
- Chincinska, I.A.; Liesche, J.; Krügel, U.; Michalska, J.; Geigenberger, P.; Grimm, B.; Kühn, C. Sucrose Transporter StSUT4 from Potato Affects Flowering, Tuberization, and Shade Avoidance Response. Plant Physiol. 2008, 146, 323–324. [Google Scholar] [CrossRef]
- Garg, V.; Hackel, A.; Kühn, C. Expression Level of Mature miR172 in Wild Type and StSUT4-Silenced Plants of Solanum tuberosum Is Sucrose-Dependent. Int. J. Mol. Sci. 2021, 22, 1455. [Google Scholar] [CrossRef]
- Islam, M.S.; Roni, M.Z.K.; Jamal Uddin, A.F.M.; Shimasaki, K. Tracing the role of sucrose in potato microtuber formation in vitro. Plant Omics 2017, 10, 15–19. [Google Scholar] [CrossRef]
- Wu, X.H.; Geng, M.T.; Fan, J.; Yao, Y.; Min, Y.; Li, R.M.; Hu, X.W.; Fu, S.P.; Guo, J.C. Effects of Sucrose on Tuberous Root Formation and Saccharide Accumulation in Manihot esculenta Crantz In Vitro. Adv. Mater. Res. 2014, 1010–1012, 225–228. [Google Scholar] [CrossRef]
- Jin, S.; Nasim, Z.; Susila, H.; Ahn, J.H. Evolution and functional diversification of Flowering Locus T/Terminal Flower 1 family genes in plants. Semin. Cell Dev. Biol. 2021, 109, 20–30. [Google Scholar] [CrossRef]
- Bernier, I.; Jollés, P. Purification and characterization of a basic 23 kDa cytosolic protein from bovine brain. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 1984, 790, 174–181. [Google Scholar] [CrossRef]
- Serre, L.; Vallée, B.; Bureaud, N.; Schoentgen, F.; Zelwer, C. Crystal structure of the phosphatidylethanolamine-binding protein from bovine brain: A novel structural class of phospholipid-binding proteins. Structure 1998, 6, 1255–1265. [Google Scholar] [CrossRef]
- Ahn, J.H.; Miller, D.; Winter, V.J.; Banfield, M.J.; Lee, J.H.; Yoo, S.Y.; Henz, S.R.; Brady, R.L.; Weigel, D. A divergent external loop confers antagonistic activity on floral regulators FT and TFL1. EMBO J. 2006, 25, 605–614. [Google Scholar] [CrossRef]
- Karlgren, A.; Gyllenstrand, N.; Källman, T.; Sundström, J.F.; Moore, D.; Lascoux, M.; Lagercrantz, U. Evolution of the PEBP Gene Family in Plants: Functional Diversification in Seed Plant Evolution. Plant Physiol. 2011, 156, 1967–1977. [Google Scholar] [CrossRef]
- Kardailsky, I.; Shukla, V.K.; Ahn, J.H.; Dagenais, N.; Christensen, S.K.; Nguyen, J.T.; Chory, J.; Harrison, M.J.; Weigel, D. Activation Tagging of the Floral Inducer FT. Science 1999, 286, 1962–1965. [Google Scholar] [CrossRef]
- Navarro, C.; Abelenda, J.A.; Cruz-Oró, E.; Cuéllar, C.A.; Tamaki, S.; Silva, J.; Shimamoto, K.; Prat, S. Control of flowering and storage organ formation in potato by Flowering Locus T. Nature 2011, 478, 119–122. [Google Scholar] [CrossRef]
- Khosa, J.; Bellinazzo, F.; Kamenetsky Goldstein, R.; Macknight, R.; Immink, R.G.H.; Melzer, R. Phosphatidylethanolamine-Binding Proteins: The conductors of dual reproduction in plants with vegetative storage organs. J. Exp. Bot. 2021, 72, 2845–2856. [Google Scholar] [CrossRef]
- Kim, W.; Park, T.I.; Yoo, S.J.; Jun, A.R.; Ahn, J.H. Generation and analysis of a complete mutant set for the Arabidopsis FT/TFL1 family shows specific effects on thermo-sensitive flowering regulation. J. Exp. Bot. 2013, 64, 1715–1729. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Yang, K.Z.; Wei, X.X.; Wang, X.Q. Revisiting the phosphatidylethanolamine-binding protein (PEBP) gene family reveals cryptic FLOWERING LOCUS T gene homologs in gymnosperms and sheds new light on functional evolution. New Phytol. 2016, 212, 730–744. [Google Scholar] [CrossRef] [PubMed]
- Klintenäs, M.; Pin, P.A.; Benlloch, R.; Ingvarsson, P.K.; Nilsson, O. Analysis of conifer Flowering Locus T/Terminal Flower1-like genes provides evidence for dramatic biochemical evolution in the angiosperm FT lineage. New Phytol. 2012, 196, 1260–1273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Jin, X.; Li, X.; Zhang, N.; Li, S.; Si, H.; Rajora, O.P.; Li, X.-Q. Genome-wide identification of PEBP gene family members in potato, their phylogenetic relationships, and expression patterns under heat stress. Mol. Biol. Rep. 2022, 49, 4683–4697. [Google Scholar] [CrossRef] [PubMed]
- Teo, C.-J.; Takahashi, K.; Shimizu, K.; Shimamoto, K.; Taoka, K.-I. Potato Tuber Induction is Regulated by Interactions Between Components of a Tuberigen Complex. Plant Cell Physiol. 2016, 58, 365–374. [Google Scholar] [CrossRef]
- Odipio, J.; Getu, B.; Chauhan, R.D.; Alicai, T.; Bart, R.; Nusinow, D.A.; Taylor, N.J. Transgenic overexpression of endogenous Flowering Locus T-like gene MeFT1 produces early flowering in cassava. PLoS ONE 2020, 15, e0227199. [Google Scholar] [CrossRef]
- Abelenda, J.A.; Cruz-Oró, E.; Franco-Zorrilla, J.M.; Prat, S. Potato StCONSTANS-like1 Suppresses Storage Organ Formation by Directly Activating the FT-like StSP5G Repressor. Curr. Biol. 2016, 26, 872–881. [Google Scholar] [CrossRef]
- Morris, W.L.; Alamar, M.C.; Lopez-Cobollo, R.M.; Castillo Cañete, J.; Bennett, M.; Van der Kaay, J.; Stevens, J.; Kumar Sharma, S.; McLean, K.; Thompson, A.J.; et al. A member of the TERMINAL FLOWER 1/CENTRORADIALIS gene family controls sprout growth in potato tubers. J. Exp. Bot. 2019, 70, 835–843. [Google Scholar] [CrossRef]
- Zhang, X.; Campbell, R.; Ducreux, L.J.M.; Morris, J.; Hedley, P.E.; Mellado-Ortega, E.; Roberts, A.G.; Stephens, J.; Bryan, G.J.; Torrance, L.; et al. TERMINAL FLOWER-1/CENTRORADIALIS inhibits tuberisation via protein interaction with the tuberigen activation complex. Plant J. 2020, 103, 2263–2278. [Google Scholar] [CrossRef]
- Dereeper, A.; Guignon, V.; Blanc, G.; Audic, S.; Buffet, S.; Chevenet, F.; Dufayard, J.F.; Guindon, S.; Lefort, V.; Lescot, M.; et al. Phylogeny.Fr: Robust Phylogenetic Analysis for the Non-Specialist. Nucleic Acids Res. 2008, 36 (Suppl. 2), W465–W469. [Google Scholar] [CrossRef]
- Zheng, X.-M.; Wu, F.-Q.; Zhang, X.; Lin, Q.-B.; Wang, J.; Guo, X.-P.; Lei, C.-L.; Cheng, Z.-J.; Zou, C.; Wan, J.-M. Evolution of the PEBP gene family and selective signature on FT-like clade. J. Syst. Evol. 2016, 54, 502–510. [Google Scholar] [CrossRef]
- Ho, W.W.H.; Weigel, D. Structural Features Determining Flower-Promoting Activity of Arabidopsis FLOWERING LOCUS T. Plant Cell 2014, 26, 552–564. [Google Scholar] [CrossRef]
- Wickland, D.P.; Hanzawa, Y. The FLOWERING LOCUS T/TERMINAL FLOWER 1 Gene Family: Functional Evolution and Molecular Mechanisms. Mol. Plant 2015, 8, 983–997. [Google Scholar] [CrossRef]
- Pin, P.A.; Benlloch, R.; Bonnet, D.; Wremerth-Weich, E.; Kraft, T.; Gielen, J.J.L.; Nilsson, O. An Antagonistic Pair of FT Homologs Mediates the Control of Flowering Time in Sugar Beet. Science 2010, 330, 1397–1400. [Google Scholar] [CrossRef]
- Pieper, R.; Tomé, F.; Pankin, A.; von Korff, M.; Wilson, Z. FLOWERING LOCUS T4 delays flowering and decreases floret fertility in barley. J. Exp. Bot. 2021, 72, 107–121. [Google Scholar] [CrossRef]
- Yoo, S.-C.; Chen, C.; Rojas, M.; Daimon, Y.; Ham, B.-K.; Araki, T.; Lucas, W.J. Phloem long-distance delivery of FLOWERING LOCUS T (FT) to the apex. Plant J. 2013, 75, 456–468. [Google Scholar] [CrossRef]
- Andrés, F.; Coupland, G. The genetic basis of flowering responses to seasonal cues. Nat. Rev. Genet. 2012, 13, 627–639. [Google Scholar] [CrossRef]
- Song, Y.H.; Shim, J.S.; Kinmonth-Schultz, H.A.; Imaizumi, T. Photoperiodic Flowering: Time Measurement Mechanisms in Leaves. Annu. Rev. Plant Biol. 2015, 66, 441–464. [Google Scholar] [CrossRef]
- Wenkel, S.; Turck, F.; Singer, K.; Gissot, L.; Le Gourrierec, J.; Samach, A.; Coupland, G. CONSTANS and the CCAAT Box Binding Complex Share a Functionally Important Domain and Interact to Regulate Flowering of Arabidopsis. Plant Cell 2006, 18, 2971–2984. [Google Scholar] [CrossRef]
- Putterill, J.; Robson, F.; Lee, K.; Simon, R.; Coupland, G. The CONSTANS gene of arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell 1995, 80, 847–857. [Google Scholar] [CrossRef]
- Huang, N.-C.; Jane, W.-N.; Chen, J.; Yu, T.-S. Arabidopsis thaliana CENTRORADIALIS homologue(ATC) acts systemically to inhibit floral initiation in Arabidopsis. Plant J. 2012, 72, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Susila, H.; Nasim, Z.; Ahn, J.H. Ambient Temperature-Responsive Mechanisms Coordinate Regulation of Flowering Time. Int. J. Mol. Sci. 2018, 19, 3196. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.V.; Lucyshyn, D.; Jaeger, K.E.; Alós, E.; Alvey, E.; Harberd, N.P.; Wigge, P.A. Transcription factor PIF4 controls the thermosensory activation of flowering. Nature 2012, 484, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Susila, H.; Juric, S.; Liu, L.; Gawarecka, K.; Chung, K.S.; Jin, S.; Kim, S.J.; Nasim, Z.; Youn, G.; Suh, M.C.; et al. Florigen sequestration in cellular membranes modulates temperature-responsive flowering. Science 2021, 373, 1137–1142. [Google Scholar] [CrossRef]
- Riboni, M.; Galbiati, M.; Tonelli, C.; Conti, L. GIGANTEA Enables Drought Escape Response via Abscisic Acid-Dependent Activation of the Florigens and Suppressor of Overexpression of Constans1. Plant Physiol. 2013, 162, 1706–1719. [Google Scholar] [CrossRef]
- Hwang, K.; Susila, H.; Nasim, Z.; Jung, J.-Y.; Ahn, J.H. Arabidopsis ABF3 and ABF4 Transcription Factors Act with the NF-YC Complex to Regulate SOC1 Expression and Mediate Drought-Accelerated Flowering. Mol. Plant 2019, 12, 489–505. [Google Scholar] [CrossRef]
- Riboni, M.; Robustelli Test, A.; Galbiati, M.; Tonelli, C.; Conti, L. Environmental stress and flowering time. Plant Signal. Behav. 2014, 9, e29036. [Google Scholar] [CrossRef]
- Bao, S.; Hua, C.; Shen, L.; Yu, H. New insights into gibberellin signaling in regulating flowering in Arabidopsis. J. Integr. Plant Biol. 2020, 62, 118–131. [Google Scholar] [CrossRef]
- Wu, G.; Park, M.Y.; Conway, S.R.; Wang, J.-W.; Weigel, D.; Poethig, R.S. The Sequential Action of miR156 and miR172 Regulates Developmental Timing in Arabidopsis. Cell 2009, 138, 750–759. [Google Scholar] [CrossRef]
- Baumann, K.; Venail, J.; Berbel, A.; Domenech, M.J.; Money, T.; Conti, L.; Hanzawa, Y.; Madueno, F.; Bradley, D. Changing the spatial pattern of TFL1 expression reveals its key role in the shoot meristem in controlling Arabidopsis flowering architecture. J. Exp. Bot. 2015, 66, 4769–4780. [Google Scholar] [CrossRef]
- Chen, Q.; Payyavula, R.S.; Chen, L.; Zhang, J.; Zhang, C.; Turgeon, R. FLOWERING LOCUS T mRNA is synthesized in specialized companion cells in Arabidopsis and Maryland Mammoth tobacco leaf veins. Proc. Natl. Acad. Sci. USA 2018, 115, 2830–2835. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, C.; Liang, Z.; Yu, H. Characterization of Multiple C2 Domain and Transmembrane Region Proteins in Arabidopsis. Plant Physiol. 2018, 176, 2119–2132. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, C.; Teo, Z.W.N.; Zhang, B.; Yu, H. The MCTP-SNARE Complex Regulates Florigen Transport in Arabidopsis. Plant Cell 2019, 31, 2475–2490. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, C.; Hou, X.; Xi, W.; Shen, L.; Tao, Z.; Wang, Y.; Yu, H. FTIP1 Is an Essential Regulator Required for Florigen Transport. PLoS Biol. 2012, 10, e1001313. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Klasfeld, S.; Jeong, C.W.; Jin, R.; Goto, K.; Yamaguchi, N.; Wagner, D. Terminal Flower 1-FD complex target genes and competition with Flowering Locus T. Nat. Commun. 2020, 11, 5118. [Google Scholar] [CrossRef]
- Jang, S.; Torti, S.; Coupland, G. Genetic and spatial interactions between FT, TSF and SVP during the early stages of floral induction in Arabidopsis. Plant J. 2009, 60, 614–625. [Google Scholar] [CrossRef]
- Ryu, J.Y.; Lee, H.-J.; Seo, P.J.; Jung, J.-H.; Ahn, J.H.; Park, C.-M. The Arabidopsis Floral Repressor BFT Delays Flowering by Competing with FT for FD Binding under High Salinity. Mol. Plant 2014, 7, 377–387. [Google Scholar] [CrossRef]
- Hou, C.-J.; Yang, C.-H. Comparative analysis of the pteridophyte Adiantum MFT ortholog reveals the specificity of combined FT/MFT C and N terminal interaction with FD for the regulation of the downstream gene AP1. Plant Mol. Biol. 2016, 91, 563–579. [Google Scholar] [CrossRef]
- Taoka, K.-i.; Ohki, I.; Tsuji, H.; Furuita, K.; Hayashi, K.; Yanase, T.; Yamaguchi, M.; Nakashima, C.; Purwestri, Y.A.; Tamaki, S.; et al. 14-3-3 proteins act as intracellular receptors for rice Hd3a florigen. Nature 2011, 476, 332–335. [Google Scholar] [CrossRef]
- Li, D.; Zhang, H.; Mou, M.; Chen, Y.; Xiang, S.; Chen, L.; Yu, D. Arabidopsis Class II TCP Transcription Factors Integrate with the FT–FD Module to Control Flowering. Plant Physiol. 2019, 181, 97–111. [Google Scholar] [CrossRef]
- Murray, F.V.N.; Cohen, J.E. Efficacy of shoot production of cassava using the multiple shoot removal technique for rapid propagation. J. Agric. Sci. 2021, 159, 177–187. [Google Scholar] [CrossRef]
- George, N.A.; Pecota, K.V.; Bowen, B.D.; Schultheis, J.R.; Yencho, G.C. Root Piece Planting in Sweetpotato—A Synthesis of Previous Research and Directions for the Future. HortTechnology 2011, 21, 703–711. [Google Scholar] [CrossRef]
- Simmonds, N.W. A review of potato propagation by means of seed, as distinct from clonal propagation by tubers. Potato Res. 1997, 40, 191–214. [Google Scholar] [CrossRef]
- Aighewi, B.A.; Asiedu, R.; Maroya, N.; Balogun, M. Improved propagation methods to raise the productivity of yam (Dioscorea rotundata Poir.). Food Secur. 2015, 7, 823–834. [Google Scholar] [CrossRef]
- McKey, D.; Elias, M.; Pujol, B.; Duputié, A. The evolutionary ecology of clonally propagated domesticated plants. New Phytol. 2010, 186, 318–332. [Google Scholar] [CrossRef]
- Consortium, T.P.G.S. Genome sequence and analysis of the tuber crop potato. Nature 2011, 475, 189–195. [Google Scholar] [CrossRef]
- Lyons, J.B.; Bredeson, J.V.; Mansfeld, B.N.; Bauchet, G.J.; Berry, J.; Boyher, A.; Mueller, L.A.; Rokhsar, D.S.; Bart, R.S. Current status and impending progress for cassava structural genomics. Plant Mol. Biol. 2021, 109, 177–191. [Google Scholar] [CrossRef]
- Charles, W.B.; Hoskinj, D.G.; Cave, P.J. Overcoming cross and self-incompatibility in Ipomoea batatas (L) Lam. and Ipomoea trichocarpa (Elliot). J. Hortic. Sci. 2015, 49, 113–121. [Google Scholar] [CrossRef]
- Rodríguez-Falcón, M.; Bou, J.; Prat, S. Seasonal Control of Tuberization in Potato: Conserved Elements with the Flowering Response. Annu. Rev. Plant Biol. 2006, 57, 151–180. [Google Scholar] [CrossRef]
- Markarov, A.M. Causes of Flowering of Long-Day Potato Species under Short-Day and Cold-Night Conditions. Russ. J. Plant Physiol. 2002, 49, 465–469. [Google Scholar] [CrossRef]
- Seibert, T.; Abel, C.; Wahl, V.; Wilson, Z. Flowering time and the identification of floral marker genes in Solanum tuberosum ssp. andigena. J. Exp. Bot. 2020, 71, 986–996. [Google Scholar] [CrossRef] [PubMed]
- Kiełbowicz-Matuk, A.; Grądzka, K.; Biegańska, M.; Talar, U.; Czarnecka, J.; Rorat, T. The StBBX24 protein affects the floral induction and mediates salt tolerance in Solanum tuberosum. Front. Plant Sci. 2022, 13, 965098. [Google Scholar] [CrossRef] [PubMed]
- González-Schain, N.D.; Suárez-López, P. Constans delays flowering and affects tuber yield in potato. Biol. Plant. 2008, 52, 251–258. [Google Scholar] [CrossRef]
- Li, F.; Sun, J.; Wang, D.; Bai, S.; Clarke, A.K.; Holm, M. The B-Box Family Gene STO (BBX24) in Arabidopsis thaliana Regulates Flowering Time in Different Pathways. PLoS ONE 2014, 9, e87544. [Google Scholar] [CrossRef] [PubMed]
- Plantenga, F.D.M.; Bergonzi, S.; Abelenda, J.A.; Bachem, C.W.B.; Visser, R.G.F.; Heuvelink, E.; Marcelis, L.F.M. The tuberization signal StSP6A represses flower bud development in potato. J. Exp. Bot. 2019, 70, 937–948. [Google Scholar] [CrossRef]
- Fornara, F.; Panigrahi, K.C.S.; Gissot, L.; Sauerbrunn, N.; Rühl, M.; Jarillo, J.A.; Coupland, G. Arabidopsis DOF Transcription Factors Act Redundantly to Reduce Constans Expression and Are Essential for a Photoperiodic Flowering Response. Dev. Cell 2009, 17, 75–86. [Google Scholar] [CrossRef]
- Song, Y.H.; Smith, R.W.; To, B.J.; Millar, A.J.; Imaizumi, T. FKF1 Conveys Timing Information for Constans Stabilization in Photoperiodic Flowering. Science 2012, 336, 1045–1049. [Google Scholar] [CrossRef]
- Imaizumi, T.; Schultz, T.F.; Harmon, F.G.; Ho, L.A.; Kay, S.A. FKF1 F-Box Protein Mediates Cyclic Degradation of a Repressor of Constans in Arabidopsis. Science 2005, 309, 293–297. [Google Scholar] [CrossRef]
- Sawa, M.; Nusinow, D.A.; Kay, S.A.; Imaizumi, T. FKF1 and Gigantea Complex Formation Is Required for Day-Length Measurement in Arabidopsis. Science 2007, 318, 261–265. [Google Scholar] [CrossRef]
- Ile, E.I.; Craufurd, P.Q.; Asiedu, R.; Battey, N.H. Duration from vine emergence to flowering suggests a long-day or rate of change of photoperiod response in white yam (Dioscorea rotundata Poir.). Environ. Exp. Bot. 2007, 60, 86–94. [Google Scholar] [CrossRef]
- Shiwachi, H.; Ayankanmi, T.; Asiedu, R. Effect of photoperiod on the development of inflorescences in white Guinea yam (Dioscorea rotundata). Trop. Sci. 2005, 45, 126–130. [Google Scholar] [CrossRef]
- Hyde, P.T.; Setter, T.L. Long-day photoperiod and cool temperature induce flowering in cassava: Expression of signaling genes. Front. Plant Sci. 2022, 13, 3413. [Google Scholar] [CrossRef]
- Campbell, G.M.; Hernandez, T.P.; Miller, J.C. The effect of temperature, photoperiod and other related treatments on flowering in Ipomoea batatas. Am. Soc. Hortic. Sci. Proc. 1963, 83, 618–622. [Google Scholar]
- Adeyemo, O.S.; Hyde, P.T.; Setter, T.L. Identification of FT family genes that respond to photoperiod, temperature and genotype in relation to flowering in cassava (Manihot esculenta, Crantz). Plant Reprod. 2018, 32, 181–191. [Google Scholar] [CrossRef]
- Lin, X.; Fang, C.; Liu, B.; Kong, F. Natural variation and artificial selection of photoperiodic flowering genes and their applications in crop adaptation. aBIOTECH 2021, 2, 156–169. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Ogawa, M.; Kuwahara, A.; Hanada, A.; Kamiya, Y.; Yamaguchi, S. Activation of Gibberellin Biosynthesis and Response Pathways by Low Temperature during Imbibition of Arabidopsis thaliana Seeds. Plant Cell 2004, 16, 367–378. [Google Scholar] [CrossRef]
- Li, Y.; Sun, M.; Xiang, H.; Liu, Y.; Li, H.; Qi, M.; Li, T. Low Overnight Temperature-Induced Gibberellin Accumulation Increases Locule Number in Tomato. Int. J. Mol. Sci. 2019, 20, 3042. [Google Scholar] [CrossRef]
- Plantenga, F.D.M.; Bergonzi, S.; Bachem, C.W.B.; Visser, R.G.F.; Heuvelink, E.; Marcelis, L.F.M. High light accelerates potato flowering independently of the FT-like flowering signal StSP3D. Environ. Exp. Bot. 2019, 160, 35–44. [Google Scholar] [CrossRef]
- Feng, P.; Guo, H.; Chi, W.; Chai, X.; Sun, X.; Xu, X.; Ma, J.; Rochaix, J.-D.; Leister, D.; Wang, H.; et al. Chloroplast retrograde signal regulates flowering. Proc. Natl. Acad. Sci. USA 2016, 113, 10708–10713. [Google Scholar] [CrossRef]
- Susila, H.; Jin, S.; Ahn, J.H. Light Intensity and Floral Transition: Chloroplast Says “Time to Flower!”. Mol. Plant 2016, 9, 1551–1553. [Google Scholar] [CrossRef]
- Mateos, J.L.; Madrigal, P.; Tsuda, K.; Rawat, V.; Richter, R.; Romera-Branchat, M.; Fornara, F.; Schneeberger, K.; Krajewski, P.; Coupland, G. Combinatorial activities of Short Vegetative Phase and Flowering Locus C define distinct modes of flowering regulation in Arabidopsis. Genome Biol. 2015, 16, 31. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ryu, H.-S.; Chung, K.S.; Posé, D.; Kim, S.; Schmid, M.; Ahn, J.H. Regulation of Temperature-Responsive Flowering by MADS-Box Transcription Factor Repressors. Science 2013, 342, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Oluwasanya, D.N.; Gisel, A.; Stavolone, L.; Setter, T.L. Environmental responsiveness of flowering time in cassava genotypes and associated transcriptome changes. PLoS ONE 2021, 16, e0253555. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, H.; Quynh, D.T.N.; Anh, N.H.; Nhan, P.T.; Matsui, A.; Takahashi, S.; Tanaka, M.; Anh, N.M.; Van Dong, N.; Ham, L.H.; et al. Field transcriptome analysis reveals a molecular mechanism for cassava-flowering in a mountainous environment in Southeast Asia. Plant Mol. Biol. 2020, 109, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Huang, F.; Wu, N.; Li, X.; Hu, H.; Xiong, L. Integrative Regulation of Drought Escape through ABA-Dependent and -Independent Pathways in Rice. Mol. Plant 2018, 11, 584–597. [Google Scholar] [CrossRef]
- Jing, S.; Sun, X.; Yu, L.; Wang, E.; Cheng, Z.; Liu, H.; Jiang, P.; Qin, J.; Begum, S.; Song, B. Transcription factor StABI5-like 1 binding to the Flowering Locus T homologs promotes early maturity in potato. Plant Physiol. 2022, 189, 1677–1693. [Google Scholar] [CrossRef]
- Shiwachi, H.; Ayankanmi, T.G.; Asiedu, R. Effect of day length on the development of tubers in yams (Dioscorea spp.). Trop. Sci. 2002, 42, 162–170. [Google Scholar]
- Vaillant, V.; Bade, P.; Constant, C. Photoperiod affects the growth and development of yam plantlets obtained by in vitro propagation. Biol. Plant. 2005, 49, 355–359. [Google Scholar] [CrossRef]
- Lowe, S.B.; Mahon, J.D.; Hunt, L.A. The effect of daylength on shoot growth and formation of root tubers in young plants of cassava (Manihot esculenta grantz). Plant Sci. Lett. 1976, 6, 57–62. [Google Scholar] [CrossRef]
- Paik, I.; Huq, E. Plant photoreceptors: Multi-functional sensory proteins and their signaling networks. Semin. Cell Dev. Biol. 2019, 92, 114–121. [Google Scholar] [CrossRef]
- Batutis, E.J.; Ewing, E.E. Far-Red Reversal of Red Light Effect during Long-Night Induction of Potato (Solanum tuberosum L.) Tuberization. Plant Physiol. 1982, 69, 672–674. [Google Scholar] [CrossRef]
- Jackson, S.D.; Heyer, A.; Dietze, J.; Prat, S. Phytochrome B mediates the photoperiodic control of tuber formation in potato. Plant J. 1996, 9, 159–166. [Google Scholar] [CrossRef]
- Zhou, T.; Song, B.; Liu, T.; Shen, Y.; Dong, L.; Jing, S.; Xie, C.; Liu, J. Phytochrome F plays critical roles in potato photoperiodic tuberization. Plant J. 2019, 98, 42–54. [Google Scholar] [CrossRef]
- Valverde, F.; Mouradov, A.; Soppe, W.; Ravenscroft, D.; Samach, A.; Coupland, G. Photoreceptor Regulation of Constans Protein in Photoperiodic Flowering. Science 2004, 303, 1003–1006. [Google Scholar] [CrossRef]
- Zhang, Z.; Ji, R.; Li, H.; Zhao, T.; Liu, J.; Lin, C.; Liu, B. CONSTANS-LIKE 7 (COL7) Is Involved in Phytochrome B (phyB)-Mediated Light-Quality Regulation of Auxin Homeostasis. Mol. Plant 2014, 7, 1429–1440. [Google Scholar] [CrossRef]
- Hamaoka, N.; Nabeshima, M.; Moriyama, T.; Kozawa, Y.; Ishibashi, Y. Photoperiodic Regulation of Tuber Enlargement in Water Yam. Agronomy 2022, 12, 2939. [Google Scholar] [CrossRef]
- Kloosterman, B.; Abelenda, J.A.; Gomez, M.d.M.C.; Oortwijn, M.; de Boer, J.M.; Kowitwanich, K.; Horvath, B.M.; van Eck, H.J.; Smaczniak, C.; Prat, S.; et al. Naturally occurring allele diversity allows potato cultivation in northern latitudes. Nature 2013, 495, 246–250. [Google Scholar] [CrossRef]
- Hannapel, D.J.; Sharma, P.; Lin, T.; Banerjee, A.K. The Multiple Signals That Control Tuber Formation. Plant Physiol. 2017, 174, 845–856. [Google Scholar] [CrossRef]
- Kondhare, K.R.; Vetal, P.V.; Kalsi, H.S.; Banerjee, A.K. BEL1-like protein (StBEL5) regulates CYCLING DOF FACTOR1 (StCDF1) through tandem TGAC core motifs in potato. J. Plant Physiol. 2019, 241, 153014. [Google Scholar] [CrossRef]
- Martin, A.; Adam, H.; Díaz-Mendoza, M.; Zurczak, M.; González-Schain, N.D.; Suárez-López, P. Graft-transmissible induction of potato tuberization by the microRNA miR172. Development 2009, 136, 2873–2881. [Google Scholar] [CrossRef]
- Bhogale, S.; Mahajan, A.S.; Natarajan, B.; Rajabhoj, M.; Thulasiram, H.V.; Banerjee, A.K. MicroRNA156: A Potential Graft-Transmissible MicroRNA That Modulates Plant Architecture and Tuberization in Solanum tuberosum ssp. andigena. Plant Physiol. 2014, 164, 1011–1027. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Lin, T.; Hannapel, D.J. Targets of the StBEL5 Transcription Factor Include the FT Ortholog StSP6A. Plant Physiol. 2016, 170, 310–324. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, T.; Kitano, M.; Yoshida, S.; Chikushi, J. Root Temperature Effects on Tuberous Root Growth of Sweetpotato (Ipomoea batatas Lam.). Direct and Indirect Effects of Temperature. Environ. Control Biol. 2003, 41, 43–49. [Google Scholar] [CrossRef]
- Alvarenga, A.A.; Valio, I.F.M. Influence of Temperature and Photoperiod on Flowering and Tuberous Root Formation of Pachyrrhizus Tuberosus. Ann. Bot. 1989, 64, 411–414. [Google Scholar] [CrossRef]
- Lehretz, G.G.; Sonnewald, S.; Hornyik, C.; Corral, J.M.; Sonnewald, U. Post-transcriptional Regulation of FLOWERING LOCUS T Modulates Heat-Dependent Source-Sink Development in Potato. Curr. Biol. 2019, 29, 1614–1624.e3. [Google Scholar] [CrossRef] [PubMed]
- Morris, W.L.; Ducreux, L.J.M.; Morris, J.; Campbell, R.; Usman, M.; Hedley, P.E.; Prat, S.; Taylor, M.A.; Lunn, J. Identification of TIMING OF CAB EXPRESSION 1 as a temperature-sensitive negative regulator of tuberization in potato. J. Exp. Bot. 2019, 70, 5703–5714. [Google Scholar] [CrossRef]
- Zhu, J.-Y.; Oh, E.; Wang, T.; Wang, Z.-Y. TOC1–PIF4 interaction mediates the circadian gating of thermoresponsive growth in Arabidopsis. Nat. Commun. 2016, 7, 13692. [Google Scholar] [CrossRef]
- Jung, J.-H.; Barbosa, A.D.; Hutin, S.; Kumita, J.R.; Gao, M.; Derwort, D.; Silva, C.S.; Lai, X.; Pierre, E.; Geng, F.; et al. A prion-like domain in ELF3 functions as a thermosensor in Arabidopsis. Nature 2020, 585, 256–260. [Google Scholar] [CrossRef]
- Gao, K.; Zhang, Z.; Zhu, T.; Coulter, J.A. The influence of flower removal on tuber yield and biomass characteristics of Helianthus tuberosus L. in a semi-arid area. Ind. Crop. Prod. 2020, 150, 112374. [Google Scholar] [CrossRef]
- Jansky, S.H.; Thompson, D.M. The Effect of Flower Removal on Potato Tuber Yield. Can. J. Plant Sci. 1990, 70, 1223–1225. [Google Scholar] [CrossRef]
- Gebregwergis, F.; Gebremicheal, M.; Gebremedhin, H.; Asefa, A. The effects of flower removal and earthing up on tuber yield and quality of potato (Solanum tuberosum L.). J. Agric. Sci. Belgrade 2021, 66, 121–137. [Google Scholar] [CrossRef]
- Adeyemo, O.S.; Chavarriaga, P.; Tohme, J.; Fregene, M.; Davis, S.J.; Setter, T.L. Overexpression of Arabidopsis FLOWERING LOCUS T (FT) gene improves floral development in cassava (Manihot esculenta, Crantz). PLoS ONE 2017, 12, e0181460. [Google Scholar] [CrossRef]
- Abelenda, J.A.; Bergonzi, S.; Oortwijn, M.; Sonnewald, S.; Du, M.; Visser, R.G.F.; Sonnewald, U.; Bachem, C.W.B. Source-Sink Regulation Is Mediated by Interaction of an FT Homolog with a SWEET Protein in Potato. Curr. Biol. 2019, 29, 1178–1186.e6. [Google Scholar] [CrossRef]
- van den Herik, B.; ten Tusscher, K. Undirected Sucrose Efflux Mitigation by the FT-Like SP6A Preferentially Enhances Tuber Resource Partitioning. Front. Plant Sci. 2022, 13, 998. [Google Scholar] [CrossRef]
- Tang, D.; Jia, Y.; Zhang, J.; Li, H.; Cheng, L.; Wang, P.; Bao, Z.; Liu, Z.; Feng, S.; Zhu, X.; et al. Genome evolution and diversity of wild and cultivated potatoes. Nature 2022, 606, 535–541. [Google Scholar] [CrossRef]
- Nicolas, M.; Torres-Pérez, R.; Wahl, V.; Cruz-Oró, E.; Rodríguez-Buey, M.L.; Zamarreño, A.M.; Martín-Jouve, B.; García-Mina, J.M.; Oliveros, J.C.; Prat, S.; et al. Spatial control of potato tuberization by the TCP transcription factor BRANCHED1b. Nat. Plants 2022, 8, 281–294. [Google Scholar] [CrossRef]
- Niwa, M.; Daimon, Y.; Kurotani, K.-i.; Higo, A.; Pruneda-Paz, J.L.; Breton, G.; Mitsuda, N.; Kay, S.A.; Ohme-Takagi, M.; Endo, M.; et al. BRANCHED1 Interacts with FLOWERING LOCUS T to Repress the Floral Transition of the Axillary Meristems in Arabidopsis. Plant Cell 2013, 25, 1228–1242. [Google Scholar] [CrossRef]
- Wang, E.; Liu, T.; Sun, X.; Jing, S.; Zhou, T.; Liu, T.; Song, B. Profiling of the Candidate Interacting Proteins of SELF-PRUNING 6A (SP6A) in Solanum tuberosum. Int. J. Mol. Sci. 2022, 23, 9126. [Google Scholar] [CrossRef]
- Xu, X.; van Lammeren, A.A.M.; Vermeer, E.; Vreugdenhil, D. The Role of Gibberellin, Abscisic Acid, and Sucrose in the Regulation of Potato Tuber Formation in Vitro. Plant Physiol. 1998, 117, 575–584. [Google Scholar] [CrossRef]
- Utsumi, Y.; Tanaka, M.; Utsumi, C.; Takahashi, S.; Matsui, A.; Fukushima, A.; Kobayashi, M.; Sasaki, R.; Oikawa, A.; Kusano, M.; et al. Integrative omics approaches revealed a crosstalk among phytohormones during tuberous root development in cassava. Plant Mol. Biol. 2020, 109, 249–269. [Google Scholar] [CrossRef]
- Susila, H.; Jurić, S.; Gawarecka, K.; Chung, K.; Jin, S.; Kim, S.; Nasim, Z.; Youn, G.; Ahn, J. In vitro Assays to Evaluate Specificity and Affinity in Protein-phospholipid Interactions. Bio-Protocol 2022, 12, e4421. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Susila, H.; Purwestri, Y.A. PEBP Signaling Network in Tubers and Tuberous Root Crops. Plants 2023, 12, 264. https://doi.org/10.3390/plants12020264
Susila H, Purwestri YA. PEBP Signaling Network in Tubers and Tuberous Root Crops. Plants. 2023; 12(2):264. https://doi.org/10.3390/plants12020264
Chicago/Turabian StyleSusila, Hendry, and Yekti Asih Purwestri. 2023. "PEBP Signaling Network in Tubers and Tuberous Root Crops" Plants 12, no. 2: 264. https://doi.org/10.3390/plants12020264
APA StyleSusila, H., & Purwestri, Y. A. (2023). PEBP Signaling Network in Tubers and Tuberous Root Crops. Plants, 12(2), 264. https://doi.org/10.3390/plants12020264





