Assessment of Morphological, Anatomical and Palynological Variation in the Medicinal Plant Disporopsis longifolia Craib (Asparagaceae) for Botanical Quality Control
Abstract
1. Introduction
2. Results
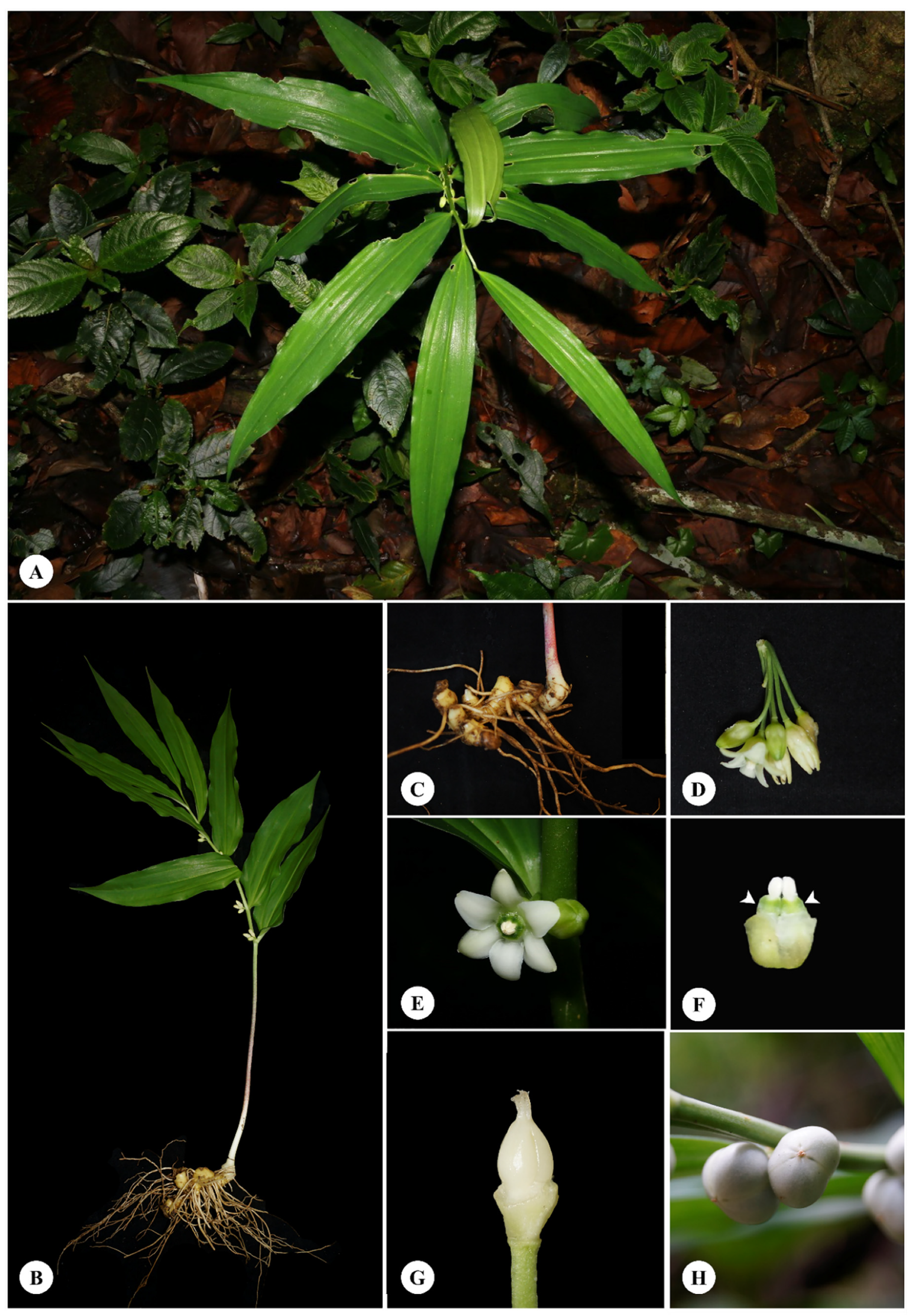
2.1. Species Description and Morphological Characters
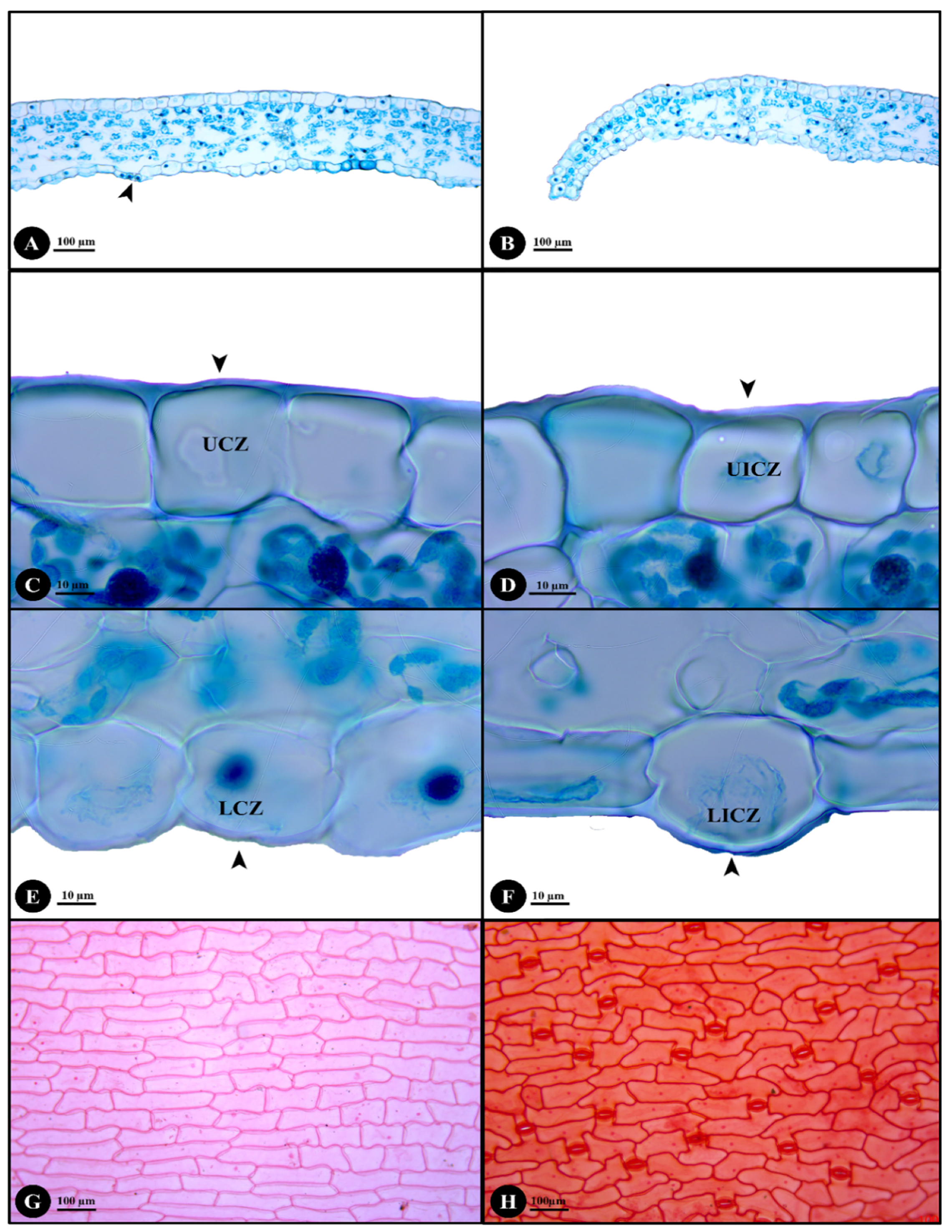
2.2. Stem, Petiole and Leaf Anatomy
2.3. Pollen Morphology
3. Discussion
4. Materials and Methods
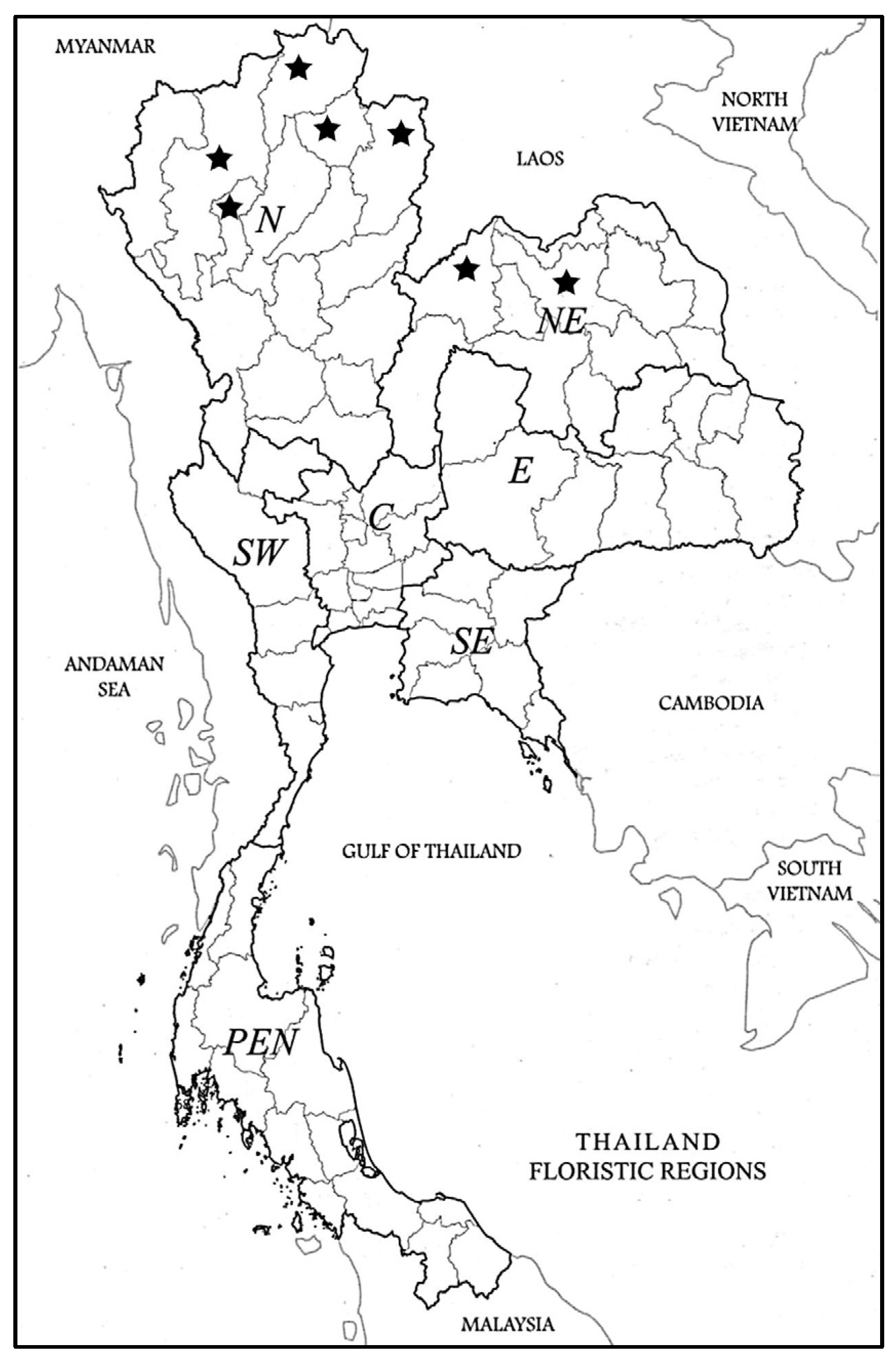
Specimens Examined
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Long, C.-L.; Li, R. Ethnobotanical studies on medicinal plants used by the Red-headed Yao People in Jinping, Yunnan Province, China. J. Ethnopharmacol. 2004, 90, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A.; Langenberger, G.; Feng, L.; Sauerborn, J. Ethnobotanical study of medicinal plants utilised by Hani ethnicity in Naban river watershed national nature reserve, Yunnan, China. J. Ethnopharmacol. 2011, 134, 651–667. [Google Scholar] [CrossRef] [PubMed]
- Sydara, K.; Xayvue, M.; Souliya, O.; Elkington, B.G.; Soejarto, D.D. Inventory of medicinal plants of Lao People’s Democratic Republic: A Mini Review. J. Med. Plant Res. 2014, 8, 1262–1274. [Google Scholar]
- Nguyen, T.S.; Xia, N.H.; Chu, T.V.; Sam, H.V. Ethnobotanical study on medicinal plants in traditional markets of Son La province, Vietnam. For. Soc. 2019, 3, 171–192. [Google Scholar] [CrossRef]
- Srisanga, P.; Wongpakam, S.; Kamkuan, W.; Pekthong, T.; Tovaranonte, J.; Yaso, T.; Nontachaiyapoom, S. Ethnobotany of Akha in Huay Yuak Pa So village, Mae Fah Luang district and Ban Mai Patthana village, Mae Suai district, Chiang Rai province. Thai J. Bot. 2011, 3, 93–114. [Google Scholar]
- Nguanchoo, V.; Srisanga, P.; Swangpol, S.; Prathanturarug, S.; Jenjittikul, T. Food plants in Hmong cuisine in Northern Thailand. Thai J. Bot. 2014, 6, 131–145. [Google Scholar]
- Du Toit, K.; Drewes, S.E.; Bodenstein, J. The chemical structures, plant origins, ethnobotany and biological activities of homoisoflavanones. Nat. Prod. Res. 2010, 24, 457–490. [Google Scholar] [CrossRef]
- Gang, F.-L.; Zhu, F.; Yang, C.-F.; Li, X.-T.; Yang, H.; Sun, M.-X.; Wu, W.-J.; Zhang, J.-W. Antifungal, anti-inflamatory and neuritogenic activity of newly-isolated compounds from Disporopsis aspersa. Nat. Prod. Res. 2018, 34, 1521–1527. [Google Scholar] [CrossRef]
- Thu Ha, T.T.; Vui, D.K.; Hoang, N.H.; Tai, B.H.; Kiem, P.V. Dispolongiosides A and B, two new fucose containing spirostanol glycosides from the rhizomes of Disporopsis longifolia Craib., and their nitric oxide production inhibitory activities. Nat. Prod. Commun. 2021, 16, 1934578X211055013. [Google Scholar] [CrossRef]
- Conran, J.G.; Tamura, M.N. Convallariaceae. In The Families and Genera of Vascular Plants 3. Monocotyledons. Lilianae (Except Orchidaceae); Kubitzki, K., Ed.; Springer: Berlin/Heidelberg, Germany, 1998; p. 190. [Google Scholar]
- Song-Yun, L.; Tamura, M.N. Disporopsis Hance. In Flora of China; Wu, Z.Y., Raven, P.H., Eds.; Science Press: Beijing, China, 2000; Volume 24, pp. 232–234. [Google Scholar]
- Hance, H.F. Disporopsis, genus novum Liliacearum. J. Bot. 1883, 21, 278. [Google Scholar]
- Hutchinson, J. The Families of Flowering Plants, 2nd ed.; Macmillan & Co., Ltd.: London, UK, 1959; p. 749. [Google Scholar]
- Conran, J.G. Cladistic analyses of some net veined Liliiflorae. Plant Syst. Evol. 1989, 168, 123–141. [Google Scholar] [CrossRef]
- Shinwari, Z.K.; Kato, H.; Terauchi, R.; Kawano, S. Phylogenetic relationships among genera in the Liliaceae-Asparagoideae-Polygonatae s.l. inferred from rbcL gene sequence data. Plant Syst. Evol. 1994, 192, 263–277. [Google Scholar] [CrossRef]
- Rudall, P.J.; Conran, J.G.; Chase, M.W. Systematics of Ruscaceae/Convallariaceae: A combined morphological and molecular investigation. Bot. J. Linn. Soc. 2000, 134, 73–92. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, D.-K.; Forest, F.; Fay, M.F.; Chase, M.W. Molecular phylogenetics of Ruscaceae sensu lato and related families (Asparagales) based on plastid and nuclear DNA sequences. Ann. Bot. 2010, 106, 775–790. [Google Scholar] [CrossRef]
- Tamura, M.N. A karyological review of the orders Asparagales and Liliales (Monocotyledonae). Feddes Repert. 1995, 106, 83–111. [Google Scholar] [CrossRef]
- The Angiosperm Phylogeny Group; Chase, M.W.; Christenhusz, M.J.; Fay, M.; Byng, J.W.; Judd, W.S.; Soltis, D.E.; Mabberley, D.J.; Sennikov, A.N.; Soltis, P.S.; et al. An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- Floden, A.J. Molecular phylogenetic studies of the genera of tribe Polygonateae (Asparagaceae: Nolinoideae): Disporopsis, Heteropolygonatum, and Polygonatum. Ph.D. Thesis, University of Tennessee, Knoxville, TN, USA, May 2017. [Google Scholar]
- Floden, A.J. A new Disporopsis (Asparagaceae) transferred from Polygonatum. Phytotaxa 2015, 222, 159–161. [Google Scholar] [CrossRef]
- Lee, Y.M. A Checklist of Plants in Lao PDR; National Agriculture and Forestry Research Institute: Vientiane, Laos, 2016; p. 77. [Google Scholar]
- Steenis, V. Flora Malesiana; The Hague Boston: London, UK, 1983; Volume 9, p. 219. [Google Scholar]
- Charoenphol, C.C. Studies in Thai Liliaceae. Thai For. Bull. Bot. 1973, 7, 87–94. [Google Scholar]
- Royal Forest Herbarium. Flora of Thailand Project; Department of National Park, Wildlife and Plant Conservation: Bangkok, Thailand, 2022. [Google Scholar]
- Xinqi, C.; Song-Yun, L.; Jiemei, X.; Tamura, M.N. Liliaceae AL Jussieu. In Flora of China; Wu, Z.Y., Raven, P.H., Eds.; Science Press: Beijing, China, 2000; Volume 24, pp. 73–263. [Google Scholar]
- Conover, M.V. Epidermal patterns of the reticulate-veined Liliiflorae and their parallel-veined allies. Bot. J. Linn. Soc. 1991, 107, 295–312. [Google Scholar] [CrossRef]
- Wang, L.; Gu, L.; Zhao, C.; Liu, J. Pollen morphology of Polygonatae and its systematic significance. Palynology 2018, 42, 255–272. [Google Scholar] [CrossRef]
- Coughlan, P.; Carolan, J.C.; Hook, I.L.I.; Kilmartin, L.; Hodkinson, T.R. Phylogenetics of Taxus Using the Internal Transcribed Spacers of Nuclear Ribosomal DNA and Plastid trnL-F Regions. Horticulturae 2020, 6, 19. [Google Scholar] [CrossRef]
- Høyer, A.K.; Hodkinson, T.R. Hidden Fungi: Combining Culture-Dependent and -Independent DNA Barcoding Reveals Inter-plant Variation in Species Richness of Endophytic Root Fungi in Elymus repens. J. Fungi 2021, 7, 466. [Google Scholar] [CrossRef] [PubMed]
- Van Welzen, P.; Madern, A.; Raes, N.; Parnell, J.; Simpson, D.; Byrne, C.; Curtis, T.; Macklin, J.; Trias-Blasi, A.; Prajaksood, A.; et al. The Current and Future Status of Floristic Provinces in Thailand. In Land Use, Climate Change and Biodiversity Modeling: Perspectives and Applications; Trisurat, Y., Shrestha, R., Alkemade, R., Eds.; IGI Global: Hershey, PA, USA, 2011; pp. 219–247. [Google Scholar]
- Thiers, B. Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff. New York Botanical Garden’s Virtual Herbarium. 2016. Available online: http://sweetgum.nybg.org/science/ih/ (accessed on 16 June 2022).
- Thammathaworn, A. Handbook by Paraffin Method; Department of Biology, Faculty of Science, Khon Kaen University: Khon Kaen, Thailand, 1995; p. 1. [Google Scholar]
- Kermanee, P. Plant Microtechnique; Kasetsart University Press: Bangkok, Thailand, 2008; p. 105. [Google Scholar]
- Erdtman, G. Pollen Morphology and Plant Taxonomy; Hafner Publishing Company: New York, NY, USA, 1972; p. 6. [Google Scholar]
- Halbritter, H.; Ulrich, S.; Grímsson, F.; Weber, M.; Zetter, R.; Hesse, M.; Buchner, R.; Svojtka, M.; Frosch-Radivo, A. Illustrated Pollen Terminology; Springer: Cham, Switzerland, 2018; p. 105. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarapan, A.; Hodkinson, T.R.; Suwanphakdee, C. Assessment of Morphological, Anatomical and Palynological Variation in the Medicinal Plant Disporopsis longifolia Craib (Asparagaceae) for Botanical Quality Control. Plants 2023, 12, 259. https://doi.org/10.3390/plants12020259
Sarapan A, Hodkinson TR, Suwanphakdee C. Assessment of Morphological, Anatomical and Palynological Variation in the Medicinal Plant Disporopsis longifolia Craib (Asparagaceae) for Botanical Quality Control. Plants. 2023; 12(2):259. https://doi.org/10.3390/plants12020259
Chicago/Turabian StyleSarapan, Anuwat, Trevor R. Hodkinson, and Chalermpol Suwanphakdee. 2023. "Assessment of Morphological, Anatomical and Palynological Variation in the Medicinal Plant Disporopsis longifolia Craib (Asparagaceae) for Botanical Quality Control" Plants 12, no. 2: 259. https://doi.org/10.3390/plants12020259
APA StyleSarapan, A., Hodkinson, T. R., & Suwanphakdee, C. (2023). Assessment of Morphological, Anatomical and Palynological Variation in the Medicinal Plant Disporopsis longifolia Craib (Asparagaceae) for Botanical Quality Control. Plants, 12(2), 259. https://doi.org/10.3390/plants12020259







