Long-Term Changes in Organic Matter Content and Soil Moisture Determine the Degree of Root and Soil Respiration
Abstract
1. Introduction
2. Results and Discussion
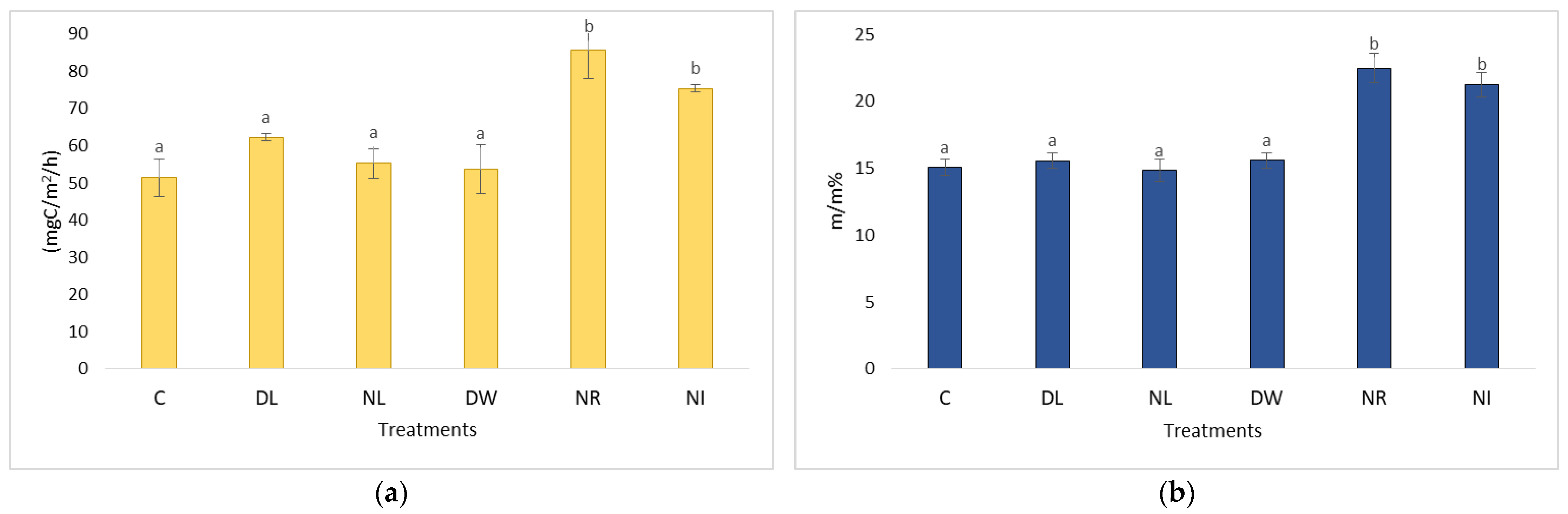
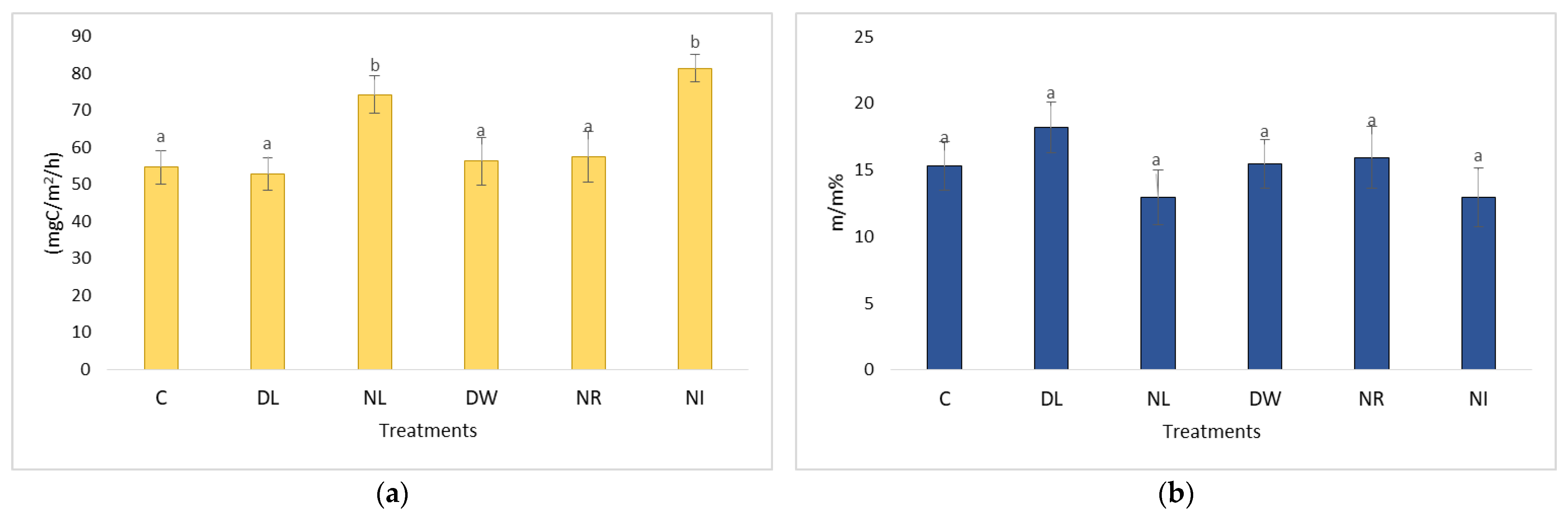
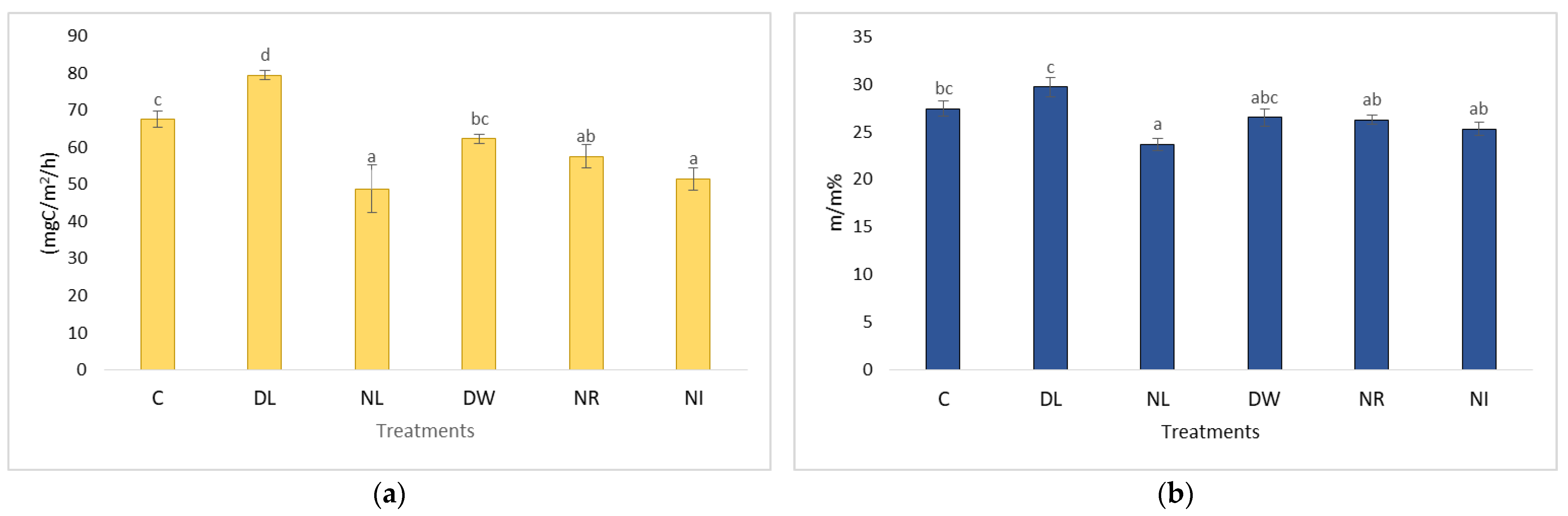
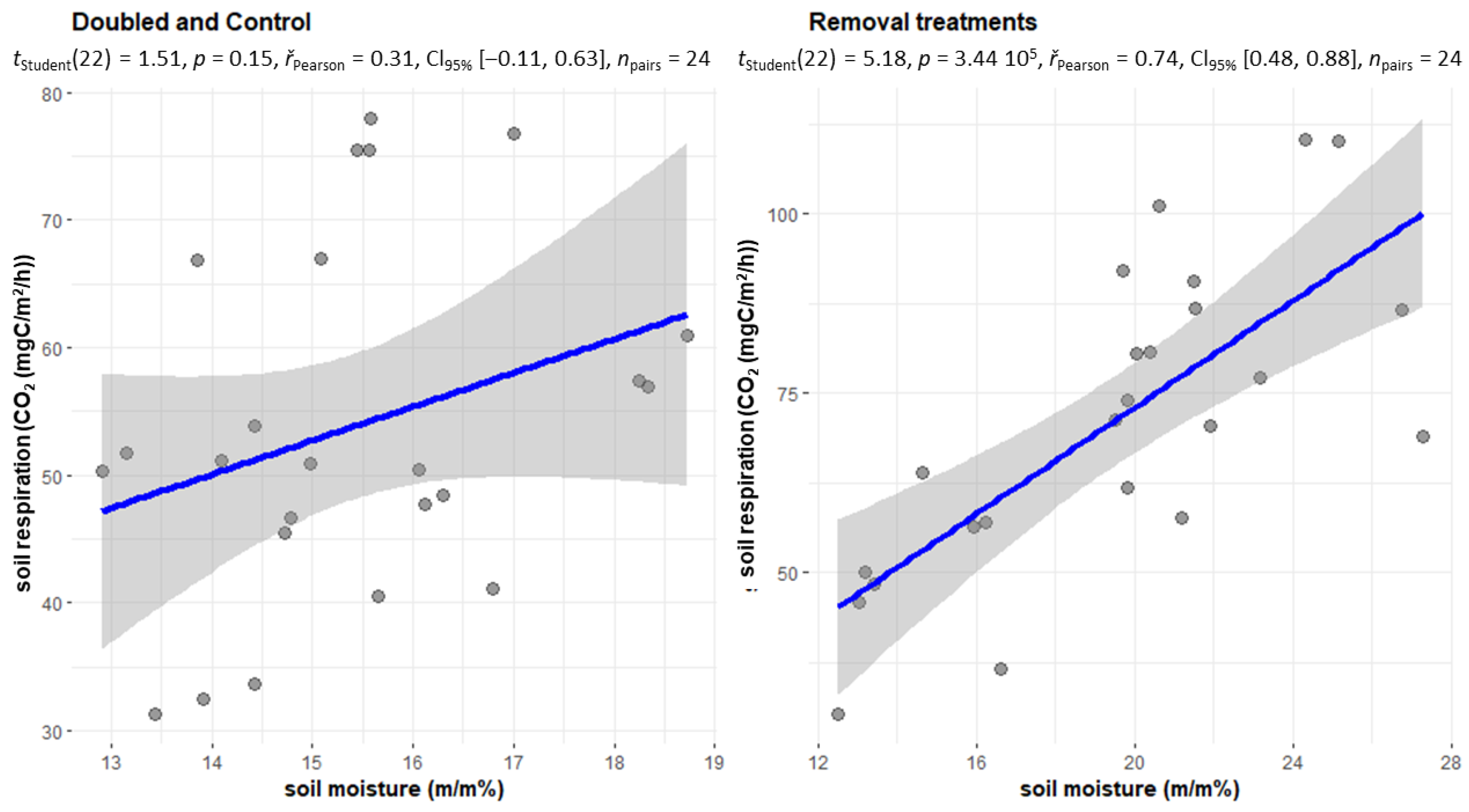
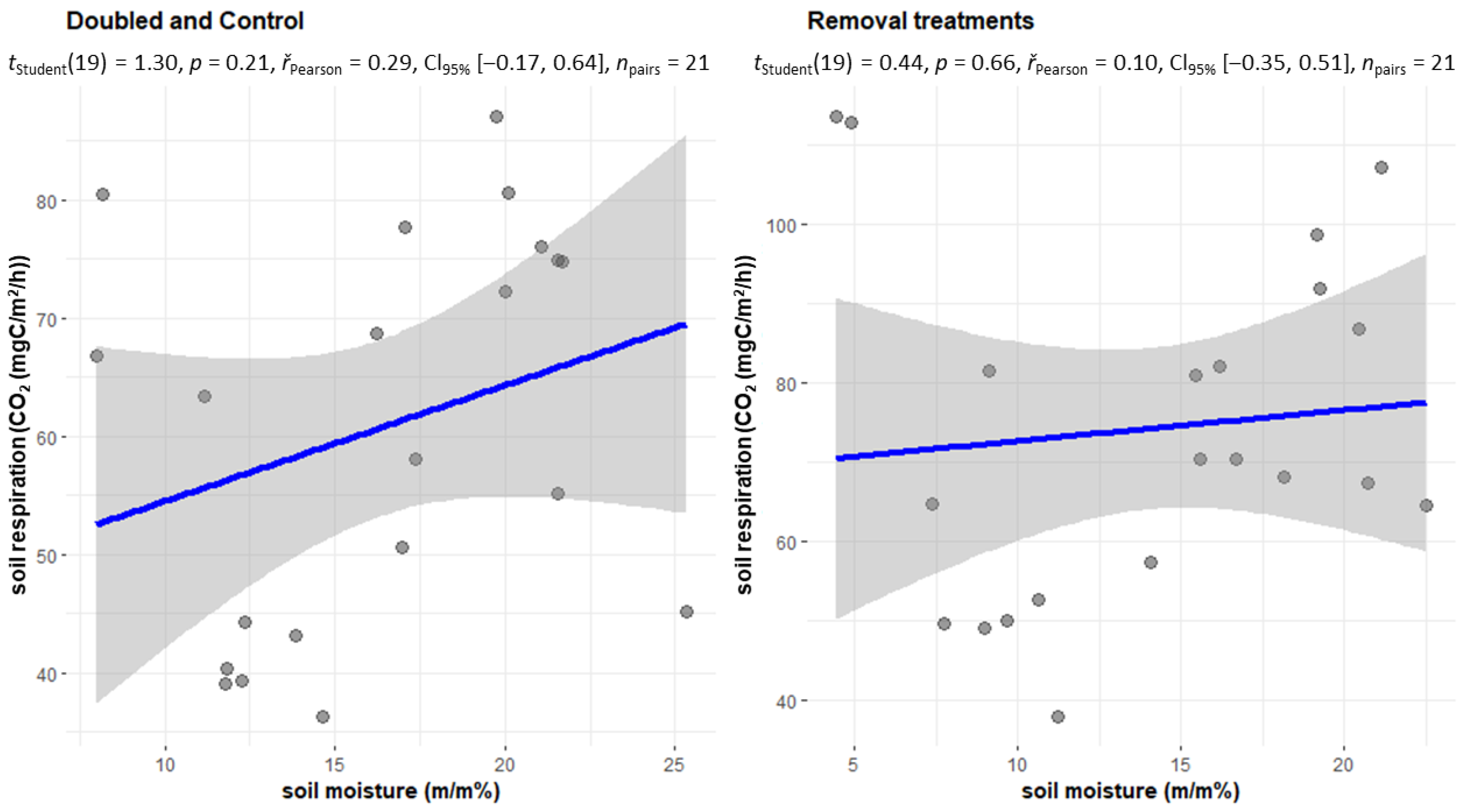
2.1. Changes in Soil Respiration during Dry Periods
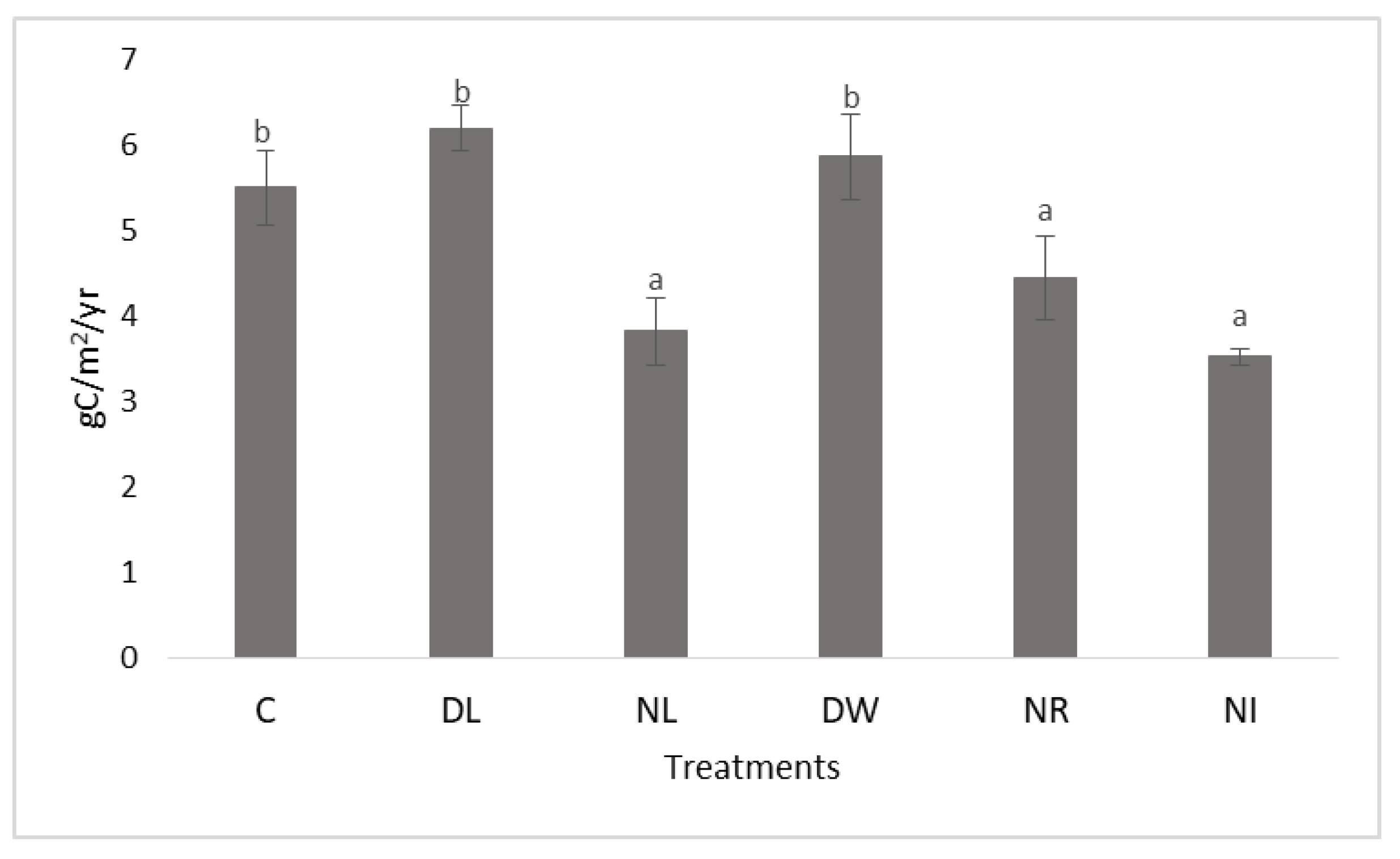
2.2. Evolution of Soil Carbon Content at the End of the Experimental Period
2.3. Changes in Soil Respiration during Dry Periods
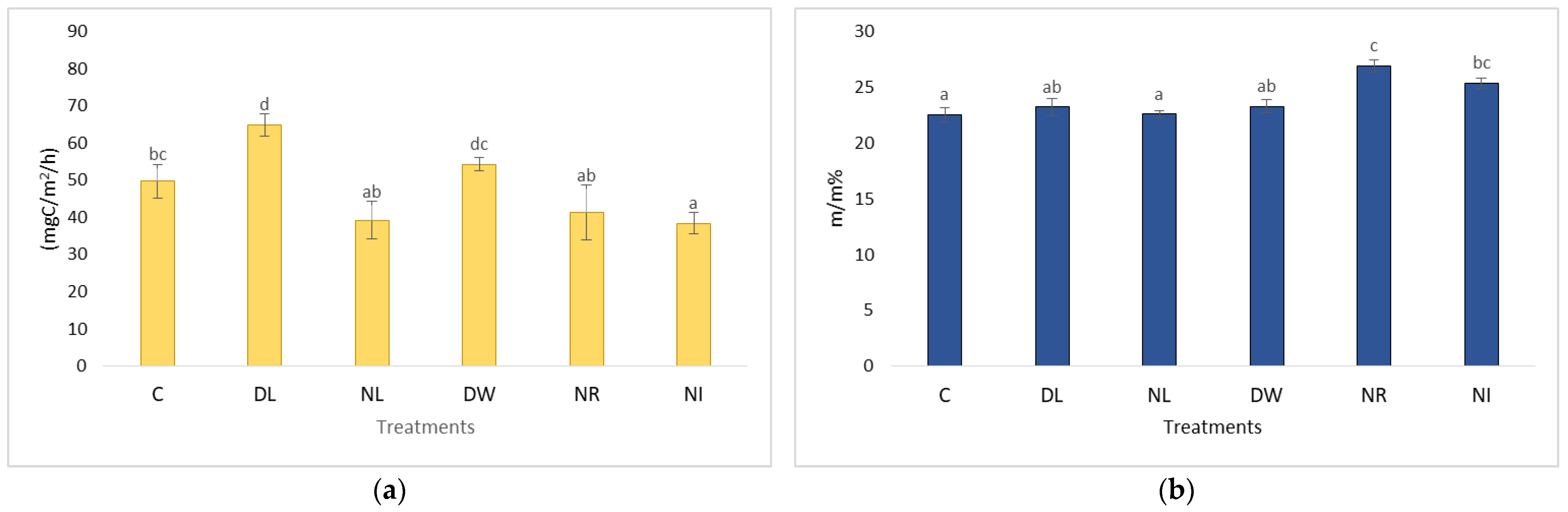
2.4. The CO2 Emissions of the Treatments Withdrawal (NL, NR, NI) and Doubling (DL, DW) and C in the Case of Soil Moisture below 20 m/m%
3. Materials and Methods
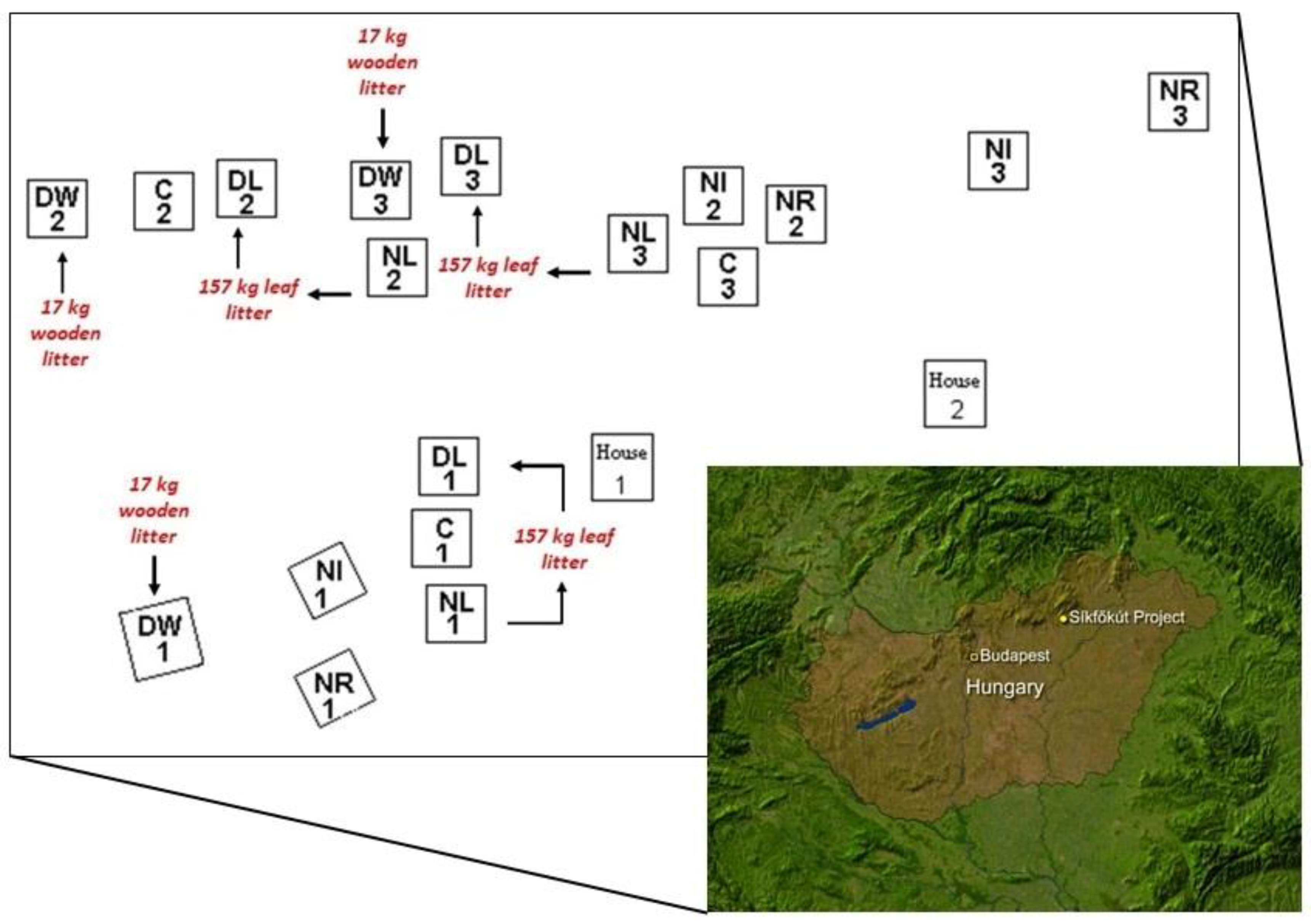
3.1. Study Area
3.2. Soil Sampling and Test Methods
3.3. Statistical Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bahn, M.; Reichstein, M.; Davidson, E.A.; Grünzweig, J.; Jung, M.; Carbone, M.S.; Epron, D.; Misson, L.; Nouvellon, Y.; Roupsard, O.; et al. Soil respiration at mean annual temperature predicts annual total across vegetation types and biomes. Biogeosciences 2010, 7, 2147–2157. [Google Scholar] [CrossRef]
- Oertel, C.; Matschullat, J.; Zurba, K.; Zimmermann, F.; Erasmi, S. Greenhouse gas emissions from soils—A review. Geochemistry 2016, 76, 327–352. [Google Scholar] [CrossRef]
- Fekete, I.; Berki, I.; Lajtha, K.; Trumbore, S.; Francioso, O.; Gioacchini, P.; Montecchio, D.; Várbiró, G.; Béni, Á.; Makádi, M.; et al. How will a drier climate change carbon sequestration in soils of the deciduous forests of Central Europe? Biogeochemistry 2021, 152, 13–32. [Google Scholar] [CrossRef]
- Kotroczó, Z.; Veres, Z.; Biró, B.; Tóth, J.A.; Fekete, I. Influence of temperature and organic matter content on soil respiration in a deciduous oak forest. Eurasian. J. Soil Sci. 2014, 3, 303–310. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Sources of CO2 efflux from soil and review of partitioning methods. Soil Biol. Biochem. 2006, 38, 425–448. [Google Scholar] [CrossRef]
- Ke, X.; Winter, K.; Filser, J. Effects of soil mesofauna and farming management on decomposition of clover litter: A microcosm experiment. Soil Biol. Biochem. 2005, 37, 731–738. [Google Scholar] [CrossRef]
- Stoyan, H.; De-Polli, H.; Böhm, S. Spatial Heterogeneity of Soil Respiration and Related Properties at the Plant Scale. Plant Soil 2000, 222, 203–214. [Google Scholar] [CrossRef]
- Kotroczó, Z.; Fekete, I.; Tóth, J.A.; Tóthmérész, B.; Balázsy, S. Effect of leaf- and root-litter manipulation for carbon-dioxide efflux in forest soil. Cer. Res. Comm. 2008, 36, 663–666. Available online: https://www.jstor.org/stable/90002791 (accessed on 20 December 2022).
- Varga, C.; Fekete, I.; Kotroczó, Z.; Krakomperger, Z.; Vincze, G. The Effect of litter on soil organic matter (SOM) turnover in Síkfőkút site. Cer. Res.Comm. 2008, 36, 547–550. Available online: https://www.jstor.org/stable/90002762 (accessed on 20 December 2022).
- Raich, J.W.; Nadelhoffer, K.J. Belowground carbon allocation in forest ecosystems: Global trends. Ecology 1989, 70, 1346–1354. [Google Scholar] [CrossRef]
- Lellei-Kovács, E.; Kovács-Láng, E.; Kalapos, T.; Botta-Dukát, Z.; Barabás, S.; Beier, C. Experimental warming does not enhance soil respiration in a semiarid temperate forest-steppe ecosystem. Community Ecol. 2008, 9, 29–37. [Google Scholar] [CrossRef]
- Gavrichkova, O.; Moscatelli, M.C.; Kuzyakov, Y.; Grego, S.; Valentini, R. Influence of defoliation on CO2 efflux from soil and microbial activity in a Mediterranean grassland. Agr. Ecosyst. Environ. 2010, 136, 87–96. [Google Scholar] [CrossRef]
- Heinemeyer, A.; Wilkinson, M.; Vargas, R.; Subke, J.; Casella, E.; Morison, J.I.L.; Ineson, P. Exploring the “overflow tap” theory: Linking forest soil CO2 fluxes and individual mycorrhizosphere components to photosynthesis. Biogeosciences 2012, 9, 79–95. [Google Scholar] [CrossRef]
- Sulzman, E.W.; Brant, J.B.; Bowden, R.D.; Lajtha, K. Contribution of aboveground litter, belowground litter, and rhizosphere respiration to total soil CO2 efflux in an old growth coniferous forest. Biogeochemistry 2005, 73, 231–256. [Google Scholar] [CrossRef]
- Bahn, M.; Janssens, I.A.; Reichstein, M.; Smith, P.; Trumbore, S.E. Soil respiration across scales: Towards an integration of patterns and processes. New Phytol. 2010, 186, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sayer, E.J.; Zhang, W.; Ye, J.; Yuan, Z.; Lin, F.; Hao, Z.; Fang, S.; Mao, Z.; Ren, J.; et al. Seasonal Influenceof Biodiversity on Soil Respirationin a Temperate Forest. Plants 2022, 11, 3391. [Google Scholar] [CrossRef] [PubMed]
- Somogyi, Z.; Horváth, B. Az 1930 óta telepített erdők szénlekötéséről. (On the carbon sequestration of forests established after 1930). Erd. Lapok 2006, 116, 257–259. [Google Scholar]
- Rieder, Á.; Madarász, B.; Szabó, J.A.; Zacháry, D.; Vancsik, A.; Ringer, M.; Szalai, Z.; Jakab, G. Soil organic matter alteration velocity due to land-use change: A case study under conservation agriculture. Sustainability 2018, 10, 943. [Google Scholar] [CrossRef]
- Madarász, B.; Jakab, G.; Szalai, Z.; Juhos, K.; Kotroczó, Z.; Tóth, J.A.; Ladányi, M. Long-term effects of conservation tillage on soil erosion in Central Europe: A random forest-based approach. Soil Till. Res. 2021, 209, 104959. [Google Scholar] [CrossRef]
- Nadelhoffer, K.; Boone, R.; Bowden, R.D.; Canary, J.; Kaye, J.; Micks, P.; Ricca, A.; McDowell, W.; Aitkenhead, J. The DIRT experiment. In Forests in Time; Foster, D.R., Aber, D.J., Eds.; Yale University Press: New Haven, CT, USA, 2004. [Google Scholar]
- Fekete, I.; Kotroczó, Z.; Varga, C.; Hargitai, R.; Townsend, K.; Csányi, G.; Várbiró, G. Variability of organic matter inputs affects soil moisture and soil biological parameters in a European detritus manipulation experiment. Ecosystems 2012, 15, 792–803. [Google Scholar] [CrossRef]
- Fekete, I.; Béni, Á.; Juhos, K.; Kotroczó, Z. Húsz éves avarmanipulációs kísérlet hatásai barna erdőtalaj szén tartalmára és vízkapacitására: Síkfőkút DIRT Project. Agrokémia És Talajt. 2022, 71, 239–253. [Google Scholar] [CrossRef]
- Karhu, K.; Auffret, M.D.; Dungait, J.A.J.; Hopkins, D.W.; Prosser, J.I.; Singh, B.K.; Subke, J.-A.; Wookey, P.A.; Ågren, G.I.; Sebastià, M.-T.; et al. Temperature sensitivity of soil respiration rates enhanced by microbial community response. Nature 2014, 513, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.; Welp, G.; Amelung, W. The temperature sensitivity (Q10) of soil respiration: Controlling factors and spatial prediction at regional scale based on environmental soil classes. Global. Biogeochem. Cy. 2018, 32, 306–323. [Google Scholar] [CrossRef]
- Fekete, I.; Kotroczó, Z.; Varga, C.; Nagy, P.T.; Várbíró, G.; Bowden, R.D.; Tóth, J.A.; Lajtha, K. Alterations in forest detritus inputs influence soil carbon concentration and soil respiration in a Central-European deciduous forest. Soil Biol. Biochem. 2014, 74, 106–114. [Google Scholar] [CrossRef]
- Zhang, B.; Li, S.; Chen, S.; Ren, T.; Yang, Z.; Zhao, H.; Liang, Y.; Han, X. Arbuscular mycorrhizal fungi regulate soil respiration and its response to precipitation change in a semiarid steppe. Sci. Rep. UK 2016, 6, 19990. [Google Scholar] [CrossRef]
- Heinemeyer, A.; Tortorella, D.; Petrovičová, B.; Gelsomino, A. Partitioning of soil CO2 flux components in a temperate grassland ecosystem. Eur. J. Soil Sci. 2012, 63, 249–260. [Google Scholar] [CrossRef]
- Matteucci, M.; Gruening, C.; Ballarin, I.G.; Seufert, G.; Cescatti, A. Components, drivers and temporal dynamics of ecosystem respiration in a Mediterranean pine forest. Soil Biol. Biochem. 2015, 88, 224–235. [Google Scholar] [CrossRef]
- Pintér, K.; Barcza, Z.; Balogh, J.; Czóbel, S.; Csintalan, Z.; Tuba, Z.; Nagy, Z. Interannual variability of grasslands’ carbon balance depends on soil type. Comm. Ecol. 2008, 9, 43–48. [Google Scholar] [CrossRef]
- Molen, M.K.; Dolman, A.J.; Ciais, P.; Eglin, T.; Gobron, N.; Law, B.E.; Meir, P.; Peters, W.; Phillips, O.L.; Reichstein, M.; et al. Drought and ecosystem carbon cycling. Agr. Forest Meteorol. 2011, 151, 765–773. [Google Scholar] [CrossRef]
- Rey, A.; Pegoraro, E.; Tedeschi, V.; De Parri, I.; Jarvis, P.G.; Valentini, R. Annual variation in soil respiration and its components in a coppice oak forest in Central Italy. Glob. Chang. Biol. 2002, 8, 851–866. [Google Scholar] [CrossRef]
- Risk, D.; Nickerson, N.; Phillips, C.L.; Kellman, L.; Moroni, M. Drought alters respired δ13CO2 from autotrophic, but not heterotrophic soil respiration. Soil Biol. Biochem. 2012, 50, 26–32. [Google Scholar] [CrossRef]
- Casals, P.; Lopez-Sangil, L.; Carrara, A.; Gimeno, C.; Nogués, S. Autotrophic and heterotrophic contributions to short-term soil CO2 efflux following simulated summer precipitation pulses in a Mediterranean dehesa. Glob. Biogeo. Cy. 2011, 25, 1–12. [Google Scholar] [CrossRef]
- Suseela, V.; Conant, R.T.; Wallenstein, M.D.; Dukes, J.S. Effects of soil moisture on the temperature sensitivity of heterotrophic respiration vary seasonally in an old-field climate change experiment. Glob. Chang. Biol. 2012, 18, 336–348. [Google Scholar] [CrossRef]
- Balogh, J.; Papp, M.; Pintér, K.; Fóti, S.; Posta, K.; Eugster, W.; Nagy, Z. Autotrophic component of soil respiration is repressed by drought more than the heterotrophic one in dry grasslands. Biogeosciences 2016, 13, 5171–5182. [Google Scholar] [CrossRef]
- Sanaullah, M.; Chabbi, A.; Rumpel, C.; Kuzyakov, Y. Carbon allocation in grassland communities under drought stress followed by 14C pulse labeling. Soil Biol. Biochem. 2012, 55, 132–139. [Google Scholar] [CrossRef]
- Nagy, Z.; Pintér, K.; Pavelka, M.; Darenová, E.; Balogh, J. Carbon fluxes of surfaces vs. ecosystems: Advantages of measuring eddy covariance and soil respiration simultaneously in dry grassland ecosystems. Biogeosciences 2011, 8, 2523–2534. [Google Scholar] [CrossRef]
- Switoniak, M.; Charzynski, P.; Novak, T.J.; Zalewska, K.; Bednarek, R. Forested hilly landscape of Büukkalja Foothill (Hungary). In Soil Sequences Atlas; Nicholaus Copernicus University Press: Toruń, Poland, 2014; pp. 169–181. [Google Scholar]
- Juhos, K.; Madarász, B.; Kotroczó, Z.; Béni, Á.; Makádi, M.; Fekete, I. Carbon sequestration of forest soils is reflected by changes in physicochemical soil indicators—A comprehensive discussion of a long-term experiment on a detritus manipulation. Geoderma 2021, 385, 114918. [Google Scholar] [CrossRef]
- Chichester, F.W.; Chaison, R.F. Analysis of carbon in calcareous soils using a two temperature dry combustion infrared instrumental procedure. Soil Sci. 1992, 153, 237–241. [Google Scholar] [CrossRef]
- Raich, J.W.; Bowden, R.D.; Steudler, P.A. Comparison of two static chamber techniques for determining carbon dioxide eflux from forest soils. Soil Sci. Soc. Am. J. 1990, 54, 1754–1757. [Google Scholar] [CrossRef]
- Grogan, P. CO2 flux measurement using soda lime: Correction for water formed during CO2 adsorption. Ecology 1998, 79, 1467–1468. [Google Scholar] [CrossRef]
| g C/m2/day | 2002–2004 | 2010–2012 | |||
|---|---|---|---|---|---|
| Treatments | Dry | Humid | Dry | Humid | |
| C | 1.23 ± 0.07 | 1.19 ± 0.06 | 1.31 ± 0.06 | 1.62 ± 0.03 | |
| NL | 1.32 ± 0.06 | 0.94 ± 0.07 | 1.78 ± 0.07 | 1.17 ± 0.09 | |
| DL | 1.49 ± 0.01 | 1.55 ± 0.04 | 1.26 ± 0.06 | 1.90 ± 0.02 | |
| NR | 2.05 ± 0.11 | 0.99 ± 0.10 | 1.37 ± 0.09 | 1.38 ± 0.04 | |
| DW | 1.29 ± 0.09 | 1.30 ± 003 | 1.35 ± 0.09 | 1.49 ± 0.02 | |
| NI | 1.81 ± 0.01 | 0.92 ± 0.04 | 1.95 ± 0.05 | 1.23 ± 0.04 | |
| Treatments | Description |
|---|---|
| Control (C) | Normal litter inputs. Average litter amount typical of the forest site. |
| No Litter (NL) | Aboveground inputs are excluded from plots. Leaf litter was removed by raking. This process was repeated continuously every year. |
| No Roots (NR) | Plant roots are excluded, there are no herbs above the ground, and growth from the side is also inhibited. The leaf litter is left on the ground. The plots were trenched around 100 cm deep with root-proof Delta MS 500 PE foil, which was 0.6 mm thick. To eliminate root production, plants were cleared (bushes had been cut out at the establishment). |
| No Inputs (NI) | Aboveground inputs are excluded from plots; the belowground inputs are provided as in NR plots. This treatment is a combination of NR + NL plots. |
| Double Wood (DW) | Aboveground wood debris inputs are doubled by adding wood to each plot. Annual wood litter amount was measured by boxes placed at the site, and double its amount was applied in the case of every DW plot. |
| Double Litter (DL) | Aboveground leaf inputs are doubled by adding litter removed from No Litter plots. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotroczó, Z.; Makádi, M.; Kocsis, T.; Béni, Á.; Várbíró, G.; Fekete, I. Long-Term Changes in Organic Matter Content and Soil Moisture Determine the Degree of Root and Soil Respiration. Plants 2023, 12, 251. https://doi.org/10.3390/plants12020251
Kotroczó Z, Makádi M, Kocsis T, Béni Á, Várbíró G, Fekete I. Long-Term Changes in Organic Matter Content and Soil Moisture Determine the Degree of Root and Soil Respiration. Plants. 2023; 12(2):251. https://doi.org/10.3390/plants12020251
Chicago/Turabian StyleKotroczó, Zsolt, Marianna Makádi, Tamás Kocsis, Áron Béni, Gábor Várbíró, and István Fekete. 2023. "Long-Term Changes in Organic Matter Content and Soil Moisture Determine the Degree of Root and Soil Respiration" Plants 12, no. 2: 251. https://doi.org/10.3390/plants12020251
APA StyleKotroczó, Z., Makádi, M., Kocsis, T., Béni, Á., Várbíró, G., & Fekete, I. (2023). Long-Term Changes in Organic Matter Content and Soil Moisture Determine the Degree of Root and Soil Respiration. Plants, 12(2), 251. https://doi.org/10.3390/plants12020251







