Modern Approaches for the Development of New Herbicides Based on Natural Compounds
Abstract
1. Introduction
2. Current Trends in Herbicide Development
2.1. Screening
2.1.1. Virtual Screening
2.1.2. Bioassay Techniques
2.2. Mechanisms of Action and Molecular Targets of Herbicides
2.3. Formulation of Herbicides
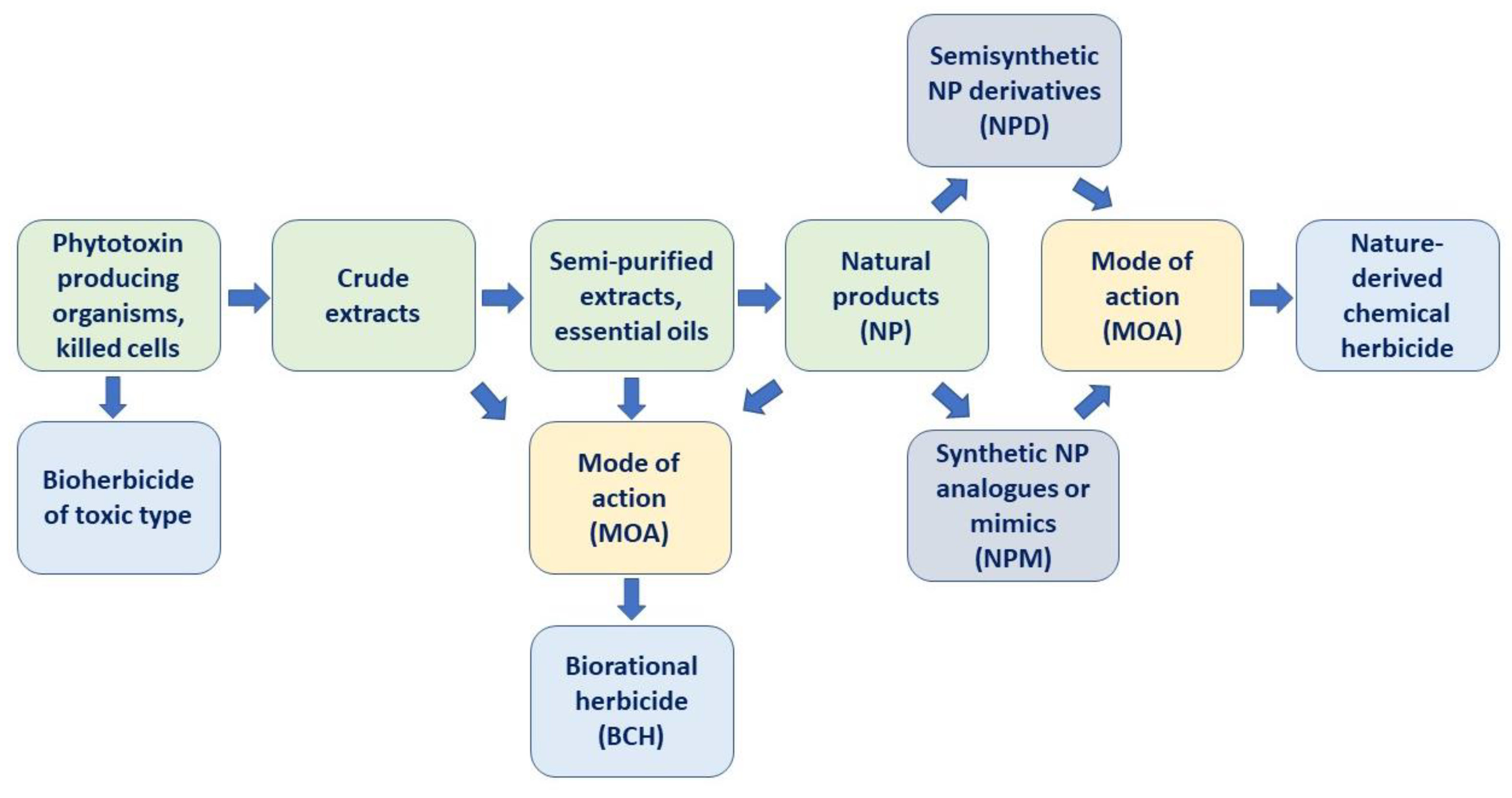
3. Perspectives of Natural Compounds as Biorational Herbicides
3.1. Promising and Commercialized Natural Compounds
3.2. Enhancement of Biorational Herbicides
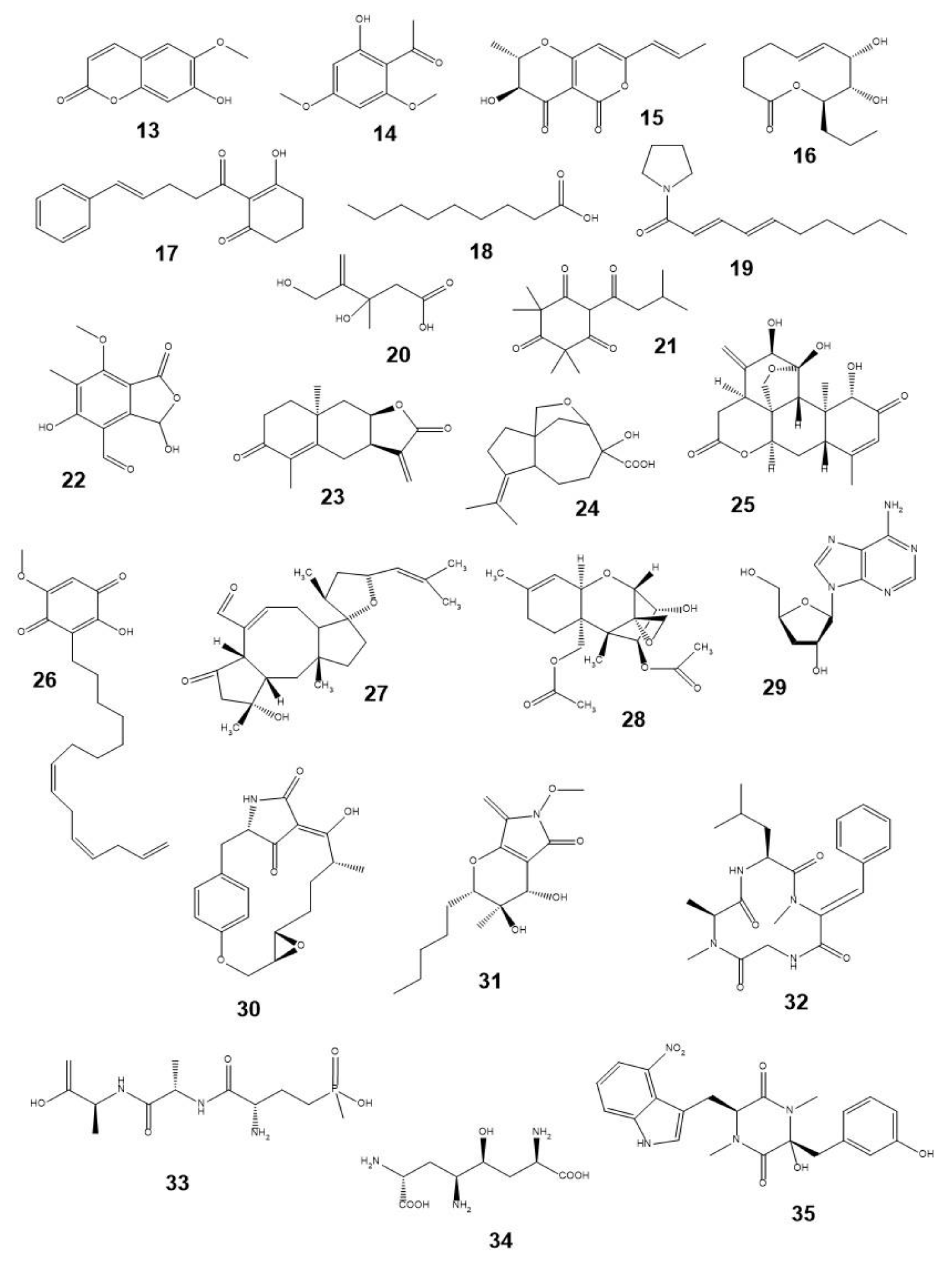
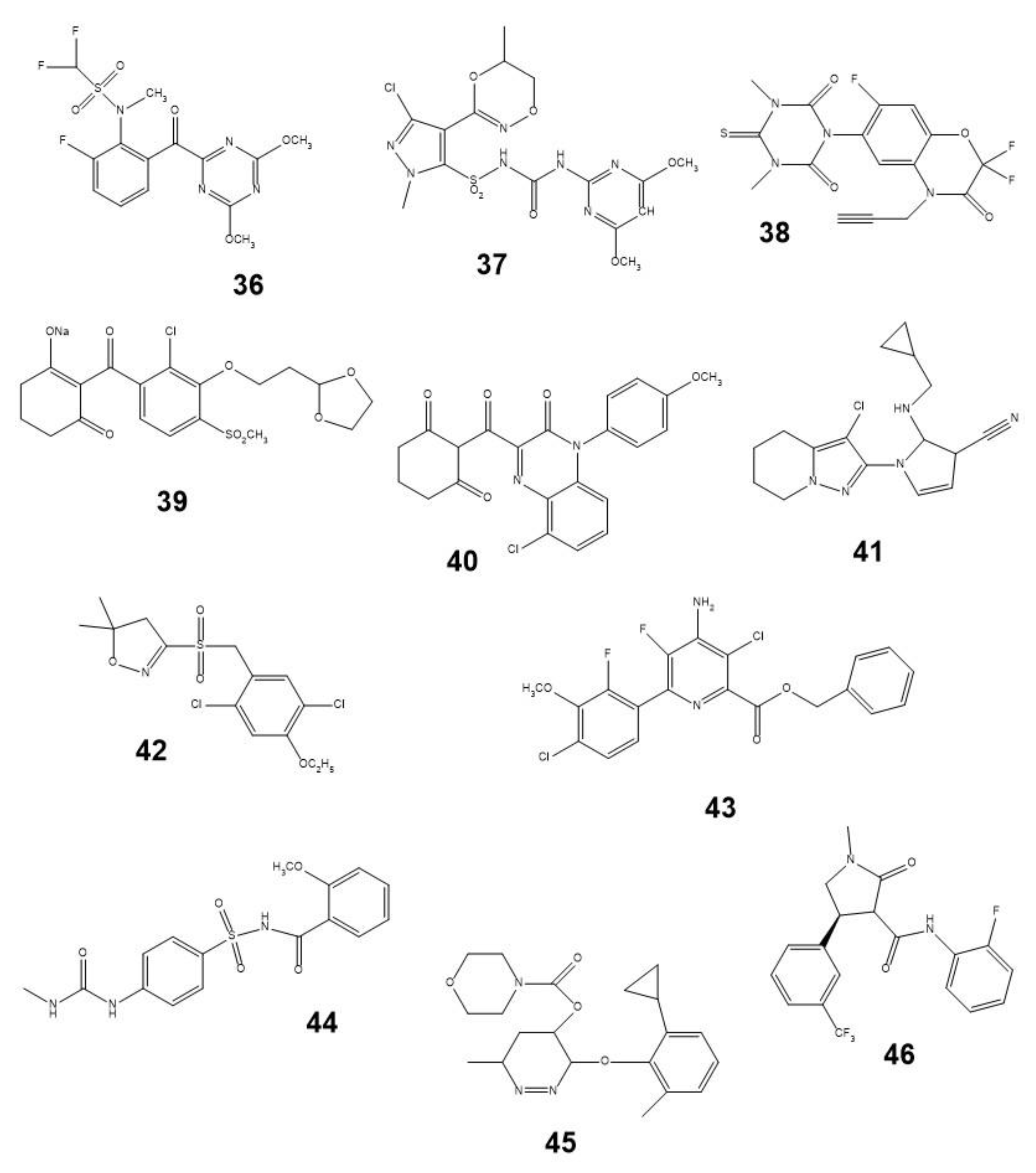
4. Natural Phytotoxins as Prototypes of Synthetic Herbicides
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perspectives on Wheat Yield Losses Due to Weeds in North America. Available online: https://wssa.net/wp-content/uploads/Wheat-yield-loss-POSTER.pdf (accessed on 11 November 2022).
- Chauhan, B.S. Grand challenges in weed management. Front. Agron. 2020, 1, 1–4. [Google Scholar] [CrossRef]
- Dayan, F.E. Current Status and Future Prospects in Herbicide Discovery. Plants 2019, 8, 341. [Google Scholar] [CrossRef] [PubMed]
- Haywood, J.; Vadlamani, G.; Stubbs, K.A.; Mylne, J.S. Antibiotic resistance lessons for the herbicide resistance crisis. Pest Manag. Sci. 2021, 77, 3807–3814. [Google Scholar] [CrossRef] [PubMed]
- MacLaren, C.; Storkey, J.; Menegat, A.; Metcalfe, H.; Dehnen-Schmutz, K. An ecological future for weed science to sustain crop production and the environment. A review. Agron. Sustain. Dev. 2020, 40, 1–29. [Google Scholar] [CrossRef]
- Westwood, J.H.; Charudattan, R.; Duke, S.O.; Fennimore, S.A.; Marrone, P.; Slaughter, D.C.; Swanton, C.; Zollinger, R. Weed Management in 2050: Perspectives on the future of weed science. Weed Sci. 2018, 66, 275–285. [Google Scholar] [CrossRef]
- Cobb, A.H.; Reade, J.P.H. Herbicides and Plant Physiology, 2nd ed.; Wiley-Blackwell: Oxford, UK, 2007; 298p. [Google Scholar]
- Mesnage, R. Coformulants in commercial herbicides. In Herbicides: Chemistry, Efficacy, Toxicology, and Environmental Impacts; Mesnage, R., Zahler, J.G., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 87–111. [Google Scholar] [CrossRef]
- Hachisu, S. Strategies for discovering resistance-breaking, safe and sustainable commercial herbicides with novel modes of action and chemotypes. Pest Manag. Sci. 2021, 77, 3042–3048. [Google Scholar] [CrossRef]
- Qu, R.Y.; He, B.; Yang, J.F.; Lin, H.Y.; Yang, W.C.; Wu, Q.Y.; Li, Q.X.; Yang, G.F. Where are the new herbicides? Pest Manag. Sci. 2021, 77, 2620–2625. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, Y.; Garcia, E.A.L.; Wang, Y.; Butch, C.J. Drug chemical space as a guide for new herbicide development: A cheminformatic analysis. J. Agric. Food Chem. 2022, 70, 9625–9636. [Google Scholar] [CrossRef]
- Schreiber, K.J.; Austin, R.S.; Gong, Y.; Zhang, J.; Fung, P.; Wang, P.W.; Guttman, D.S.; Desveax, D. Forward chemical genetic screens in Arabidopsis identify genes that influence sensitivity to the phytotoxic compound sulfamethoxazole. BMC Plant Biol. 2012, 12, 226. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, W.; Wang, X.; Yang, L.; Han, S.; Chen, S.; Strasser, R.J.; Valverde, B.E.; Qiang, S. Comparative phytotoxicity of usnic acid, salicylic acid, cinnamic acid and benzoic acid on photosynthetic apparatus of Chlamydomonas reinhardtii. Plant Physiol. Biochem. 2018, 128, 1–12. [Google Scholar] [CrossRef]
- Abdullah, H.S.T.S.H.; Chi, P.W.; Omar, D.; Chuah, T.S. Herbicidal properties of antihypertensive drugs: Calcium channel blockers. Sci. Rep. 2021, 11, 14227. [Google Scholar] [CrossRef]
- Sukhoverkov, K.V.; Corral, M.G.; Leroux, J.; Haywood, J.; Johnen, P.; Newton, T.; Stubbs, K.A.; Mylne, J.S. Improved herbicide discovery using physico-chemical rules refined by antimalarial library screening. RSC Adv. 2021, 11, 8459–8467. [Google Scholar] [CrossRef]
- Haywood, J.; Breese, K.J.; Zhang, J.; Waters, M.T.; Bond, C.S.; Stubbs, K.A.; Mylne, J.S. A fungal tolerance trait and selective inhibitors proffer HMG-CoA reductase as a herbicide mode-of-action. Nat. Commun. 2022, 13, 5563. [Google Scholar] [CrossRef]
- Herbicides-Like Library. Available online: https://otavachemicals.com/products/screening-compounds-for-agrochemical-discovery/herbicides-like-library (accessed on 17 November 2022).
- AgroChemical Screening Libraries. Available online: https://lifechemicals.com/screening-libraries/targeted-and-focused-screening-libraries/agrochemical-library (accessed on 17 November 2022).
- Guan, A.; Liu, C.; Yang, X.; Dekeyser, M. Application of the intermediate derivatization approach in agrochemical discovery. Chem. Rev. 2014, 114, 7079–7107. [Google Scholar] [CrossRef]
- Jeschke, P. Manufacturing approaches of new halogenated agrochemicals. EuroJOC 2022, 2022, e202101513. [Google Scholar] [CrossRef]
- Wang, Q.; Song, H.; Wang, Q. Fluorine-containing agrochemicals in the last decade and approaches for fluorine incorporation. Chin. Chem. Lett. 2022, 33, 626–642. [Google Scholar] [CrossRef]
- Gandy, M.N.; Corral, M.G.; Mylne, J.S.; Stubbs, K.A. An interactive database to explore herbicide physicochemical properties. Org. Biomol. Chem. 2015, 13, 5586–5590. [Google Scholar] [CrossRef]
- Zhang, Y.; Lorsbach, B.A.; Castetter, S.; Lambert, W.T.; Kister, J.; Wang, N.X.; Klittich, C.J.R.; Roth, J.; Sparks, T.C.; Loso, M.R. Physicochemical property guidelines for modern agrochemicals. Pest Manag. Sci. 2018, 74, 1979–1991. [Google Scholar] [CrossRef]
- Chen, D.; Hao, G.; Song, B. Finding the missing property concepts in pesticide-likeness. J. Agric. Food Chem. 2022, 70, 10090–10099. [Google Scholar] [CrossRef]
- Huang, J.; Wang, F.; Ouyang, Y.; Huang, Y.; Jia, C.; Zhong, H.; Hao, G. HerbiPAD: A free web platform to comprehensively analyze constitutive property and herbicide-likeness to estimate chemical bioavailability. Pest Manag. Sci. 2021, 77, 1273–1281. [Google Scholar] [CrossRef]
- Oršolić, D.; Pehar, V.; Šmuc, T.; Stepanić, V. Comprehensive machine learning based study of the chemical space of herbicides. Sci. Rep. 2021, 11, 11479. [Google Scholar] [CrossRef] [PubMed]
- Filimonov, D.; Druzhilovskiy, D.; Lagunin, A.; Gloriozova, T.; Rudik, A.; Dmitriev, A.; Pogodin, P.; Poroikov, V. Computer-aided Prediction of Biological Activity Spectra for Chemical Compounds: Opportunities and Limitations. Biomed. Chem. Res. Methods 2018, 1, e00004. [Google Scholar] [CrossRef]
- Clark, R.D. Predicting mammalian metabolism and toxicity of pesticides in silico. Pest Manag. Sci. 2018, 74, 1992–2003. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.E.V.; Stubbs, K.A.; Mylne, J.S.; Ascher, D.B. cropCSM: Designing safe and potent herbicides with graph-based signatures. Brief. Bioinform. 2022, 23, bbac042. [Google Scholar] [CrossRef] [PubMed]
- Chiddarwar, R.K.; Rohrer, S.G.; Wolf, A.; Tresch, S.; Wollenhaupt, S.; Bender, A. In silico target prediction for elucidating the mode of action of herbicides including prospective validation. J. Mol. Graph. Model. 2017, 71, 70–79. [Google Scholar] [CrossRef]
- Flieller, G.; Riffault-Valois, L.; Bergaentzlé, M.; Ennahar, S. Fast and reproducible 96-well plate-based method for the evaluation of the antigerminative potential of plant extracts and phytotoxic compounds. J. Agric. Food Chem. 2022, 70, 7842–7850. [Google Scholar] [CrossRef]
- Macías, F.A.; Castellano, D.; Molinillo, J.M.G. Search for a Standard Phytotoxic Bioassay for Allelochemicals. Selection of Standard Target Species. J. Agric. Food Chem. 2000, 48, 2512–2521. [Google Scholar] [CrossRef]
- Mendes, P.M.; Ribeiro, J.A.; Martins, G.A.; Lucia, T., Jr.; Araujo, T.R.; Fuentes-Guevara, M.D.; Corrêa, L.B.; Corrêa, É.K. Phytotoxicity test in check: Proposition of methodology for comparison of different method adaptations usually used worldwide. J. Environ. Manag. 2021, 291, 112698. [Google Scholar] [CrossRef]
- Belz, R.G.; Hurle, K.A. Novel laboratory screening bioassay for crop seedling allelopathy. J. Chem. Ecol. 2004, 30, 175–198. [Google Scholar] [CrossRef]
- Dayan, F.E.; Romagni, J.G.; Duke, S.O. Investigating the mode of action of natural phytotoxins. J. Chem. Ecol. 2000, 26, 2079–2094. [Google Scholar] [CrossRef]
- Messelhäuser, M.H.; Linn, A.I.; Mathes, A.; Sievernich, B.; Gerhards, R. Development of an Agar Bioassay Sensitivity Test in Alopecurus myosuroides for the Pre-Emergence Herbicides Cinmethylin and Flufenacet. Agronomy 2021, 11, 1408. [Google Scholar] [CrossRef]
- Park, J.; Brown, M.T.; Depuydt, S.; Kim, J.K.; Won, D.-S.; Han, T. Comparing the acute sensitivity of growth and photosynthetic endpoints in three Lemna species exposed to four herbicides. Environ. Pollut. 2017, 220, 818–827. [Google Scholar] [CrossRef]
- Loll, A.; Reinwald, H.; Ayobahan, S.U.; Göckener, B.; Salinas, G.; Schäfers, C.; Schlich, K.; Hamscher, G.; Eilebrecht, S. Short-term test for toxicogenomic analysis of ecotoxic modes of action in Lemna minor. Environ. Sci. Technol. 2022, 56, 11504–11515. [Google Scholar] [CrossRef]
- Li, B.; Yuan, H.; Fang, J.; Tao, L.; Huang, Q.; Qian, X.; Fan, Z. Recent progress of highly efficient in vivo biological screening for novel agrochemicals in China. Pest Manag. Sci. 2010, 66, 238–247. [Google Scholar] [CrossRef]
- Hess, F.D.; Anderson, R.J.; Reagan, J.D. High throughput synthesis and screening: The partner of genomics for discovery of new chemicals for agriculture. Weed Sci. 2001, 49, 249–256. [Google Scholar] [CrossRef]
- Ma, J.; Xu, L.; Wang, S. A quick, simple, and accurate method of screening herbicide activity using green algae cell suspension cultures. Weed Sci. 2002, 50, 555–559. Available online: http://www.jstor.org/stable/4046689 (accessed on 11 November 2022). [CrossRef][Green Version]
- Alfred, S.E.; Surendra, A.; Le, C.; Lin, K.; Mok, A.; Wallace, I.M.; Proctor, M.; Urbanus, M.L.; Giaever, G.; Nislow, C. A phenotypic screening platform to identify small molecule modulators of Chlamydomonas reinhardtii growth, motility and photosynthesis. Genome Biol. 2012, 13, R105. [Google Scholar] [CrossRef]
- Deng, C.; Shao, H.; Pan, X.; Wang, S.; Zhang, D. Herbicidal effects of harmaline from Peganum harmala on photosynthesis of Chlorella pyrenoidosa: Probed by chlorophyll fluorescence and thermoluminescence. Pestic. Biochem. Physiol. 2014, 115, 23–31. [Google Scholar] [CrossRef]
- Jiang, M.; Yang, Q.; Wang, H.; Luo, Z.; Guo, Y.; Shi, J.; Wang, X.; Qiang, S.; Strasser, R.J.; Chen, S. Effect of Mycotoxin cytochalasin A on Photosystem II in Ageratina adenophora. Plants 2022, 11, 2797. [Google Scholar] [CrossRef]
- Wilkinson, A.D.; Collier, C.J.; Flores, F.; Mercurio, P.; O’Brien, J.; Ralph, P.J.; Negri, A.P. A miniature bioassay for testing the acute phytotoxicity of photosystem II herbicides on seagrass. PLoS ONE 2015, 10, e0117541. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kwon, O.K. fluorescence assay for high efficient mass screening of the herbicides inducing rapid membrane peroxidation. Weed Turfgrass Sci. 2015, 4, 308–314. [Google Scholar] [CrossRef]
- Minges, A.; Janßen, D.; Offermann, S.; Groth, G. Efficient In Vivo Screening Method for the Identification of C4 Photosynthesis Inhibitors Based on Cell Suspensions of the Single-Cell C4 Plant Bienertia sinuspersici. Front. Plant Sci. 2019, 10, 1350. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.-X.; Zhang, Z.-Y.Z.; Guo, W.-Y.; Dai, Y.-J.; Wang, Z.-Y.; Yang, W.-C.; Yang, G.-F. In vivo fluorescent screening for HPPD-targeted herbicide discovery. Pest Manag. Sci. 2022, 78, 4947–4955. [Google Scholar] [CrossRef] [PubMed]
- Székács, A. Herbicide mode of action. In Herbicides: Chemistry, Efficacy, Toxicology, and Environmental Impacts; Mesnage, R., Zahler, J.G., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 41–86. [Google Scholar] [CrossRef]
- Grossmann, K. What it takes to get a herbicide’s mode of action. Physionomics, a classical approach in a new complexion. Pest Manag. Sci. 2005, 61, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Dayan, F.E.; Owens, D.K.; Corniani, N.; Silva, F.M.L.; Watson, S.B.; Howell, J.; Shaner, D.L. biochemical markers and enzyme assays for herbicide mode of action and resistance studies. Weed Sci. 2015, 63, 23–63. [Google Scholar] [CrossRef]
- Aliferis, K.A.; Chrysayi-Tokousbalides, M. Metabolomics in pesticide research and development: Review and future perspectives. Metabolomics 2011, 7, 35–53. [Google Scholar] [CrossRef]
- Grossmann, K.; Christiansen, N.; Looser, R.; Tresch, S.; Hutzler, J.; Pollmann, S.; Ehrhardt, T. Physionomics and metabolomics-two key approaches in herbicidal mode of action discovery. Pest Manag. Sci. 2012, 68, 494–504. [Google Scholar] [CrossRef]
- Duke, S.O.; Bajsa, J.; Pan, Z. Omics methods for probing the mode of action of natural and synthetic phytotoxins. J. Chem. Ecol. 2013, 39, 333–347. [Google Scholar] [CrossRef]
- Duke, S.O.; Stidham, M.A.; Dayan, F.E. A novel genomic approach to herbicide and herbicide mode of action discovery. Pest Manag. Sci. 2018, 75, 314–317. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, Q.; Zang, X.; Yuan, S.; Bat-Erdene, U.; Nguyen, C.; Gan, J.; Zhou, J.; Jacobsen, S.E.; Tang, Y. Resistance-gene-directed discovery of a natural-product herbicide with a new mode of action. Nature 2018, 559, 415–418. [Google Scholar] [CrossRef]
- Dayan, F.E.; Duke, S.O. Discovery for new herbicide sites of action by quantification of plant primary metabolite and enzyme pools. Engineering 2020, 6, 509–514. [Google Scholar] [CrossRef]
- Tanetani, Y.; Kaku, K.; Kawai, K.; Fujioka, T.; Shimizu, T. Action mechanism of a novel herbicide, pyroxasulfone. Pestic. Biochem. Phys. 2009, 95, 47–55. [Google Scholar] [CrossRef]
- Bettiol, C.; De Vettori, S.; Minervini, G.; Zuccon, E.; Marchetto, D.; Ghirardini, A.V.; Argese, E. Assessment of phenolic herbicide toxicity and mode of action by different assays. Environ. Sci. Pollut. Res. 2015, 23, 7398–7408. [Google Scholar] [CrossRef]
- Takano, H.K.; Beffa, R.; Preston, C.; Westra, P.; Dayan, F.E. A novel insight into the mode of action of glufosinate: How reactive oxygen species are formed. Photosynth. Res. 2020, 144, 361–372. [Google Scholar] [CrossRef]
- Campe, R.; Hollenbach, E.; Kämmerer, L.; Hendriks, J.; Höffken, H.W.; Kraus, H.; Lerchl, J.; Mietzner, T.; Tresch, S.; Witschel, M.; et al. A new herbicidal site of action: Cinmethylin binds to acyl-ACP thioesterase and inhibits plant fatty acid biosynthesis. Pestic. Biochem. Physiol. 2018, 148, 116–125. [Google Scholar] [CrossRef]
- Shino, M.; Hamada, T.; Shigematsu, Y.; Hirase, K.; Banba, S.; Tsukamoto, Y.; Kadotani, J. Discovery and mode of action of cyclopyrimorate: A new paddy rice herbicide. In Recent Highlights in the Discovery and Optimization of Crop Protection Products; Maienfisch, P., Mangelinckx, S., Eds.; Academic Press: London, UK, 2021; pp. 451–457. [Google Scholar] [CrossRef]
- Gressel, J. Perspective: Present pesticide discovery paradigms promote the evolution of resistance—Learn from nature and prioritize multi-target site inhibitor design. Pest Manag. Sci. 2020, 76, 421–425. [Google Scholar] [CrossRef]
- Gaines, T.A.; Busi, R.; Küpper, A. Can new herbicide discovery allow weed management to outpace resistance evolution? Pest Manag. Sci. 2021, 77, 3036–3041. [Google Scholar] [CrossRef]
- Duke, S.O.; Dayan, F.E. The search for new herbicide mechanisms of action: Is there a ‘holy grail’? Pest Manag. Sci. 2022, 78, 1303–1313. [Google Scholar] [CrossRef]
- Mackie, E.R.R.; Barrow, A.S.; Christoff, R.M.; Abbott, B.M.; Gendall, A.R.; Soares da Costa, T.P. A dual-target herbicidal inhibitor of lysine biosynthesis. Elife 2022, 11, e78235. [Google Scholar] [CrossRef]
- Epp, J.B.; Alexander, A.L.; Balko, T.W.; Buysse, A.M.; Brewster, W.K.; Bryan, K.; Yerkes, C.N. The discovery of Arylex™ active and Rinskor™ active: Two novel auxin herbicides. Bioorg. Med. Chem. 2016, 24, 362–371. [Google Scholar] [CrossRef]
- Herrera, R.; Weimer, M.R.; Morell, M.; Havens, P.L.; Meregalli, G.; Papineni, S.; Laughlin, L.A.; Shan, G. Rinskor active herbicide—A new environment-friendly tool for weed management in rice and aquatic environments. In Recent Highlights in the Discovery and Optimization of Crop Protection Products; Maienfisch, P., Mangelinckx, S., Eds.; Academic Press: London, UK, 2021; pp. 511–523. [Google Scholar] [CrossRef]
- Xu, J.; Liu, X.; Napier, R.; Dong, L.; Li, J. Mode of action of a novel synthetic auxin herbicide halauxifen-methyl. Agronomy 2022, 12, 1659. [Google Scholar] [CrossRef]
- Ahrens, H. Indaziflam: An innovative broad spectrum herbicide. In Discovery and Synthesis of Crop Protection Products; American Chemical Society: Washington, DC, USA, 2015; pp. 233–245. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Zhang, X.; Bai, S.; Jin, T.; Liu, W.; Wang, J. Unravelling phytotoxicity and mode of action of tripyrasulfone, a novel herbicide. J. Agric. Food Chem. 2021, 69, 7168–7177. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Zhao, B.; Zhang, Z.; Xing, J.; Zhang, J.; Dong, J.; Fan, Z. structure-based discovery and synthesis of potential transketolase inhibitors. Molecules 2018, 23, 2116. [Google Scholar] [CrossRef]
- Pinneh, E.C.; Mina, J.G.; Stark, M.J.R.; Lindell, S.D.; Luemmen, P.; Knight, M.R.; Steel, P.G.; Denny, P.W. The identification of small molecule inhibitors of the plant inositol phosphorylceramide synthase which demonstrate herbicidal activity. Sci. Rep. 2019, 9, 8083. [Google Scholar] [CrossRef] [PubMed]
- Tadros, T.F. Formulation Science and Technology; Walter de Gruyter GmbH: Berlin, Germany; Boston, MA, USA, 2018; 301p. [Google Scholar]
- Knowles, A. Recent developments of safer formulations of agrochemicals. Environmentalist 2008, 28, 35–44. [Google Scholar] [CrossRef]
- Sukhoverkov, K.V.; Mylne, J.S. Systematic, small-scale screening with Arabidopsis reveals herbicides synergies that extend to lettuce. Pest Manag. Sci. 2021, 77, 4930–4941. [Google Scholar] [CrossRef]
- Ndou, V.; Phiri, E.E.; Pieterse, P.J. Screening herbicides and herbicide mixtures to identify alternative chemical controls for resistant Plantago biotypes. S. Afr. J. Plant Soil 2022, 39, 198–203. [Google Scholar] [CrossRef]
- Comont, D.; Lowe, C.; Hull, R.; Crook, L.; Hicks, H.L.; Onkokesung, N.; Beffa, R.; Childs, D.Z.; Edwards, R.; Freckleton, R.P.; et al. Evolution of generalist resistance to herbicide mixtures reveals a trade-off in resistance management. Nat. Commun. 2020, 11, 3086. [Google Scholar] [CrossRef]
- Bo, A.B.; Won, O.J.; Sin, H.T.; Lee, J.J.; Park, K.W. Mechanisms of herbicide resistance in weeds. Korean J. Agric. Sci. 2017, 44, 001–015. [Google Scholar] [CrossRef]
- Torra, J.; Osuna, M.D.; Merotto, A.; Vila-Aiub, M. Editorial: Multiple herbicide-resistant weeds and non-target site resistance mechanisms: A global challenge for food Production. Front. Plant Sci. 2021, 12, 763212. [Google Scholar] [CrossRef]
- Riemens, M.; Sønderskov, M.; Moonen, A.-C.; Storkey, J.; Kudsk, P. An Integrated Weed Management framework: A pan-European perspective. Europ. J. Agron. 2022, 133, 126443. [Google Scholar] [CrossRef]
- Kirkwood, R.C. Use and mode of action of adjuvants for herbicides: A review of some current work. Pestic. Sci. 1993, 38, 93–102. [Google Scholar] [CrossRef]
- Green, J.M.; Foy, C.L. Adjuvants. In Weed Biology and Management; Inderjit, Ed.; Springer: Dordrecht, The Netherlands, 2004. [Google Scholar] [CrossRef]
- Pacanoski, Z. Herbicides and Adjuvants. In Herbicides, Physiology of Action, and Safety; Price, A., Kelton, J., Sarunaite, L., Eds.; IntechOpen: London, UK, 2015. [Google Scholar] [CrossRef]
- Zimdahl, R.L. Herbicide Formulation. In Fundamentals of Weed Science, 5th ed.; Academic Press: London, UK, 2018. [Google Scholar] [CrossRef]
- Mesnage, R.; Defarge, N.; Spiroux de Vendômois, J.; Séralini, G.E. Major pesticides are more toxic to human cells than their declared active principles. Biomed. Res. Int. 2014, 2014, 179691. [Google Scholar] [CrossRef]
- Gayathiri, E.; Prakash, P.; Karmegam, N.; Varjani, S.; Awasthi, M.K.; Ravindran, B. Biosurfactants: Potential and Eco-Friendly Material for Sustainable Agriculture and Environmental Safety—A Review. Agronomy 2022, 12, 662. [Google Scholar] [CrossRef]
- Compendium of Herbicide Adjuvants. Available online: https://ppp.purdue.edu/wp-content/uploads/2016/11/PPP-115.pdf (accessed on 17 November 2022).
- Singh, M.; Tan, S.; Sharma, S.D. Adjuvants enhance weed control efficacy of foliar-applied diuron. Weed Technol. 2002, 16, 74–78. [Google Scholar] [CrossRef]
- Bunting, J.A.; Sprague, C.L.; Riechers, D.E. Proper adjuvant selection for foramsulfuron activity. Crop Prot. 2004, 23, 361–366. [Google Scholar] [CrossRef]
- Zhang, J.; Jaeck, O.; Menegat, A.; Zhang, Z.; Gerhards, R.; Ni, H. The mechanism of methylated seed oil on enhancing biological efficacy of topramezone on weeds. PLoS ONE 2013, 8, e74280. [Google Scholar] [CrossRef][Green Version]
- Palma-Bautista, C.; Vazquez-Garcia, J.G.; Travlos, I.; Tataridas, A.; Kanatas, P.; Domínguez-Valenzuela, J.A.; De Prado, R. Effect of Adjuvant on Glyphosate Effectiveness, Retention, Absorption and Translocation in Lolium rigidum and Conyza canadensis. Plants 2020, 9, 297. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, Y.; Zhang, C.; Zhang, P.; Jia, C.; Zhao, E. Early evaluation of adjuvant effects on topramezone efficacy under different temperature conditions using chlorophyll fluorescence tests. Front. Plant Sci. 2022, 13, 920902. [Google Scholar] [CrossRef]
- Singh, A.; Dhiman, N.; Kar, A.K.; Singh, D.; Purohit, M.P.; Ghosh, D.; Patnaik, S. Advances in controlled release pesticide formulations: Prospects to safer integrated pest management and sustainable agriculture. J. Hazard. Mater. 2020, 385, 121525. [Google Scholar] [CrossRef]
- Li, N.; Sun, C.; Jiang, J.; Wang, A.; Wang, C.; Shen, Y.; Huang, B.; An, C.; Cui, B.; Zhao, X.; et al. Advances in controlled-release pesticide formulations with improved efficacy and targetability. J. Agric. Food Chem. 2021, 69, 12579–12597. [Google Scholar] [CrossRef] [PubMed]
- Sopeña, F.; Maqueda, C.; Morillo, E. Controlled release formulations of herbicides based on micro-encapsulation. Cienc. Investig. Agrar. 2009, 36, 27–42. [Google Scholar] [CrossRef]
- Campos, E.V.R.; de Oliveira, J.L.; Fraceto, L.F.; Singh, B. Polysaccharides as safer release systems for agrochemicals. Agron. Sustain. Dev. 2014, 35, 47–66. [Google Scholar] [CrossRef]
- Rao, J.; Chandrani, A.N.; Powar, A.; Chandra, S. Preparation of microcapsule suspension of herbicide oxyfluorfen polyurea and its effects on phytotoxicity on rice crop. J. Dispers. Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Yılmaz, H.; Enginar, H.; Çifci, C. Microencapsulation of pendimethalin with polyurethane-urea and determination of its stability. J. Taibah Univ. Sci. 2021, 15, 685–694. [Google Scholar] [CrossRef]
- Kiselev, E.G.; Boyandin, A.N.; Zhila, N.O.; Prudnikova, S.V.; Shumilova, A.A.; Baranovskiy, S.V.; Shishatskaya, E.I.; Thomas, S.; Volova, T.G. Constructing sustained-release herbicide formulations based on poly-3-hydroxybutyrate and natural materials as a degradable matrix. Pest Manag. Sci. 2019, 76, 1772–1785. [Google Scholar] [CrossRef]
- Volova, T.; Shumilova, A.; Zhila, N.; Sukovatyi, A.; Shishatskaya, E.; Thomas, S. Efficacy of slow-release formulations of metribuzin and tribenuron methyl herbicides for controlling weeds of various species in wheat and barley stands. ACS Omega 2020, 5, 25135–25147. [Google Scholar] [CrossRef]
- Prudnikova, S.; Streltsova, N.; Volova, T. The effect of the pesticide delivery method on the microbial community of field soil. Environ. Sci. Pollut. Res. Int. 2021, 28, 8681–8697. [Google Scholar] [CrossRef]
- Cao, L.; Liu, Y.; Xu, C.; Zhou, Z.; Zhao, P.; Niu, S.; Huang, Q. Biodegradable poly(3-hydroxybutyrate-co-4-hydroxybutyrate) microcapsules for controlled release of trifluralin with improved photostability and herbicidal activity. Mater. Sci. Eng. C 2019, 102, 134–141. [Google Scholar] [CrossRef]
- De Oliveira, J.L.; Campos, E.V.R.; Camara, M.C.; de Carvalho, L.B.; Monteiro, R.A.; de Lima, R.; Fraceto, L.F. Nanotechnology-based delivery systems: Highlights in agricultural applications. J. Sib. Fed. Univ. Biol. 2019, 12, 311–328. [Google Scholar] [CrossRef]
- Huang, B.; Chen, F.; Shen, Y.; Qian, K.; Wang, Y.; Sun, C.; Zhao, X.; Cui, B.; Gao, F.; Zeng, Z.; et al. Advances in Targeted Pesticides with Environmentally Responsive Controlled Release by Nanotechnology. Nanomaterials 2018, 8, 102. [Google Scholar] [CrossRef]
- Ma, E.; Chen, K.; Sun, L.; Fu, Z.; Guo, J.; Liu, J.; Zhao, J.; Liu, Z.; Lei, Z.; Li, L.; et al. Rapid construction of green nanopesticide delivery systems using sophorolipids as surfactants by flash nanoprecipitation. J. Agric. Food Chem. 2022, 70, 4912–4920. [Google Scholar] [CrossRef]
- Forini, M.M.L.; Pontes, M.S.; Antunes, D.R.; de Lima, P.H.C.; Santos, J.S.; Santiago, E.F.; Grillo, R. Nano-enabled weed management in agriculture: From strategic design to enhanced herbicidal activity. Plant Nano Biol. 2022, 1, 100008. [Google Scholar] [CrossRef]
- Walker, G.W.; Kookana, R.S.; Smith, N.E.; Kah, M.; Doolette, C.L.; Reeves, P.T.; Lovell, W.; Anderson, D.J.; Turney, T.W.; Navarro, D.A. Ecological Risk Assessment of Nano-enabled Pesticides: A Perspective on Problem Formulation. J. Agric. Food Chem. 2018, 66, 6480–6486. [Google Scholar] [CrossRef]
- De Albuquerque, F.P.; Preisler, A.C.; Fraceto, L.F.; Oliveira, H.C.; de Castro, V.L.S.S. Overview of Nanopesticide Environmental Safety Aspects and Regulatory Issues: The Case of Nanoatrazine. In Nanopesticides; Fraceto, L.F., de Castro, V.L.S.S., Grillo, R., Ávila, D., Caixeta Oliveira, H., Lima, R., Eds.; Springer: Cham, Switzerland, 2020; pp. 281–298. [Google Scholar] [CrossRef]
- Chaud, M.; Souto, E.B.; Zielinska, A.; Severino, P.; Batain, F.; Oliveira-Junior, J.; Alves, T. Nanopesticides in Agriculture: Benefits and Challenge in Agricultural Productivity, Toxicological Risks to Human Health and Environment. Toxics 2021, 9, 131. [Google Scholar] [CrossRef]
- Pontes, M.S.; Antunes, D.R.; Oliveira, I.P.; Forini, M.M.L.; Santos, J.S.; Arruda, G.J.; Caires, A.R.L.; Santiago, E.F.; Grillo, R. Chitosan/tripolyphosphate nanoformulation carrying paraquat: Insights on its enhanced herbicidal activity. Environ. Sci. Nano 2021, 8, 1336–1351. [Google Scholar] [CrossRef]
- Artusio, F.; Casà, D.; Granetto, M.; Tosco, T.; Pisano, R. Alginate nanohydrogels as a biocompatible platform for the controlled release of a hydrophilic herbicide. Processes 2021, 9, 1641. [Google Scholar] [CrossRef]
- Wu, J.; Zhai, Y.; Monikh, F.A.; Arenas-Lago, D.; Grillo, R.; Vijver, M.G.; Peijnenburg, W.J.G.M. The Differences between the effects of a nanoformulation and a conventional form of atrazine to lettuce: Physiological responses, defense mechanisms, and nutrient displacement. J. Agric. Food Chem. 2021, 69, 12527–12540. [Google Scholar] [CrossRef]
- Takeshita, V.; Carvalhol, L.B.; Galhardil, J.A.; Munhoz-Garcial, G.V.; Pimpinatol, R.F.; Oliveiral, H.C.; Tornisielol, V.L.; Fracetol, L.F. Development of a preemergent nanoherbicide: From efficiency evaluation to the assessment of environmental fate and risks to soil microorganisms. ACS Nanosci. 2022, 2, 307–323. [Google Scholar] [CrossRef]
- Shan, P.; Lu, Y.; Lu, W.; Yin, X.; Liu, H.; Li, D.; Lian, X.; Wang, W.; Li, Z.; Li, Z. Biodegradable and light-responsive polymeric nanoparticles for environmentally safe herbicide delivery. ACS Appl. Mater. Interfaces 2022, 14, 43759–43770. [Google Scholar] [CrossRef]
- Hasan, M.; Ahmad-Hamdani, M.S.; Rosli, A.M.; Hamdan, H. Bioherbicides: An Eco-Friendly Tool for Sustainable Weed Management. Plants 2021, 10, 1212. [Google Scholar] [CrossRef] [PubMed]
- De Souza Barros, V.M.; Pedrosa, J.L.F.; Gonçalves, D.R.; de Medeiros, F.C.L.; Carvalho, G.R.; Gonçalves, A.H.; Teixeira, P.V.V.Q. Herbicides of biological origin: A review. J. Hortic. Sci. Biotechnol. 2021, 96, 288–296. [Google Scholar] [CrossRef]
- Korres, N.E.; Burgosa, N.R.; Travlos, I.; Vurro, M.; Gitsopoulos, T.K.; Varanasi, V.K.; Duke, S.O.; Kudsk, P.; Brabhama, C.; Rouseh, C.E.; et al. New directions for integrated weed management: Modern technologies, tools and knowledge discovery. Adv. Agron. 2019, 155, 243–319. [Google Scholar] [CrossRef]
- Duke, S.O.; Pan, Z.; Bajsa-Hirschel, J.; Boyette, C.D. The potential future roles of natural compounds and microbial bioherbicides in weed management in crops. Adv. Weed Sci. 2022, 40, e020210054. [Google Scholar] [CrossRef] [PubMed]
- Vurro, M.; Boari, A.; Casella, F.; Zonno, M.C. Fungal phytotoxins in sustainable weed management. Curr. Med. Chem. 2018, 25, 268–286. [Google Scholar] [CrossRef]
- Chaïb, S.; Pistevos, J.C.A.; Bertrand, C.; Bonnard, I. Allelopathy and allelochemicals from microalgae: An innovative source for bio-herbicidal compounds and biocontrol research. Algal Res. 2021, 54, 102213. [Google Scholar] [CrossRef]
- Kalra, R.; Conlan, X.A.; Goel, M. Lichen allelopathy: A new hope for limiting chemical herbicide and pesticide use. Biocontrol. Sci. Technol. 2021, 31, 773–796. [Google Scholar] [CrossRef]
- Palanivel, H.; Tilaye, G.; Belliathan, S.K.; Benor, S.; Abera, S.; Kamaraj, M. Allelochemicals as Natural Herbicides for Sustainable Agriculture to Promote a Cleaner Environment. In Strategies and Tools for Pollutant Mitigation; Aravind, J., Kamaraj, M., Prashanthi Devi, M., Rajakumar, S., Eds.; Springer: Cham, Switzerland, 2021; pp. 93–116. [Google Scholar] [CrossRef]
- Seigler, D. Basic pathways for the origin of allelopathic compounds. In Allelopathy; Reigosa, M., Pedrol, N., González, L., Eds.; Springer: Dordrecht, The Netherland, 2006; pp. 11–61. [Google Scholar] [CrossRef]
- Xu, D.; Xue, M.; Shen, Z.; Jia, X.; Hou, X.; Lai, D.; Zhou, L. Phytotoxic secondary metabolites from fungi. Toxins 2021, 13, 261. [Google Scholar] [CrossRef]
- Lorenzo, P.; Reboredo-Durán, J.; Muñoz, L.; Freitas, H.; González, L. Herbicidal properties of the commercial formulation of methyl cinnamate, a natural compound in the invasive silver wattle (Acacia dealbata). Weed Sci. 2019, 68, 1–37. [Google Scholar] [CrossRef]
- Masi, M.; Freda, F.; Sangermano, F.; Calabrò, V.; Cimmino, A.; Cristofaro, M.; Meyer, S.; Evidente, A. Radicinin, a fungal phytotoxin as a target-specific bioherbicide for invasive buffelgrass (Cenchrus ciliaris) Control. Molecules 2019, 24, 1086. [Google Scholar] [CrossRef]
- Pannacci, E.; Masi, M.; Farneselli, M.; Tei, F. Evaluation of Mugwort (Artemisia vulgaris L.) Aqueous Extract as a Potential Bioherbicide to Control Amaranthus retroflexus L. in Maize. Agriculture 2020, 10, 642. [Google Scholar] [CrossRef]
- Zonno, M.C.; Vurro, M. Inhibition of germination of Orobanche ramose seeds by Fusarium toxins. Phytoparasitica 2002, 30, 519–524. [Google Scholar] [CrossRef]
- Anteyi, W.O.; Klaiber, I.; Rasche, F. Diacetoxyscirpenol, a Fusarium exometabolite, prevents efficiently the incidence of the parasitic weed Striga hermonthica. BMC Plant Biol. 2022, 22, 84. [Google Scholar] [CrossRef]
- Vurro, M.; Boari, A.; Evidente, A.; Andolfi, A.; Zermane, N. Natural metabolites for parasitic weed management. Pest Manag. Sci. 2009, 65, 566–571. [Google Scholar] [CrossRef]
- Zwanenburg, B.; Mwakaboko, A.S.; Kannan, C. Suicidal germination for parasitic weed control. Pest Manag. Sci. 2016, 72, 2016–2025. [Google Scholar] [CrossRef]
- Quy, T.N.; Xuan, T.D.; Andriana, Y.; Tran, H.; Khanh, T.D.; Teschke, R. Cordycepin isolated from Cordyceps militaris: Its newly discovered herbicidal property and potential plant-based novel alternative to glyphosate. Molecules 2019, 24, 2901. [Google Scholar] [CrossRef]
- Li, F.; Ye, Z.; Huang, Z.; Chen, X.; Sun, W.; Gao, W.; Zhang, S.; Cao, F.; Wang, J.; Hu, Z.; et al. New α-pyrone derivatives with herbicidal activity from the endophytic fungus Alternaria brassicicola. Bioorg. Chem. 2021, 117, 105452. [Google Scholar] [CrossRef]
- Rivero-Cruz, J.F.; Garcia-Aguirre, G.; Cerda-Garcia-Rojas, C.M.; Mata, R. conformational behavior and absolute stereostructure of two phytotoxic nonenolides from the fungus Phoma herbarum. Tetrahedron 2000, 56, 5337–5344. [Google Scholar] [CrossRef]
- Dalinova, A.; Dubovik, V.R.; Chisty, L.; Kochura, D.; Ivanov, A.; Smirnov, S.; Petrova, M.; Zolotarev, A.; Evidente, A.; Berestetskiy, A. Stagonolides J and K and Stagochromene A, two New Natural Substituted Nonenolides and a New Disubstituted Chromene-4,5-Dione Isolated from Stagonospora cirsii S-47 Proposed for the Biocontrol of Sonchus arvensis. J. Agric. Food Chem. 2019, 67, 13040–13050. [Google Scholar] [CrossRef]
- Yin, M.; Fasoyin, O.E.; Wang, C.; Yue, Q.; Zhang, Y.; Dun, B.; Xu, Y.; Zhang, L. Herbicidal efficacy of harzianums produced by the biofertilizer fungus, Trichoderma brevicompactum. AMB Express 2020, 10, 118. [Google Scholar] [CrossRef]
- Durán, A.G.; Benito, J.; Macías, F.A.; Simonet, A.M. Agave steroidal saponins as potential bioherbicides. Agronomy 2021, 11, 2404. [Google Scholar] [CrossRef]
- Da Cruz-Silva, C.T.; Cantrell, C.L.; Nobrega, L.P.; Ali, A.; Duke, S. Bioassay-guided isolation of phytotoxins from three salvia species. Allelopath. J. 2021, 54, 13–24. [Google Scholar] [CrossRef]
- Wu, H.; Ma, L.; Li, X.; Liu, T. Selective phytotoxic effects of sesquiterpenoids from Sonchus arvensis as a preliminary approach for the biocontrol of two problematic weeds of wheat. J. Agric. Food Chem. 2022, 70, 9412–9420. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O.; Pan, Z.; Bajsa-Hirschel, J. Proving the Mode of Action of Phytotoxic Phytochemicals. Plants 2020, 9, 1756. [Google Scholar] [CrossRef] [PubMed]
- Kastanias, M.A.; Chrysayi-Tokousbalides, M. Herbicidal potential of pyrenophorol isolated from Drechslera avenae pathotype. Pest Manag. Sci. 2000, 56, 227–232. [Google Scholar] [CrossRef]
- Aliferis, K.A.; Chrysayi-Tokousbalides, M. Metabonomic Strategy for the Investigation of the Mode of Action of the Phytotoxin (5S,8R,13S,16R)-(−)-Pyrenophorol Using1H Nuclear Magnetic Resonance Fingerprinting. J. Agric. Food Chem. 2006, 54, 1687–1692. [Google Scholar] [CrossRef]
- King, R.R.; Lawrence, C.H.; Gray, J.A. Herbicidal properties of the thaxtomin group of phytotoxins. J. Agric. Food Chem. 2001, 49, 2298–2301. [Google Scholar] [CrossRef]
- Loria, R.; Kers, J.; Joshi, M. Evolution of Plant Pathogenicity in Streptomyces. Annu. Rev. Phytopath. 2006, 44, 469–487. [Google Scholar] [CrossRef]
- Duke, S.O.; Evidente, A.; Fiore, M.; Rimando, A.M.; Dayan, F.E.; Vurro, M.; Christiansen, N.; Looser, R.; Hutzler, J.; Grossmann, K. Effects of the aglycone of ascaulitoxin on amino acid metabolism in Lemna paucicostata. Pestic. Biochem. Phys. 2011, 100, 41–50. [Google Scholar] [CrossRef]
- Dayan, F.E.; Owens, D.K.; Watson, S.B.; Asolkar, R.N.; Boddy, L.G. Sarmentine, a natural herbicide from Piper species with multiple herbicide mechanisms of action. Front. Plant Sci. 2015, 6, 222. [Google Scholar] [CrossRef]
- Hubbard, M.; Hynes, R.K.; Bailey, K.L. Impact of macrocidins, produced by Phoma macrostoma, on carotenoid profiles of plants. Biol. Control 2015, 89, 11–22. [Google Scholar] [CrossRef]
- Hubbard, M.; Taylor, W.G.; Bailey, K.L.; Hynes, R.K. The dominant modes of action of macrocidins, bioherbicidal metabolites of Phoma macrostoma, differ between susceptible plant species. Environ. Exp. Bot. 2016, 132, 80–91. [Google Scholar] [CrossRef]
- Chen, S.; Qiang, S. Recent advances in tenuazonic acid as a potential herbicide. Pestic. Biochem. Physiol. 2017, 143, 252–257. [Google Scholar] [CrossRef]
- Guo, Y.; Cheng, J.; Lu, Y.; Wang, H.; Gao, Y.; Shi, J.; Yin, C.; Wang, X.; Chen, S.; Strasser, R.J.; et al. Novel action targets of natural product gliotoxin in photosynthetic apparatus. Front. Plant Sci. 2020, 10, 1688. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, W.; Wang, H.; Wang, X.; Qiang, S.; Kalaji, H.M.; Strasser, R.J.; Chen, S. Action Mode of the Mycotoxin Patulin as a Novel Natural Photosystem II Inhibitor. J. Agric. Food Chem. 2021, 69, 7313–7323. [Google Scholar] [CrossRef]
- Samperna, S.; Masi, M.; Vurro, M.; Evidente, A.; Marra, M. Cyclopaldic Acid, the Main Phytotoxic Metabolite of Diplodia cupressi, Induces Programmed Cell Death and Autophagy in Arabidopsis thaliana. Toxins 2022, 14, 474. [Google Scholar] [CrossRef]
- Verdeguer, M.; Sánchez-Moreiras, A.M.; Araniti, F. Phytotoxic effects and mechanism of action of essential oils and terpenoids. Plants 2020, 9, 1571. [Google Scholar] [CrossRef]
- Marrone, P. Prospects for Bioherbicides. Outlooks Pest Manag. 2021, 32, 214–217. [Google Scholar] [CrossRef]
- Cabrera-Pérez, C.; Royo-Esnal, A.; Recasens, J. Herbicidal Effect of Different Alternative Compounds to Control Conyza bonariensis in Vineyards. Agronomy 2022, 12, 960. [Google Scholar] [CrossRef]
- Duke, S.O.; Dayan, F.E. Modes of action of microbially-produced phytotoxins. Toxins 2011, 3, 1038–1064. [Google Scholar] [CrossRef]
- Duke, S.O.; Dayan, F.E. Discovery of New Herbicide Modes of Action with Natural Phytotoxins. Discovery and Synthesis of Crop Protection Products. In Discovery and Synthesis of Crop Protection Products; Maienfisch, P., Stevenson, T.M., Eds.; ACS Symposium Series; ACS: Washington, DC, USA, 2015; pp. 79–92. [Google Scholar] [CrossRef]
- Cordeau, S.; Triolet, M.; Wayman, S.; Steinberg, C.; Guillemin, J. Bioherbicides: Dead in the water? A review of the existing products for integrated weed management. Crop Prot. 2016, 87, 44–49. [Google Scholar] [CrossRef]
- Koivunen, M.; Marrone, P. Uses of Thaxtomin and Thaxtomin Compositions as Herbicides. US Patent 8476195B2, 30 December 2009. [Google Scholar]
- O’Sullivan, J.; Van Acker, R.; Grohs, R.; Riddle, R. Improved herbicide efficacy for organically grown vegetables. Org. Agric. 2015, 5, 315–322. [Google Scholar] [CrossRef]
- Wolfe, J.C.; Neal, J.C.; Harlow, C.D. Selective Broadleaf Weed Control in Turfgrass with the Bioherbicides Phoma macrostoma and Thaxtomin A. Weed Technol. 2016, 30, 688–700. [Google Scholar] [CrossRef]
- Wolfe, J.C.; Neal, J.C.; Harlow, C.D.; Gannon, T.W. Efficacy of the Bioherbicide Thaxtomin A on Smooth Crabgrass and Annual Bluegrass and Safety in Cool-Season Turfgrasses. Weed Technol. 2016, 30, 733–742. [Google Scholar] [CrossRef]
- Graupner, P.R.; Gerwick, B.C.; Siddall, T.L.; Carr, A.W.; Clancy, E.; Gilbert, J.R.; Bailey, K.L.; Derby, J.-A. Chlorosis Inducing Phytotoxic Metabolites: New Herbicides from Phoma macrostoma. In Natural Products for Pest Management; Rimando, A.M., Duke, S.O., Eds.; ACS: Washington, DC, USA, 2006; pp. 37–47. [Google Scholar] [CrossRef]
- Irvine, N.M.; Yerkes, C.N.; Graupner, P.R.; Roberts, R.E.; Hahn, D.R.; Pearce, C.; Gerwick, B.C. Synthesis and characterization of synthetic analogs of cinnacidin, a novel phytotoxin from Nectria sp. Pest Manag. Sci. 2008, 64, 891–899. [Google Scholar] [CrossRef]
- Hahn, D.R.; Graupner, P.R.; Chapin, E.; Gray, J.; Heim, D.; Gilbert, J.R.; Gerwick, B.C. Albucidin: A novel bleaching herbicide from Streptomyces albus subsp. chlorinus NRRL B-24108. J. Antibiot. 2009, 62, 191–194. [Google Scholar] [CrossRef]
- Gerwick, B.C.; Brewster, W.K.; deBoer, G.J.; Fields, S.C.; Graupner, P.R.; Hahn, D.R.; Pearce, C.J.; Webster, J.D. Mevalocidin: A Novel, Phloem Mobile Phytotoxin from Fusarium DA056446 and Rosellinia DA092917. J. Chem. Ecol. 2013, 39, 253–261. [Google Scholar] [CrossRef]
- Marrone Bio Advances Novel Herbicides. Available online: https://marronebio.com/marrone-bio-advances-novel-herbicides/ (accessed on 17 November 2022).
- Hasan, M.; Mokhtar, A.S.; Rosli, A.M.; Hamdan, H.; Motmainna, M.; Ahmad-Hamdani, M.S. Weed control efficacy and crop-weed selectivity of a new bioherbicide WeedLock. Agronomy 2021, 11, 1488. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, H.; Meng, B.; Wei, R.; Wang, L.; An, C.; Chen, S.; Yang, C.; Qiang, S. An evaluation of tenuazonic acid, a potential biobased herbicide in cotton. Pest Manag. Sci. 2019, 75, 2482–2489. [Google Scholar] [CrossRef]
- Varejão, E.V.V.; Demuner, A.J.; Barbosa, L.C.A.; Barreto, R.W. The search for new natural herbicides–Strategic approaches for discovering fungal phytotoxins. Crop Prot. 2013, 48, 41–50. [Google Scholar] [CrossRef]
- Cimmino, A.; Masi, M.; Evidente, M.; Superchi, S.; Evidente, A. Fungal phytotoxins with potential herbicidal activity: Chemical and biological characterization. Nat. Prod. Rep. 2015, 32, 1629–1653. [Google Scholar] [CrossRef]
- Berestetskiy, A.O.; Poluektova, E.V.; Sabashuk, Y.A.; Pervushin, A.L. Development of chromatography techniques for analysis and preparative isolation of phytotoxic metabolites produced by Stagonospora cirsii. Appl. Biochem. Microbiol. 2019, 55, 684–690. [Google Scholar] [CrossRef]
- Wang, L.; Wang, M.; Fu, Y.; Huang, P.; Kong, D.; Niu, G. Engineered biosynthesis of thaxtomin phytotoxins. Crit. Rev. Biotech. 2020, 40, 1–9. [Google Scholar] [CrossRef]
- Masi, M.; Meyer, S.; Clement, S.; Cimmino, A.; Evidente, A. Effect of cultural conditions on the production of radicinin, a specific fungal phytotoxin for buffelgrass (Cenchrus ciliaris) biocontrol, by different Cochlioboulus australiensis strains. Nat. Prod. Res. 2021, 35, 99–107. [Google Scholar] [CrossRef]
- Seger, C.; Erlebach, D.; Stuppner, H.; Griesser, U.; Strasser, H. physicochemical properties of oosporein, the major secreted metabolite of the entomopathogenic fungus Beauveria brongniartii. Helv. Chim. Acta 2005, 88, 802–810. [Google Scholar] [CrossRef]
- Ahonsi, M.O.; Boss, D.; Maurhofer, M.; Défago, G. Potential environmental fate of elsinochrome A, a perylenequinone toxin produced in culture by bindweed biocontrol fungus Stagonospora convolvuli LA39. Environmentalist 2006, 26, 183–193. [Google Scholar] [CrossRef][Green Version]
- Trivella, A.; Stawinoga, M.; Dayan, F.E.; Cantrell, C.L.; Mazellier, P.; Richard, C. Photolysis of natural β-triketonic herbicides in water. Water Res. 2015, 78, 28–36. [Google Scholar] [CrossRef]
- Scheepmaker, J.W.A.; Busschers, M.; Sundh, I.; Eilenberg, J.; Butt, T.M. Sense and nonsense of the secondary metabolites data requirements in the EU for beneficial microbial control agents. Biol. Control 2019, 136, 104005. [Google Scholar] [CrossRef]
- Kiseleva, M.; Chalyy, Z.; Sedova, I.; Aksenov, I. Stability of mycotoxins in individual stock and multi-analyte standard solutions. Toxins 2020, 12, 94. [Google Scholar] [CrossRef]
- Bordin, E.R.; Frumi Camargo, A.; Stefanski, F.S.; Scapini, T.; Bonatto, C.; Zanivan, J.; Treichel, H. Current production of bioherbicides: Mechanisms of action and technical and scientific challenges to improve food and environmental security. Biocatal. Biotransfor. 2020, 39, 346–359. [Google Scholar] [CrossRef]
- Uddin, M.R.; Park, S.U.; Dayan, F.E.; Pyon, J.Y. Herbicidal activity of formulated sorgoleone, a natural product of sorghum root exudate. Pest Manag. Sci. 2013, 70, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Todero, I.; Confortin, T.C.; Luft, L.; Brun, T.; Ugalde, G.A.; de Almeida, T.C.; Arnemann, J.A.; Zabot, G.L.; Mazutti, M.A. Formulation of a bioherbicide with metabolites from Phoma sp. Sci. Hortic. 2018, 241, 285–292. [Google Scholar] [CrossRef]
- Gressel, J.; Shaaltiel, Y. Biorational Herbicide Synergists. In Biotechnology for Crop Protection; Hedin, P., Menn, J.J., Hollingworth, R.M., Eds.; American Chemical Society: Washington, DC, USA, 1988; pp. 4–24. [Google Scholar] [CrossRef]
- Dilipkumar, M.; Chuah, T.S. Is combination ratio an important factor to determine synergistic activity of allelopathic crop extract and herbicide? Int. J. Agric. Biol. 2013, 15, 259–265. [Google Scholar]
- Pardo-Muras, M.; Puig, C.G.; Pedrol, N. Complex synergistic interactions among volatile and phenolic compounds underlie the effectiveness of allelopathic residues added to the soil for weed control. Plants 2022, 11, 1114. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Chen, M.; Ye, H.-C.; Zhang, Z.-K.; Li, H.; Chen, L.-L.; Chen, X.-L.; Yan, C.; Zhang, J. Herbicidal activities of compounds isolated from the medicinal plant Piper sarmentosum. Ind. Crops Prod. 2019, 132, 41–47. [Google Scholar] [CrossRef]
- Poluektova, E.; Tokarev, Y.; Sokornova, S.; Chisty, L.; Evidente, A.; Berestetskiy, A. Curvulin and Phaeosphaeride A from Paraphoma sp. VIZR 1.46 Isolated from Cirsium arvense as Potential Herbicides. Molecules 2018, 23, 2795. [Google Scholar] [CrossRef]
- Dubovik, V.; Dalinova, A.; Berestetskiy, A. Effect of adjuvants on herbicidal activity and selectivity of three phytotoxins produced by the fungus, Stagonospora cirsii. Plants 2020, 11, 1621. [Google Scholar] [CrossRef]
- Jamil, M.; Wang, J.Y.; Yonli, D.; Patil, R.H.; Riyazaddin, M.; Gangashetty, P.; Berqdar, L.; Chen, G.-T.E.; Traore, H.; Margueritte, O.; et al. A New Formulation for strigolactone suicidal germination agents, towards successful Striga management. Plants 2022, 11, 808. [Google Scholar] [CrossRef]
- de Almeida, T.C.; Spannemberg, S.S.; Brun, T.; Schmaltz, S.; Escobar, O.; Sanchotene, D.M.; Dornelles, S.H.B.; Zabot, G.L.; Tres, M.V.; Kuhn, R.C.; et al. Development of a solid bioherbicide formulation by spray drying technology. Agriculture 2020, 10, 215. [Google Scholar] [CrossRef]
- Moeini, A.; Masi, M.; Zonno, M.C.; Boari, A.; Cimmino, A.; Tarallo, O.; Vurrp, M.; Evidente, A. Encapsulation of inuloxin A, a plant germacrane sesquiterpene with potential herbicidal activity, in β-cyclodextrins. Org. Biomol. Chem. 2019, 17, 2508–2515. [Google Scholar] [CrossRef]
- Serino, N.; Boari, A.; Santagata, G.; Masi, M.; Malinconico, M.; Evidente, A.; Vurro, M. Biodegradable polymers as carriers for tuning the release and improve the herbicidal effectiveness of Dittrichia viscosa plant organic extracts. Pest Manag. Sci. 2021, 77, 646–658. [Google Scholar] [CrossRef]
- Galán-Pérez, J.A.; Gámiz, B.; Celis, R. Granulated organoclay as a sorbent to protect the allelochemical scopoletin from rapid biodegradation in soil. Environ. Technol. Innov. 2022, 28, 102707. [Google Scholar] [CrossRef]
- Kremer, R.J. Bioherbicides and nanotechnology: Current status and future trends. In Nano-Biopesticides Today and Future Perspectives; Koul, O., Ed.; Academic Press: London, UK, 2019; pp. 353–366. [Google Scholar] [CrossRef]
- Vurro, M.; Miguel-Rojas, C.; Pérez-de-Luque, A. Safe nanotechnologies for increasing the effectiveness of environmentally friendly natural agrochemicals. Pest Manag. Sci. 2019, 75, 2403–2412. [Google Scholar] [CrossRef]
- Abdollahdokht, D.; Gao, Y.; Faramarz, S.; Poustforoosh, A.; Abbasi, M.; Asadikaram, G.; Nematollahi, M.H. Conventional agrochemicals towards nano-biopesticides: An overview on recent advances. Chem. Biol. Technol. Agric. 2022, 9, 13. [Google Scholar] [CrossRef]
- Taban, A.; Saharkhiz, M.J.; Khorram, M. Formulation and assessment of nano-encapsulated bioherbicides based on biopolymers and essential oil. Ind. Crops Prod. 2020, 149, 112348. [Google Scholar] [CrossRef]
- Vurro, M.; Townley, H.E.; Morrison, R.; Boari, A.; Masi, M.; Evidente, A. Augmented phytotoxic effect of nanoencapsulated ophiobolin A. Nat. Prod. Res. 2020, 36, 1143–1150. [Google Scholar] [CrossRef]
- Demasi, S.; Caser, M.; Caldera, F.; Kumar Dhakar, N.; Vidotto, F.; Trotta, F.; Scariot, V. Functionalized dextrin-based nanosponges as effective carriers for the herbicide ailanthone. Ind. Crops Prod. 2021, 164, 113346. [Google Scholar] [CrossRef]
- Somala, N.; Laosinwattana, C.; Teerarak, M. Formulation process, physical stability and herbicidal activities of Cymbopogon nardus essential oil-based nanoemulsion. Sci. Rep. 2022, 12, 10280. [Google Scholar] [CrossRef]
- Permatasari, G.W.; Putranto, R.A.; Widiastuti, H. Structure-based virtual screening of bioherbicide candidates for weeds in sugarcane plantation using in silico approaches. Menara Perkeb. 2020, 88, 100–110. [Google Scholar] [CrossRef]
- de Oliveira, M.V.D.; Fernandes, G.M.B.; da Costa, K.S.; Vakal, S.; Lima, A.H. Virtual screening of natural products against 5-enolpyruvylshikimate-3-phosphate synthase using the Anagreen herbicide-like natural compound library. RSC Adv. 2022, 12, 18834–18847. [Google Scholar] [CrossRef]
- Sparks, T.C.; Bryant, R.J. Impact of natural products on discovery of, and innovation in, crop protection compounds. Pest Manag. Sci. 2022, 78, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Takano, H.K.; Dayan, F.E. Glufosinate-ammonium: A review of the current state of knowledge. Pest Manag. Sci. 2020, 76, 3911–3925. [Google Scholar] [CrossRef] [PubMed]
- Aucique-Pérez, C.E.; Resende, R.S.; Cruz Neto, L.B.; Dornelas, F.; Da Matta, F.M.; Rodrigues, F.Á. Picolinic acid spray stimulates the antioxidative metabolism and minimizes impairments on photosynthesis on wheat leaves infected by Pyricularia oryzae. Physiol. Plant. 2019, 167, 628–644. [Google Scholar] [CrossRef] [PubMed]
- Riemer, M.; Uzunova, V.V.; Riemer, N.; Clarkson, G.J.; Pereira, N.; Napier, R.; Shipman, M. Phyllostictine A: Total synthesis, structural verification and determination of substructure responsible for plant growth inhibition. Chem. Commun. 2018, 54, 7211–7214. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Reisenauer, K.N.; Masi, M.; Evidente, A.; Taube, J.H.; Romo, D. pharmacophore-directed retrosynthesis applied to ophiobolin A: Simplified bicyclic derivatives displaying anticancer activity. Org. Lett. 2020, 22, 8307–8312. [Google Scholar] [CrossRef] [PubMed]
- Treiber, L.; Pezolt, C.; Zeng, H.; Schrey, H.; Jungwirth, S.; Shekhar, A.; Stadler, M.; Bilitewski, U.; Erb-Brinkmann, M.; Schobert, R. Dual Agents: Fungal Macrocidins and Synthetic Analogues with Herbicidal and Antibiofilm Activities. Antibiotics 2021, 10, 1022. [Google Scholar] [CrossRef]
- Takano, H.K.; Patterson, E.L.; Nissen, S.J.; Dayan, F.E.; Gaines, T.A. Predicting herbicide movement across semi-permeable membranes using three phase partitioning. Pestic. Biochem. Physiol. 2019, 159, 22–26. [Google Scholar] [CrossRef]
- Krähmer, H.; Walter, H.; Jeschke, P.; Haaf, K.; Baur, P.; Evans, R. What makes a molecule a pre- or a post-herbicide–How valuable are physicochemical parameters for their design? Pest Manag. Sci. 2021, 77, 4863–4873. [Google Scholar] [CrossRef]
- Fujiwara, T.; O’Hagan, D. Successful fluorine-containing herbicide agrochemicals. J. Fluor. Chem. 2014, 167, 16–29. [Google Scholar] [CrossRef]
- Ogawa, Y.; Tokunaga, E.; Kobayashi, O.; Hirai, K.; Shibata, N. Current contributions of organofluorine compounds to the agrochemical industry. iScience 2020, 23, 101467. [Google Scholar] [CrossRef]
- Koradin, C.; Schröder, H.; Oyama, K.; Ōmura, S. Chemistry and biology connected: The development of Inscalis. In Recent Highlights in the Discovery and Optimization of Crop Protection Products; Maienfisch, P., Mangelinckx, S., Eds.; Academic Press: London, UK, 2021; pp. 231–239. [Google Scholar] [CrossRef]
- Clark, R.D. A perspective on the role of quantitative structure-activity and structure-property relationships in herbicide discovery. Pest Manag. Sci. 2012, 68, 513–518. [Google Scholar] [CrossRef]
- Wang, Y.L.; Li, J.Y.; Shi, X.X.; Wang, Z.; Hao, G.-F.; Yang, G.-F. Web-based quantitative structure–activity relationship resources facilitate effective drug discovery. Top Curr. Chem. (Z) 2021, 379, 37. [Google Scholar] [CrossRef]
- Truax, N.J.; Romo, D. Bridging the gap between natural product synthesis and drug discovery. Nat. Prod. Rep. 2020, 37, 1436–1453. [Google Scholar] [CrossRef]
- Karageorgis, G.; Foley, D.J.; Laraia, L.; Brakmann, S.; Waldmann, H. Pseudo natural products—Chemical evolution of natural product structure. Angew. Chem. Int. Ed. 2021, 60, 15705–15723. [Google Scholar] [CrossRef]
- Sparks, T.C.; Duke, S.O. Structure simplification of natural products as a lead generation approach in agrochemical discovery. J. Agric. Food Chem. 2021, 69, 8324–8346. [Google Scholar] [CrossRef]
- Ben Nejma, A.; Znati, M.; Daich, A.; Othman, M.; Lawson, A.M.; Ben Jannet, H. Design and semisynthesis of new herbicide as 1,2,3-triazole derivatives of the natural maslinic acid. Steroids 2018, 38, 102–107. [Google Scholar] [CrossRef]
- Rodrigues, R.P.; Baroni, A.C.M.; Carollo, C.A.; Demarque, D.P.; Pardo, L.F.L.; de Rezende, L.M.P.; dos Santos, F.J.L.; Lima, W.G.; de Siqueira, J.M. Synthesis, phytotoxic evaluation and in silico studies for the development of novel natural products-inspired herbicides. Biocatal. Agric. Biotechnol. 2020, 24, 101559. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Z.; Peng, J.; Zou, Y.; Hui, Y.; Chen, Y.; Gao, S.; Fu, Y.; Ye, F. Design, synthesis, and herbicidal activity of novel phenoxypyridine derivatives containing natural product coumarin. Pest Manag. Sci. 2021, 77, 4785–4798. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, T.; Bi, X.; Yang, S.; Huang, J.; Zhou, L. Laboratory bioassay, greenhouse experiment and 3D-QSAR studies on berberine analogues: A search for new herbicides based on natural products. Pest Manag. Sci. 2021, 77, 2054–2067. [Google Scholar] [CrossRef]
- Yang, Z.; Li, Q.; Yin, J.; Liu, R.; Tian, H.; Duan, L.; Li, Z.; Wang, B.; Tan, W.; Liu, S. Design, synthesis and mode of action of novel 3-chloro-6-pyrazolyl picolinate derivatives as herbicide candidates. Pest Manag. Sci. 2021, 77, 2252–2263. [Google Scholar] [CrossRef]
- Renaud, J.B.; DesRochers, N.; Hoogstra, S.; Garnham, C.P.; Sumarah, M.W. Structure Activity Relationship for Fumonisin Phytotoxicity. Chem. Res. Toxic. 2021, 34, 1604–1611. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Freda, F.; Clement, S.; Cimmino, A.; Cristofaro, M.; Meyer, S.; Evidente, A. Phytotoxic Activity and Structure-Activity Relationships of Radicinin Derivatives against the Invasive Weed Buffelgrass (Cenchrus ciliaris). Molecules 2019, 24, 2793. [Google Scholar] [CrossRef] [PubMed]
- Dalinova, A.; Fedorov, A.; Dubovik, V.; Voitsekhovskaja, O.; Tyutereva, E.; Smirnov, S.; Kochura, D.; Chisty, L.; Senderskiy, I.; Berestetskiy, A. Structure–Activity Relationship of Phytotoxic Natural 10-Membered Lactones and Their Semisynthetic Derivatives. J. Fungi 2021, 7, 829. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yao, Q.; Guo, Y.; Zhang, Q.; Wang, Z.; Strasser, R.J.; Valverde, B.E.; Chen, S.; Qiang, S.; Kalaji, H.M. Structure-based ligand design and discovery of novel tenuazonic acid derivatives with high herbicidal activity. J. Adv. Res. 2022, 40, 29–44. [Google Scholar] [CrossRef]
- Ooka, J.K.; Correia, M.V.; Scotti, M.T.; Fokoue, H.H.; Yamaguchi, L.F.; Kato, M.J.; Dayan, F.E.; Owens, D.K. Synthesis and Activity of 2-Acyl-cyclohexane-1,3-dione Congeners Derived from Peperomia Natural Products against the Plant p-Hydroxyphenylpyruvate Dioxygenase Herbicidal Molecular Target Site. Plants 2022, 11, 2269. [Google Scholar] [CrossRef]
- Chotpatiwetchkul, W.; Chotsaeng, N.; Laosinwattana, C.; Charoenying, P. Structure–activity relationship study of xanthoxyline and related small methyl ketone herbicides. ACS Omega 2022, 7, 29002–29012. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berestetskiy, A. Modern Approaches for the Development of New Herbicides Based on Natural Compounds. Plants 2023, 12, 234. https://doi.org/10.3390/plants12020234
Berestetskiy A. Modern Approaches for the Development of New Herbicides Based on Natural Compounds. Plants. 2023; 12(2):234. https://doi.org/10.3390/plants12020234
Chicago/Turabian StyleBerestetskiy, Alexander. 2023. "Modern Approaches for the Development of New Herbicides Based on Natural Compounds" Plants 12, no. 2: 234. https://doi.org/10.3390/plants12020234
APA StyleBerestetskiy, A. (2023). Modern Approaches for the Development of New Herbicides Based on Natural Compounds. Plants, 12(2), 234. https://doi.org/10.3390/plants12020234






