Calcium Involved in the Enrichment of γ-Aminobutyric Acid (GABA) in Broccoli Sprouts under Fructose Treatment
Abstract
1. Introduction
2. Results
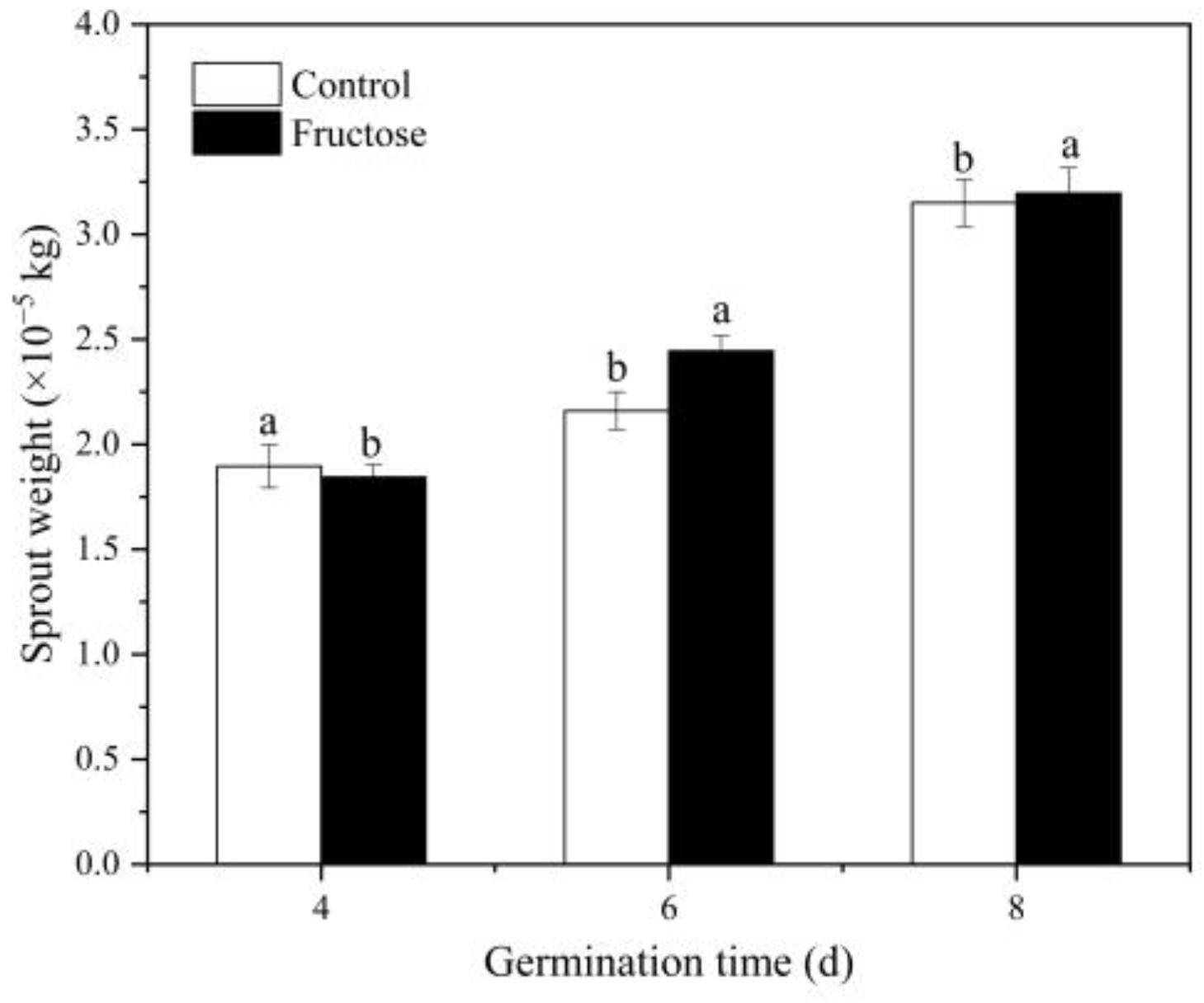
2.1. Effect of Fructose Treatment on Growth of Broccoli Sprouts
2.2. Fructose Treatment Increased the Contents of GABA and Glutamate in Broccoli Sprouts
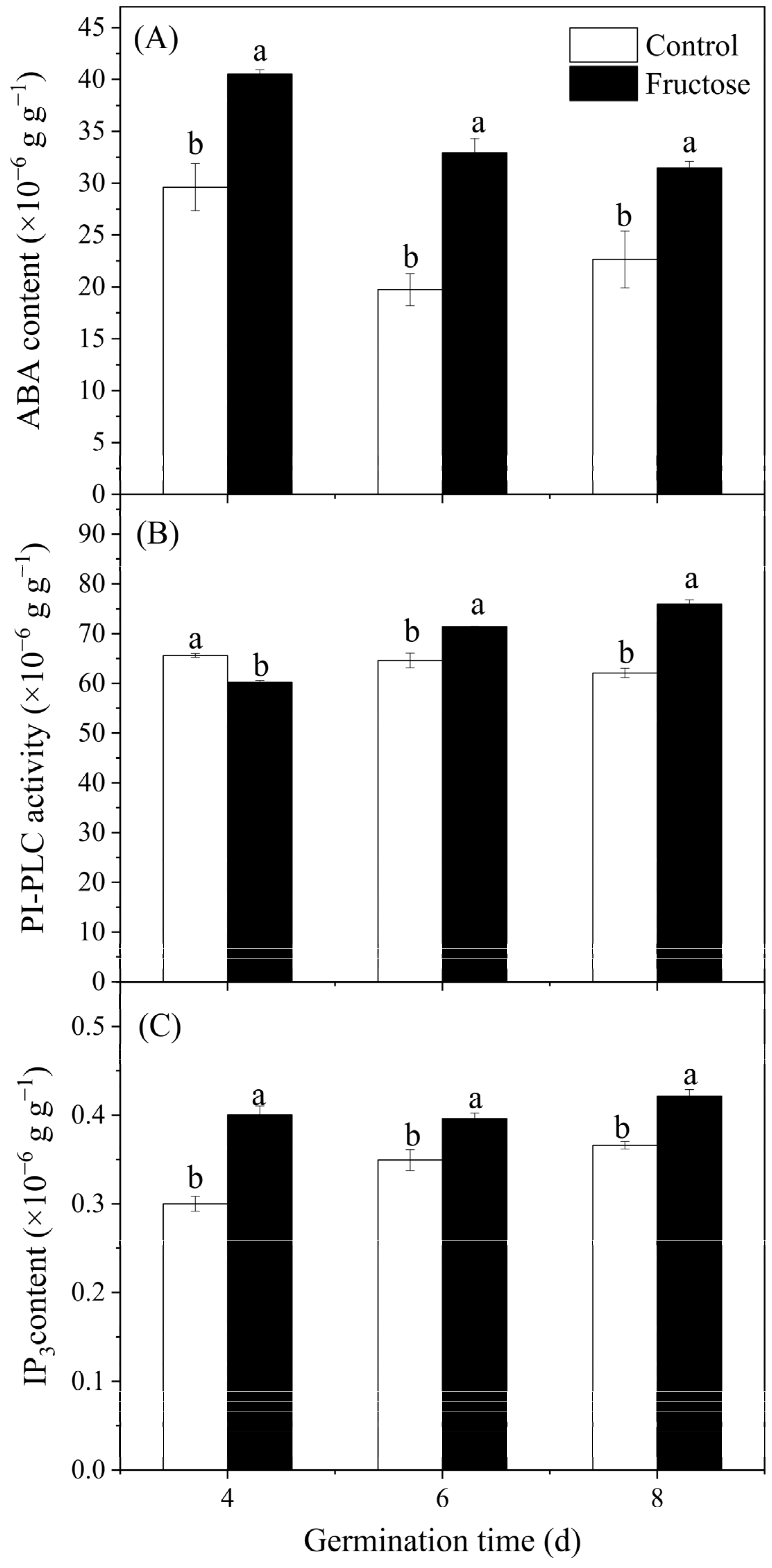
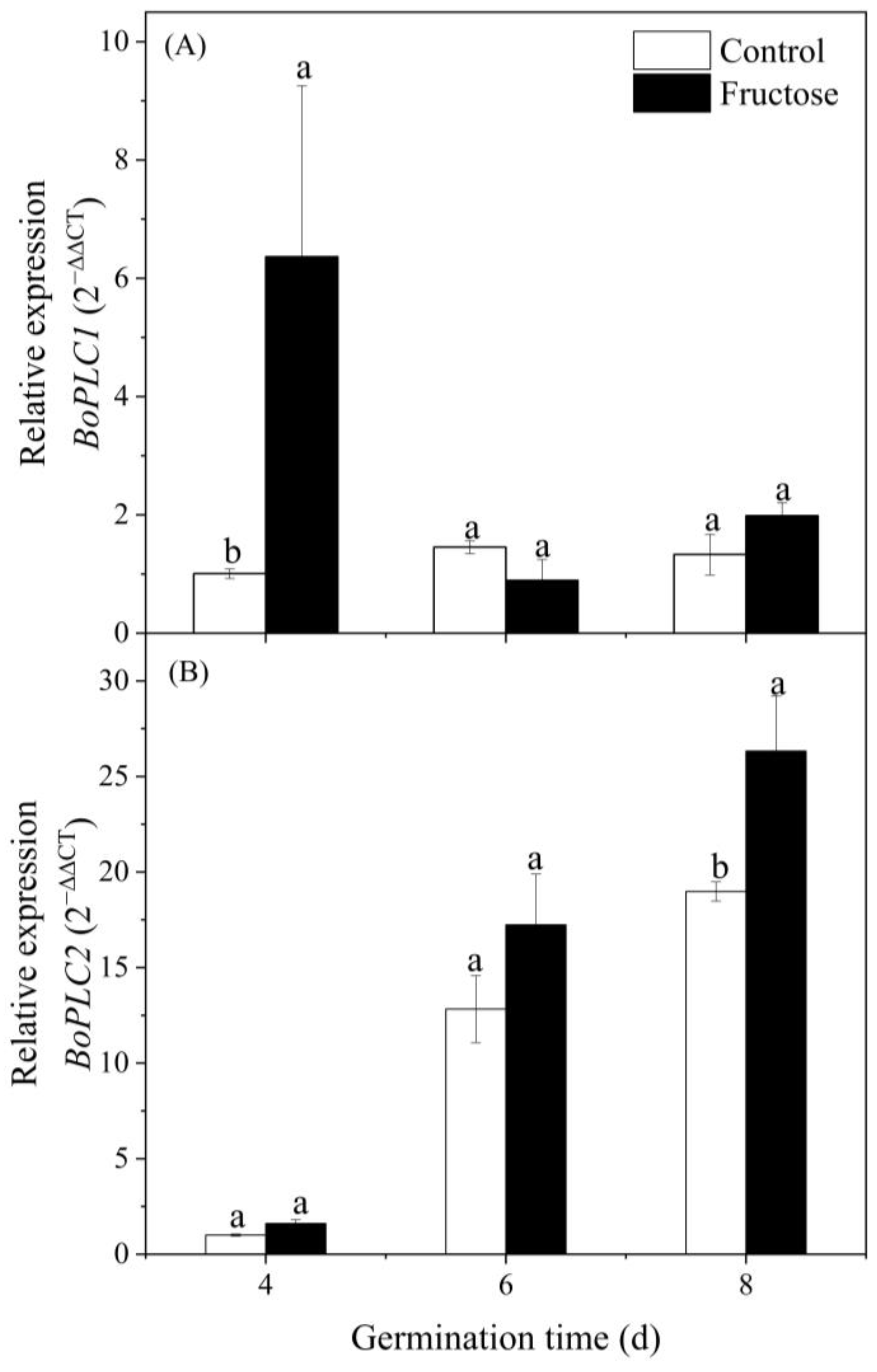
2.3. Effect of Fructose Treatment on ABA, IP3 Contents, PI-PLC Activity, and BoPLC Expression in Broccoli Sprouts
2.4. Fructose Treatment Improved Ca2+ and CaM Contents in Broccoli Sprouts
2.5. Effect of Fructose Treatment on the Activities of GAD and GABA-T
2.6. GABA Shunt Metabolism Genes Expression under Fructose Treatment
3. Discussion
4. Materials and Methods
4.1. Seed and Cultivation Conditions
4.2. Sprouts Stem Length and Fresh Weight Measurement
4.3. Determination of GABA Content
4.4. Analysis of Glutamate Content
4.5. Abscisic Acid (ABA) Content Determination
4.6. Determination of Phosphatidylinositol-Specific Phospholipase C (PI-PLC) Activity
4.7. Inositol Trisphosphate (IP3) Content Assay
4.8. Analysis of Ca2+ and CaM Contents
4.9. GAD and GABA-T Activities Assay
4.10. Gene Expression Analysis
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Luo, Z.; Huang, X.; Yang, K.; Gao, S.; Du, R. Effect of exogenous γ-aminobutyric acid (GABA) treatment on chilling injury and antioxidant capacity in banana peel. Sci. Hortic. 2014, 168, 132–137. [Google Scholar] [CrossRef]
- Ren, L.; Wang, F.; Xu, Z.; Chan, W.M.; Zhao, C.; Xue, H. GABAA receptor subtype selectivity underlying anxiolytic effect of 6-hydroxyflavone. Biochem. Pharmacol. 2010, 79, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Abdou, A.M.; Higashiguchi, S.; Horie, K.; Kim, M.; Hatta, H.; Yokogoshi, H. Relaxation and immunity enhancement effects of γ-Aminobutyric acid (GABA) administration in humans. BioFactors 2006, 26, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Sugishita, T.; Murakami, T.; Murai, H.; Saikusa, T.; Horino, T.; Onoda, A.; Kajimoto, O.; Takahashi, R.; Takahashi, T. Effect of the defatted rice germ enriched with GABA for sleeplessness, depression, autonomic disorder by oral administration. J. Jpn. Soc. Food Sci. 2000, 47, 8. [Google Scholar] [CrossRef]
- Shelp, B.J.; Bozzo, G.G.; Trobacher, C.P.; Zarei, A.; Deyman, K.L.; Brikis, C.J. Hypothesis/review: Contribution of putrescine to 4-aminobutyrate (GABA) production in response to abiotic stress. Plant Sci. 2012, 193–194, 130–135. [Google Scholar] [CrossRef]
- Bouché, N.; Fromm, H. GABA in plants: Just a metabolite? Trends Plant Sci. 2004, 9, 110–115. [Google Scholar] [CrossRef]
- Liao, J.; Wu, X.; Xing, Z.; Li, Q.; Duan, Y.; Fang, W.; Zhu, X. γ-Aminobutyric Acid (GABA) accumulation in tea (Camellia sinensis L.) through the GABA shunt and polyamine degradation pathways under anoxia. J. Agric. Food Chem. 2017, 65, 3013–3018. [Google Scholar] [CrossRef]
- Kinnersley, A.M.; Turano, F.J. Gamma aminobutyric acid (GABA) and plant responses to stress. Crit. Rev. Plant Sci. 2000, 19, 479–509. [Google Scholar] [CrossRef]
- Wang, K.; Xu, F.; Cao, S.; Wang, H.; Wei, Y.; Shao, X.; Zhou, W.; Zheng, Y. Effects of exogenous calcium chloride (CaCl2) and ascorbic acid (AsA) on the γ-aminobutyric acid (GABA) metabolism in shredded carrots. Postharvest Biol. Technol. 2019, 152, 111–117. [Google Scholar] [CrossRef]
- Chi, Z.; Dai, Y.; Cao, S.; Wei, Y.; Shao, X.; Huang, X.; Xu, F.; Wang, H. Exogenous calcium chloride (CaCl2) promotes γ-aminobutyric acid (GABA) accumulation in fresh-cut pears. Postharvest Biol. Technol. 2021, 174, 111446. [Google Scholar] [CrossRef]
- Carvacho, H.B.; Perez, C.; Zuniga, G.; Mahn, A. Effect of methyl jasmonate, sodium selenate and chitosan as exogenous elicitors on the phenolic compounds profile of broccoli sprouts. J. Sci. Food Agric. 2014, 94, 2555–2561. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Xu, K.; Zhu, Y.; Wang, F.; Xiao, J.; Guo, L. Calcium affects glucoraphanin metabolism in broccoli sprouts under ZnSO4 stress. Food Chem. 2021, 334, 127520. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, A.; Saei, A.; McKenzie, M.J.; Matich, A.J.; Babalar, M.; Hunter, D.A. Preferentially enhancing anti-cancer isothiocyanates over glucosinolates in broccoli sprouts: How NaCl and salicylic acid affect their formation. Plant Physiol. Biochem. 2017, 115, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Song, S.; Nirasawa, S.; Hung, Y.-C.; Jiang, Z.; Liu, H. Slightly acidic electrolyzed water treatment enhances the main bioactive phytochemicals content in broccoli sprouts via changing metabolism. J. Agric. Food Chem. 2019, 67, 606–614. [Google Scholar] [CrossRef]
- Li, C.; Song, S.; He, Y.; Zhang, X.; Liu, H. CaCl2-HCl electrolyzed water affects glucosinolate metabolism and improves the quality of broccoli sprouts. Food Res. Int. 2021, 150 Pt B, 110807. [Google Scholar] [CrossRef]
- Xie, K.; Wu, C.; Chi, Z.; Wang, J.; Wang, H.; Wei, Y.; Shao, X.; Xu, F. Enhancement of γ-aminobutyric acid (GABA) and other health-promoting metabolites in germinated broccoli by mannose treatment. Sci. Hortic. 2021, 276, 109706. [Google Scholar] [CrossRef]
- Jin, L.; Cai, Y.; Sun, C.; Huang, Y.; Yu, T. Exogenous l-glutamate treatment could induce resistance against penicillium expansum in pear fruit by activating defense-related proteins and amino acids metabolism. Postharvest Biol. Technol. 2019, 150, 148–157. [Google Scholar] [CrossRef]
- Zhang, D.; Wei, X.; Liu, Z.; Wu, X.; Bao, C.; Sun, Y.; Su, N.; Cui, J. Transcriptome analysis reveals the molecular mechanism of GABA accumulation during quinoa (chenopodium quinoa willd.) germination. J. Agric. Food Chem. 2021, 69, 12171–12186. [Google Scholar] [CrossRef]
- Yang, R.; Guo, Y.; Wang, S.; Gu, Z. Ca2+ and aminoguanidine on γ-aminobutyric acid accumulation in germinating soybean under hypoxia–NaCl stress. J. Food Drug Anal. 2015, 23, 287–293. [Google Scholar] [CrossRef]
- Arazi, T.; Baum, G.; Snedden, W.A.; Shelp, B.J.; Fromm, H. Molecular and Biochemical Analysis of Calmodulin Interactions with the Calmodulin-Binding Domain of Plant Glutamate Decarboxylase. Plant Physiol. Biochem. 1995, 108, 551–561. [Google Scholar] [CrossRef]
- Chin, D.; Means, A.R. Calmodulin: A prototypical calcium sensor. Trends Cell Biol. 2000, 10, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Vitrac, X.; Larronde, F.; Krisa, S.; Decendit, A.; Deffieux, G.; Mérillon, J.-M. Sugar sensing and Ca2+–calmodulin requirement in Vitis vinifera cells producing anthocyanins. Phytochemistry 2000, 53, 659–665. [Google Scholar] [CrossRef]
- Furuichi, T.; Muto, S. H+-coupled sugar transporter, an initiator of sugar-induced Ca2+-signaling in plant cells. Z. Naturforsch. C 2005, 60, 764–768. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, H.; Chiltz, A.; Madani, S.; Vatsa, P.; Schoefs, B.; Pugin, A.; Garcia-Brugger, A. Calcium signatures and signaling in cytosol and organelles of tobacco cells induced by plant defense elicitors. Cell Calcium 2012, 51, 434–444. [Google Scholar] [CrossRef]
- Reggiani, R.; Aurisano, N.; Mattana, M.; Bertani, A. ABA induces 4-aminobutyrate accumulation in wheat seedlings. Phytochemistry 1993, 34, 605–609. [Google Scholar] [CrossRef]
- Hartung, W.; Schraut, D.; Jiang, F. Physiology of abscisic acid (ABA) in roots under stress—a review of the relationship between root ABA and radial water and ABA flows. Aust. J. Agric. Res. 2005, 56, 1253–1259. [Google Scholar] [CrossRef]
- Akhtar, S.; Wahid, A.; Rasul, E. Emergence, growth and nutrient composition of sugarcane sprouts under NaCl salinity. Biol. Plant 2003, 46, 113–116. [Google Scholar] [CrossRef]
- Lee, Y.; Choi, Y.B.; Suh, S.; Lee, J.; Assmann, S.M.; Joe, C.O.; Kelleher, J.F.; Crain, R.C. Abscisic Acid-Induced Phosphoinositide Turnover in Guard Cell Protoplasts of Vicia faba. Plant Physiol. 1996, 110, 987–996. [Google Scholar] [CrossRef]
- Mikoshiba, K. Inositol 1,4,5-trisphosphate IP3 receptors and their role in neuronal cell function. J. Neurochem. 2006, 97, 1627–1633. [Google Scholar] [CrossRef]
- Sanders, D.; Pelloux, J.; Brownlee, C.; Harper, J.F. Calcium at the crossroads of signaling. Plant Cell 2002, 14 (Suppl. 1), S401–S417. [Google Scholar] [CrossRef]
- Parre, E.; Ghars, M.A.; Leprince, A.-S.; Thiery, L.; Lefebvre, D.; Bordenave, M.; Richard, L.; Mazars, C.; Abdelly, C.; Savouré, A. Calcium signaling via phospholipase C is essential for proline accumulation upon Ionic but not nonionic hyperosmotic stresses in arabidopsis. Plant Physiol. 2007, 144, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Jiao, C.; Yang, R.; Gu, Z. Cyclic ADP-ribose and IP3 mediate abscisic acid-induced isoflavone accumulation in soybean sprouts. Biochem. Bioph. Res. Commun. 2016, 479, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Al-Quraan, N.A.; Locy, R.D.; Singh, N.K. Implications of paraquat and hydrogen peroxide-induced oxidative stress treatments on the GABA shunt pathway in Arabidopsis thaliana calmodulin mutants. Plant Biotechnol. Rep. 2011, 5, 225–234. [Google Scholar] [CrossRef]
- Hu, X.; Xu, Z.; Xu, W.; Li, J.; Zhao, N.; Zhou, Y. Application of gamma-aminobutyric acid demonstrates a protective role of polyamine and GABA metabolism in muskmelon seedlings under Ca(NO3)2 stress. Plant Physiol. Biochem. 2015, 92, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bethke, P.C.; Jones, R.L. Cell death of barley aleurone protoplasts is mediated by reactive oxygen species. Plant J. 2001, 25, 19–29. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Hong, Q.; Lin, Y.; Mao, W.; Zhou, S. Reactive oxygen species triggering systemic programmed cell death process via elevation of nuclear calcium ion level in tomatoes resisting tobacco mosaic virus. Plant Sci. 2018, 270, 166–175. [Google Scholar] [CrossRef]





| Gene | Accession No. | Primer | Primer Sequence (5′-3′) |
|---|---|---|---|
| 18S | AF513990 | 18S-F | CGAGACCTCAGCCTGCTAACT |
| 18S-R | CAGAACATCTAAGGGCATCACA | ||
| BoGAD1 | LOC106329129 | GAD1-F | GGTGACGGTGAAGAAAACCG |
| GAD1-R | CCTTCAAATCTCCGAATTAGTGC | ||
| BoGAD2 | LOC106300456 | GAD2-F | ATCTCGCTATGTCCGCACTG |
| GAD2-R | TCTGGAGCTCGGTAGTGACA | ||
| BoGAD4 | LOC106325355 | GAD4-F | AGGGTTCACGCTAAGATGGC |
| GAD4-R | CCATGGGAGAAAGGGCTTCA | ||
| BoGABA-T | LOC106391515 | GABA-T-F | TTGATTCTGGGAACTGAG |
| GABA-T-R | TGAGATAATAAGCGGTGG | ||
| BoPLC1 | LOC106322918 | PLC1-F | CGTGGACCCGATTTAGTG |
| PLC1-R | TGCATATTGAAGGCAACC | ||
| BoPLC2 | LOC106312862 | PLC2-F | CCCGATTTCTACGCAAGGGT |
| PLC2-R | ACGCAATGGGAACTCGAACT | ||
| BoCaM1 | LOC106317776 | CaM1-F | CTCTTCGACAAGGATGGTGAC |
| CaM1-R | GGTTTTGCCCTAGAGACCTCA | ||
| BoCaM2 | LOC106327158 | CaM2-F | AGTTCCTGAACCTGATGGCG |
| CaM2-R | TCAGCTTCTCCCCGAGGTTA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Q.; Xie, K.; Wang, H.; Shao, X.; Wei, Y.; Chen, Y.; Jiang, S.; Cao, M.; Chen, J.; Xu, F. Calcium Involved in the Enrichment of γ-Aminobutyric Acid (GABA) in Broccoli Sprouts under Fructose Treatment. Plants 2023, 12, 224. https://doi.org/10.3390/plants12020224
Wei Q, Xie K, Wang H, Shao X, Wei Y, Chen Y, Jiang S, Cao M, Chen J, Xu F. Calcium Involved in the Enrichment of γ-Aminobutyric Acid (GABA) in Broccoli Sprouts under Fructose Treatment. Plants. 2023; 12(2):224. https://doi.org/10.3390/plants12020224
Chicago/Turabian StyleWei, Qinling, Keqin Xie, Hongfei Wang, Xingfeng Shao, Yingying Wei, Yi Chen, Shu Jiang, Mengze Cao, Jisuan Chen, and Feng Xu. 2023. "Calcium Involved in the Enrichment of γ-Aminobutyric Acid (GABA) in Broccoli Sprouts under Fructose Treatment" Plants 12, no. 2: 224. https://doi.org/10.3390/plants12020224
APA StyleWei, Q., Xie, K., Wang, H., Shao, X., Wei, Y., Chen, Y., Jiang, S., Cao, M., Chen, J., & Xu, F. (2023). Calcium Involved in the Enrichment of γ-Aminobutyric Acid (GABA) in Broccoli Sprouts under Fructose Treatment. Plants, 12(2), 224. https://doi.org/10.3390/plants12020224







