Exogenous Hydrogen Sulfide Supplementation Alleviates the Salinity-Stress-Mediated Growth Decline in Wheat (Triticum aestivum L.) by Modulating Tolerance Mechanisms
Abstract
:1. Introduction
2. Results
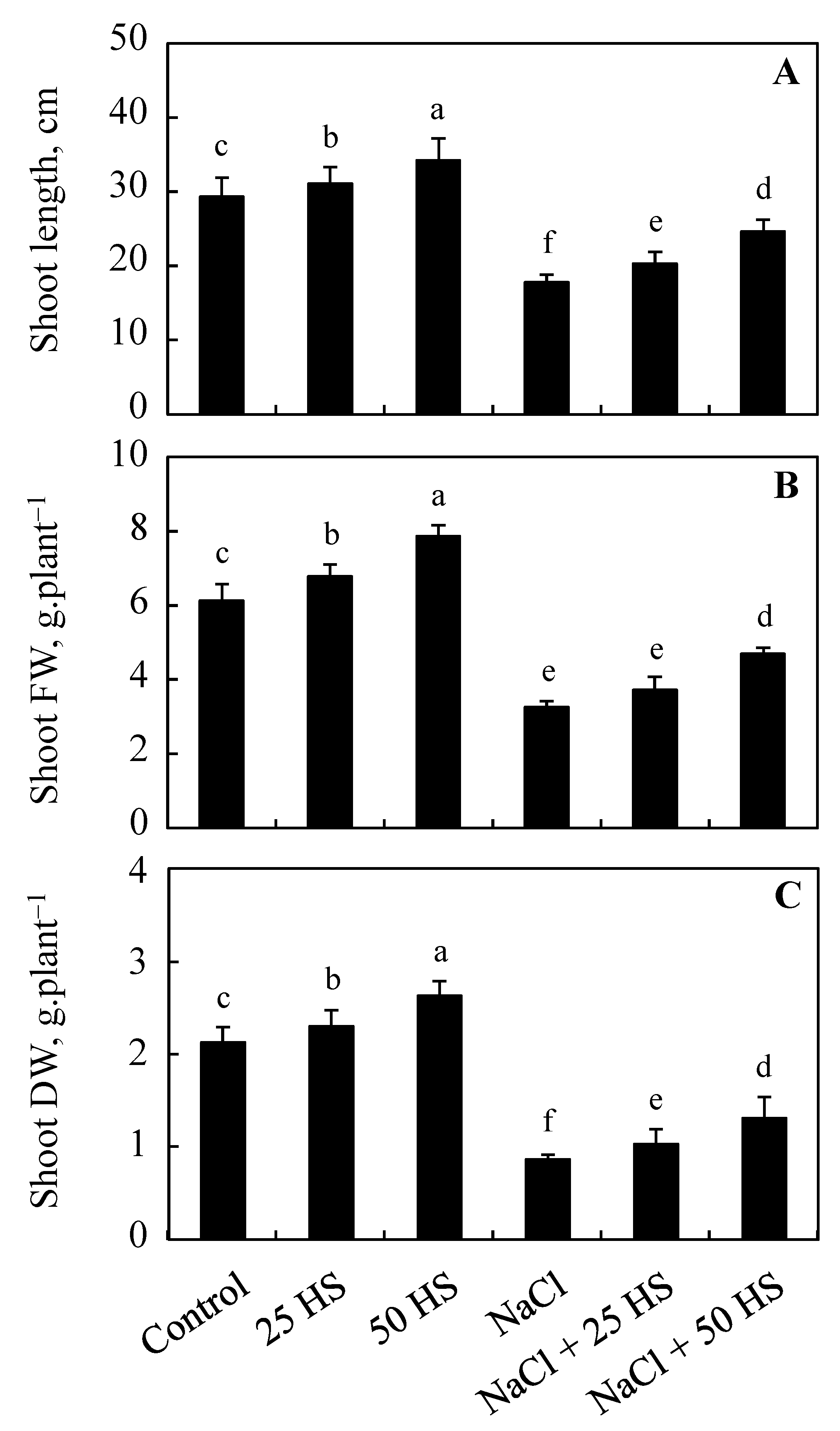
2.1. Effect of HS on the Height, Fresh, and Dry Weight of Shoot
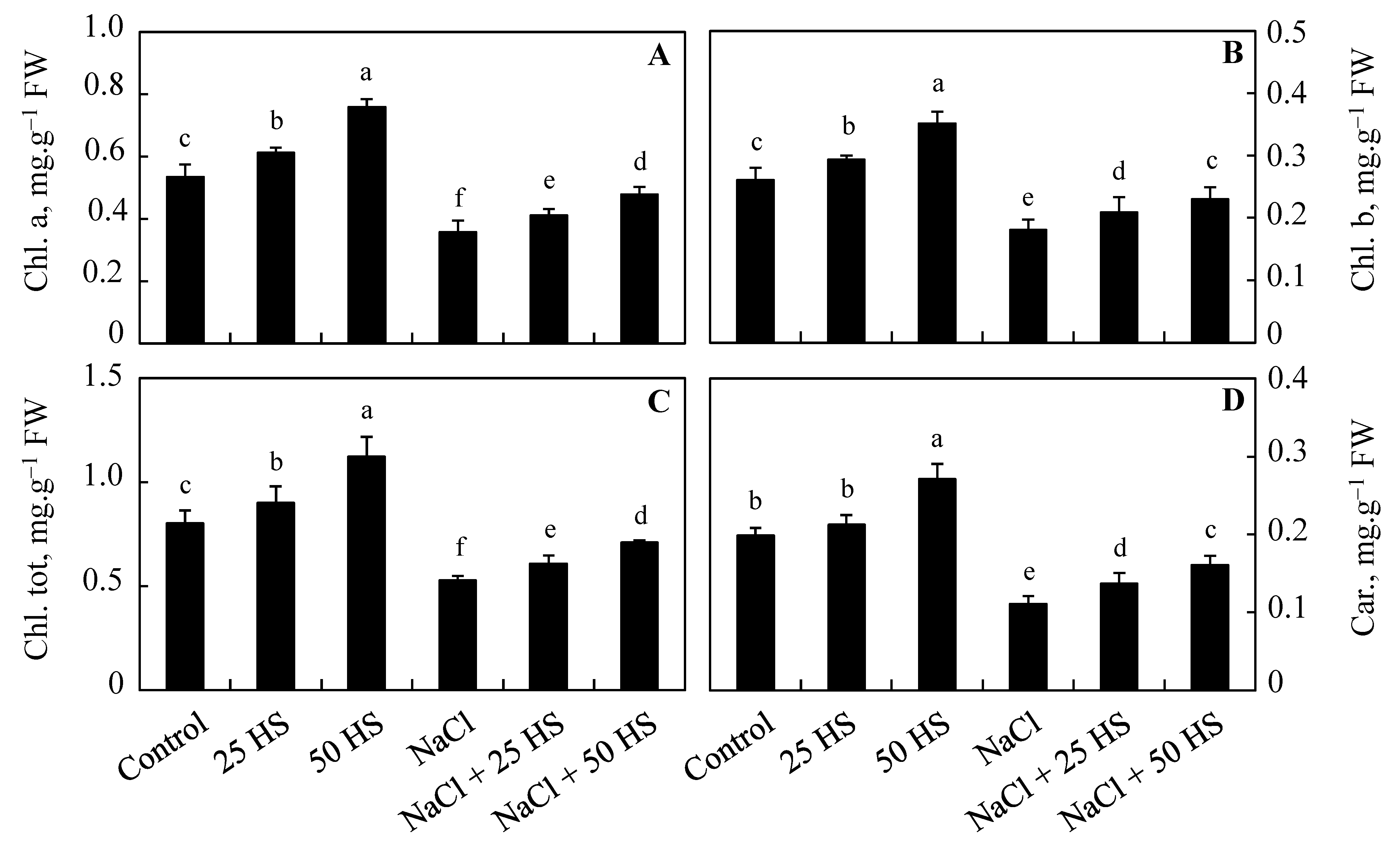
2.2. Effect of Exogenous HS on Photosynthetic Pigments
2.3. The Impact of HS Treatment and NaCl Stress on Oxidative Stress Parameters
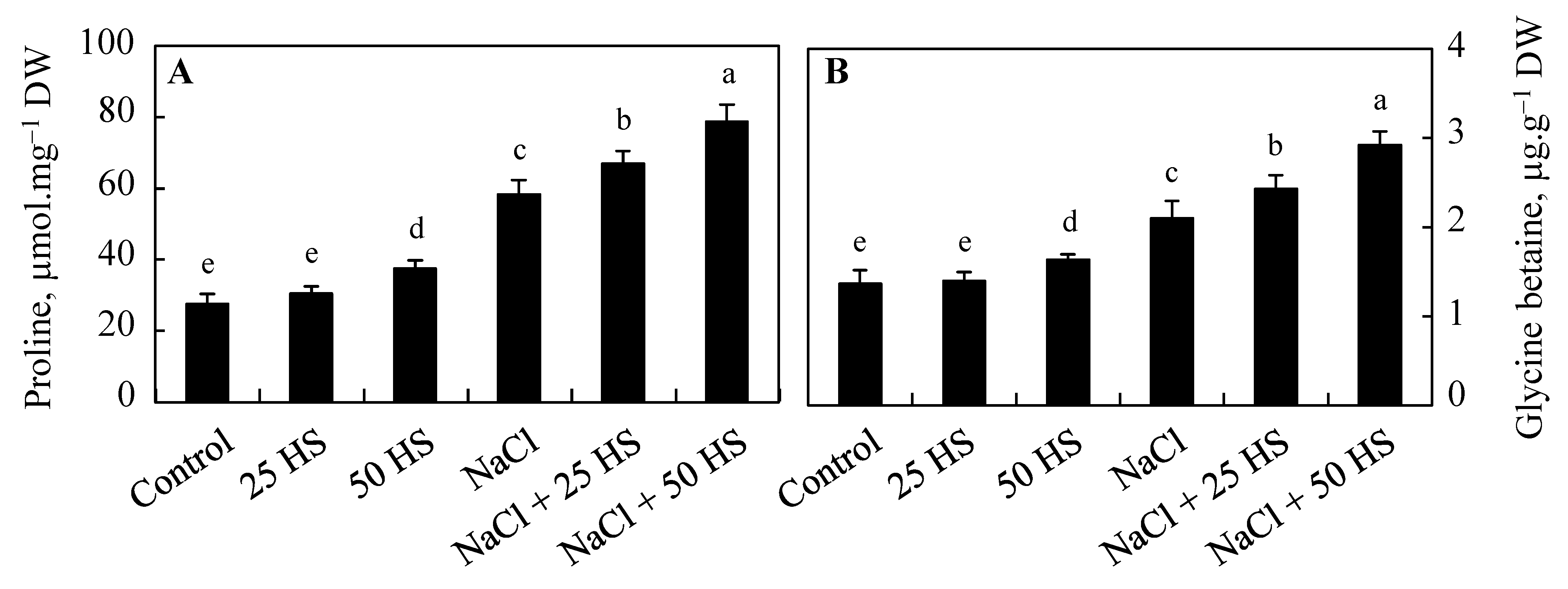
2.4. Effect of HS on Proline and Glycine Betaine
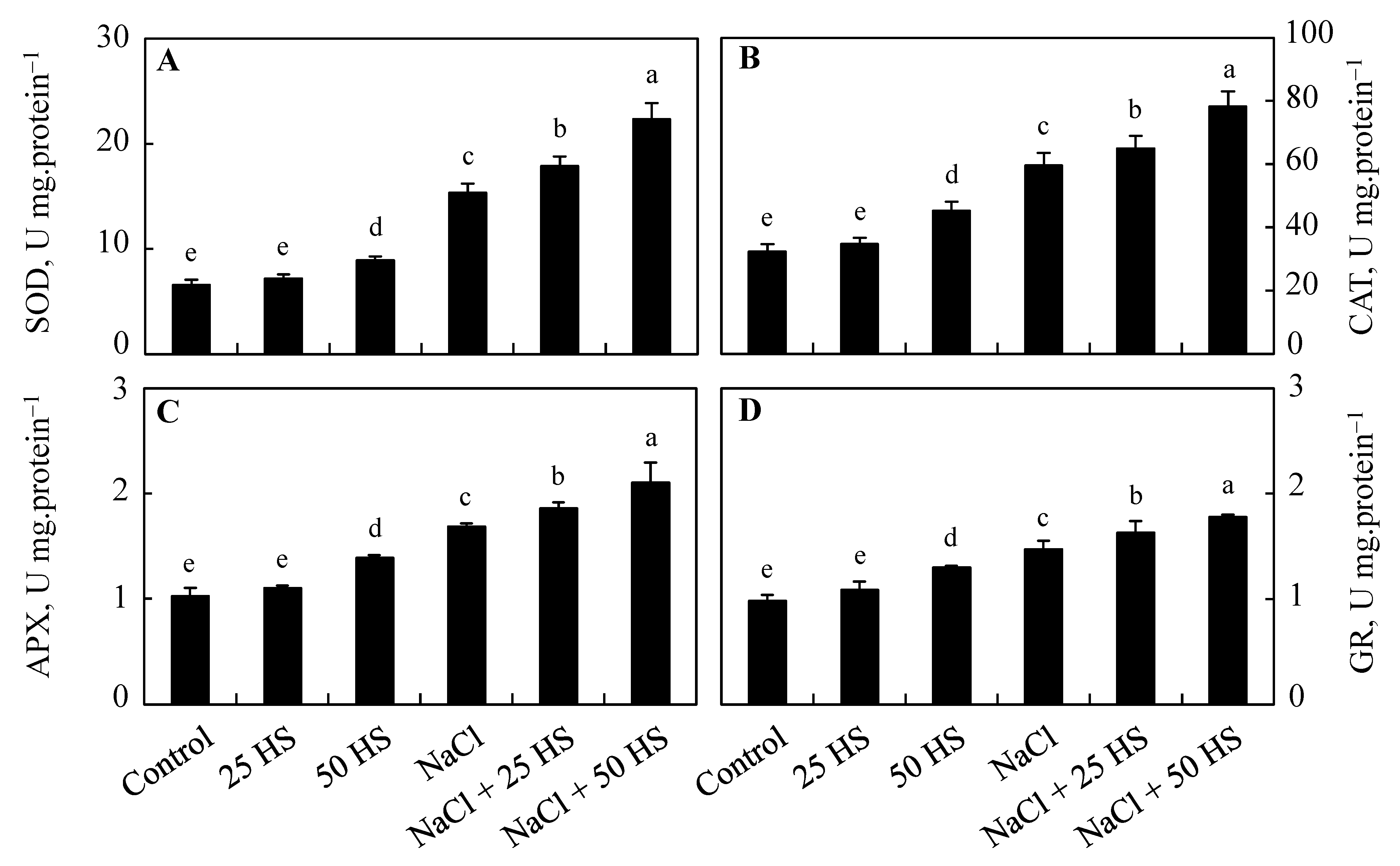
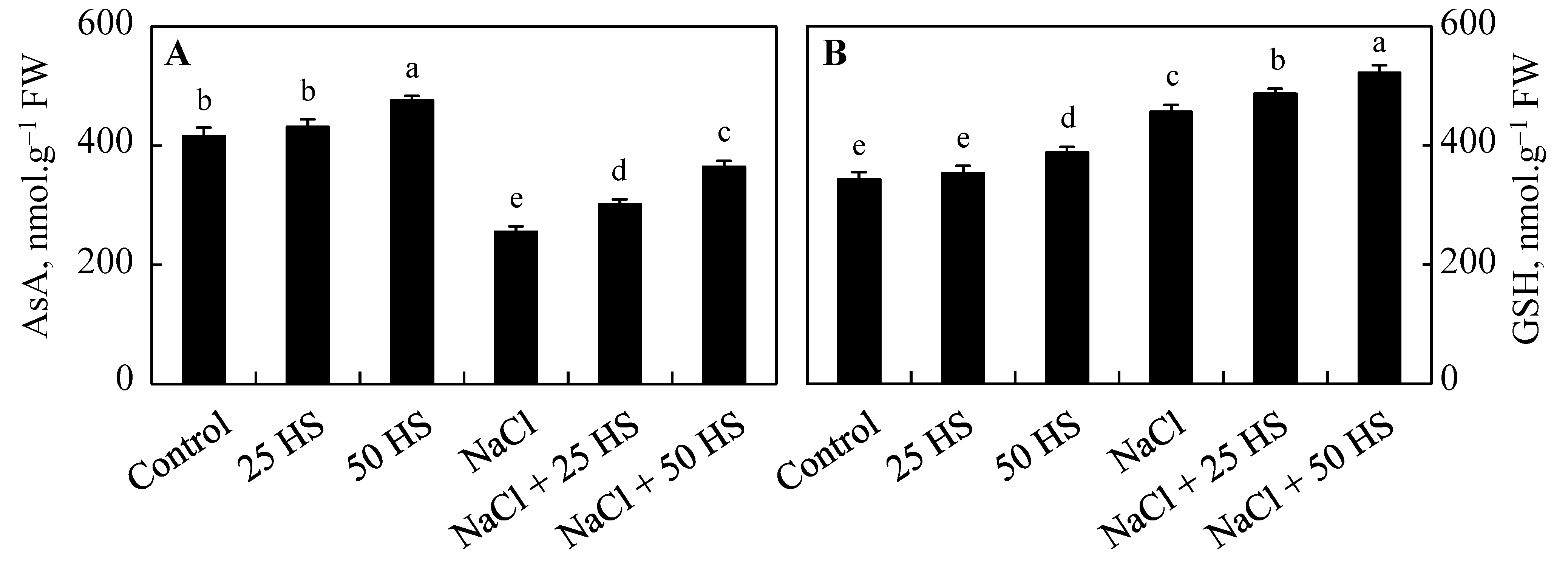
2.5. Effect of HS and NaCl Stress on the Antioxidant System
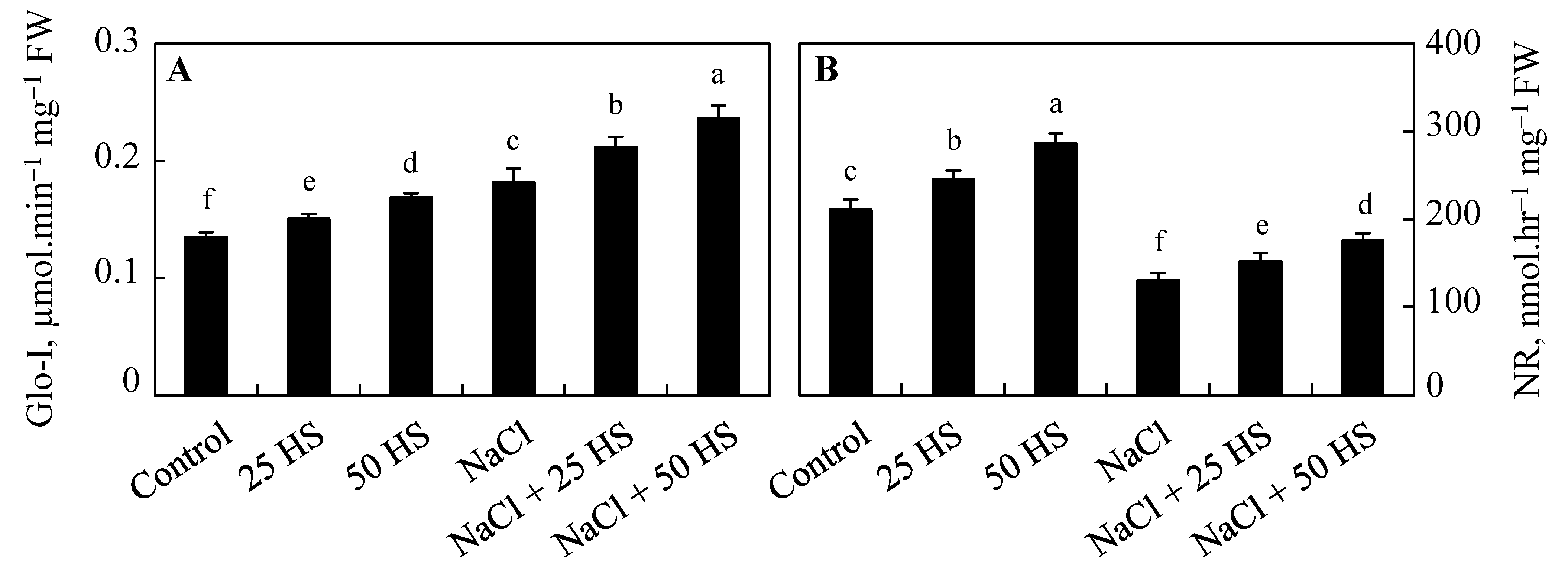
2.6. Influence of HS and NaCl on Glyoxylase I Activity
2.7. Effect of HS and NaCl on Nitrate Reductase Activity
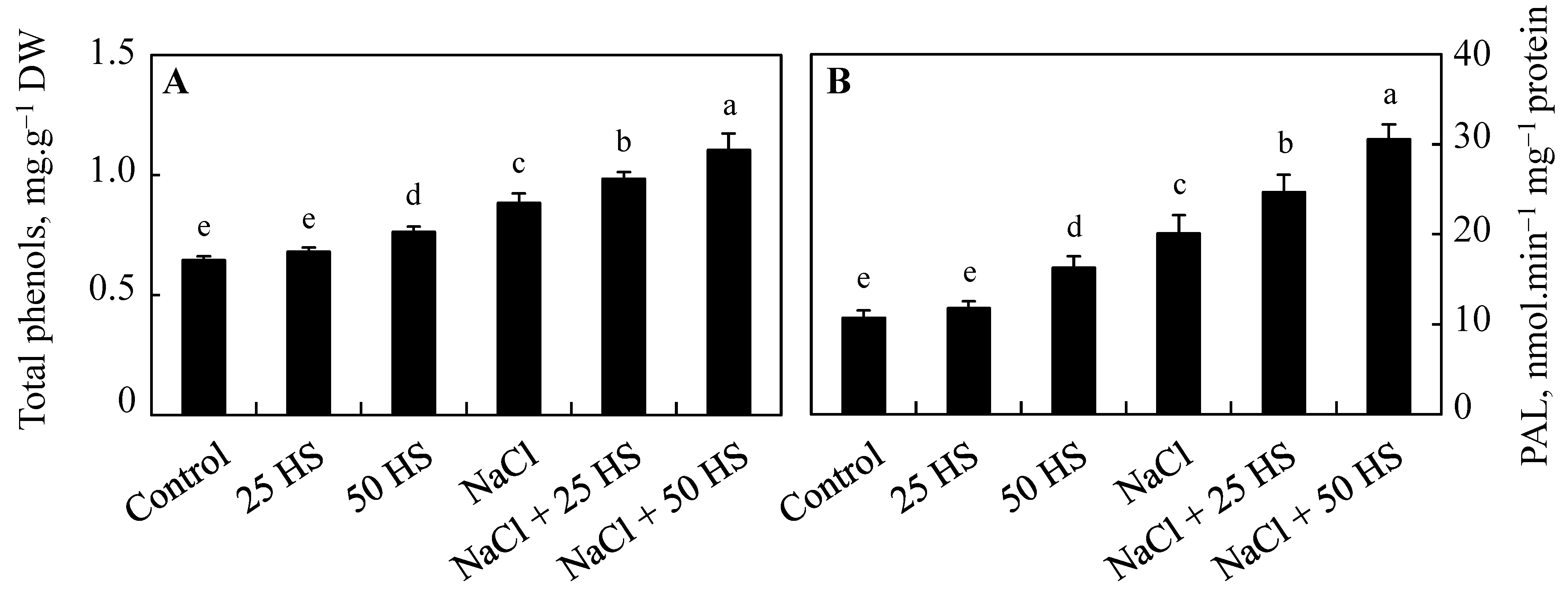
2.8. Effect of HS and NaCl on the Content of Phenol and Activity of Phenylalanine Ammonia-Lyase
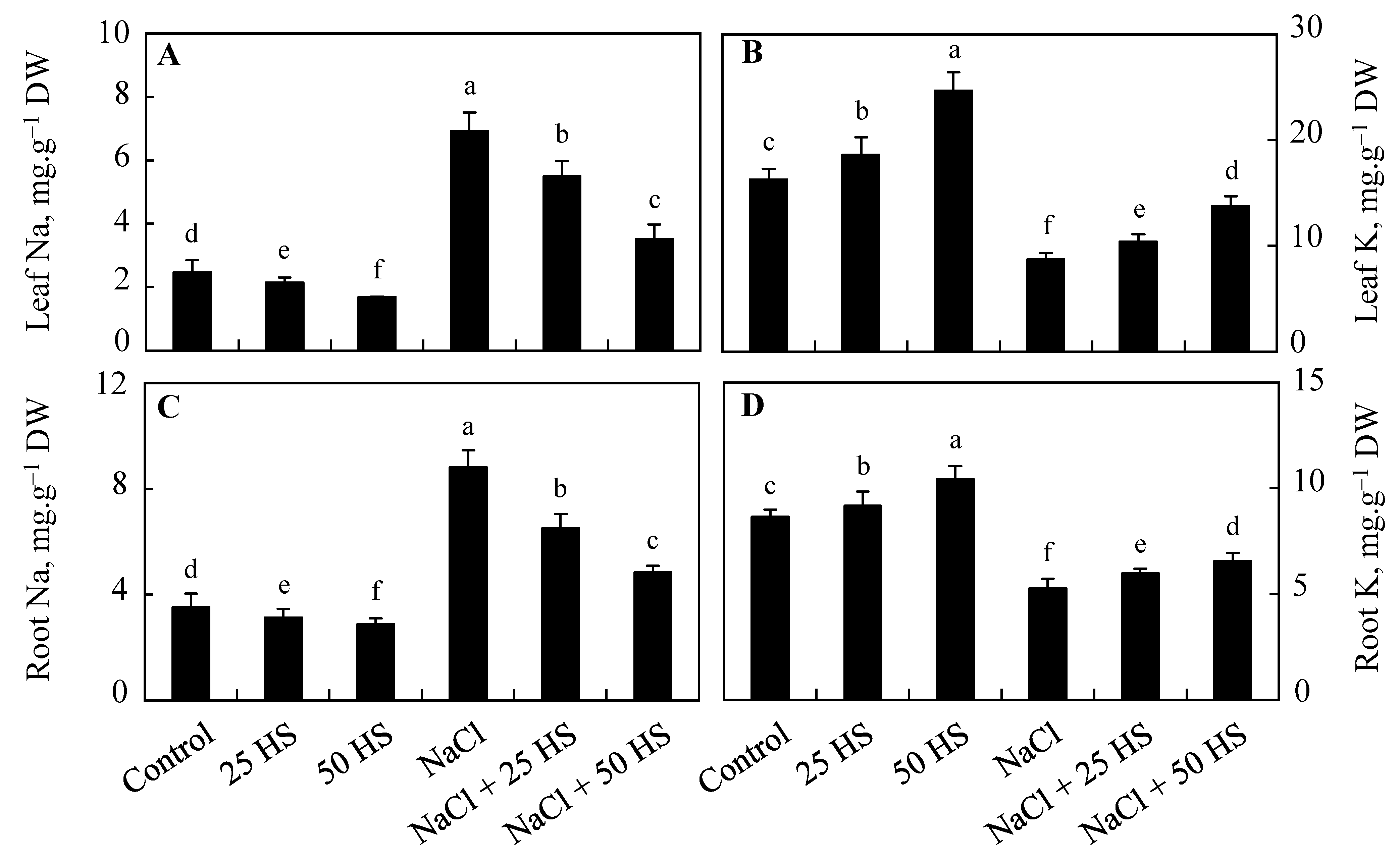
2.9. Influence of NaCl and HS on the Content of Sodium (Na) and Potasssium (K)
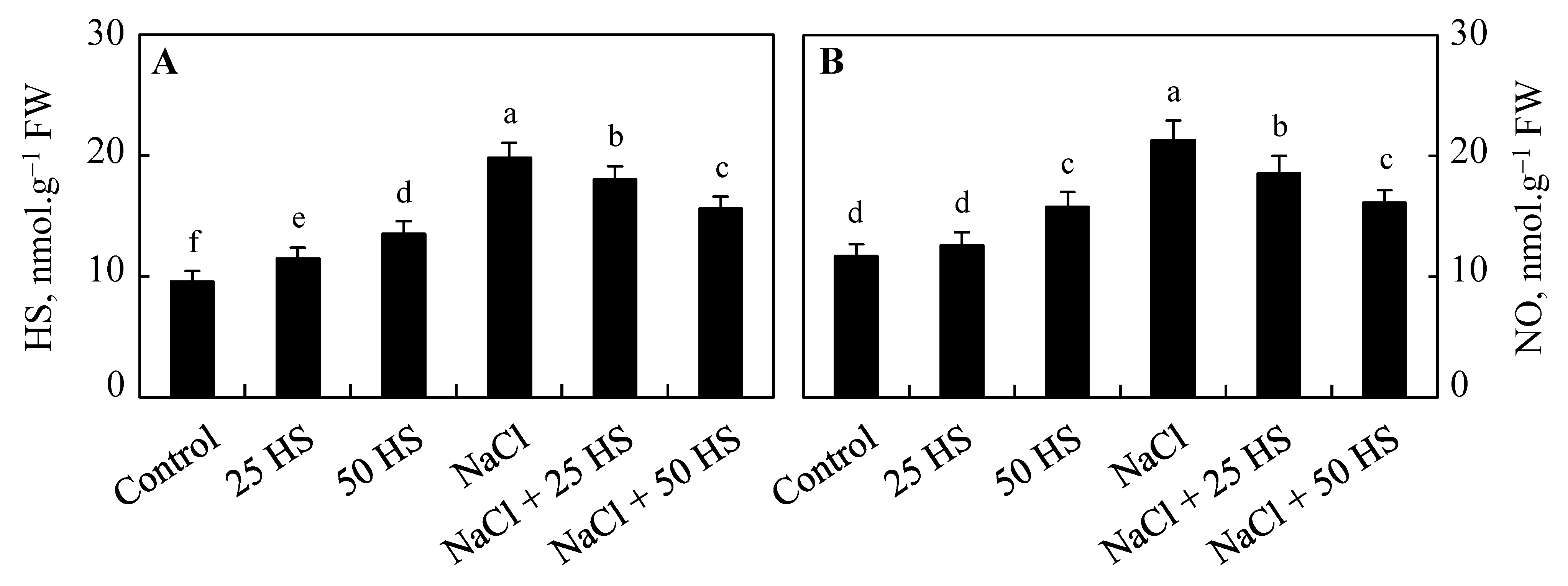
2.10. Effect of NaCl and HS on Endogenous HS and Nitric Oxide
3. Discussion
4. Material and Methods
4.1. Experimental Design, Growth Conditions, and Treatments
4.2. Growth Parameters
4.3. Measurement of Chlorophyll and Carotenoid Content
4.4. Measurement of Hydrogen Peroxide, Methylglyoxal, Lipid Peroxidation, and the Membrane Stability Index (MSI)
4.5. Determination of Proline and Glycine Betaine
4.6. Assay of Antioxidant Enzymes and Ascorbate and Reduced Glutathione
4.7. Activity of Glyoxalase I and Nitrate Reductase
4.8. Determination of Total Phenols and Activity of Phenylalanine Ammonia-Lyase
4.9. Estimation of Na and K
4.10. Estimation of Hydrogen Sulfide and Nitric Oxide
4.11. Statistical Analysis
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alamer, K.H.; Attia, H. UV-C seed priming improves tomato plant growth against salt stress. J. Taibah Univ. Sci. 2022, 16, 1181–1191. [Google Scholar] [CrossRef]
- Attia, H.; Alamer, K.; Algethami, B.; Zorrig, W.; Hessini, K.; Gupta, K.; Gupta, B. Gibberellic acid interacts with salt stress on germination, growth and polyamines gene expression in fennel (Foeniculum vulgare Mill.) seedlings. Physiol. Mol. Biol. Plants 2022, 28, 607–622. [Google Scholar] [CrossRef] [PubMed]
- Attia, H.; Alamer, K.; Algethami, B.; Ellouzi, H.; Lachaâl, M. Effects of growth and salinity on some morphological parameters, pigment and flavonoid concentrations in basil. Agrochimica 2020, 65, 2. [Google Scholar] [CrossRef]
- Ahmad, P.; Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alam, P.; Ashraf, M. Mitigation of sodium chloride toxicity in Solanum lycopersicum L. by supplementation of jasmonic acid and nitric oxide. J. Plant Int. 2018, 13, 64–72. [Google Scholar]
- Ahanger, M.A.; Tomar, N.S.; Tittal, M.; Argal, S.; Agarwal, R.M. Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol. Mol. Biol. Plants 2017, 23, 731–744. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Ahmad, P.; Alyemeni, M.N.; Wijaya, L.; Ahanger, M.A.; Ashraf, M.; Alam, P.; Paray, B.A.; Rinklebe, J. Nitric oxide donor, sodium nitroprusside, mitigates mercury toxicity in different cultivars of soybean. J. Hazard. Mater. 2021, 408, 124852. [Google Scholar] [CrossRef]
- Iqbal, N.; Umar, S.; Khan, N.A.; Corpas, F.J. Nitric oxide and hydrogen sulfide coordinately reduce glucose sensitivity and decrease oxidative stress via ascorbate-glutathione cycle in heat-stressed wheat (Triticum aestivum L.) plants. Antioxidants 2021, 10, 108. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Qin, C.; Begum, N.; Maodong, Q.; Dong, X.X.; El-Esawi, M.; El-Sheikh, M.A.; Alatar, A.A.; Zhang, L. Nitrogen availability prevents oxidative effects of salinity on wheat growth and photosynthesis by up-regulating the antioxidants and osmolytes metabolism, and secondary metabolite accumulation. BMC Plant Biol. 2019, 19, 479. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Qin, C.; Maodong, Q.; Dong, X.X.; Ahmad, P.; Abd_Allah, E.F.; Zhang, L. Spermine application alleviates salinity induced growth and photosynthetic inhibition in Solanum lycopersicum by modulating osmolyte and secondary metabolite accumulation and differentially regulating antioxidant metabolism. Plant Physiol. Biochem. 2019, 144, 1–13. [Google Scholar] [CrossRef]
- Qin, C.; Shen, J.; Ahanger, M.A. Supplementation of nitric oxide and spermidine alleviates the nickel stress induced damage to growth, chlorophyll metabolism, and photosynthesis by up-regulating ascorbate–glutathione and glyoxalase cycle functioning in tomato. Front. Plant Sci. 2022, 13, 1039480. [Google Scholar] [CrossRef] [PubMed]
- Toit, A.D. Metabolism: The health benefits of hydrogen sulphide. Nat. Rev. Mol. Cell Biol. 2015, 16, 68. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Saegusa, D.; Fujita, M.; Tran, L.S. Hydrogen sulfide regulates salt tolerance in rice by maintaining Na+/K+ balance, mineral homeostasis and oxidative metabolism under excessive salt stress. Front. Plant Sci. 2015, 6, 1055. [Google Scholar] [CrossRef]
- Raju, A.D.; Prasad, S.M. Hydrogen sulfide implications on easing NaCl induced toxicity in eggplant and tomato seedlings. Plant Physiol. Biochem. 2021, 164, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.S.; Islam, F.; Ye, Y.; Ashline, M.; Wang, D.; Zhao, B.; Fu, Z.Q.; Chen, J. The Interplay between Hydrogen Sulfide and Phytohormone Signaling Pathways under Challenging Environments. Int. J. Mol. Sci. 2022, 23, 4272. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Li, L.; Cui, W.T.; Xu, S.; Shen, W.B.; Wang, R. Hydrogen sulfide enhances alfalfa (Medicago sativa) tolerance against salinity during seed germination by nitric oxide pathway. Plant Soil. 2012, 351, 107–119. [Google Scholar] [CrossRef]
- Deng, Y.Q.; Bao, J.; Yuan, F.; Liang, X.; Feng, Z.T.; Wang, B.S. Exogenous hydrogen sulfide alleviates salt stress in wheat seedlings by decreasing Na+ content. Plant Growth Regul. 2016, 79, 391–399. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Liu, J.; Liu, T.; Xue, S. Hydrogen sulfide (H2S) signaling in plant development and stress responses. aBIOTECH 2021, 2, 32–63. [Google Scholar] [CrossRef]
- Singh, V.P.; Singh, S.; Kumar, J.; Prasad, S.M. Hydrogen sulfide alleviates toxic effects of arsenate in pea seedlings through up-regulation of the ascorbate-glutathione cycle: Possible involvement of nitric oxide. J. Plant Physiol. 2015, 181, 20–29. [Google Scholar] [CrossRef]
- Guo, L.; Ling, L.; Wang, X.; Cheng, T.; Wang, H.; Ruan, Y. Exogenous hydrogen sulfide and methylglyoxal alleviate cadmium-induced oxidative stress in Salix matsudana Koidz by regulating glutathione metabolism. BMC Plant Biol. 2023, 23, 73. [Google Scholar] [CrossRef]
- Naz, R.; Batool, S.; Shahid, M.; Keyani, R.; Yasmin, H.; Nosheen, A.; Hassan, M.N.; Mumtaz, S.; Siddiqui, M.H. Exogenous silicon and hydrogen sulfide alleviates the simultaneously occurring drought stress and leaf rust infection in wheat. Plant Physiol. Biochem. 2021, 166, 558–571. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Ahanger, M.A.; Zhou, J.; Ahmed, N.; Wei, C.; Yuan, S.; Ashraf, M.; Zhang, L. Beneficial role of acetylcholine in chlorophyll metabolism and photosynthetic gas exchange in Nicotiana benthamiana seedlings under salinity stress. Plant Biol. 2020, 22, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Ahanger, M.A.; Lin, B.; Huang, Z.; Zhou, J.; Ahmed, N.; Ai, S.; Mustafa, N.S.A.; Ashraf, M.; Zhang, L. Comparative transcriptomic analysis reveals the regulatory effects of acetylcholine on salt tolerance of Nicotiana benthamiana. Phytochemistry 2021, 181, 112582. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Zhang, F. Cell cycle regulation in the plant response to stress. Front. Plant Sci. 2020, 30, 1765. [Google Scholar] [CrossRef]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef]
- Chen, J.; Wang, W.H.; Wu, F.H.; He, E.M.; Liu, X.; Shangguan, Z.P.; Zheng, H.L. Hydrogen sulfide enhances salt tolerance through nitric oxide-mediated maintenance of ion homeostasis in barley seedling roots. Sci. Rep. 2015, 5, 12516. [Google Scholar] [CrossRef]
- Jiang, J.L.; Tian, Y.; Li, L.; Yu, M.; Hou, R.P.; Ren, X.M. H2S alleviates salinity stress in cucumber by maintaining the Na+/K+ balance and regulating h2s metabolism and oxidative stress response. Front. Plant Sci. 2019, 10, 678. [Google Scholar] [CrossRef]
- Dawood, M.F.A.; Sohag, A.A.M.; Tahjib-ul-Arif, M.; Abdel Lateef, A.A.H. Hydrogen sulfide priming can enhance the tolerance of artichoke seedlings to individual and combined saline-alkaline and aniline stresses. Plant Physiol. Biochem. 2021, 159, 347–362. [Google Scholar] [CrossRef]
- Turan, S.; Tripathy, B.C. Salt-stress induced modulation of chlorophyll biosynthesis during de-etiolation of rice seedlings. Physiol. Plant. 2015, 53, 477–491. [Google Scholar] [CrossRef]
- Chen, J.; Shang, Y.T.; Wang, W.H.; Chen, X.Y.; He, E.M.; Zheng, H.L.; Shangguan, Z. Hydrogen sulfide-mediated polyamines and sugar changes are involved in hydrogen sulfide-induced drought tolerance in Spinacia oleracea seedlings. Front. Plant Sci. 2016, 7, 1173. [Google Scholar] [CrossRef]
- Uarrota, V.G.; Stefen, D.L.V.; Leolato, L.S.; Gindri, D.M.; Nerling, D. Revisiting carotenoids and their role in plant stress responses: From biosynthesis to plant signaling mechanisms during stress. In Antioxidants and Antioxidant Enzymes in Higher Plants; Springer: Berlin/Heidelberg, Germany, 2018; pp. 207–232. [Google Scholar]
- Hasanuzzaman, M.; Hossainm, M.A.; Fujita, M. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol. Rep. 2011, 5, 353–365. [Google Scholar] [CrossRef]
- Soliman, M.; Alhaithloul, H.A.; Hakeem, K.R.; Alharbi, B.M.; El-Esawi, M.; Elkelish, A. Exogenous nitric oxide mitigates nickel-induced oxidative damage in eggplant by upregulating antioxidants, osmolyte metabolism, and glyoxalase systems. Plants 2019, 8, 562. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.S.; Wong, S.K.; Ismail, N.H.; Zegin, G.; Duangjai, A.; Saokaew, S.; Phisalprapa, P.; Tan, K.W.; Goh, B.H.; Tang, S.Y. Mitigation of environmental stress-impacts in plants: Role of sole and combinatory exogenous application of glutathione. Front. Plant Sci. 2021, 22, 791205. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Li, Z.; Zhu, L.; Wang, J.; Zhang, B.; Zheng, F.; Zhao, B.; Zhang, H.; Wang, Y.; Zhang, Z. The multiple roles of ascorbate in the abiotic stress response of plants: Antioxidant, cofactor, and regulator. Front. Plant Sci. 2021, 12, 598173. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F.; Ashraf, M. Proline Alleviates Abiotic Stress Induced Oxidative Stress in Plants. J. Plant Growth Reg. 2023, 42, 4629–4651. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Nazir, F.; Asgher, M.; Per, T.S.; Khan, N.A. Selenium and sulfur influence ethylene formation and alleviate cadmium-induced oxidative stress by improving proline and glutathione production in wheat. J. Plant Physiol. 2015, 173, 9–18. [Google Scholar] [CrossRef]
- Kumar, S.; Ahanger, M.A.; Alshaya, H.; Jan, B.L.; Yerramilli, V. Salicylic acid mitigates salt induced toxicity through the modifications of biochemical attributes and some key antioxidants in capsicum annuum. Saudi J. Bio. Sci. 2022, 29, 1337–1347. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, X.; Sun, M.; Peng, F. Hydrogen sulfide alleviates waterlogging-induced damage in peach seedlings via enhancing antioxidative system and inhibiting ethylene synthesis. Front. Plant Sci. 2020, 11, 696. [Google Scholar] [CrossRef]
- Ghori, N.H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altaym, V.; Ozturk, M. Heavy metal stress and responses in plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Jia, H.; Wang, X.; Dou, Y.; Liu, D.; Si, W.; Fang, H.; Zhao, C.; Chen, S.; Xi, J.; Li, J. Hydrogen sulfide-cysteine cycle system enhances cadmium tolerance through alleviating cadmium-induced oxidative stress and ion toxicity in Arabidopsis roots. Sci. Rep. 2016, 6, 39702. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.G.; Yang, S.Z.; Long, W.B.; Yang, G.X.; Shen, Z.Z. Hydrogen sulphide may be a novel downstream signal molecule in nitric oxide-induced heat tolerance of maize (Zea mays L.) seedlings. Plant Cell Environ. 2013, 36, 1564–1572. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplast polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Wild, R.; Ooi, L.; Srikanth, V.; Münch, G. A quick, convenient and economical method for the reliable determination of methylglyoxal in milimolar concentrations: The N-acetyl- L-cysteine assay. Anal. Bioanal. Chem. 2012, 403, 2577–2581. [Google Scholar] [CrossRef]
- Sairam, R.K. Effect of moisture stress on physiological activities of two contrasting wheat genotypes. Indian J. Exp. Biol. 1994, 32, 584–593. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil. 1983, 70, 303. [Google Scholar] [CrossRef]
- Bayer, W.F.; Fridovich, J.L. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Meth. Enzymol. 1984, 105, 121–126. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplast: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.P.; Choudhuri, M.A. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulphydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, E.G. Nitrate reductase assay in intact plant tissue. Biochem. Bioph. Res. Co. 1971, 43, 1274–1279. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A., Jr. Colorimetry of total phenolics with phosphor-molybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–153. [Google Scholar] [CrossRef]
- Zucker, M. Induction of phenylalanine deaminase by light and its relation to chlorogenic acid synthesis in potato tuber tissue. Plant Physiol. 1965, 40, 779. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, C.; Lai, D.; Sun, Y.; Samma, M.; Zhang, J.; Shen, W. Hydrogen sulfide delays GA-triggered programmed cell death in wheat aleurone layers by the modulation of glutathione homeostasis and heme oxygenase-1 expression. J. Plant Physiol. 2014, 171, 53–62. [Google Scholar] [CrossRef]
- Zhou, B.; Guo, Z.; Xing, J.; Huang, B. Nitric oxide is involved in abscisic acid-induced antioxidant activities in Stylosanthes guianensis. J. Exp. Bot. 2005, 56, 3223–3228. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alamer, K.H. Exogenous Hydrogen Sulfide Supplementation Alleviates the Salinity-Stress-Mediated Growth Decline in Wheat (Triticum aestivum L.) by Modulating Tolerance Mechanisms. Plants 2023, 12, 3464. https://doi.org/10.3390/plants12193464
Alamer KH. Exogenous Hydrogen Sulfide Supplementation Alleviates the Salinity-Stress-Mediated Growth Decline in Wheat (Triticum aestivum L.) by Modulating Tolerance Mechanisms. Plants. 2023; 12(19):3464. https://doi.org/10.3390/plants12193464
Chicago/Turabian StyleAlamer, Khalid H. 2023. "Exogenous Hydrogen Sulfide Supplementation Alleviates the Salinity-Stress-Mediated Growth Decline in Wheat (Triticum aestivum L.) by Modulating Tolerance Mechanisms" Plants 12, no. 19: 3464. https://doi.org/10.3390/plants12193464
APA StyleAlamer, K. H. (2023). Exogenous Hydrogen Sulfide Supplementation Alleviates the Salinity-Stress-Mediated Growth Decline in Wheat (Triticum aestivum L.) by Modulating Tolerance Mechanisms. Plants, 12(19), 3464. https://doi.org/10.3390/plants12193464





