Resprouting Response among Savanna Tree Species in Relation to Stem Size, Woody Removal Intensity and Herbicide Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Research Design
2.3. Data Analysis
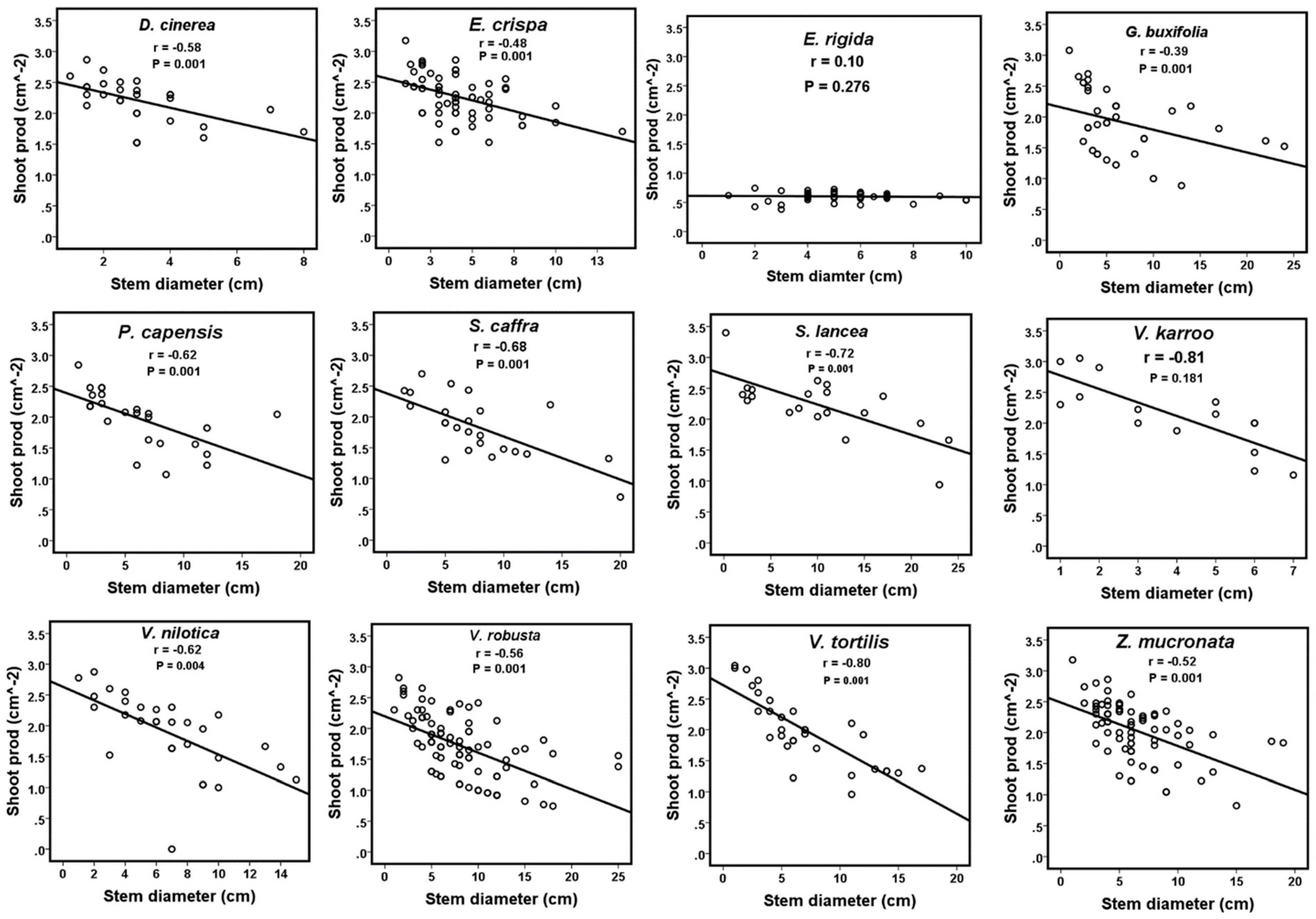
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffman, M.T.; Ashwell, A. Nature Divided: Land Degradation in South Africa; University of Cape Town Press: Cape Town, South Africa, 2001; p. 176. [Google Scholar]
- O’Connor, T.G.; Puttick, J.R.; Hoffman, M.T. Bush encroachment in southern Africa: Changes and causes. Afr. J. Range For. Sci. 2014, 31, 67–88. [Google Scholar] [CrossRef]
- Smit, G.N. Tree thinning as an option to increase herbaceous yield of an encroached semi-arid savanna in South Africa. BMC Ecol. 2005, 5, e4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ward, D. Do we understand the causes of bush encroachment in African savannas. Afr. J. Range For. Sci. 2005, 22, 101–105. [Google Scholar] [CrossRef]
- Ward, D.; Hoffman, M.T.; Collocott, S.J. A century of woody plant encroachment in the dry Kimberley savanna of South Africa. Afr. J. Range For. Sci. 2014, 39, 107–121. [Google Scholar] [CrossRef]
- Dreber, N.; van Rooyen, S.E.; Kellner, K. One savanna, many shapes: How bush control affects the woody layer in the southern Kalahari. S. Afri. J. Bot. 2019, 125, 511–520. [Google Scholar] [CrossRef]
- Kellner, K.; Mangani, R.T.; Sebitloane, T.J.K.; Chirima, J.G.; Meyer, N.; Coetzee, H.C.; Malan, P.W.; Koch, J. Restoration after bush control in selected rangeland areas of semi-arid savannas in South Africa. Bothalia 2021, 51, a7. [Google Scholar] [CrossRef]
- Tiawoun, M.A.P.; Malan, P.W.; Comole, A.A. Composition and structural patterns of encroaching woody plant species along riparian zones of the Molopo River, North-West Province, South Africa. S. Afr. J. Bot. 2022, 147, 652–658. [Google Scholar] [CrossRef]
- Warren, K.; Hugo, W.; Wilson, H. Preliminary Report and Data on Bush Encroachment and Land Cover Change, Released to DEA, DEA Consultants, and Selected Collaborators; Department of Environment, Forestry and Fisheries: Pretoria, South Africa, 2018.
- Monegi, P.; Mkhize, N.R.; Tjelele, T.J.; Ward, D.; Tsvuura, Z. The impact of tree removal on standing grass biomass, seedling establishment and growth of woody species. Rangel J. 2022, 44, 25–32. [Google Scholar] [CrossRef]
- Mndela, M.; Madakadze, I.C.; Nherera-Chokuda, F.; Dube, S.; Ramoelo, A.; Mangwane, M.; Tjelele, J. Short-term responses of herbaceous vegetation to bush clearing in semi-arid rangelands of South Africa. Pastor. Res. Policy Pract. 2022, 12, 17. [Google Scholar] [CrossRef]
- Kambatuku, J.R.; Cramer, M.D.; Ward, D. Nitrogen fertilisation reduces grass-induced N2 fixation of tree seedlings from semi-arid savannas. Plant Soil 2013, 365, 307–320. [Google Scholar] [CrossRef]
- Kambatuku, J.R.; Cramer, M.D.; Ward, D. Overlap in soil water sources of savanna woody seedlings and grasses. Ecohydrology 2013, 6, 464–473. [Google Scholar] [CrossRef]
- Ward, D.; Wiegand, K.; Getzin, S. Walter’s two-layer hypothesis revisited: Back to the roots. Oecologia 2013, 172, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Mureva, A.; Ward, D.; Pillay, T.; Chivenge, P.; Cramer, M. Soil organic carbon increases in semi-arid regions while it decreases in humid regions due to woody-plant encroachment of grasslands in South Africa. Sci. Rep 2018, 8, e15506. [Google Scholar] [CrossRef] [PubMed]
- Marquart, A.; Geissler, K.; Heblack, J.; Lobas, C.; Münch, E.; Blaum, N. Individual shrubs, large scale grass cover and seasonal rainfall explain invertebrate-derived macropore density in a semi-arid Namibian savannah. J. Arid Environ. 2020, 176, 104101. [Google Scholar] [CrossRef]
- Mureva, A.; Ward, D. Spatial patterns of encroaching shrub species under different grazing regimes in a semi-arid savanna, eastern Karoo, South Africa. Afr. J. Range For. Sci. 2016, 33, 77–89. [Google Scholar] [CrossRef]
- Pillay, T.; Ward, D.; Mureva, A.; Cramer, M. Differential effects of nutrient addition and woody plant encroachment on grassland soil, litter and plant dynamics across a precipitation gradient. Pedobiologia 2021, 85/86, e150726. [Google Scholar] [CrossRef]
- Smit, G.N. An approach to tree thinning to structure southern African savannas for long-term restoration from bush encroachment. J. Environ. Manag. 2004, 71, 179–191. [Google Scholar] [CrossRef]
- Harmse, C.J.; Kellner, K.; Dreber, N. Restoring productive rangelands: A comparative assessment of selective and non-selective chemical bush control in a semi-arid Kalahari savanna. J. Arid. Environ. 2016, 135, 39–49. [Google Scholar] [CrossRef]
- Ndhlovu, T.; Milton, S.J.; Esler, K.J. Effect of Prosopis (mesquite) invasion and clearing on vegetation cover in semi-arid Nama Karoo rangeland, South Africa. Afr. J. Range For. 2016, 33, 11–19. [Google Scholar] [CrossRef]
- Archer, S.R. Rangeland conservation and shrub encroachment: New perspectives on an old problem. In Wild Rangelands: Conserving Wildlife While Maintaining Livestock in Semi-Arid Ecosystems; Du Toit, J.T., Kock, R., Deutsch, J.C., Eds.; John Wiley and Sons: Chichester, UK, 2010; pp. 53–97. [Google Scholar]
- du Toit, J.C.O.; Sekwadi, K.P. Tebuthiuron residues remain active in soil for at least eight years in a semi-arid grassland, South Africa. Afr. J. Range Forage Sci. 2012, 29, 85–90. [Google Scholar] [CrossRef]
- Eldridge, D.J.; Ding, J. Limited long-term effectiveness of roller-chopping for managing woody encroachment. Restor. Ecol. 2021, 29, e13274. [Google Scholar] [CrossRef]
- Moyo, H.P.M.; Scholes, M.C.; Twine, W.C. The effects of repeated cutting on coppice response of Terminalia sericea. Trees 2015, 29, 161–169. [Google Scholar] [CrossRef]
- Pausas, J.G.; Keeley, J.E. Epicormic resprouting in fire-prone ecosystems’. Trends Plant Sci. 2017, 22, 1008–1015. [Google Scholar] [CrossRef]
- Shackleton, C.M. Stump size and the number of coppice shoots for selected savanna tree species. S. Afr. J. Bot. 2000, 66, 124–127. [Google Scholar] [CrossRef]
- Higgins, S.I.; Bond, W.J.; Trollope, W.S.W. Fire, resprouting and variability: A recipe for grass-tree coexistence in savannah. J. Ecol. 2000, 88, 213–229. [Google Scholar] [CrossRef]
- Clarke, P.J.; Lawes, M.J.; Midgley, J.J.; Lamont, B.B.; Ojeda, F.; Burrows, G.E.; Enright, N.J.; Knox, K.J.E. Resprouting as a key functional trait: How buds, protection and resources drive persistence after fire. N. Phytol. 2013, 197, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Nzunda, E.F.; Griffiths, M.E.; Lawes, M.J. Resource allocation and storage relative to resprouting ability in wind disturbed coastal forest trees. Evol. Ecol. 2014, 28, 735–749. [Google Scholar] [CrossRef]
- Dietze, M.C.; Clarke, J.S. Changing the gap dynamics paradigm: Vegetative regeneration control on forest response to disturbance. Ecol. Monogr. 2008, 78, 331–347. [Google Scholar] [CrossRef]
- Poorter, L.; Kitajima, K.; Mercado, P.; Chubina, J.; Melgar, I.; Prins, H.H.T. Resprouting as a persistence strategy of tropical forest trees: Relations with carbohydrate storage and shade tolerance. Ecology 2010, 91, 2613–2627. [Google Scholar] [CrossRef]
- Nano, C.E.; Clarke, P.J. Woody-grass ratios in a grassy arid system are limited by multi-causal interactions of abiotic constraint, competition and fire. Oecologia 2010, 162, 719–732. [Google Scholar] [CrossRef]
- Pausas, J.G.; Keeley, J.E. Evolutionary ecology. of resprouting and seeding in fire-prone ecosystems. N. Phytol. 2014, 204, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Shackleton, C.M. Managing regrowth of an indigenous savanna tree species (Terminalia sericea) for fuelwood: The influence of stump dimensions and post-harvest coppice pruning. Biomass Bioenerg. 2001, 20, 261–270. [Google Scholar] [CrossRef]
- Schutz, A.E.N.; Bond, W.J.; Cramer, M.D. Defoliation depletes the carbohydrate reserves of resprouting Acacia saplings in an African savanna. Plant Ecol. 2011, 212, 2047–2055. [Google Scholar] [CrossRef]
- Bond, W.J.; Midgley, J.J. Ecology of sprouting in woody plants: The persistence niche. Trends Ecol. Evol. 2001, 16, 45–51. [Google Scholar] [CrossRef]
- Casals, P.; Rios, A.I. Burning intensity and low light availability reduce resprouting ability and vigor of Buxus sempervirens L. after clearing. Sci. Total Environ. 2018, 627, 403–416. [Google Scholar] [CrossRef]
- Burch, P.L.; Zedaker, S.M. Removing the invasive tree Ailanthus altissima and restoring natural cover. J. Arboric. Urban For. 2003, 29, 18–24. [Google Scholar] [CrossRef]
- Ansley, R.J.; Castellano, M.J. Strategies for savanna restoration in the southern Great Plains: Effects of fire and herbicides. Restor. Ecol. 2006, 14, 420–428. [Google Scholar] [CrossRef]
- Enloe, S.F.; O’Sullivan, S.E.; Loewenstein, N.J.; Brantley, E.; Lauer, D.K. The influence of treatment timing and shrub size on Chinese privet (Ligustrum sinense) control with cut stump herbicide treatments in the southeastern United States. Invasive Plant Sci. Manag. 2018, 11, 49–55. [Google Scholar] [CrossRef]
- Shultz, A.E.N.; Bond, W.J.; Cramer, M.D. Juggling carbon: Allocation patterns of a dominant tree in a fire-prone savanna. Oecologia 2009, 160, 235–246. [Google Scholar] [CrossRef]
- Vesk, P.A.; Westoby, M. Funding the bud bank: A review of the costs of buds. Oikos 2004, 106, 200–208. [Google Scholar] [CrossRef]
- Neke, K.S.; Owen-Smith, N.; Witkowski, E.T.F. Comparative resprouting response of savanna woody plant species following harvesting: The value of persistence. For. Ecol. Manag. 2006, 232, 114–123. [Google Scholar] [CrossRef]
- Keeley, J.E. Fire severity and plant age in postfire resprouting of woody plants in sage scrub and chaparral. Madrono 2006, 53, 373–379. [Google Scholar] [CrossRef]
- Nzunda, E.F.; Griffiths, M.E.; Lawes, M.J. Sprouting by remobilization of above-ground resources ensures persistence after disturbance of coastal dune forest trees. Funct. Ecol. 2008, 22, 577–582. [Google Scholar] [CrossRef]
- Mostacedo, B.; Putz, F.E.; Fredericksen, T.S.; Villca, A.; Palacios, T. Contributions of root and stump sprouts to natural regeneration of a logged tropical dry forest in Bolivia. For. Ecol. Manag. 2009, 258, 978–985. [Google Scholar] [CrossRef]
- Sands, B.A.; Abrams, M.D. Effects of stump diameter on sprout number and size for three oak species in a Pennsylvania clearcut. N. J. Appl. For. 2009, 26, 122–125. [Google Scholar] [CrossRef]
- Gould, P.J.; Fei, S.; Steiner, K.C. Modeling sprout-origin oak regeneration in the central Appalachians. Can. J. For. Res. 2007, 37, 170–177. [Google Scholar] [CrossRef]
- Wigley, B.J.; Staver, A.C.; Zytkowiak, R.; Jagodzinski, A.M.L.; Wigley-Coetsee, C. Root trait variation in African savannas. Plant Soil 2009, 441, 555–565. [Google Scholar] [CrossRef]
- Vesk, P.A. Plant size and resprouting ability: Trading tolerance and avoidance of damage. J. Ecol. 2006, 94, 1027–1034. [Google Scholar] [CrossRef]
- Baudena, M.; Dekker, S.C.; van Bodegom, P.M.; Cuesta, B.; Higgins, S.I.; Lehsten, V.; Reick, C.H.; Rietkerk, M.; Scheiter, S.; Yin, Z.; et al. Forests, savannas, and grasslands: Bridging the knowledge gap between ecology and Dynamic Global Vegetation Models. Biogeosciences 2015, 12, 1833–1848. [Google Scholar] [CrossRef]
- Zhou, Y.; Wigley, B.J.; Case, M.F.; Coetsee, C.; Staver, A.C. Rooting depth as a key woody functional trait in savannas. N. Phytol. 2020, 227, 1350–1361. [Google Scholar] [CrossRef]
- Tomlinson, K.W.; Van Langevelde, F.; Ward, D.; Bongers, F.; Da Silva, D.A.; Prins, H.H.T.; De Bie, S.; Sterck, F.J. Deciduous and evergreen trees differ in juvenile biomass allometries because of differences in allocation to root storage. Ann. Bot. 2013, 112, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Mucina, I.; Rutherford, M.C. The Vegetation of South Africa, Lesotho and Swaziland. In Strelitzia 19; South Africa National Biodiversity Institute: Pretoria, South Africa, 2006. [Google Scholar]
- Coates-Palgrave, M. Keith Coates-Palgrave Trees of Southern Africa, 3rd ed.; Struik Publishers: Cape Town, South Africa, 2005. [Google Scholar]
- Kyalangalilwa, B.; Boatwright, J.S.; Daru, B.H.; Maurin, O.; van der Bank, M. Phylogenetic position and revised classification of Acacia s.l. (Fabaceae: Mimosoideae) in Africa, including new combinations in Vachellia and Senegalia. Bot. J. Linn. Soc. 2013, 172, 500–523. [Google Scholar] [CrossRef]
- Van Oudtshoorn, F. Guide to Grasses of Southern Africa, 2nd ed.; Briza Publications: Pretoria, South Africa, 2006. [Google Scholar]
- Teague, W.R.; Killilea, D.M. The application of various Picloram formulations to stumps of Brachystegia spiciformis Benth., Julbernardia globiflora (Benth). Troupin, Terminalia sericea Burch. ex DC. and Acacia karroo Hayne trees. J. Grassl. Soc. S. Afr. 1990, 7, 125–132. [Google Scholar] [CrossRef]
- IBM SPSS. IBM SPSS Statistics for Windows, version 26.0; IBM Corp.: Armonk, NY, USA, 2019. [Google Scholar]
- Ward, D.; Pillay, T.; Mbongwa, S.; Kirkman, K.; Hansen, E.; Van Achterbergh, M. Reinvasion of native invasive trees after a tree-thinning experiment in an African savanna. Range Ecol. Manag. 2022, 81, 69–77. [Google Scholar] [CrossRef]
- Mwavu, E.N.; Witkowski, E.T.F. Sprouting of woody species following cutting and tree-fall in a lowland semi-deciduous tropical rainforest, north-western Uganda. For. Ecol. Manag. 2008, 255, 982–992. [Google Scholar] [CrossRef]
- Bellingham, P.J.; Sparrow, A.D. Multi-stemmed trees in montane rain forests: Their frequency and demography in relation to elevation, soil nutrients and disturbance. J. Ecol. 2009, 97, 472–483. [Google Scholar] [CrossRef]
- del Tredici, P. Sprouting in temperate trees: A morphological and ecological review. Bot. Rev. 2001, 67, 121–140. [Google Scholar] [CrossRef]
- Enloe, S.F.; Loewenstein, N.J.; Streett, D.; Lauer, D.K. Herbicide treatment and application method influence root sprouting in Chinese tallowtree (Triadica sebifera). Invasive Plant Sci. Manag. 2015, 8, 160–168. [Google Scholar] [CrossRef]
- Badalamenti, E.; Barone, E.; La Mantia, T. Seasonal effects on mortality rates and resprouting of stems treated with glyphosate in the invasive tree of heaven (Ailanthus altissima (Mill.) Swingle). Arboric. J. 2015, 37, 180–195. [Google Scholar] [CrossRef]
- Enloe, S.F.; O’Sullivan, S.E.; Loewenstein, N.J.; Brantley, E.F.; Lauer, D.K. Triclopyr application timing and concentration influence low-volume basal bark efficacy on Chinese privet (Ligustrum sinense). Invasive Plant Sci. Manag. 2016, 9, 235–241. [Google Scholar] [CrossRef]
- Pillay, T.; Ward, D. Spatial pattern analysis and competition between Acacia karroo trees in humid savannas. Plant Ecol. 2012, 213, 1609–1619. [Google Scholar] [CrossRef]
| Species | Stem Diameter (cm) | n | Shoot Production (cm−2) |
|---|---|---|---|
| D. cinerea | 3.8 ± 0.2 | 72 | 81.1 ± 16.4 |
| E. crispa | 4.7 ± 0.2 | 151 | 102.4 ± 15.9 |
| E. rigida | 5.2 ± 0.1 | 100 | 157.4 ± 20.7 |
| G. buxifolia | 6.7 ± 0.6 | 82 | 65.3 ± 18.3 |
| P. capensis | 6.9 ± 0.7 | 35 | 101.8 ± 23.8 |
| S. lancea | 9.7 ± 0.8 | 42 | 147.1 ± 60.4 |
| S. caffra | 7.8 ± 0.7 | 40 | 70.5 ± 17.4 |
| V. karroo | 4.8 ± 0.3 | 55 | 79.4 ± 30.8 |
| V. nilotica | 8.4 ± 0.5 | 70 | 62.1 ± 15.4 |
| V. robusta | 9.6 ± 0.3 | 201 | 41.4 ± 6.4 |
| V. tortilis | 9.1 ± 0.8 | 47 | 144.8 ± 39.1 |
| Z. mucronata | 7.1 ± 0.3 | 140 | 93.1 ± 14.9 |
| Species | Treatment | Significance of Wilks’s λ in MANCOVA (p-Value) | Diameter of the Leader Shoot (Mean ± SE) | Length of the Leader Shoot (Mean ± SE) | Shoot Production (Mean ± SE) |
|---|---|---|---|---|---|
| D. cinerea | Herbicide Control | 0.058 | 0.03 ± 0.01 0.49 ± 0.07 | 6.20 ± 3.13 68.03 ± 9.39 | 13.5 ± 0.8 193.3 ± 32.6 * |
| E. crispa | Herbicide Control | 0.225 | 0.03 ± 0.01 0.31 ± 0.04 | 3.79 ± 1.44 37.60 ± 3.61 | 25.32 ± 10.67 204.44 ± 29.79 |
| E. rigida | Herbicide Control | 0.001 | 0.04 ± 701.24 0.75 ± 0.07 * | 4.81 ± 3.22 65.85 ± 5.93 * | 20.6 ± 9.2 317.9 ± 29.6 * |
| G. buxifolia | Herbicide Control | 0.138 | 0.05 ± 0.02 0.33 ± 0.05 | 2.35 ± 1.03 22.76 ± 3.34 | 9.0 ± 3.7 149.8 ± 42.4 |
| P. capensis | Herbicide Control | 0.099 | 0.08 ± 0.02 0.37 ± 0.09 | 6.54 ± 2.58 119.66 ± 87.26 | 44.5 ± 21.8 156.2 ± 37.8 |
| S. lancea | Herbicide Control | 0.347 | 0.12 ± 0.06 2.81 ± 1.82 | 9.64 ± 4.89 89.00 ± 9.71 | 114.7 ± 100.1 194.9 ± 28.5 |
| V. caffra | Herbicide Control | 0.122 | 0.21 ± 0.07 0.45 ± 0.08 | 3.70 ± 10.06 62.29 ± 10.19 | 28.1 ± 9.7 134.3 ± 36.9 |
| V. karroo | Herbicide Control | 0.158 | 0.01 ± 0.01 0.35 ± 0.07 | 1.89 ± 1.36 42.22 ± 4.68 | 10.1 ± 6.2 221.7 ± 85.3 |
| V. nilotica | Herbicide Control | 0.083 | 0.04 ± 0.03 0.36 ± 0.05 | 2.09 ± 1.23 42.28 ± 5.45 | 3.9 ± 2.9 130.1 ± 29.5 |
| V. robusta | Herbicide Control | 0.004 | 0.09 ± 0.02 0.42 ± 0.04 * | 5.84 ± 1.15 39.64 ± 3.08 * | 6.8 ± 1.7 155.7 ± 15.2 * |
| V. tortilis | Herbicide Control | 0.038 | 0.15 ± 0.05 0.65 ± 0.12 | 17.67 ± 4.75 61.94 ± 6.67 * | 98.6 ± 48.2 212.9 ± 63.5 |
| Z. mucronata | Herbicide Control | 0.001 | 0.07 ± 0.02 1.05 ± 0.08 * | 7.02 ± 2.44 104.92 ± 5.89 * | 4.3 ± 1.5 192.4 ± 26.8 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monegi, P.; Mkhize, N.R.; Tjelele, J.T.; Ward, D.; Tsvuura, Z. Resprouting Response among Savanna Tree Species in Relation to Stem Size, Woody Removal Intensity and Herbicide Application. Plants 2023, 12, 3451. https://doi.org/10.3390/plants12193451
Monegi P, Mkhize NR, Tjelele JT, Ward D, Tsvuura Z. Resprouting Response among Savanna Tree Species in Relation to Stem Size, Woody Removal Intensity and Herbicide Application. Plants. 2023; 12(19):3451. https://doi.org/10.3390/plants12193451
Chicago/Turabian StyleMonegi, Piet, Ntuthuko Raphael Mkhize, Julius Tlou Tjelele, David Ward, and Zivanai Tsvuura. 2023. "Resprouting Response among Savanna Tree Species in Relation to Stem Size, Woody Removal Intensity and Herbicide Application" Plants 12, no. 19: 3451. https://doi.org/10.3390/plants12193451
APA StyleMonegi, P., Mkhize, N. R., Tjelele, J. T., Ward, D., & Tsvuura, Z. (2023). Resprouting Response among Savanna Tree Species in Relation to Stem Size, Woody Removal Intensity and Herbicide Application. Plants, 12(19), 3451. https://doi.org/10.3390/plants12193451






