Effect of Eco-Friendly Application of Bee Honey Solution on Yield, Physio-Chemical, Antioxidants, and Enzyme Gene Expressions in Excessive Nitrogen-Stressed Common Bean (Phaseolus vulgaris L.) Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trial Location and Soil Analyses
2.2. Planting, Treatments, and Experimental Layout
2.3. Plant Sampling
2.4. Evaluation of Growth, Green Pod Yield, and Pod Quality
2.5. Estimation of Photosynthesis Efficiency and Leaf Contents of Nutrients
2.6. Assessment of Leaf Tissue Integrity and Biomarkers of Oxidative Stress
2.7. Assessment of Nitrate (NO3−) Content, Nitrate Reductase (NR) Activity, Osmoregulatory Compounds and Antioxidants
2.8. Assay of Antioxidant Enzyme Activities, Enzyme Gene Expression, and Hormonal Content
2.9. Statistical Analysis Tests
3. Results
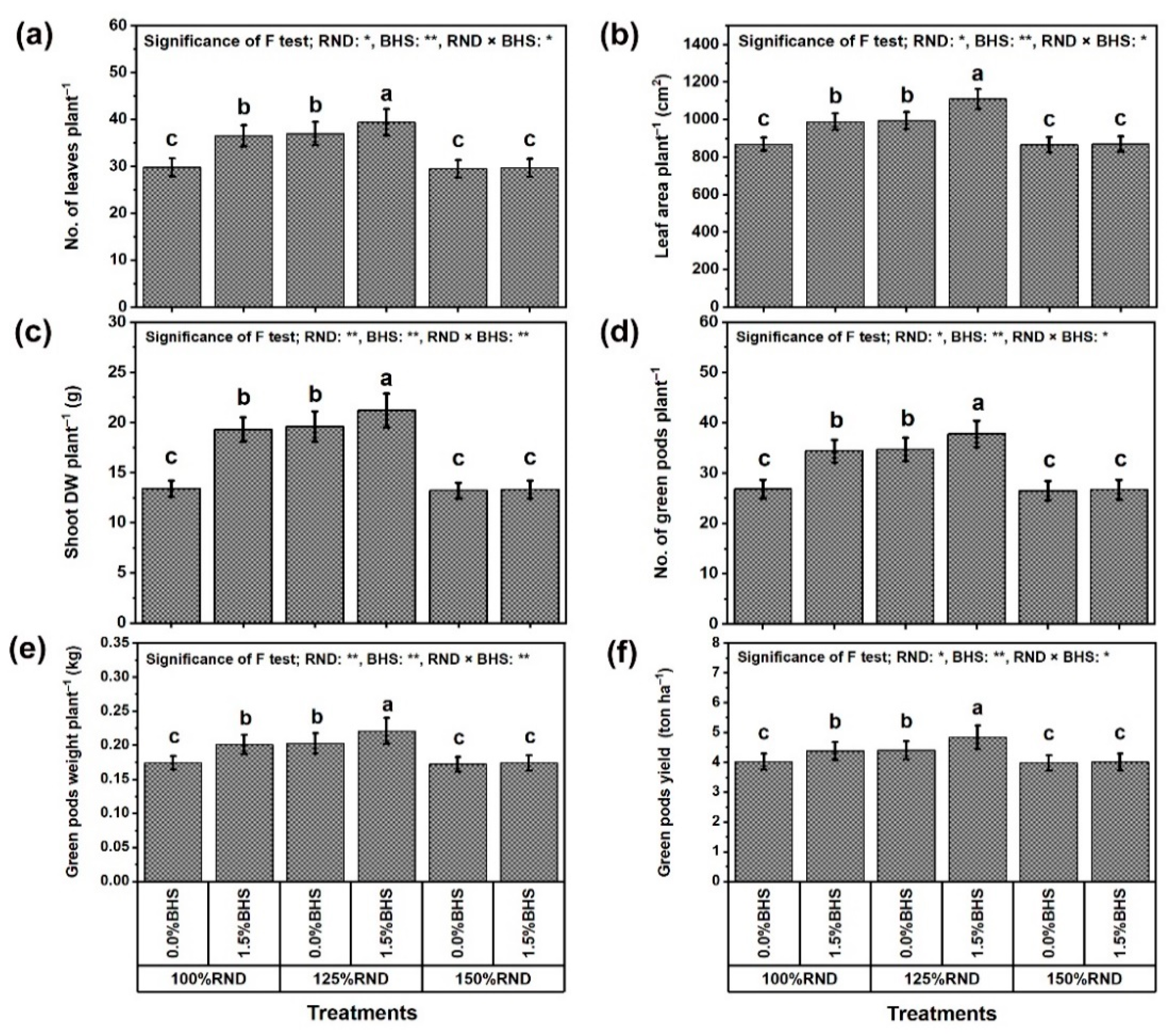
3.1. Growth, Yield, and Pod Quality Traits
3.2. Photosynthetic Machinery Efficiency
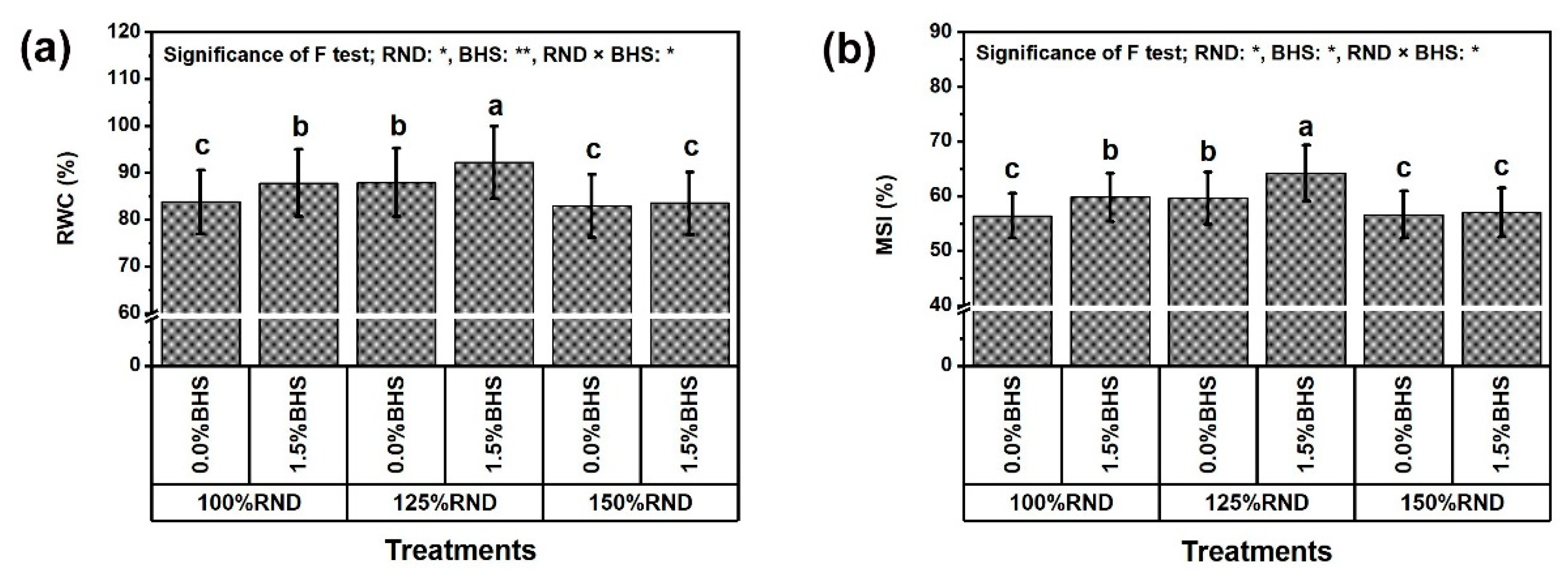
3.3. Leaf Mineral Contents and Integrity
3.4. Oxidative Stress Damage and Biomarkers
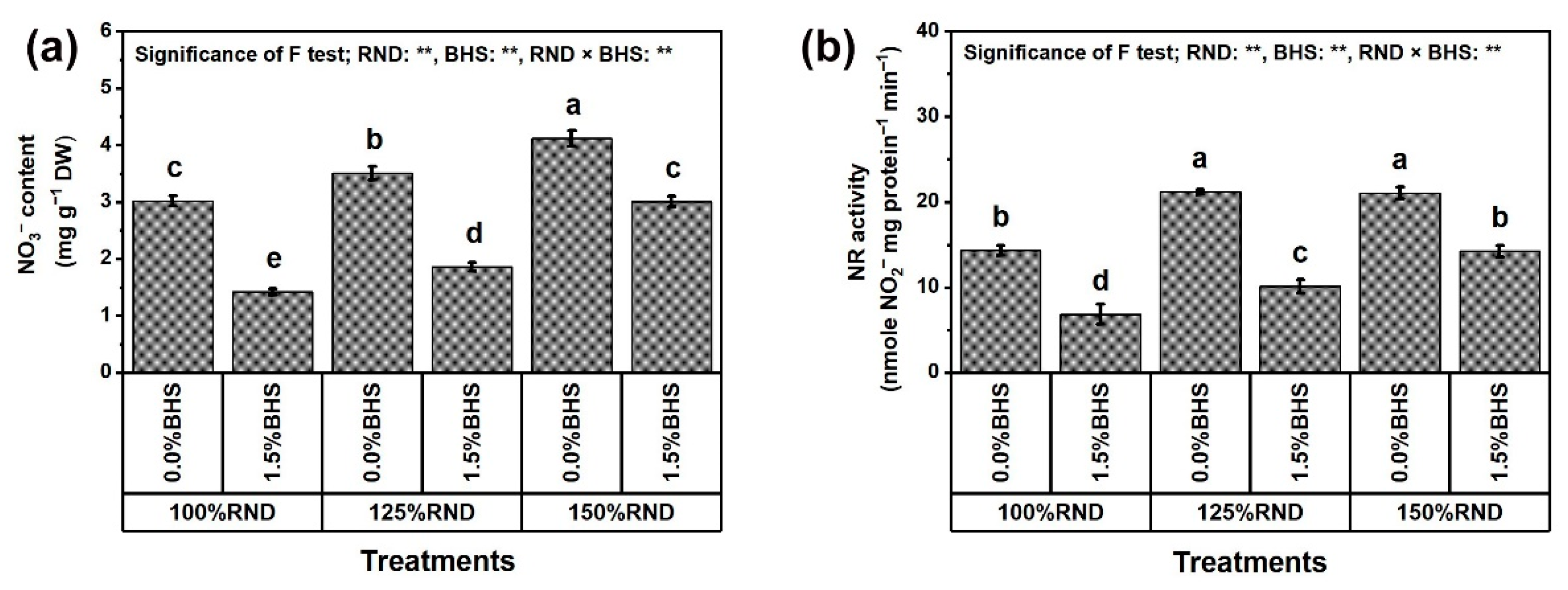
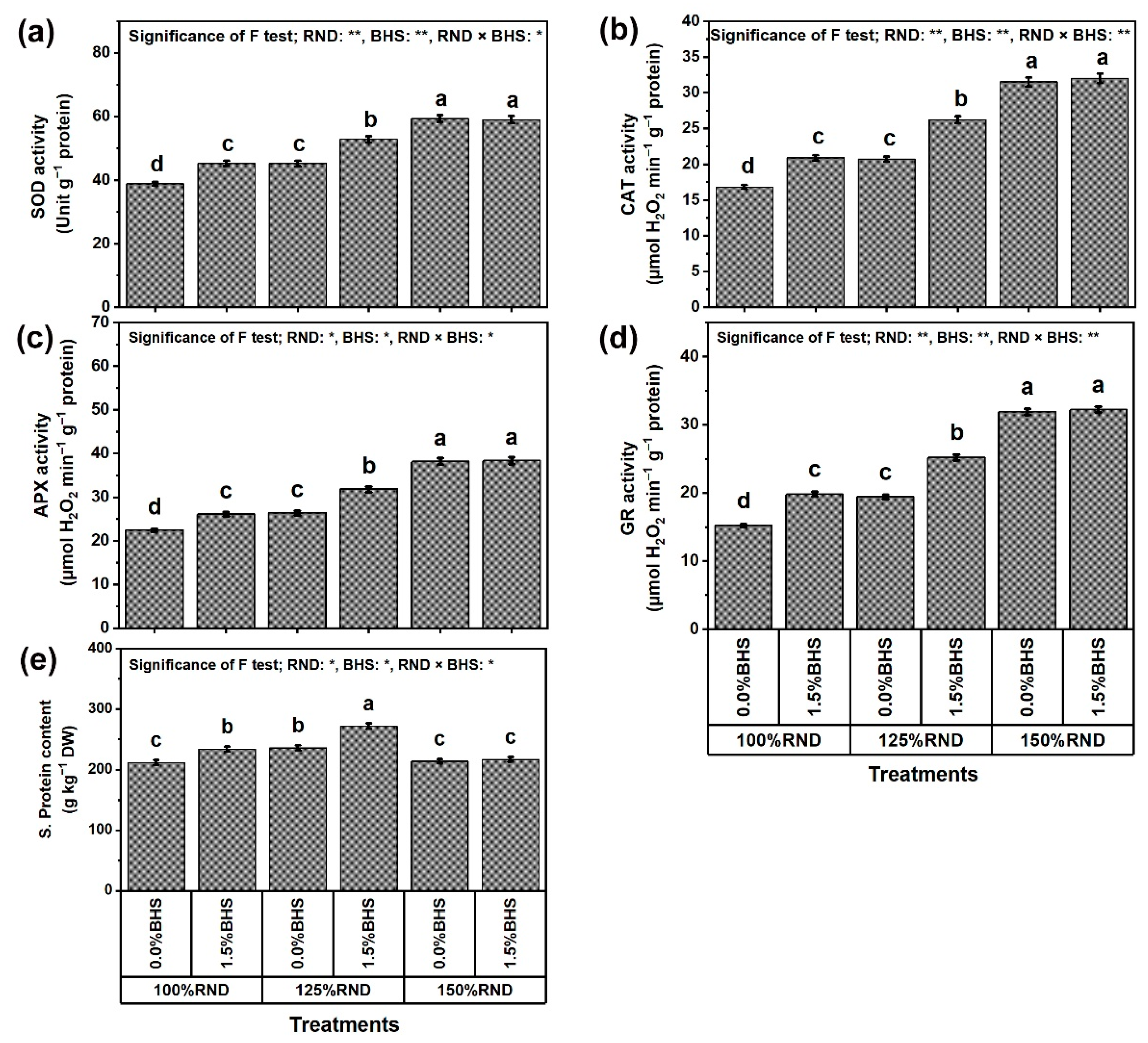
3.5. Osmoregulatory Compounds (ORCs), Non-Enzymatic and Enzymatic Antioxidants
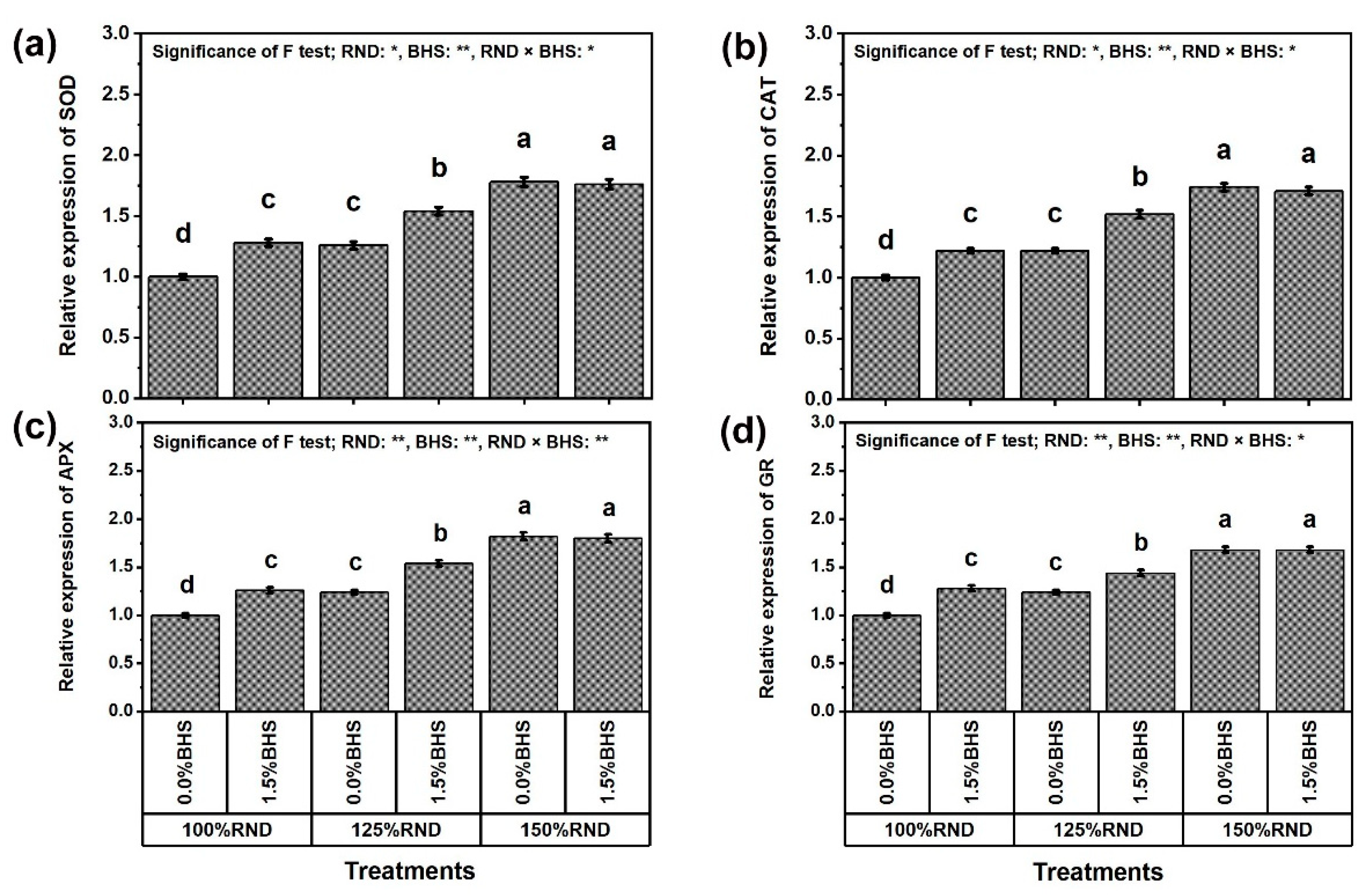
3.6. Gene Transcriptional Level of Antioxidant Enzymes
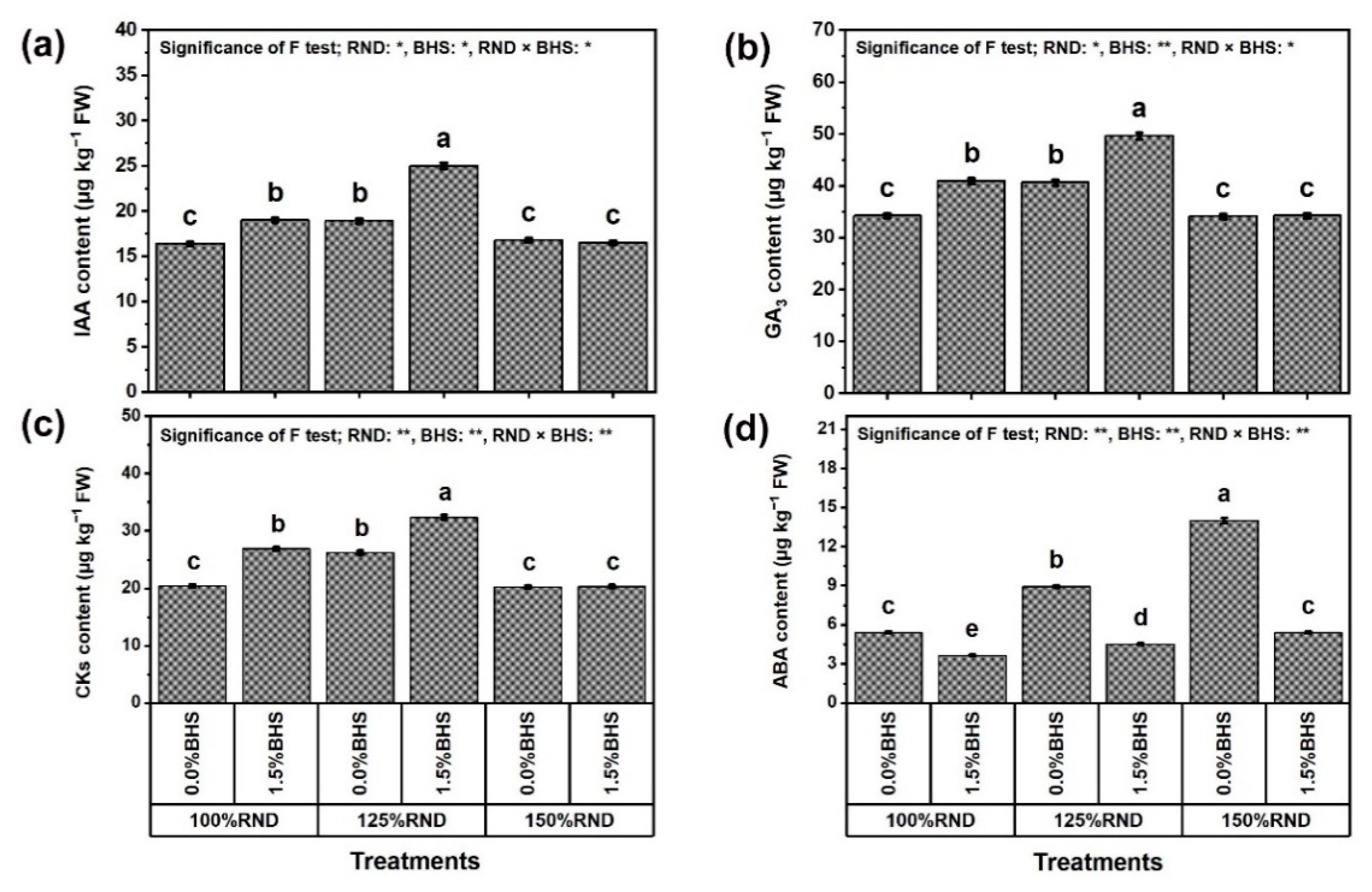
3.7. Phytohormone Contents
3.8. Correlation Analysis between the Studied Traits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Broughton, W.J.; Hernández, G.; Blair, M.; Beebe, S.; Gepts, P.; Vanderleyden, J. Beans (Phaseolus spp.)-model food legumes. Plant Soil 2003, 252, 55–128. [Google Scholar] [CrossRef]
- Xiong, Q.; Tang, G.; Zhong, L.; He, H.; Chen, X. Response to nitrogen deficiency and compensation on physiological characteristics, yield formation, and nitrogen utilization of rice. Front. Plant Sci. 2018, 9, 1075. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef] [PubMed]
- Schroder, J.L.; Zhang, H.; Girma, K.; Raun, W.R.; Penn, C.J.; Payton, M.E. Soil acidification from long-term use of nitrogen fertilizers on winter wheat. Soil Sci. Soc. Am. J. 2011, 75, 957–964. [Google Scholar] [CrossRef]
- Ju, X.T.; Kou, C.L.; Zhang, F.S.; Christie, P. Nitrogen balance and groundwater nitrate contamination: Comparison among three intensive cropping systems on the North China Plain. Environ. Pollut. 2006, 143, 117–125. [Google Scholar] [CrossRef]
- Chen, X.; Cui, Z.; Fan, M.; Vitousek, P.; Zhao, M.; Ma, W.; Wang, Z.; Zhang, W.; Yan, X.; Yang, J.; et al. Producing more grain with lower environmental costs. Nature 2014, 514, 486–489. [Google Scholar] [CrossRef]
- Hord, N.G.; Tang, Y.; Bryan, N.S. Food sources of nitrates and nitrites: The physiologic context for potential health benefits. Am. J. Clin. Nutr. 2009, 90, 1–10. [Google Scholar] [CrossRef]
- Zhu, G.; Peng, S.; Huang, J.; Cui, K.; Nie, L.; Wang, F. Genetic improvements in rice yield and concomitant increases in radiation-and nitrogen-use efficiency in middle reaches of Yangtze river. Sci. Rep. 2016, 6, 21049. [Google Scholar] [CrossRef]
- Shilpha, J.; Song, J.; Jeong, B.R. Ammonium Phytotoxicity and Tolerance: An Insight into Ammonium Nutrition to Improve Crop Productivity. Agronomy 2023, 13, 1487. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Semida, W.M.; Rady, M.M. Pre-soaking in 24-epibrassinolide or salicylic acid improves seed germination, seedling growth, and anti-oxidant capacity in Phaseolus vulgaris L. grown under NaCl stress. J. Hortic. Sci. Biotechnol. 2014, 89, 344–383. [Google Scholar] [CrossRef]
- Semida, W.M.; Taha, R.S.; Abdelhamid, M.T.; Rady, M.M. Foliar-applied α-tocopherol enhances salt-tolerance in Vicia faba L. plants grown under saline conditions. S. Afr. J. Bot. 2014, 95, 24–31. [Google Scholar] [CrossRef]
- Taha, R.S.; Alharby, H.F.; Bamagoos, A.A.; Medani, R.A.; Rady, M.M. Elevating tolerance of drought stress in Ocimum basilicum using pollen grains extract; a natural biostimulant by regulation of plant performance and antioxidant defense system. S. Afr. J. Bot. 2020, 128, 42–53. [Google Scholar] [CrossRef]
- Alghamdi, S.A.; Alharby, H.F.; Bamagoos, A.A.; Zaki, S.-n.S.; Abdelhamed, A.M.; Desoky, E.-S.M.; Mohamed, I.A.A.; Rady, M.M. Rebalancing nutrients, reinforcing antioxidant and osmoregulatory capacity, and improving yield quality in drought-stressed phaseolus vulgaris by foliar application of a bee-honey solution. Plants 2023, 12, 63. [Google Scholar] [CrossRef]
- Tarfayah, D.A.M.M.; Ahmed, S.M.A.; Rady, M.M.; Mohamed, I.A.A. Alleviating saline-calcareous stress in Atriplex nummularia seedlings by foliar spraying with silymarin-enriched bee-honey solution. Labyrinth Fayoum J. Sci. Interdiscipl. Stud. 2023, 1, 11–20. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef]
- Teklić, T.; Parađiković, N.; Špoljarević, M.; Zeljković, S.; Lončarić, Z.; Lisjak, M. Linking abiotic stress, plant metabolites, biostimulants and functional food. Ann. Appl. Biol. 2021, 178, 169–191. [Google Scholar] [CrossRef]
- Desoky, E.M.; Elrys, A.S.; Mansour, E.; Eid, R.S.M.; Selem, E.; Ali, E.F.; Mersal, G.A.M.; Rady, M.M.; Semida, W.M. Application of biostimulants promotes growth and productivity by fortifying the antioxidant machinery and suppressing oxidative stress in faba bean under various abiotic stresses. Sci. Hortic. 2021, 288, 110340. [Google Scholar] [CrossRef]
- Desoky, E.S.; Mansour, E.; Ali, M.M.A.; Yasin, M.A.T.; Abdul-Hamid, M.I.E.; Rady, M.M.; Ali, E.F. Exogenously used 24-epibrassinolide promotes drought tolerance in maize hybrids by improving plant and water productivity in an arid environment. Plants 2021, 10, 354. [Google Scholar] [CrossRef] [PubMed]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 1–12. [Google Scholar] [CrossRef]
- Caradonia, F.; Battaglia, V.; Righi, L.; Pascali, G.; La Torre, A. Plant biostimulant regulatory framework: Prospects in Europe and current situation at international level. J. Plant Growth Regul. 2019, 38, 438–448. [Google Scholar] [CrossRef]
- Semida, W.M.; Abd El-Mageed, T.A.; Hemida, K.A.; Rady, M.M. Natural bee-honey based biostimulants confer salt tolerance in onion via modulation of the antioxidant defence system. J. Hortic. Sci. Biotechnol. 2019, 94, 632–642. [Google Scholar] [CrossRef]
- Rady, M.M.; Boriek, S.H.; Abd El-Mageed, T.A.; Seif El-Yazal, M.A.; Ali, E.F.; Hassan, F.A.; Abdelkhalik, A. Exogenous gibberellic acid or dilute bee honey boosts drought stress tolerance in Vicia faba by rebalancing osmoprotectants, antioxidants, nutrients, and phytohormones. Plants 2021, 10, 748. [Google Scholar] [CrossRef] [PubMed]
- Isla, M.I.; Craig, A.; Ordoñez, R.; Zampini, C.; Sayago, J.; Bedascarrasbure, E.; Alvarez, A.; Salomón, V.; Maldonado, L. Physicochemical and bioactive properties of honeys from Northwestern Argentina. LWT-Food Sci. Technol. 2011, 44, 1922–1930. [Google Scholar] [CrossRef]
- Saxena, S.; Gautam, S.; Sharma, A. Physical, biochemical and antioxidant properties of some Indian honeys. Food Chem. 2010, 118, 391–397. [Google Scholar] [CrossRef]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis Part. 2. Chemical and Microbiological Properties; American Society of Agronomy, Inc.: Madison, WI, USA, 1982. [Google Scholar] [CrossRef]
- Klute, A. Methods of Soil Analysis: Part 1 Physical and Mineralogical Methods, 2nd ed.; American Society of Agronomy, Inc.: Madison, WI, USA; Soil Science Society of America, Inc.: Madison, WI, USA, 1986. [Google Scholar] [CrossRef]
- Dahnke, W.C.; Whitney, D.A. Measurement of soil salinity. In Recommended Chemical Soil Test Procedures for the North Central Region; North Central Regional Publication 221, Dahnke, W.C., Eds.; North Dakota Agricultural Experiment Station Bulletin: Fargo, ND, USA, 1988; Volume 499, pp. 32–34. [Google Scholar]
- Association of Official Analytical Chemists. Official Methods of Analysis, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Sadasivam, S.; Manickam, A. Biochemical Methods, 2nd ed.; New Age International: New Delhi, India, 2004. [Google Scholar]
- Rai, S.N.; Mudgal, V.D. Synergistic effect of sodium hydroxide and steam pressure treatment on composition changes and fibre utilization of wheat straw. Biol. Wastes 1988, 24, 105–113. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall of India Pvt. Ltd.: New Delhi, India, 1967. [Google Scholar]
- Galeeva, E.I.; Trifonova, T.V.; Ponomareva, A.A.; Viktorova, L.V.; Minibayeva, F.V. Nitrate reductase from Triticum aestivum leaves: Regulation of activity and possible role in production of nitric oxide. Biochemistry 2012, 77, 404–410. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Haroon, M.; Schrader, L.E.; Young, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Clark, A.J.; Landolt, W.; Bucher, J.B.; Strasser, R.J. Beech (Fagus sylvatica) response to ozone exposure assessed with a chlorophyll a fluorescence performance index. Environ. Pollut. 2000, 109, 501–507. [Google Scholar] [CrossRef]
- Johnson, C.M.; Ulrich, A. Analytical Methods for Use in Plant Analysis; Bulletin (Agricultural Experiment Station, Berkeley, California); University of California: Berkeley, CA, USA, 1959. [Google Scholar]
- Osman, A.S.; Rady, M.M. Effect of humic acid as an additive to growing media to enhance the production of eggplant and tomato transplants. J. Hortic. Sci. Biotechnol. 2014, 89, 237–244. [Google Scholar] [CrossRef]
- Rady, M.M. Effect of 24-epibrassinolide on growth, yield, antioxidant system and cadmium content of bean (Phaseolus vulgaris L.) plants under salinity and cadmium stress. Sci. Hortic. 2011, 129, 232–237. [Google Scholar] [CrossRef]
- Rady, M.M.; ur Rehman, H. Supplementing organic biostimulants into growing media enhances growth and nutrient uptake of tomato transplants. Sci. Hortic. 2016, 203, 192–198. [Google Scholar] [CrossRef]
- Kubiś, J. Exogenous spermidine differentially alters activities of some scavenging system enzymes, H2O2 and superoxide radical levels in water-stressed cucumber leaves. J. Plant Physiol. 2008, 165, 397–406. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Qu, W. Experimental Guidance of Plant Physiology; High Education: Beijing, China, 2004. [Google Scholar]
- Irigoyen, J.J.; Einerich, D.W.; Sánchez-Díaz, M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol. Plant. 1992, 84, 55–60. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 207, 205–207. [Google Scholar] [CrossRef]
- Huang, C.; He, W.; Guo, J.; Chang, X.; Su, P.; Zhang, L. Increased sensitivity to salt stress in an ascorbate-deficient Arabidopsis mutant. J. Exp. Bot. 2005, 56, 3041–3049. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, A.; Berardino, R.; de Pinto, M.C.; Sanità Di Toppi, L.; Storelli, M.M.; Tommasi, F.; De Gara, L. Increase in ascorbate-glutathione metabolism as local and precocious systemic responses induced by cadmium in durum wheat plants. Plant Cell Physiol. 2008, 49, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Nagy, Z.; Daood, H.; Koncsek, A.; Molnár, H.; Helyes, L. The simultaneous determination of capsaicinoids, tocopherols, and carotenoids in pungent pepper powder. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 199–209. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Havir, E.A.; McHale, N.A. Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol. 1987, 84, 450–455. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Foster, J.G.; Hess, J.L. Responses of Superoxide Dismutase and Glutathione Reductase Activities in Cotton Leaf Tissue Exposed to an Atmosphere Enriched in Oxygen. Plant Physiol. 1980, 66, 482–487. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ruijter, J.M.; Ramakers, C.; Hoogaars, W.M.H.; Karlen, Y.; Bakker, O.; van den Hoff, M.J.B.; Moorman, A.F.M. Amplification efficiency: Linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009, 37, e45. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Nehela, Y.; Hijaz, F.; Elzaawely, A.A.; El-Zahaby, H.M.; Killiny, N. Phytohormone profiling of the sweet orange (Citrus sinensis (L.) Osbeck) leaves and roots using GC-MS-based method. J. Plant Physiol. 2016, 199, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Rady, M.M.; Talaat, N.B.; Abdelhamid, M.T.; Shawky, B.T.; Desoky, E.S.M. Maize (Zea mays L.) grains extract mitigates the deleterious effects of salt stress on common bean (Phaseolus vulgaris L.) growth and physiology. J. Hortic. Sci. Biotechnol. 2019, 94, 777–789. [Google Scholar] [CrossRef]
- Ünyayar, S.; Topcuoglu, S.F.; Ünyayar, A. A modified method for extraction and identification of indole-3-acetic acid (IAA), gibberellic acid (GA3), abscisic acid (ABA) and zeatin produced by phanerochaete chrysosporium ME446. Bulg. J. Plant Physiol. 1996, 22, 105–110. [Google Scholar]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley & Sons: New York, NY, USA, 1984. [Google Scholar]
- Levene, H. Robust tests for equality of variances. In Contributions to Probability and Statistics. Essays in Honor of Harold Hotelling; Stanford University Press: Palo Alto, CA, USA, 1961; pp. 279–292. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Abou-Sreea, A.I.B.; Azzam, C.R.; Al-Taweel, S.; Abdel-Aziz, R.M.; Belal, H.E.E.; Rady, M.M.; Abdel-Kader, A.A.S.; Majrashi, A.; Khaled, K.A.M. Natural Biostimulant Attenuates Salinity Stress Effects in Chili Pepper by Remodeling the Antioxidant, Ion and Phytohormone balances, and Augment Gene Expression. Plants 2021, 10, 2316. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Ashraf, M. Exogenous application of ascorbic acid stimulates growth and photosynthesis of wheat (Triticum aestivum L.) under drought. Soil Environ. 2012, 31, 72–77. [Google Scholar]
- Rady, M.M.; Hemida, K.A. Sequenced application of ascorbate-proline-glutathione improves salt tolerance in maize seedlings. Ecotoxicol. Environ. Saf. 2016, 133, 252–259. [Google Scholar] [CrossRef]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble sugars: Metabolism, sensing and abiotic stress: A complex network in the life of plants. Plant Signal. Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef]
- Javanmardi, J.; Emami, S. Application of sucrose on tomato seedlings improves transplant quality, crop establishment, cold and dark hardiness. Adv. Hort. Sci. 2013, 27, 122–126. [Google Scholar]
- Zhang, Y.; Fu, C.; Yan, Y.; Fan, X.; Wang, Y.A.; Li, M. Foliar application of sugar alcohol zinc increases sugar content in apple fruit and promotes activity of metabolic enzymes. Hortic. Sci. 2014, 49, 1067–1070. [Google Scholar] [CrossRef]
- Lastdrager, J.; Hanson, J.; Smeekens, S. Sugar signals and the control of plant growth and development. J. Exp. Bot. 2014, 65, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Zahid, M.; Iqbal, N.; Muhammad, S.; Faisal, S.; Mahboob, W.; Hussain, M. Efficacy of exogenous applications of glucose in improving wheat crop (Triticum aestivum L.) performance under drought stress. Pak. J. Agric. Sci. 2018, 31, 264–273. [Google Scholar] [CrossRef]
- Tan, C.; Zhang, L.; Duan, X.; Chai, X.; Huang, R.; Kang, Y.; Yang, X. Effects of exogenous sucrose and selenium on plant growth, quality, and sugar metabolism of pea sprouts. J. Sci. Food Agric. 2022, 102, 2855–2863. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, G.P.S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Alterations in photosynthetic pigments, protein, and carbohydrate metabolism in a wild plant Coronopus didymus L. (Brassicaceae) under lead stress. Acta Physiol. Plant. 2017, 39, 1–9. [Google Scholar] [CrossRef]
- Hamani, A.K.M.; Wang, G.; Soothar, M.K.; Shen, X.; Gao, Y.; Qiu, R.; Mehmood, F. Responses of leaf gas exchange attributes, photosynthetic pigments and antioxidant enzymes in NaCl-stressed cotton (Gossypium hirsutum L.) seedlings to exogenous glycine betaine and salicylic acid. BMC Plant Biol. 2020, 20, 1–14. [Google Scholar] [CrossRef]
- Tan, S.L.; Yang, Y.J.; Liu, T.; Zhang, S.B.; Huang, W. Responses of photosystem I compared with photosystem II to combination of heat stress and fluctuating light in tobacco leaves. Plant Sci. 2020, 292, 110371. [Google Scholar] [CrossRef]
- ALKahtani, M.D.F.; Attia, K.A.; Hafez, Y.M.; Khan, N.; Eid, A.M.; Ali, M.A.M.; Abdelaal, K.A.A. Chlorophyll fluorescence parameters and antioxidant defense system can display salt tolerance of salt acclimated sweet pepper plants treated with chitosan and plant growth promoting rhizobacteria. Agronomy 2020, 10, 1180. [Google Scholar] [CrossRef]
- Tian, F.; Hou, M.; Qiu, Y.; Zhang, T.; Yuan, Y. Salinity stress effects on transpiration and plant growth under different salinity soil levels based on thermal infrared remote (TIR) technique. Geoderma 2020, 357, 113961. [Google Scholar] [CrossRef]
- Stitt, M.; Hurry, V. A plant for all seasons: Alterations in photosynthetic carbon metabolism during cold acclimation in Arabidopsis. Curr. Opin. Plant Biol. 2002, 5, 199–206. [Google Scholar] [CrossRef]
- Wang, X.; Vignjevic, M.; Liu, F.; Jacobsen, S.; Jiang, D.; Wollenweber, B. Drought priming at vegetative growth stages improves tolerance to drought and heat stresses occurring during grain filling in spring wheat. Plant Growth Regul. 2015, 75, 677–687. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, S.; Li, Z.; Chang, T.; Xu, Q.; Xu, H.; Zhang, J. Effects of excessive nitrogen fertilizer and soil moisture deficiency on antioxidant enzyme system and osmotic adjustment in tomato seedlings. Int. J. Agric. Biol. Eng. 2022, 15, 127–134. [Google Scholar] [CrossRef]
- Zhu, J.J.; Zhang, J.L.; Liu, H.C.; Cao, K.F. Photosynthesis, non-photochemical pathways and activities of antioxidant enzymes in a resilient evergreen oak under different climatic conditions from a valley-savanna in Southwest China. Physiol. Plant. 2009, 135, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, Y.; Guo, K.; Fan, D.; Li, G.; Zheng, Y.; Yu, L.; Yang, R. Effect of drought on pigments, osmotic adjustment and antioxidant enzymes in six woody plant species in karst habitats of southwestern China. Environ. Exp. Bot. 2011, 71, 174–183. [Google Scholar] [CrossRef]
- Jiang, W.; Ding, M.; Duan, Q.; Zhou, Q.; Huang, D. Exogenous glucose preserves the quality of watermelon (Citrullus lanatus) plug seedlings for low temperature storage. Sci Hort. 2012, 148, 23–29. [Google Scholar] [CrossRef]
- Jahan, M.S.; Shu, S.; Wang, Y.; Hasan, M.M.; El-Yazied, A.A.; Alabdallah, N.M.; Hajjar, D.; Altaf, M.A.; Sun, J.; Guo, S. Melatonin pretreatment confers heat tolerance and repression of heat-induced senescence in tomato through the modulation of ABA- and GA-mediated pathways. Front. Plant Sci. 2021, 12, 650955. [Google Scholar] [CrossRef]
- Rehman, H.; Alharby, H.F.; Alzahrani, Y.; Rady, M.M. Magnesium and organic biostimulant integrative application induces physiological and biochemical changes in sunflower plants and its harvested progeny on sandy soil. Plant Physiol. Biochem. 2018, 126, 97–105. [Google Scholar] [CrossRef]
- Rady, M.M.; Kusvuran, A.; Alharby, H.F.; Alzahrani, Y.; Kusvuran, S. Pretreatment with proline or an organic bio-stimulant induces salt tolerance in wheat plants by improving antioxidant redox state and enzymatic activities and reducing the oxidative stress. J. Plant Growth Regul. 2019, 38, 449–462. [Google Scholar] [CrossRef]
- Acikgoz, F.E.; Adiloglu, A.; Daglioglu, F.; Adiloglu, S.; Celikyurt, G.; Karakas, O. The effect of increasing doses of nitrogen (N) application for some nutrient elements, vitamin C and protein contents of komatsuna (Brassica rapa var. perviridis) plant. Bulg. J. Agric. Sci. 2014, 20, 321–324. [Google Scholar]
- Mekdad, A.A.A.; Rady, M.M. Response of Beta vulgaris L. to nitrogen and micronutrients in a dry environment. Plant Soil Environ. 2016, 62, 23–29. [Google Scholar] [CrossRef]
- Mekdad, A.A.A.; Rady, M.M.; Ali, E.F.; Hassan, F.A.S. Early Sowing Combined with Adequate Potassium and Sulfur Fertilization: Promoting Beta vulgaris (L.) Yield, Yield Quality, and K- and S-Use Efficiency in a Dry Saline Environment. Agronomy 2021, 11, 806. [Google Scholar] [CrossRef]
- Suprasanna, P.; Nikalje, G.C.; Rai, A.N. Osmolyte accumulation and implications in plant abiotic stress tolerance. In Osmolytes and Plants Acclimation to Changing Environment; Emerging Omics Technologies; Springer: New Delhi, India, 2016; pp. 1–12. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Akram, N.A.; Ashraf, M. Osmoprotection in plants under abiotic stresses: New insights into a classical phenomenon. Planta 2020, 251, 3. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, Y.; Rady, M.M. Compared to antioxidants and polyamines, the role of maize grain-derived organic biostimulants in improving cadmium tolerance in wheat plants. Ecotoxicol. Environ. Saf. 2019, 182, 109378. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.S.; Wang, Y.; Shu, S.; Zhong, M.; Chen, Z.; Wu, J.Q.; Sun, J.; Guo, S. Exogenous salicylic acid increases the heat tolerance in tomato (Solanum lycopersicum L.) by enhancing photosynthesis efficiency and improving antioxidant defense system through scavenging of reactive oxygen species. Sci. Hortic. 2019, 247, 421–429. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Howard, A.C.; McNeil, A.K.; McNeil, P.L. Promotion of plasma membrane repair by Vitamin E. Nat. Commun. 2011, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Rahman, A.; Alam, M.; Mahmud, J.A.; Suzuki, T.; Fujita, M. Polyamines confer salt tolerance in mung bean (Vigna radiata L.) by reducing sodium uptake, improving nutrient homeostasis, antioxidant defense, and methylglyoxal detoxification systems. Front. Plant Sci. 2016, 7, 1104. [Google Scholar] [CrossRef]
- Pitzschke, A.; Forzani, C.; Hirt, H. Reactive oxygen species signaling in plants. Antioxid. Redox Signal. 2006, 8, 1757–1764. [Google Scholar] [CrossRef]
- Bian, S.; Jiang, Y. Reactive oxygen species, antioxidant enzyme activities and gene expression patterns in leaves and roots of Kentucky bluegrass in response to drought stress and recovery. Sci. Hortic. 2009, 120, 264–270. [Google Scholar] [CrossRef]
- Melchiorre, M.; Robert, G.; Trippi, V.; Racca, R.; Lascano, H.R. Superoxide dismutase and glutathione reductase overexpression in wheat protoplast: Photooxidative stress tolerance and changes in cellular redox state. Plant Growth Regul. 2009, 57, 57–68. [Google Scholar] [CrossRef]
- Li, L.; Yi, H. Effect of sulfur dioxide on ROS production, gene expression and antioxidant enzyme activity in Arabidopsis plants. Plant Physiol. Biochem. 2012, 58, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Xin, X.; Song, C.; Chen, X.; Zhang, J.; Wu, S.; Li, R.; Liu, X.; Lu, X. Activity levels and expression of antioxidant enzymes in the ascorbate-glutathione cycle in artificially aged rice seed. Plant Physiol Biochem. 2014, 80, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Harb, A.; Awad, D.; Samarah, N. Gene expression and activity of antioxidant enzymes in barley (Hordeum vulgare L.) under controlled severe drought. J. Plant Interact. 2015, 10, 109–116. [Google Scholar] [CrossRef]
- Kong, L.; Huo, H.; Mao, P. Antioxidant response and related gene expression in aged oat seed. Front. Plant Sci. 2015, 6, 158. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Wen, D.; Gong, B.; Sun, S.; Liu, S.; Wang, X.; Wei, M.; Yang, F.; Shi, Q. Promoting roles of melatonin in adventitious root development of Solanum lycopersicum L. by regulating auxin and nitric oxide signaling. Front. Plant Sci. 2016, 7, 718. [Google Scholar] [CrossRef]
- Merwad, A.M.A.; Desoky, E.M.; Rady, M.M. Response of water deficit-stressed Vigna unguiculata performances to silicon, proline or methionine foliar application. Sci. Hortic. 2018, 228, 132–144. [Google Scholar] [CrossRef]
- Peña Calzada, K.; Olivera Viciedo, D.; Habermann, E.; Calero Hurtado, A.; Lupino Gratão, P.; De Mello Prado, R.; Lata-Tenesaca, L.F.; Martinez, C.A.; Ajila Celi, G.E.; Rodríguez, J.C. Exogenous Application of Amino Acids Mitigates the Deleterious Effects of Salt Stress on Soybean Plants. Agronomy 2022, 12, 2014. [Google Scholar] [CrossRef]
- Ould said, C.; Boulahia, K.; Eid, M.A.M.; Rady, M.M.; Djebbar, R.; Abrous-Belbachir, O. Exogenously-used proline offers potent antioxidative and osmoprotective strategies to re-balance growth and physio-biochemical attributes in herbicide-stressed Trigonella foenum-graecum. J. Soil Sci. Plant Nutr. 2021, 21, 3254–3268. [Google Scholar] [CrossRef]
- Hemida, K.A.; Eloufey, A.Z.A.; Hassan, G.M.; Rady, M.M.; El-Sadek, A.N.; Abdelfattah, M.A. Integrative NPK soil and foliar application improves growth, yield, antioxidant, and nutritional status of Capsicum annuum L. in sandy soils under semi-arid condition. J. Plant Nutr. 2023, 46, 1091–1107. [Google Scholar] [CrossRef]
- Al-Taweel, S.K.; Belal, H.E.E.; El Sowfy, D.M.; Desoky, E.-S.M.; Rady, M.M.; Mazrou, K.E.; Maray, A.R.M.; El-Sharnouby, M.E.; Alamer, K.H.; Ali, E.F.; et al. Integrative seed and leaf treatment with ascorbic acid extends the planting period by improving tolerance to late sowing influences in parsley. Horticulturae 2022, 8, 334. [Google Scholar] [CrossRef]
- Zamanipour, M. Effects of Pyridoxine, Thiamine and Folic acid on Growth, Reproductive and Biochemical Characteristics of Delphus Tomato. J. Hortic. Sci. 2021, 35, 283–300. [Google Scholar] [CrossRef]
- Alsamadany, H.; Alharby, H.F.; Al-Zahrani, H.S.; Kuşvuran, A.; Kuşvuran, S.; Rady, M.M. Selenium fortification stimulates antioxidant- and enzyme gene expression-related defense mechanisms in response to saline stress in Cucurbita pepo. Sci. Hortic. 2023, 312, 111886. [Google Scholar] [CrossRef]
- Rady, M.M.; Belal, H.E.E.; Gadallah, F.M.; Semida, W.M. Selenium application in two methods promotes drought tolerance in Solanum lycopersicum plant by inducing the antioxidant defense system. Sci. Hortic. 2020, 266, 109290. [Google Scholar] [CrossRef]
- Hassan, M.S.M.F.; Belal, H.E.E.; Abou-Sreea, A.I.B.; Rady, M.M. Exogenous application of selenium or iodine improves the growth, yield and antioxidant status of Capsicum annuum L. Labyrinth: Fayoum J. Sci. Interdiscip. Stud. 2023, 1, 76–83. [Google Scholar] [CrossRef]
- Medrano-Macías, J.; Leija-Martínez, P.; González-Morales, S.; Juárez-Maldonado, A.; Benavides-Mendoza, A. Use of iodine to biofortify and promote growth and stress tolerance in crops. Front. Plant Sci. 2016, 7, 114. [Google Scholar] [CrossRef]








| Component. | Unit | Value | Component | Unit | Value | |
|---|---|---|---|---|---|---|
| Proteins | % | 0.30 ± 0.01 | Osmoprotectants: | |||
| Organic acids | 0.51 ± 0.02 | Proline | mg kg−1 FW | 50.1 ± 2.04 | ||
| pH | 4.02 ± 0.14 | Total soluble sugars | % | 81.9 ± 2.42 | ||
| Minerals: | Antioxidants and Vitamins: | |||||
| K | mg kg−1 FW | 460 ± 11.2 | Vitamin | C | mg kg−1 FW | 30.1 ± 1.02 |
| P | 49.8 ± 1.85 | B1 | 0.15 ± 0.00 | |||
| Mg | 83.6 ± 2.61 | B2 | 0.19 ± 0.00 | |||
| Ca | 70.2 ± 1.92 | B3 | 1.70 ± 0.07 | |||
| Fe | 69.5 ± 1.84 | B5 | 1.05 ± 0.05 | |||
| Mn | 8.62 ± 0.30 | B6 | 2.21 ± 0.11 | |||
| Zn | 5.64 ± 0.18 | B9 | 0.22 ± 0.01 | |||
| Cu | 4.58 ± 0.15 | DPPH radical-scavenging activity | % | 89.5 ± 3.01 | ||
| Iodine | 80.8 ± 2.44 | |||||
| Selenium | 0.94 ± 0.04 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belal, H.E.E.; Abdelpary, M.A.M.; Desoky, E.-S.M.; Ali, E.F.; Al Kashgry, N.A.T.; Rady, M.M.; Semida, W.M.; Mahmoud, A.E.M.; Sayed, A.A.S. Effect of Eco-Friendly Application of Bee Honey Solution on Yield, Physio-Chemical, Antioxidants, and Enzyme Gene Expressions in Excessive Nitrogen-Stressed Common Bean (Phaseolus vulgaris L.) Plants. Plants 2023, 12, 3435. https://doi.org/10.3390/plants12193435
Belal HEE, Abdelpary MAM, Desoky E-SM, Ali EF, Al Kashgry NAT, Rady MM, Semida WM, Mahmoud AEM, Sayed AAS. Effect of Eco-Friendly Application of Bee Honey Solution on Yield, Physio-Chemical, Antioxidants, and Enzyme Gene Expressions in Excessive Nitrogen-Stressed Common Bean (Phaseolus vulgaris L.) Plants. Plants. 2023; 12(19):3435. https://doi.org/10.3390/plants12193435
Chicago/Turabian StyleBelal, Hussein E. E., Mostafa A. M. Abdelpary, El-Sayed M. Desoky, Esmat F. Ali, Najla Amin T. Al Kashgry, Mostafa M. Rady, Wael M. Semida, Amr E. M. Mahmoud, and Ali A. S. Sayed. 2023. "Effect of Eco-Friendly Application of Bee Honey Solution on Yield, Physio-Chemical, Antioxidants, and Enzyme Gene Expressions in Excessive Nitrogen-Stressed Common Bean (Phaseolus vulgaris L.) Plants" Plants 12, no. 19: 3435. https://doi.org/10.3390/plants12193435
APA StyleBelal, H. E. E., Abdelpary, M. A. M., Desoky, E.-S. M., Ali, E. F., Al Kashgry, N. A. T., Rady, M. M., Semida, W. M., Mahmoud, A. E. M., & Sayed, A. A. S. (2023). Effect of Eco-Friendly Application of Bee Honey Solution on Yield, Physio-Chemical, Antioxidants, and Enzyme Gene Expressions in Excessive Nitrogen-Stressed Common Bean (Phaseolus vulgaris L.) Plants. Plants, 12(19), 3435. https://doi.org/10.3390/plants12193435










