Genome-Wide Identification and Expression Analysis of the TCP Gene Family Related to Developmental and Abiotic Stress in Ginger
Abstract
1. Introduction
2. Results
2.1. Identification and Characterization of TCP Gene Family in Ginger
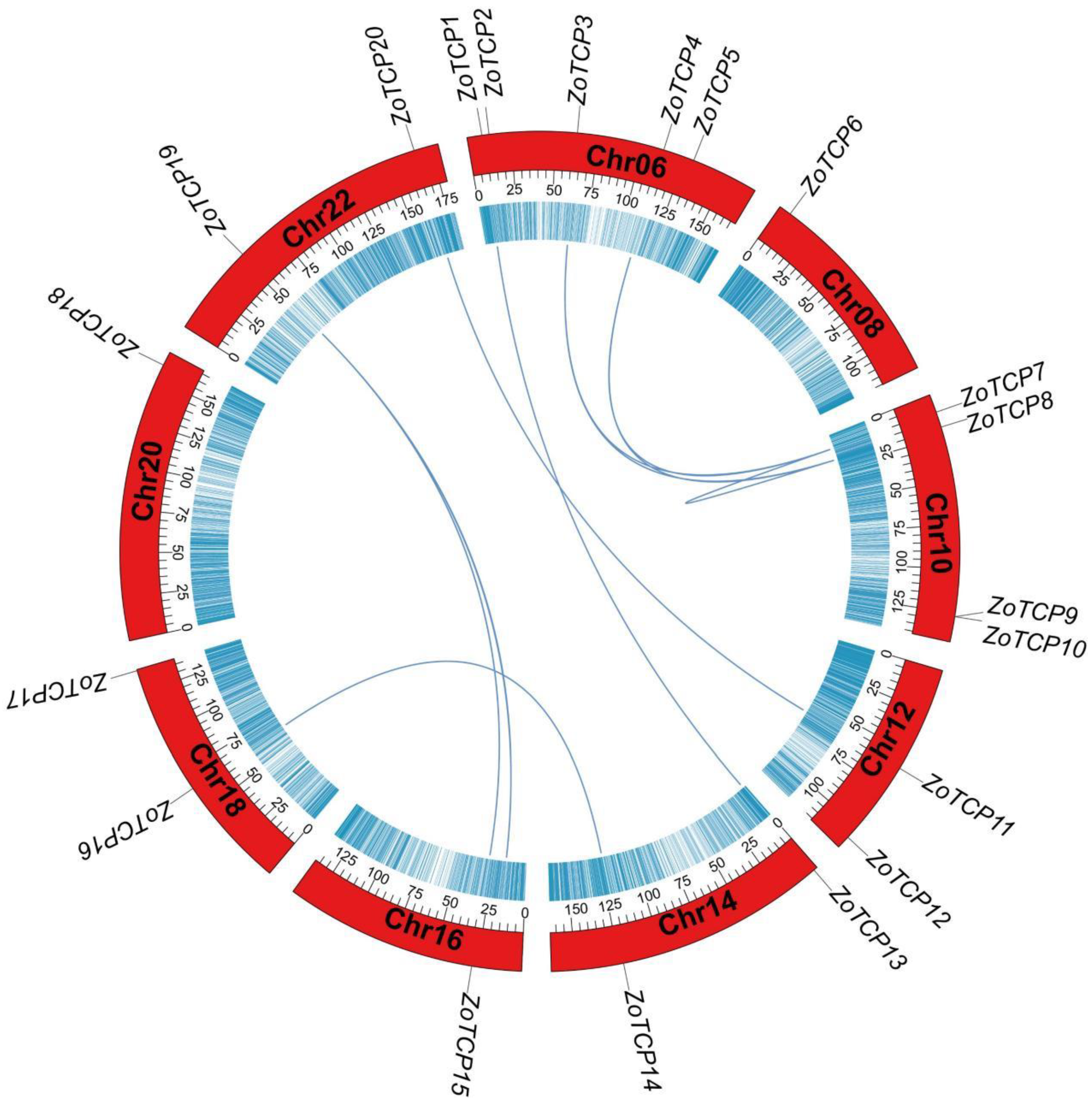
2.2. Chromosomal Localization Analysis of TCP Gene in Ginger
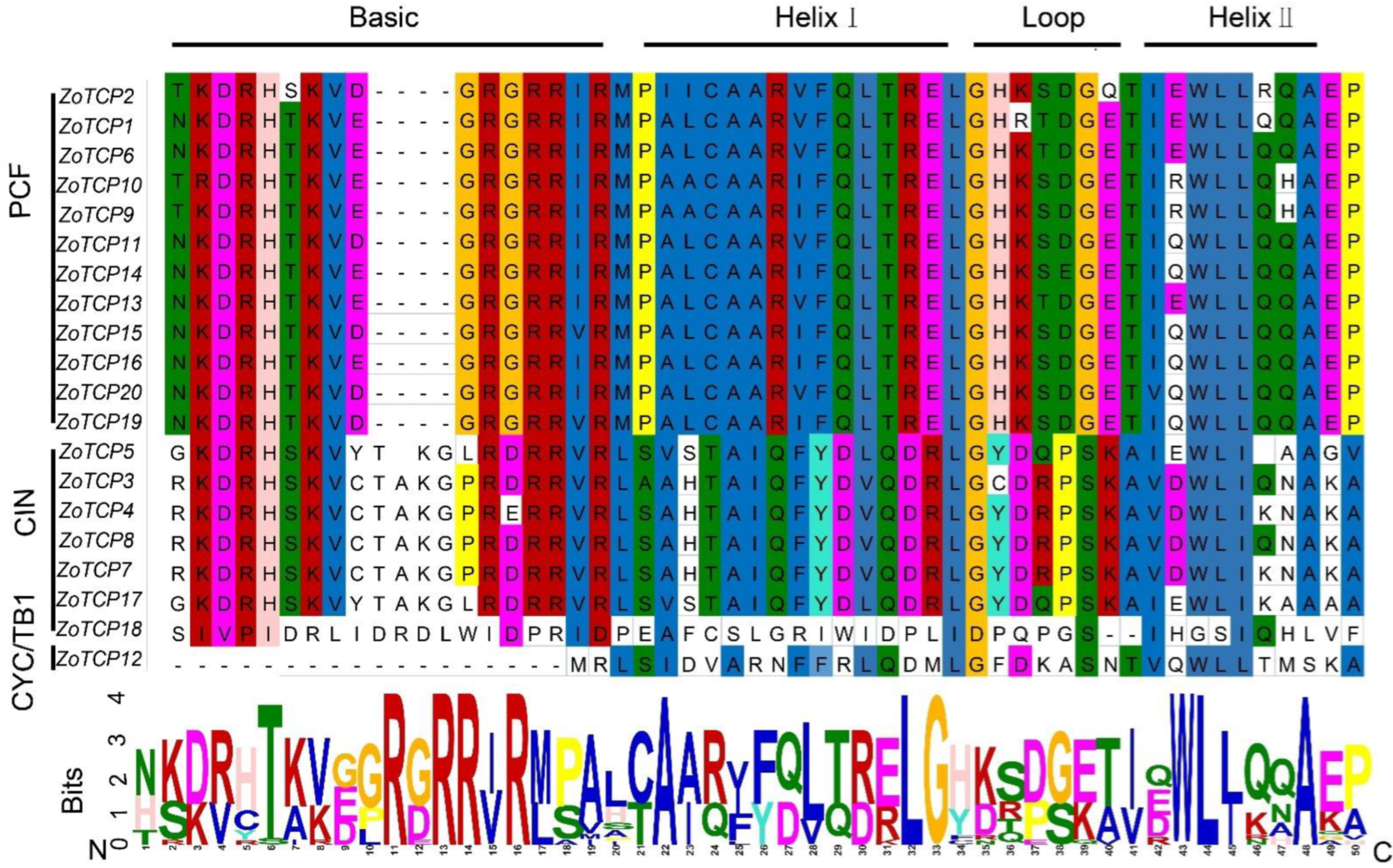
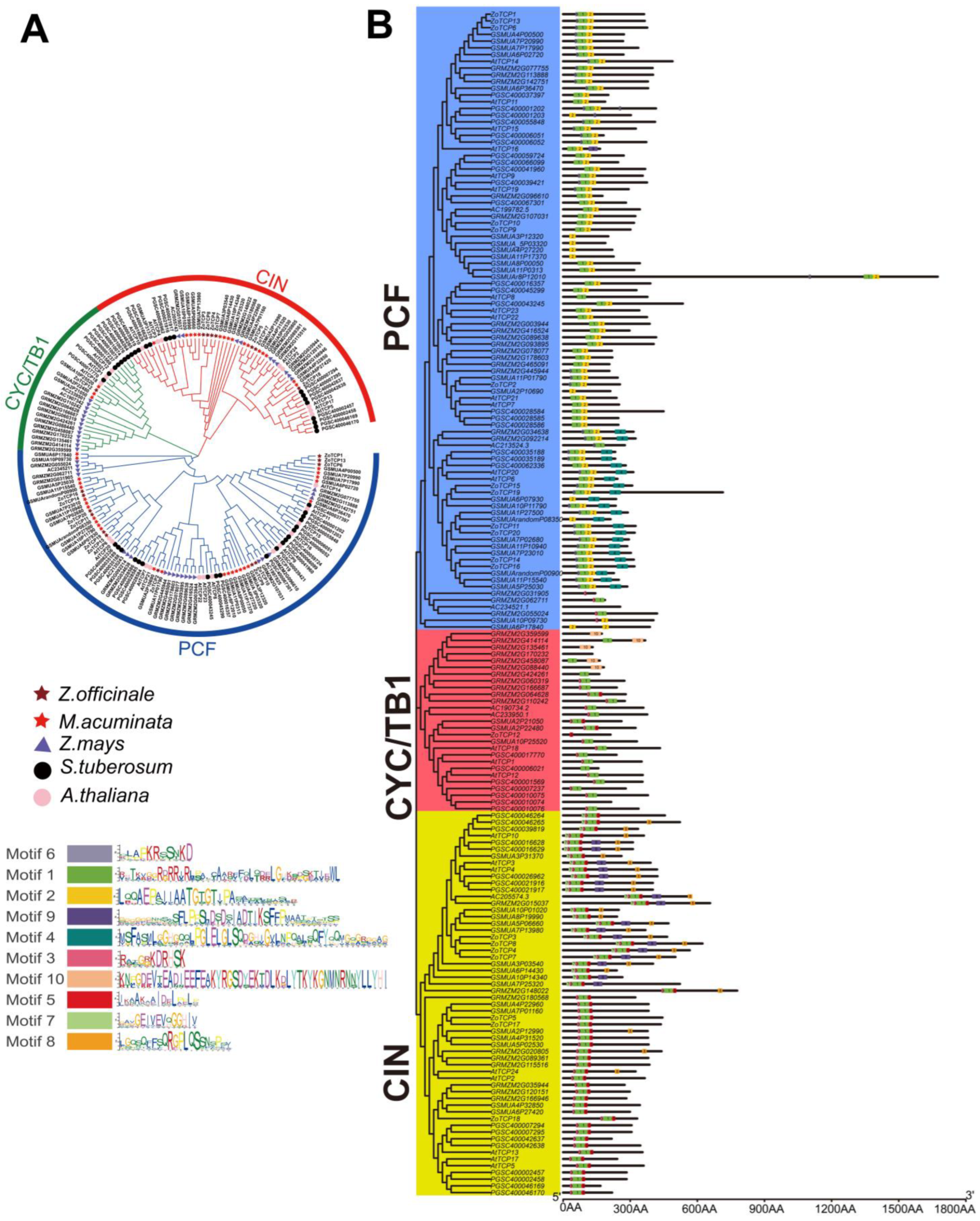
2.3. Evolutionary Analysis of Ginger TCP Gene Family
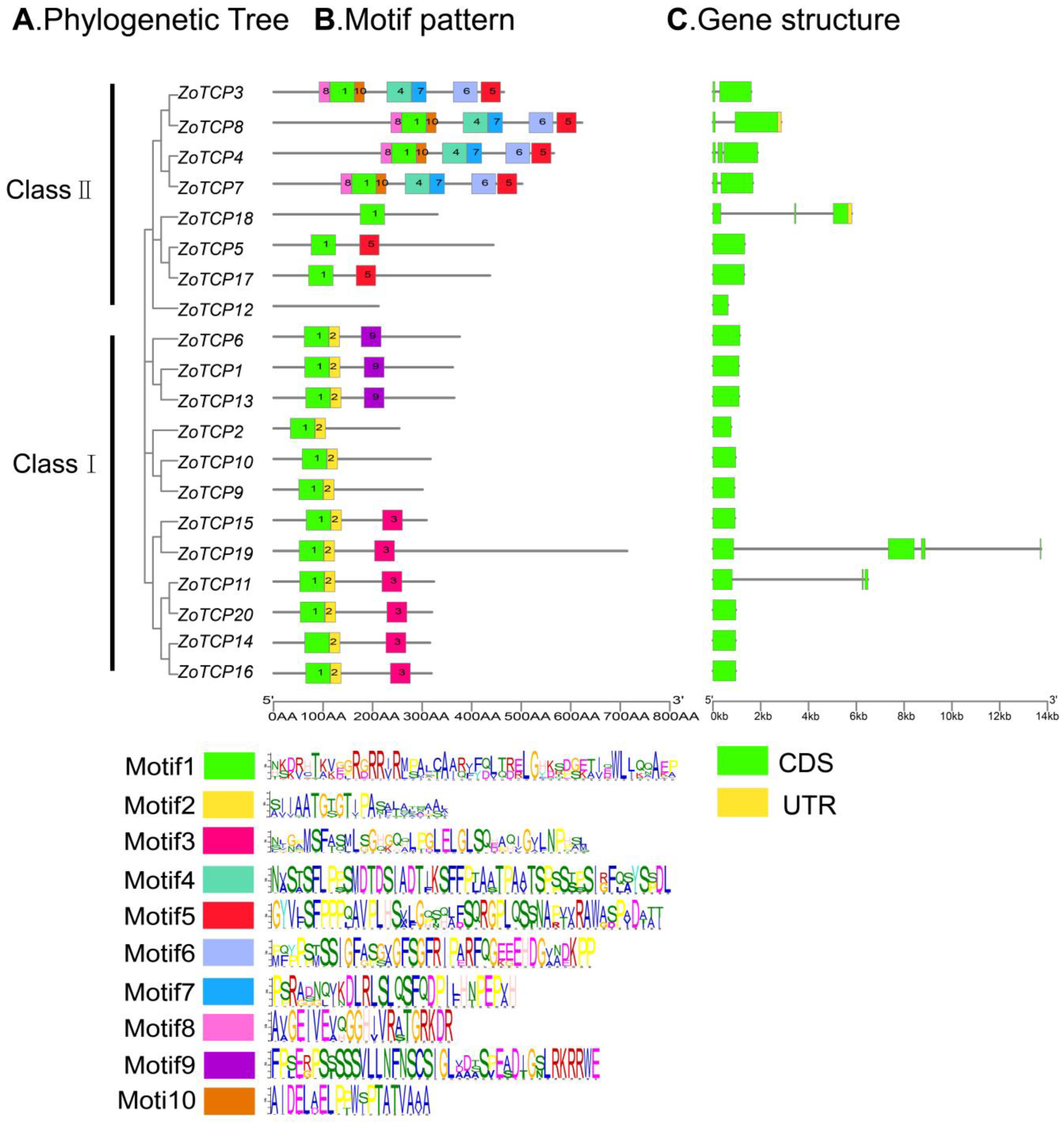
2.4. ZoTCP Gene Structure and Conserved Motif Analysis
2.5. Evolutionary Analysis of ZoTCP Gene
2.6. Cis-Acting Element Analysis
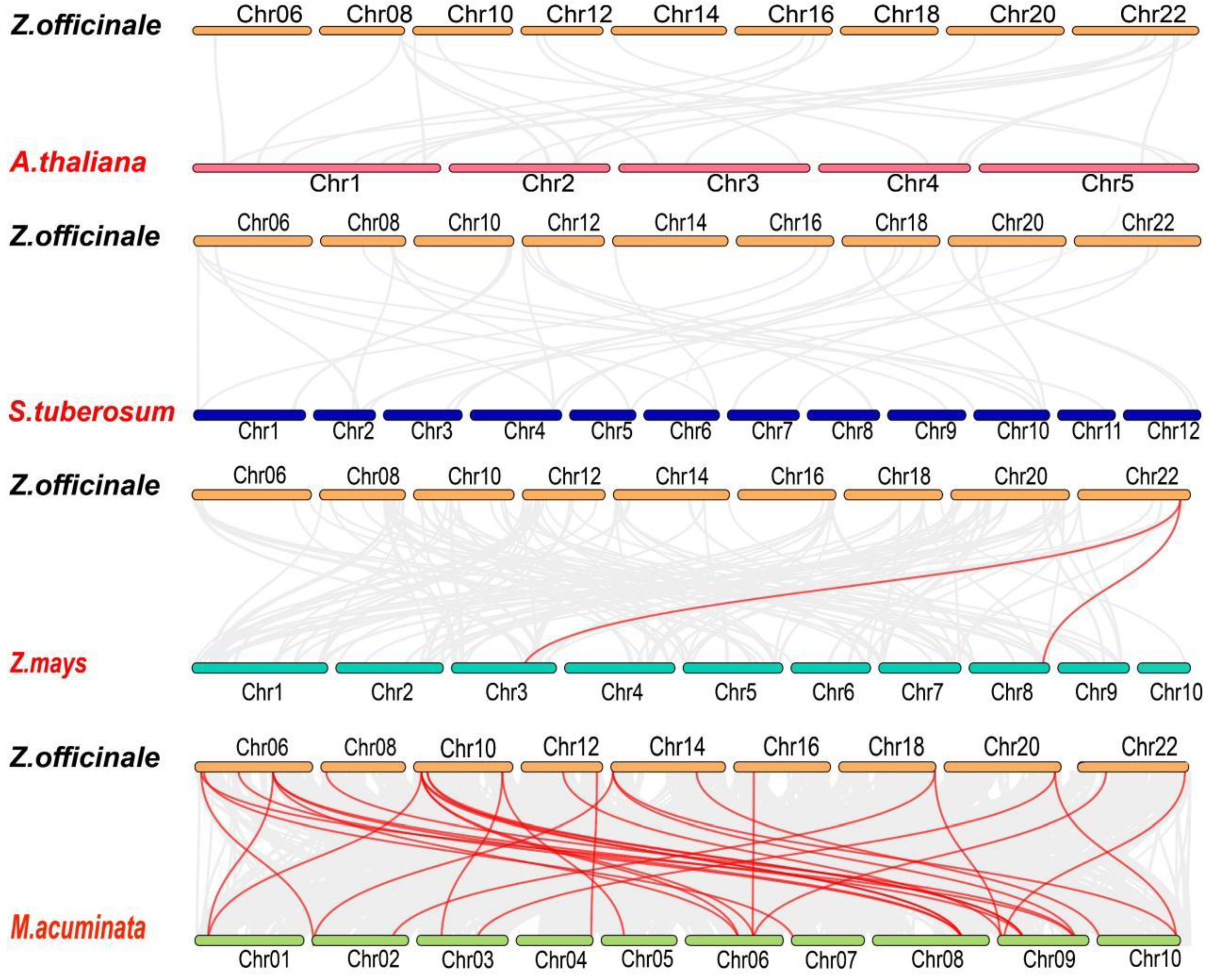
2.7. ZoTCP Gene Replication Events and Collinearity Analysis
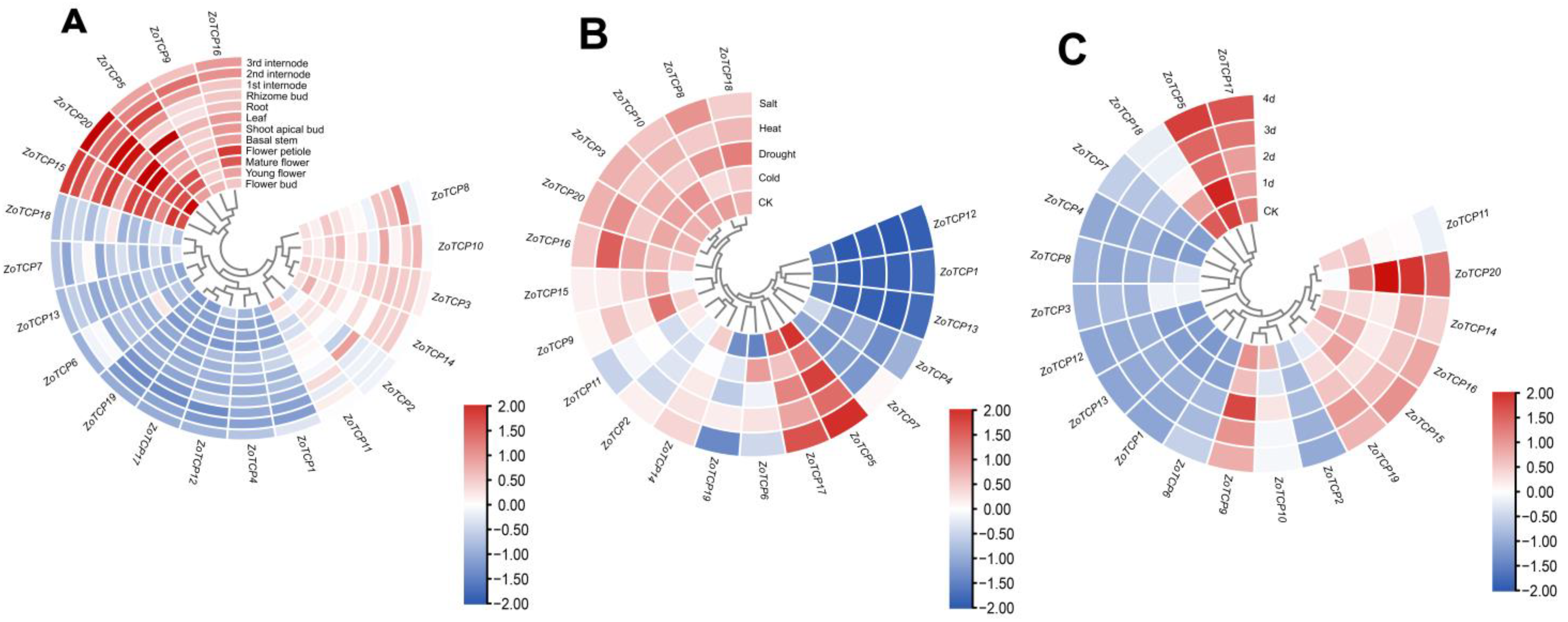
2.8. Expression Analysis of ZoTCP in Different Tissues of Ginger
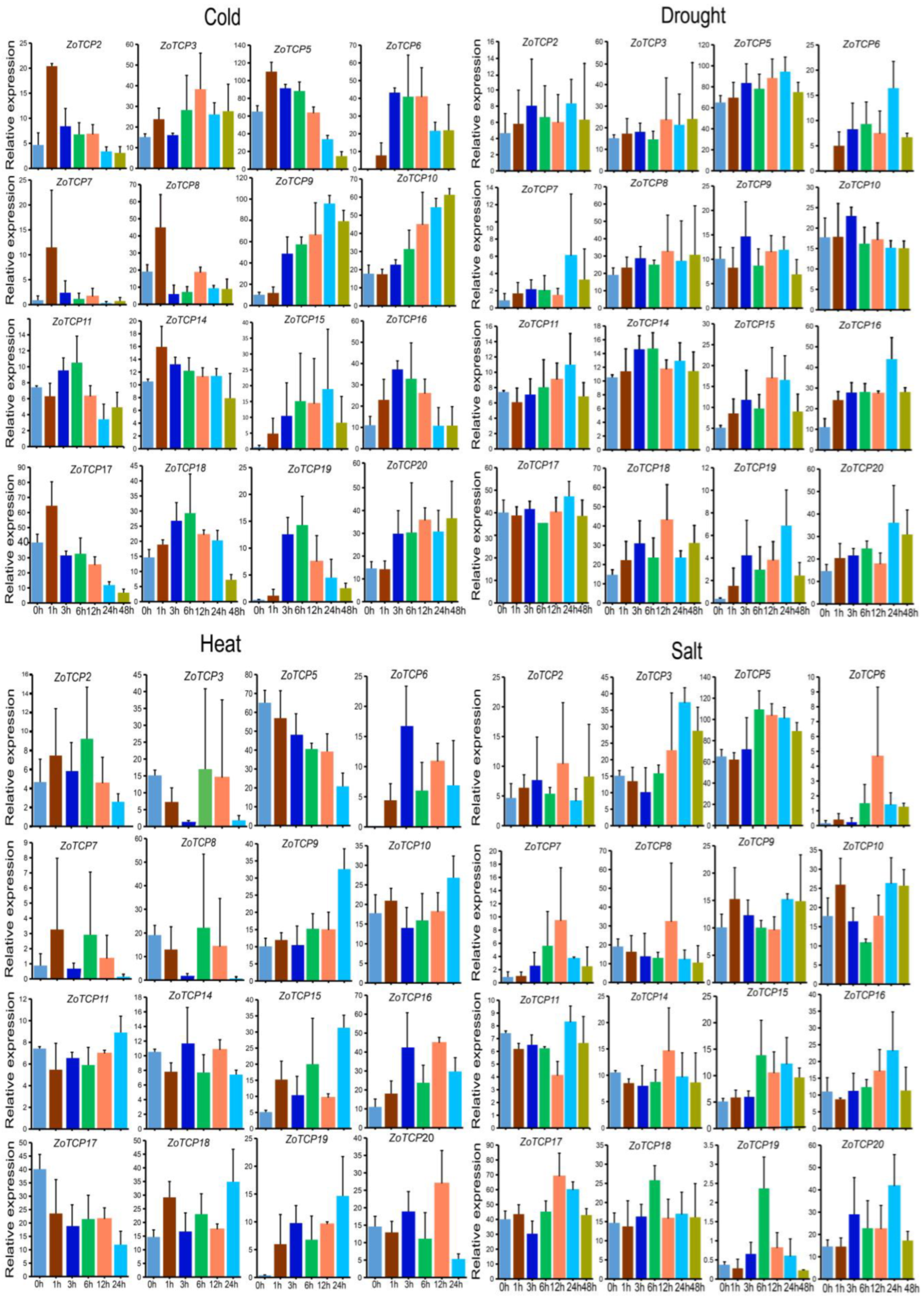
2.9. Analysis of ZoTCP Expression Patterns under Cold, Drought, Heat, Salt, High-Temperature, and Intense Light Stresses
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Identification and Physicochemical Properties Analysis of TCP Gene Family Members in Ginger
4.3. Multiple Sequence Alignment and Phylogenetic Tree Construction of Ginger TCP Protein
4.4. Analysis of TCP Gene Structure and Conserved Elements in Ginger
4.5. Analysis of Cis-Acting Elements of Ginger TCP Gene
4.6. Collinearity Analysis
4.7. ZoTCP Gene Expression and qRT-PCR Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katagiri, F.; Chua, N.-H. Plant transcription factors: Present knowledge and future challenges. Trends Genet. 1992, 8, 22–27. [Google Scholar] [CrossRef]
- Huang, F.; Shi, C.; Zhang, Y.; Hou, X. Genome-Wide Identification and Characterization of TCP Family Genes in Pak-Choi [Brassica campestris (syn. Brassica rapa) ssp. chinensis var. communis]. Front. Plant Sci. 2022, 13, 854171. [Google Scholar] [CrossRef]
- Zhao, M.; Peng, X.; Chen, N.; Shen, S. Genome-wide identification of the TCP gene family in Broussonetia papyrifera and functional analysis of BpTCP8, 14 and 19 in shoot branching. Plants 2020, 9, 1301. [Google Scholar] [CrossRef]
- Cubas, P.; Lauter, N.; Doebley, J.; Coen, E. The TCP domain: A motif found in proteins regulating plant growth and development. Plant J. 1999, 18, 215–222. [Google Scholar] [CrossRef]
- Mondragón-Palomino, M.; Trontin, C. High time for a roll call: Gene duplication and phylogenetic relationships of TCP-like genes in monocots. Ann. Bot. 2011, 107, 1533–1544. [Google Scholar] [CrossRef]
- Navaud, O.; Dabos, P.; Carnus, E.; Tremousaygue, D.; Hervé, C. TCP transcription factors predate the emergence of land plants. J. Mol. Evol. 2007, 65, 23–33. [Google Scholar] [CrossRef]
- Citerne, H.L.; Le Guilloux, M.; Sannier, J.; Nadot, S.; Damerval, C. Combining phylogenetic and syntenic analyses for understanding the evolution of TCP ECE genes in eudicots. PLoS ONE 2013, 8, e74803. [Google Scholar] [CrossRef]
- Doebley, J.; Stec, A.; Gustus, C. Teosinte branched1 and the origin of maize: Evidence for epistasis and the evolution of dominance. Genetics 1995, 141, 333–346. [Google Scholar] [CrossRef]
- Dixon, L.E.; Greenwood, J.R.; Bencivenga, S.; Zhang, P.; Cockram, J.; Mellers, G.; Ramm, K.; Cavanagh, C.; Swain, S.M.; Boden, S.A. TEOSINTE BRANCHED1 regulates inflorescence architecture and development in bread wheat (Triticum aestivum). Plant Cell 2018, 30, 563–581. [Google Scholar] [CrossRef]
- Luo, D.; Carpenter, R.; Vincent, C.; Copsey, L.; Coen, E. Origin of floral asymmetry in Antirrhinum. Nature 1996, 383, 794–799. [Google Scholar] [CrossRef]
- Hileman, L.C. Bilateral flower symmetry—How, when and why? Curr. Opin. Plant Biol. 2014, 17, 146–152. [Google Scholar] [CrossRef]
- Kosugi, S.; Ohashi, Y. PCF1 and PCF2 specifically bind to cis elements in the rice proliferating cell nuclear antigen gene. Plant Cell 1997, 9, 1607–1619. [Google Scholar]
- Martín-Trillo, M.; Cubas, P. TCP genes: A family snapshot ten years later. Trends Plant Sci. 2010, 15, 31–39. [Google Scholar] [CrossRef]
- Manassero, N.G.U.; Viola, I.L.; Welchen, E.; Gonzalez, D.H. TCP transcription factors: Architectures of plant form. Biomol. Concepts 2013, 4, 111–127. [Google Scholar] [CrossRef]
- Broholm, S.K.; Tähtiharju, S.; Laitinen, R.A.E.; Albert, V.A.; Teeri, T.H.; Elomaa, P. A TCP domain transcription factor controls flower type specification along the radial axis of the Gerbera (Asteraceae) inflorescence. Proc. Natl. Acad. Sci. USA 2008, 105, 9117–9122. [Google Scholar] [CrossRef]
- Nag, A.; King, S.; Jack, T. MiR319a targeting of TCP4 is critical for petal growth and development in Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 22534–22539. [Google Scholar] [CrossRef]
- Palatnik, J.F.; Allen, E.; Wu, X.; Schommer, C.; Schwab, R.; Carrington, J.C.; Weigel, D. Control of leaf morphogenesis by microRNAs. Nature 2003, 425, 257–263. [Google Scholar] [CrossRef]
- Aguilar-Martínez, J.A.; Poza-Carrión, C.; Cubas, P. Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. Plant Cell 2007, 19, 458–472. [Google Scholar] [CrossRef]
- Resentini, F.; Felipo-Benavent, A.; Colombo, L.; Blázquez, M.A.; Alabadí, D.; Masiero, S. TCP14 and TCP15 mediate the promotion of seed germination by gibberellins in Arabidopsis thaliana. Mol. Plant 2015, 8, 482–485. [Google Scholar] [CrossRef]
- Sugio, A.; Kingdom, H.N.; MacLean, A.M.; Grieve, V.M.; Hogenhout, S.A. Phytoplasma protein effector SAP11 enhances insect vector reproduction by manipulating plant development and defense hormone biosynthesis. Proc. Natl. Acad. Sci. USA 2011, 108, e1254–e1263. [Google Scholar] [CrossRef]
- Giraud, E.; Ng, S.; Carrie, C.; Duncan, O.; Low, J.; Lee, C.P.; Van Aken, O.; Millar, A.H.; Murcha, M.; Whelan, J. TCP transcription factors link the regulation of genes encoding mitochondrial proteins with the circadian clock in Arabidopsis thaliana. Plant cell 2010, 22, 3921–3934. [Google Scholar] [CrossRef]
- Steiner, E.; Efroni, I.; Gopalraj, M.; Saathoff, K.; Tseng, T.S.; Kieffer, M.; Eshed, Y.; Olszewski, N.; Weiss, D. The Arabidopsis O-linked N-acetylglucosamine transferase SPINDLY interacts with class I TCPs to facilitate cytokinin responses in leaves and flowers. Plant Cell 2012, 24, 96–108. [Google Scholar] [CrossRef]
- González-Grandío, E.; Pajoro, A.; Franco-Zorrilla, J.M.; Tarancón, C.; Immink, R.G.H.; Cubas, P. Abscisic acid signaling is controlled by a BRANCHED1/HD-ZIP I cascade in Arabidopsis axillary buds. Proc. Natl. Acad. Sci. USA 2017, 114, e245–e254. [Google Scholar] [CrossRef]
- Kieffer, M.; Master, V.; Waites, R.; Davies, B. TCP14 and TCP15 affect internode length and leaf shape in Arabidopsis. Plant J. 2011, 68, 147–158. [Google Scholar] [CrossRef]
- Davière, J.-M.; Wild, M.; Regnault, T.; Baumberger, N.; Eisler, H.; Genschik, P.; Achard, P. Class I TCP-DELLA interactions in inflorescence shoot apex determine plant height. Curr. Biol. 2014, 24, 1923–1928. [Google Scholar] [CrossRef]
- Danisman, S.; Van der Wal, F.; Dhondt, S.; Waites, R.; de Folter, S.; Bimbo, A.; van Dijk, A.D.; Muino, J.M.; Cutri, L.; Dornelas, M.C. Arabidopsis class I and class II TCP transcription factors regulate jasmonic acid metabolism and leaf development antagonistically. Plant Physiol. 2012, 159, 1511–1523. [Google Scholar] [CrossRef]
- Howarth, D.G.; Donoghue, M.J. Duplications in CYC-like genes from Dipsacales correlate with floral form. Int. J. Plant Sci. 2005, 166, 357–370. [Google Scholar] [CrossRef][Green Version]
- Bresso, E.G.; Chorostecki, U.; Rodriguez, R.E.; Palatnik, J.F.; Schommer, C. Spatial control of gene expression by miR319-regulated TCP transcription factors in leaf development. Plant Physiol. 2018, 176, 1694–1708. [Google Scholar] [CrossRef]
- Wei, W.; Hu, Y.; Cui, M.-Y.; Han, Y.-T.; Gao, K.; Feng, J.-Y. Identification and transcript analysis of the TCP transcription factors in the diploid woodland strawberry Fragaria vesca. Front. Plant Sci. 2016, 7, 1937. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Tyagi, A.K. Erratum: OsTCP19 influences developmental and abiotic stress signaling by modulating ABI4-mediated pathways. Sci. Rep. 2015, 5, 12381. [Google Scholar] [CrossRef]
- Wang, S.-t.; Sun, X.-l.; Hoshino, Y.; Yu, Y.; Jia, B.; Sun, Z.-w.; Sun, M.-z.; Duan, X.-b.; Zhu, Y.-m. MicroRNA319 positively regulates cold tolerance by targeting OsPCF6 and OsTCP21 in rice (Oryza sativa L.). PLoS ONE 2014, 9, e91357. [Google Scholar] [CrossRef]
- Almeida, D.M.; Gregorio, G.B.; Oliveira, M.M.; Saibo, N.J. Five novel transcription factors as potential regulators of OsNHX1 gene expression in a salt tolerant rice genotype. Plant Mol. Biol. 2017, 93, 61–77. [Google Scholar] [CrossRef]
- Guan, P.; Ripoll, J.-J.; Wang, R.; Vuong, L.; Bailey-Steinitz, L.J.; Ye, D.; Crawford, N.M. Interacting TCP and NLP transcription factors control plant responses to nitrate availability. Proc. Natl. Acad. Sci. USA 2017, 114, 2419–2424. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, H.; Gao, Y.; Xiong, R.; Wu, M.; Zhang, K.; Xiang, Y. The TCP transcription factor PeTCP10 modulates salt tolerance in transgenic Arabidopsis. Plant Cell Rep. 2021, 40, 1971–1987. [Google Scholar] [CrossRef]
- Ballester, P.; Cerdá, B.; Arcusa, R.; Marhuenda, J.; Yamedjeu, K.; Zafrilla, P. Effect of ginger on inflammatory diseases. Molecules 2022, 27, 7223. [Google Scholar] [CrossRef]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A.M. Gingerols and shogaols: Important nutraceutical principles from ginger. Phytochemistry 2015, 117, 554–568. [Google Scholar] [CrossRef]
- Li, H.-L.; Wu, L.; Dong, Z.; Jiang, Y.; Jiang, S.; Xing, H.; Li, Q.; Liu, G.; Tian, S.; Wu, Z. Haplotype-resolved genome of diploid ginger (Zingiber officinale) and its unique gingerol biosynthetic pathway. Hort. Res. 2021, 8, 189. [Google Scholar] [CrossRef]
- Hwarari, D.; Guan, Y.; Li, R.; Movahedi, A.; Chen, J.; Yang, L. Comprehensive bioinformatics and expression analysis of TCP transcription factors in Liriodendron chinense reveals putative abiotic stress regulatory roles. Forests 2022, 13, 1401. [Google Scholar] [CrossRef]
- Yao, X.; Ma, H.; Wang, J.; Zhang, D. Genome-wide comparative analysis and expression pattern of TCP gene families in Arabidopsis thaliana and Oryza sativa. J. Integr. Plant Biol. 2007, 49, 885–897. [Google Scholar] [CrossRef]
- Ding, S.; Cai, Z.; Du, H.; Wang, H. Genome-wide analysis of TCP family genes in Zea mays L. identified a role for ZmTCP42 in drought tolerance. Int. J. Mol. Sci. 2019, 20, 2762. [Google Scholar] [CrossRef]
- Feng, Z.-J.; Xu, S.-C.; Liu, N.; Zhang, G.-W.; Hu, Q.-Z.; Gong, Y.-M. Soybean TCP transcription factors: Evolution, classification, protein interaction and stress and hormone responsiveness. Plant Physiol. Biochem. 2018, 127, 129–142. [Google Scholar] [CrossRef]
- Das Gupta, M.; Aggarwal, P.; Nath, U. CINCINNATA in A Ntirrhinum majus directly modulates genes involved in cytokinin and auxin signaling. New Phytol. 2014, 204, 901–912. [Google Scholar] [CrossRef]
- Jiu, S.; Xu, Y.; Wang, J.; Wang, L.; Wang, S.; Ma, C.; Guan, L.; Abdullah, M.; Zhao, M.; Xu, W. Genome-wide identification, characterization, and transcript analysis of the TCP transcription factors in Vitis vinifera. Front. Genet. 2019, 10, 1276. [Google Scholar] [CrossRef]
- Oliver, K.R.; McComb, J.A.; Greene, W.K. Transposable elements: Powerful contributors to angiosperm evolution and diversity. Genome Biol. Evol. 2013, 5, 1886–1901. [Google Scholar] [CrossRef]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Paterson, A.H. Genome and gene duplications and gene expression divergence: A view from plants. Ann. N. Y. Acad. Sci. 2012, 1256, 1–14. [Google Scholar] [CrossRef]
- Zhou, Y.; Tao, J.; Ahammed, G.J.; Li, J.; Yang, Y. Genome-wide identification and expression analysis of aquaporin gene family related to abiotic stress in watermelon. Genome 2019, 62, 643–656. [Google Scholar] [CrossRef]
- Xing, H.; Jiang, Y.; Zou, Y.; Long, X.; Wu, X.; Ren, Y.; Li, Y.; Li, H.-L. Genome-wide investigation of the AP2/ERF gene family in ginger: Evolution and expression profiling during development and abiotic stresses. BMC Plant Biol. 2021, 21, 561. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Feng, G.; Xu, X.; Huang, L.; Nie, G.; Li, D.; Zhang, X. Genome-Wide Identification, Characterization, and Expression of TCP Genes Family in Orchardgrass. Genes 2023, 14, 925. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Dong, Q.; Ji, Z.; Chi, F.; Cong, P.; Zhou, Z. Genome-wide identification and analysis of the MADS-box gene family in apple. Gene 2015, 555, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Seven, M.; Akdemir, H. DOF, MYB and TCP transcription factors: Their possible roles on barley germination and seedling establishment. Gene Expr. Patterns 2020, 37, 119116. [Google Scholar] [CrossRef]
- Bu, X.; Wang, X.; Yan, J.; Zhang, Y.; Zhou, S.; Sun, X.; Yang, Y.; Ahammed, G.J.; Liu, Y.; Qi, M. Genome-wide characterization of B-box gene family and its roles in responses to light quality and cold stress in tomato. Front. Plant Sci. 2021, 12, 698525. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cui, D.; Liu, C.; Zhao, J.; Liu, J.; Liu, N.; Tang, D.; Hu, Y. TCP transcription factors interact with ZED1-related kinases as components of the temperature-regulated immunity. Plant Cell Environ. 2019, 42, 2045–2056. [Google Scholar] [CrossRef]
- Camoirano, A.; Alem, A.L.; Gonzalez, D.H.; Viola, I.L. The N-terminal region located upstream of the TCP domain is responsible for the antagonistic action of the Arabidopsis thaliana TCP8 and TCP23 transcription factors on flowering time. Plant Sci. 2023, 328, 111571. [Google Scholar] [CrossRef]
- Danisman, S. TCP transcription factors at the interface between environmental challenges and the plant’s growth responses. Front. Plant Sci. 2016, 7, 1930. [Google Scholar] [CrossRef]
- Arab, M.M.; Marrano, A.; Abdollahi-Arpanahi, R.; Leslie, C.A.; Askari, H.; Neale, D.B.; Vahdati, K. Genome-wide patterns of population structure and association mapping of nut-related traits in Persian walnut populations from Iran using the Axiom J. regia 700K SNP array. Sci. Rep. 2019, 9, 6376. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Jeffryes, M.; Bateman, A.; Finn, R.D. The HMMER web server for protein sequence similarity search. Curr. Protoc. Bioinformatics 2017, 60, 3.15.1–13.15.23. [Google Scholar] [CrossRef] [PubMed]
- Larralde, M.; Zeller, G. PyHMMER: A Python library binding to HMMER for efficient sequence analysis. Bioinformatics 2023, 39, btad214. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Hall, T.; Biosciences, I.; Carlsbad, C. BioEdit: An important software for molecular biology. GERF Bull. Biosci. 2011, 2, 60–61. [Google Scholar]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Tian, F.; Yang, D.-C.; Meng, Y.-Q.; Kong, L.; Luo, J.; Gao, G. PlantTFDB 4.0: Toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2016, 45, D1040–D1045. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Jin, J.; Guo, A.-Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Contreras-Moreira, B.; Naamati, G.; Rosello, M.; Allen, J.E.; Hunt, S.E.; Muffato, M.; Gall, A.; Flicek, P. Scripting Analyses of Genomes in Ensembl Plants. In Plant Bioinformatics: Methods and Protocols; Springer: New York, NY, USA, 2022; pp. 27–55. [Google Scholar]
- KL, L. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar]
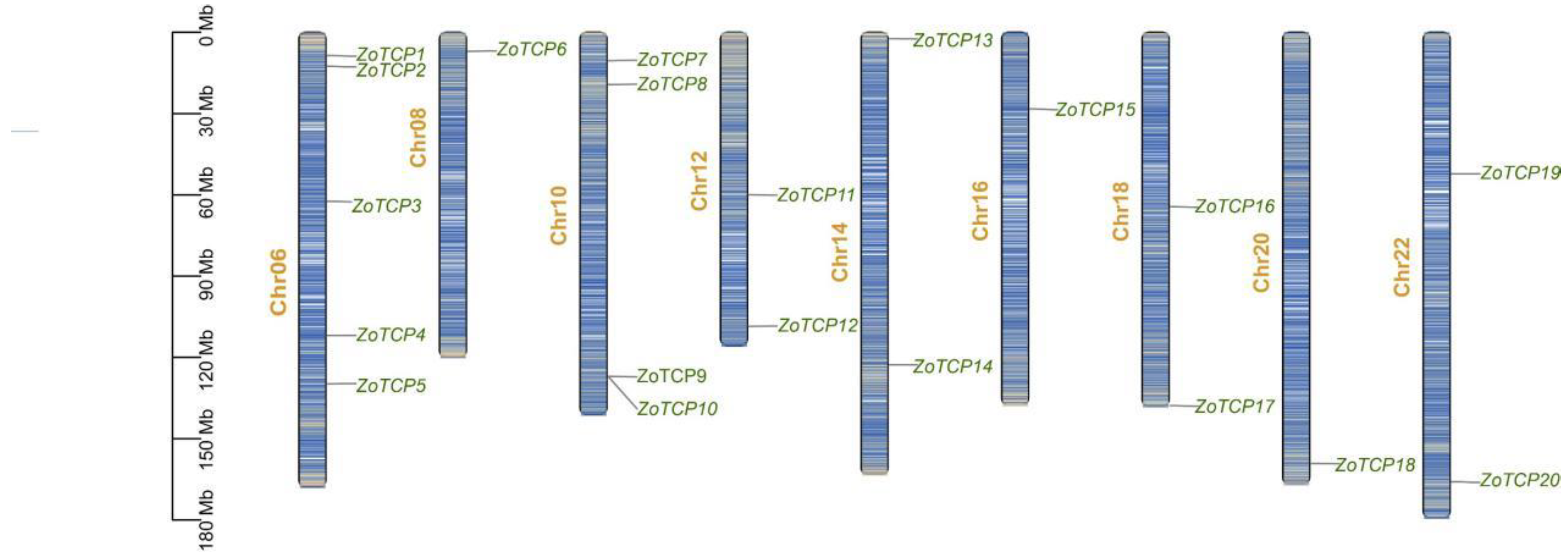


| Gene Name | Gene ID | Chromosome | Localization (bp) | Animo Acid Number | Molecular Weight (kD) | PI |
|---|---|---|---|---|---|---|
| ZoTCP1 | ZOFF_071857 | Chr.06 | 8,518,591–8,519,676 (+) | 361 | 38.71 | 6.72 |
| ZoTCP2 | ZOFF_071376 | Chr.06 | 12,551,671–12,552,432 (+) | 253 | 25.82 | 9.16 |
| ZoTCP3 | ZOFF_058948 | Chr.06 | 62,385,964–62,387,566 (−) | 464 | 49.34 | 9.38 |
| ZoTCP4 | ZOFF_070666 | Chr.06 | 111,911,935–111,913,806 (−) | 565 | 61.24 | 6.73 |
| ZoTCP5 | ZOFF_055205 | Chr.06 | 129,782,255–129,783,586 (−) | 443 | 48.45 | 7.00 |
| ZoTCP6 | ZOFF_035032 | Chr.08 | 6,982,140–6,983,267 (−) | 375 | 39.81 | 8.47 |
| ZoTCP7 | ZOFF_033278 | Chr.10 | 10,437,715–10,439,388 (+) | 501 | 54.58 | 6.68 |
| ZoTCP8 | ZOFF_009476 | Chr.10 | 19,251,872–19,254,729 (−) | 622 | 66.94 | 8.24 |
| ZoTCP9 | ZOFF_010045 | Chr.10 | 127,022,793–127,023,695 (−) | 300 | 30.89 | 5.77 |
| ZoTCP10 | ZOFF_009968 | Chr.10 | 127,027,323–127,028,273 (−) | 316 | 32.45 | 6.01 |
| ZoTCP11 | ZOFF_005937 | Chr.12 | 59,942,137–59,948,621 (+) | 323 | 33.88 | 7.21 |
| ZoTCP12 | ZOFF_075892 | Chr.12 | 108,521,821–108,522,456 (+) | 211 | 23.49 | 8.83 |
| ZoTCP13 | ZOFF_038801 | Chr.14 | 122,725,201–122,726,148 (−) | 364 | 39.35 | 7.17 |
| ZoTCP14 | ZOFF_035356 | Chr.14 | 2,292,226–2,293,320 (−) | 315 | 32.74 | 7.90 |
| ZoTCP15 | ZOFF_068839 | Chr.16 | 28,238,917–28,239,843 (+) | 308 | 33.20 | 6.87 |
| ZoTCP16 | ZOFF_008749 | Chr.18 | 64,415,581–64,416,537 (+) | 318 | 33.08 | 6.67 |
| ZoTCP17 | ZOFF_057997 | Chr.18 | 137,811,656–137,812,966 (+) | 436 | 47.31 | 8.95 |
| ZoTCP18 | ZOFF_000424 | Chr.20 | 159,161,052–159,166,863 (+) | 330 | 37.05 | 8.63 |
| ZoTCP19 | ZOFF_047522 | Chr.22 | 52,215,302–52,229,031 (−) | 713 | 78.70 | 8.34 |
| ZoTCP20 | ZOFF_003424 | Chr.22 | 165,907,234–165,908,193 (−) | 319 | 33.27 | 6.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Jiang, D.; Xia, M.; Gong, M.; Li, H.; Xing, H.; Zhu, X.; Li, H.-L. Genome-Wide Identification and Expression Analysis of the TCP Gene Family Related to Developmental and Abiotic Stress in Ginger. Plants 2023, 12, 3389. https://doi.org/10.3390/plants12193389
Jiang Y, Jiang D, Xia M, Gong M, Li H, Xing H, Zhu X, Li H-L. Genome-Wide Identification and Expression Analysis of the TCP Gene Family Related to Developmental and Abiotic Stress in Ginger. Plants. 2023; 12(19):3389. https://doi.org/10.3390/plants12193389
Chicago/Turabian StyleJiang, Yajun, Dongzhu Jiang, Maoqin Xia, Min Gong, Hui Li, Haitao Xing, Xuedong Zhu, and Hong-Lei Li. 2023. "Genome-Wide Identification and Expression Analysis of the TCP Gene Family Related to Developmental and Abiotic Stress in Ginger" Plants 12, no. 19: 3389. https://doi.org/10.3390/plants12193389
APA StyleJiang, Y., Jiang, D., Xia, M., Gong, M., Li, H., Xing, H., Zhu, X., & Li, H.-L. (2023). Genome-Wide Identification and Expression Analysis of the TCP Gene Family Related to Developmental and Abiotic Stress in Ginger. Plants, 12(19), 3389. https://doi.org/10.3390/plants12193389







