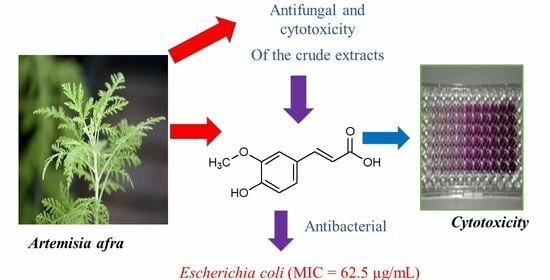
Extractives from Artemisia afra with Anti-Bacterial and Anti-Fungal Properties
Abstract
1. Introduction
2. Results and Discussions
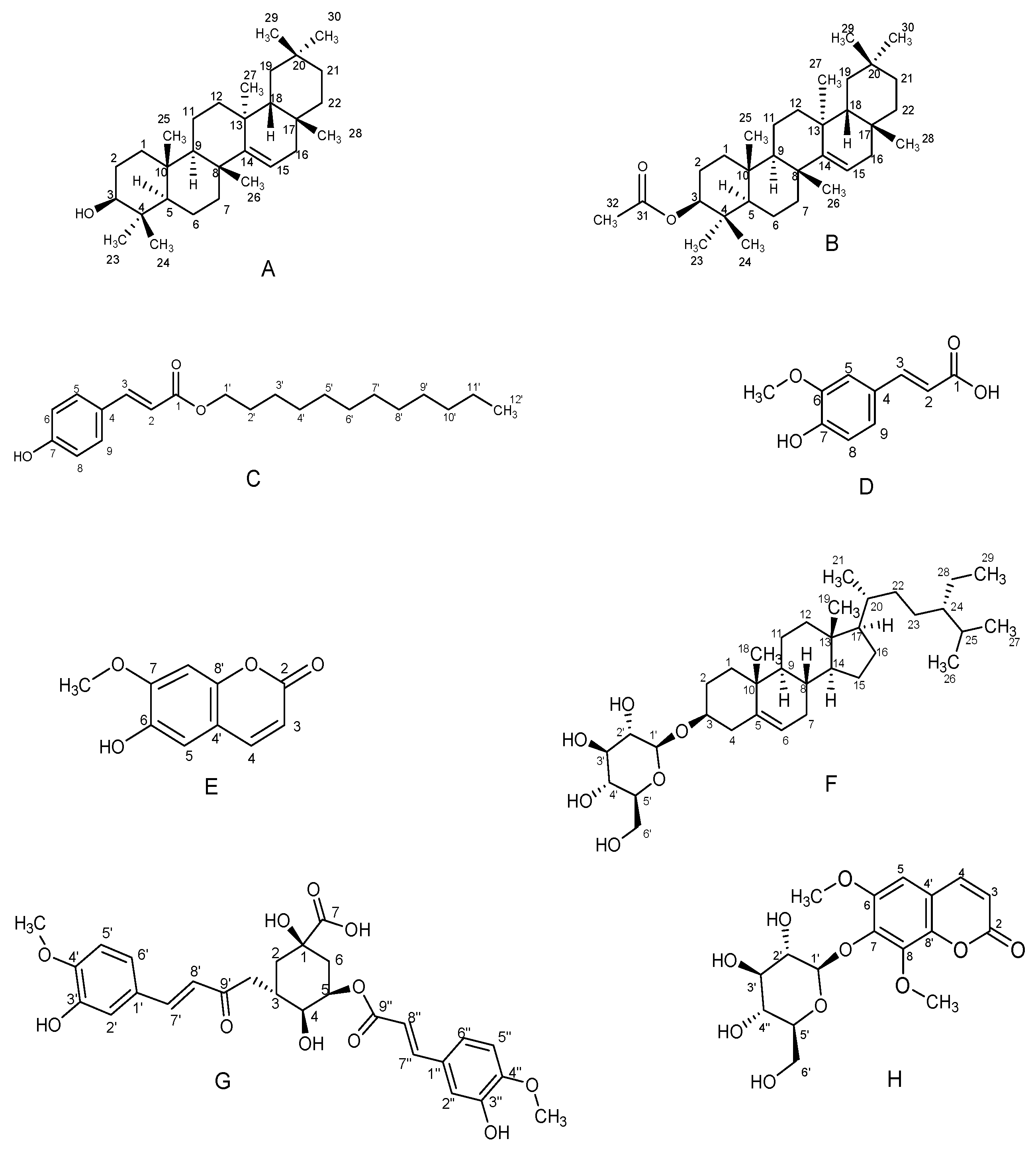
2.1. Isolated Compounds from A. afra
2.2. Anti-Bacterial, Anti-Fungal, and Cytotoxicity Studies of A. afra Crude Extracts and Isolated Compounds
3. Materials and Methods
3.1. Collection and Identification
3.2. Sample Preparation and Extraction
3.3. Fractionation and Purification of Crude Extracts
3.3.1. Fractionation and Purification of DCM/Ethyl Acetate Crude Extracts
3.3.2. Fractionation and Purification of Ethanol Crude Extract
3.4. Spectral Data of Isolated Compounds
3.4.1. Compound A: 3β-Taraxerol
3.4.2. Compound B: 3β-Taraxerol Acetate
3.4.3. Compound C: Dodecyl-p-coumarate
3.4.4. Compound D: Ferulic Acid
3.4.5. Compound E: Scopoletin
3.4.6. Compound F: Sitosterol-3-O-β-D-glucopyranoside
3.4.7. Compound G: 3,5-Di-O-feruloylquinic Acid
3.4.8. Compound H: Isofraxidin-7-O-β-D-glucopyranoside
3.5. Minimum Inhibitory Assay for Quantitative Antimicrobial Test
3.6. Cytotoxicity Assay of Isolated Compounds and Crude Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richardson, J.P.; Moyes, D.L. Adaptive immune responses to Candida albicans infection. Virulence 2015, 6, 327–337. [Google Scholar] [CrossRef]
- World Health Organization; Antimicrobial Resistance Division; Control of Neglected Tropical Diseases; Global Coordination and Partnership; Alastruey-Izquierdo, A. WHO Fungal Priority Pathogens List to Guide Research, Development, and Public Health Action; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- WHO. Antimicrobial Resistance. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 6 November 2022).
- Ruifang, Z.; Karen, E.; Vincent, R.; Richard, J.Z. Antibiotic resistance as a global threat: Evidence from China, Kuwait, and the United States. Glob. Health 2006, 2, 6. [Google Scholar]
- Nibret, E.; Wink, M. Volatile components of four Ethiopian Artemisia species extracts and their in vitro antitrypanosomal and cytotoxic activities. Phytomedicine 2010, 17, 369–374. [Google Scholar] [CrossRef]
- Adugna, M.; Feyera, T.; Taddese, W.; Admasu, P. In vivo antimalarial activity of crude extract of aerial part of Artemisia abyssinica against Plasmodium berghei in mice. Glob. J. Pharmacol. 2014, 8, 460–468. [Google Scholar]
- Bora, K.S.; Sharma, A. The genus Artemisia: A comprehensive review. Pharm. Biol. 2011, 49, 101–109. [Google Scholar] [CrossRef]
- Hussain, A.; Hayat, M.Q.; Sahreen, S.; ul Ain, Q.; Bokhari, S.A. Pharmacological promises of genus Artemisia (Asteraceae): A review: Pharmacological promises of genus Artemisia. Proc. Pak. Acad. Sci. B 2017, 54, 265–287. [Google Scholar]
- Muyima, N.Y.O.; Zulu, G.; Bhengu, T.; Popplewell, D. The potential application of some novel essential oils as natural cosmetic preservatives in an aqueous cream formulation. Flavour Fragr. J. 2002, 17, 258–266. [Google Scholar] [CrossRef]
- Liu, N.Q.; Van der Kooy, F.; Verpoorte, R. Artemisia afra: A potential flagship for African medicinal plants? S. Afr. J. Bot. 2009, 75, 185–195. [Google Scholar] [CrossRef]
- Suliman, S.; Van Vuuren, S.F.; Viljoen, A.M. Validating the in vitro antimicrobial activity of Artemisia afra in polyherbal combinations to treat respiratory infections. S. Afr. J. Bot. 2010, 76, 655–661. [Google Scholar] [CrossRef]
- More, G.; Lall, N.; Husein, A.; Tshikalange, T.E. Antimicrobial constituents of Artemisia afra jacq. ex willd. against periodontal pathogens. Evid. Based Complement. Altern. Med. 2012, 2012, 252758. [Google Scholar] [CrossRef] [PubMed]
- Venables, L.; Koekemoer, T.C.; Van de Venter, M.; Goosen, E.D. Isoalantolactone, a sesquiterpene lactone from Artemisia afra Jacq. Ex Willd and its in vitro mechanism of induced cell death in HeLa cells. S. Afr. J. Bot. 2016, 103, 216–221. [Google Scholar] [CrossRef]
- Appalasamy, S.; Lo, K.Y.; Ch’ng, S.J.; Nornadia, K.; Othman, A.S.; Chan, L.K. Antimicrobial activity of artemisinin and precursor derived from in vitro plantlets of Artemisia annua L. BioMed Res. Int. 2014, 2014, 215872. [Google Scholar] [CrossRef] [PubMed]
- Moyo, P.; Kunyane, P.; Selepe, M.A.; Eloff, J.N.; Niemand, J.; Louw, A.I.; Maharaj, V.J.; Birkholtz, L.M. Bioassay-guided isolation and identification of gametocytocidal compounds from Artemisia afra (Asteraceae). Malar. J. 2019, 18, 65. [Google Scholar] [CrossRef]
- Oladoye, S.O.; Ayodele, E.T.; Abdul-Hammed, M.; Idowu, O.T. Characterisation and Identification of Taraxerol and Taraxer-14-en-3-one from Jatropha tanjorensis (Ellis and Saroja) Leaves. Pak. J. Sci. Ind. Res. A Phys. Sci. 2015, 58, 46–50. [Google Scholar] [CrossRef]
- Abouelela, M.E.; Orabi, M.A.A.; Abdelhamid, R.A.; Abdelkader, M.S.A.; Darwish, M.M.F. Chemical and cytotoxic investigation of non-polar extract from Ceiba pentandra (L.) Gaertn: A study supported by computer-based screening. J. Appl. Pharm. Sci. 2018, 8, 057–064. [Google Scholar]
- Rehan, M. Cytotoxicity of oleanane type triterpene from leaf extract of Pterospermum acerifolium (in vitro) and theoretical investigation of the inhibitory signaling pathway. Chin. Herb. Med. 2021, 13, 124–130. [Google Scholar] [CrossRef]
- Ahmad, V.U. Handbook of Natural Products Data: Pentacyclic Triterpenoids; Elsevier: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Singh, N.K.; Singh, V.P. Isolation, characterization and antioxidant activity of dodecyl-p-coumarate from Ipomoea sepiaria. J. Chem. Pharm. Res. 2014, 6, 564–569. [Google Scholar]
- Sajjadi, S.; Shokoohinia, Y.; Moayedi, N. Isolation and identification of ferulic acid from aerial parts of Kelussia odoraatissima mozaff. Jundishapur J. Nat. Pharm. Prod. 2012, 7, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Napiroon, T.; Bacher, M.; Balslev, H.; Tawaitakham, K.; Santimaleeworagun, W.; Vajrodaya, S. Scopoletin from Lasianthus lucidus Blume (Rubiaceae): A potential antimicrobial against multidrug-resistant Pseudomonas aeruginosa. J. Appl. Pharm. Sci. 2018, 8, 001–006. [Google Scholar]
- Gakuba, E. Isolation and Characterization of Secondary Metabolites of Two Asteraceae Species, Artemisia afra, and Elytropappus rhinocerotis. Master’s Dissertation, University of KwaZulu-Natal, Durban, South Africa, 2009. [Google Scholar]
- Khan, N.M.U.; Hossain, M.S. Scopoletin and β-sitosterol glucopyranoside from roots of Ipomoea digitata. J. Pharmacogn. Phytochem. 2015, 4, 5–7. [Google Scholar]
- Swift, L.J. Isolation of β-sitosteryl-D-glucopyranoside from the juice of Florida Valencia oranges (Citrus sinensis, L.). J. Am. Chem. Soc. 1952, 74, 1099–1100. [Google Scholar] [CrossRef]
- Backhouse, N.; Delporte, C.; Negrete, R.; Suárez, S.; Cassels, B.K.; Breitmaier, E.; Schneider, C. Anti-inflammatory and antipyretic metabolites of Acaena splendens. Pharm. Biol. 1997, 35, 49–54. [Google Scholar]
- Wan, C.; Li, S.; Liu, L.; Chen, C.; Fan, S. Caffeoylquinic Acids from the Aerial Parts of Chrysanthemum coronarium L. Plants 2017, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Ko, H.J.; Chowdhury, M.A.; Chang, Y.S.; Woo, E.R. Chemical constituents on the aerial parts of Artemisia selengensis and their IL-6 inhibitory activity. Arch. Pharm. Res. 2015, 38, 1059–1065. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Masoko, P.; Picard, J.; Eloff, J.N. Antifungal activities of six South African Terminalia species (Combretaceae). J. Ethnopharmacol. 2005, 99, 301–308. [Google Scholar] [CrossRef]
- Eloff, J.N. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998, 64, 711–713. [Google Scholar] [CrossRef] [PubMed]
- Sartoratto, A.; Machado, A.L.M.; Delarmelina, C.; Figueira, G.M.; Duarte, M.C.T.; Rehder, V.L.G. Composition and antimicrobial activity of essential oils from aromatic plants used in Brazil. Braz. J. Microbiol. 2004, 35, 275–280. [Google Scholar] [CrossRef]
- Boik, J. Natural Compounds in Cancer Therapy, 1st ed.; Silvine, F., Ed.; Oregon Medical Press: Princeton, NJ, USA, 2001. [Google Scholar]
- Elisha, I.L.; Botha, F.S.; McGaw, L.J.; Eloff, J.N. The antibacterial activity of extracts of nine plant species with good activity against Escherichia coli against five other bacteria and cytotoxicity of extracts. BMC Complement Altern. Med. 2017, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Naika, H.R.; Krishna, V.; Harish, B.G.; Khadeer Ahamed, B.M.; Mahadevan, K.M. Antimicrobial Activity of Bioactive Constituents Isolated from the Leaves of Naravelia zeylanica (L.) DC. Int. J. Res. Pharm. Biomed. Sci. 2007, 1, 153–159. [Google Scholar]
- Koay, Y.C.; Wong, K.C.; Hasnah, O.; Ibrahim, E.M.S.; Mohammad, Z.A. Chemical constituents and biological activities of Strobilanthes crispus L. Rec. Nat. Prod. 2013, 7, 59–64. [Google Scholar]
- Duarte, M.C.T.; Figueira, G.M.; Sartoratto, A.; Rehder, V.L.G.; Delarmelina, C. Anti-Candida activity of Brazilian medicinal plants. J. Ethnopharmacol. 2005, 97, 305–311. [Google Scholar] [CrossRef] [PubMed]
- ur Rahman, U.; Durrani, S.; Ubaidullah, S.A.; ur Rahman, S. Anti-pyretic activity of taraxerol acetate. KJMS 2016, 9, 165. [Google Scholar]
- Aguilar-Guadarrama, B.; Navarro, V.; León-Rivera, I.; Rios, M.Y. Active Compounds against Tinea Pedis Dermatophytes from Ageratina pichinchensis var. bustamenta. Nat. Prod. Res. 2009, 23, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Mus, A.A.; Goh, L.P.W.; Marbawi, H.; Gansau, J.A. The Biosynthesis and Medicinal Properties of Taraxerol. Biomedicines 2022, 10, 807. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Sahu, P.M.; Sharma, M.K. Anti-inflammatory and antimicrobial activities of triterpenoids from Strobilanthes callosus Nees. Phytomedicine 2002, 9, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Csupor-Löffler, B.; Hajdú, Z.; Zupkó, I.; Molnár, J.; Forgo, P.; Vasas, A.; Kele, Z.; Hohmann, J. Antiproliferative Constituents of the Roots of Conyza canadensis. Planta Med. 2011, 77, 1183–1188. [Google Scholar] [CrossRef]
- Rehman, U.U.; Shah, J.; Khan, M.A.; Shah, M.R.; Khan, I. Molecular docking of taraxerol acetate as a new COX inhibitor. Bangladesh J. Pharmacol. 2013, 8, 194–197. [Google Scholar] [CrossRef][Green Version]
- Vo, T.K.; Ta, Q.T.H.; Chu, Q.T.; Nguyen, T.T.; Vo, V.G. Anti-Hepatocellular-Cancer Activity Exerted by β-Sitosterol and β-Sitosterol-Glucopyranoside from Indigofera zollingeriana Miq. Molecules 2020, 25, 3021. [Google Scholar] [CrossRef]
- Midori, T.; Takao, K.; Harukuni, T.; Kazuo, M.; Yoko, A.; Kenji, S.; Hiroyuki, A. Anti-carcinogenic activity of Taraxacum plant. II. Biol. Pharm. Bull. 1999, 22, 606–610. [Google Scholar]
- Kuljanabhagavad, T.; Suttisri, R.; Pengsuparp, T.; Ruangrungsi, N. Chemical structure and antiviral activity of aerial part from laggera pterodonta. J. Health Res. 2009, 23, 175–177. [Google Scholar]
- Lewis, D.A.; Hanson, D. Anti-ulcer drugs of plant origin. In Progress in Medicinal Chemistry; Ellis, G.P., West, G.B., Eds.; Elsevier Science Publishers B.V.: Amsterdam, The Netherlands, 1991; Volume 28, pp. 201–231. [Google Scholar]
- Navarrete, A.; Trejo-Miranda, J.L.; Reyes-Trejo, L. Principles of root bark of Hippocratea excelsa (Hippocrataceae) with gastroprotective activity. J. Ethnopharmacol. 2002, 79, 383–388. [Google Scholar] [CrossRef]
- Nandi, D.; Besra, S.E.; Vedasiromoni, J.R.; Giri, V.S.; Rana, P.; Jaisankar, P. Anti-leukemic activity of Wattakaka volubilis leaf extract against human myeloid leukemia cell lines. J. Ethnopharmacol. 2012, 144, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Thaeder, C.; Stanek, J.; Couvreur, J.; Borrego, C.; Brunissen, F.; Allais, F.; Flourat, A.L.; Cordelier, S. Chemo-Enzymatic Synthesis and Biological Assessment of p-Coumarate Fatty Esters: New Antifungal Agents for Potential Plant Protection. Molecules 2023, 28, 5803. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.P.; Yepes, L.M.; Pérez-Castillo, Y.; Robledo, S.M.; de Sousa, D.P. Alkyl and aryl derivatives based on p-coumaric acid modification and inhibitory action against Leishmania braziliensis and Plasmodium falciparum. Molecules 2020, 25, 3178. [Google Scholar] [CrossRef]
- Bodede, O.; Shaik, S.; Singh, M.; Moodley, R. Phytochemical analysis with antioxidant and cytotoxicity studies of the bioactive principles from Zanthoxylum capense (Small Knobwood). Anti-Cancer Agents Med. Chem. 2017, 17, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.; Kwok, K. Ferulic acid: Pharmaceutical functions, preparation, and applications in foods. J. Sci. Food Agric. 2004, 84, 1261–1269. [Google Scholar] [CrossRef]
- Mancuso, C.; Santangelo, R. Ferulic acid: Pharmacological and toxicological aspects. Food Chem. Toxicol. 2014, 65, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Wijayanti, E.D.; Safitri, A.; Siswanto, D.; Triprisila, L.F.; Fatchiyah, F. Antimicrobial activity of ferulic acid in Indonesian purple rice through toll-like receptor signaling. Makara J. Sci. 2021, 25, 7. [Google Scholar]
- Singh Tuli, H.; Kumar, A.; Ramniwas, S.; Coudhary, R.; Aggarwal, D.; Kumar, M.; Sharma, U.; Chaturvedi Parashar, N.; Haque, S.; Sak, K. Ferulic Acid: A Natural Phenol That Inhibits Neoplastic Events through Modulation of Oncogenic Signaling. Molecules 2022, 27, 7653. [Google Scholar] [CrossRef]
- Tal, B.; Robeson, D.J. The induction, by fungal inoculation of ayapin and scopoletin biosynthesis in Helianthus annuus. Phytochemistry 1986, 25, 77–79. [Google Scholar] [CrossRef]
- Ojewole, J.A.O.; Adesina, S.K. Cardiovascular and neuromuscular actions of scopoletin from Tetrapleura tetraptera. Planta Med. 1983, 49, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Dai, Y.; Wang, Q.; Liang, H. Determination of scopoletin in rat plasma by high-performance liquid chromatography method with UV detection and its application to a pharmacokinetic study. J. Chromatogr. A 2007, 857, 332–336. [Google Scholar]
- Gnonlonfin, G.J.B.; Sanni, A.; Brimer, L. Review scopoletin—A coumarin phytoalexin with medicinal properties. Crit. Rev. Plant Sci. 2012, 31, 47–56. [Google Scholar] [CrossRef]
- Buathong, R.; Chamchumroon, V.; Schinnerl, J.; Bacher, M.; Santimaleeworagun, W.; Kraichak, E.; Vajrodaya, S. Chemovariation and antibacterial activity of extracts and isolated compounds from species of Ixora and Greenea (Ixoroideae, Rubiaceae). PeerJ 2019, 7, e6893. [Google Scholar] [CrossRef] [PubMed]
- Antika, L.; Tasfiyati, A.; Hikmat, H.; Septama, A. Scopoletin: A review of its source, biosynthesis, methods of extraction, and pharmacological activities. Z. Naturforsch. C 2022, 77, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Mogana, R.; Teng-Jin, K.; Wiart, C. Anti-inflammatory, anticholinesterase, and antioxidant Potential of scopoletin isolated from Canarium patentinervium Miq. (Burseraceae Kunth). Evid. Based Complement. Altern. Med. 2013, 2013, 734824. [Google Scholar] [CrossRef]
- Subramaniam, S.; Keerthiraja, M.; Sivasubramanian, A. Synergistic antibacterial action of β-sitosterol-D-glucopyranoside isolated from Desmostachya bipinnata leaves with antibiotics against common human pathogens. Rev. Bras. Farmacogn. 2014, 24, 44–50. [Google Scholar] [CrossRef]
- NCCLS. 2007. Available online: http://www.nccls.org (accessed on 15 March 2022).
- Njinga, N.S.; Sule, M.I.; Pateh, U.U.; Hassan, H.S.; Abdullahi, S.T.; Ache, R.N. Isolation and antimicrobial activity of β-sitosterol-3-O-glucopyranoside from Lannea kerstingii engl. & K. Krause (Anacardiacea). J. Health Allied Sci. NU 2016, 6, 4–8. [Google Scholar]
- Yuan, K.; Zhu, J.; Si, J.; Cai, H.; Ding, X.; Pan, Y. Studies on chemical constituents and antibacterial activity from n-butanol extract of Sarcandra glabra. Zhongguo Zhong Yao Za Zhi 2008, 33, 1843–1846. [Google Scholar] [PubMed]
- McGaw, L.J.; Van Der Merwe, D.; Eloff, J.N. In vitro anthelmintic, antibacterial, and cytotoxic effects of extracts from plants used in South African ethnoveterinary medicine. Vet. J. 2007, 173, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]

| Crude Extracts | E. coli | E. faecalis | P. aeruginosa | S. aureus | S. Typhimurium | C. albicans | LC50 |
|---|---|---|---|---|---|---|---|
| HEX | >2.50 (<0.152) | 0.156 (2.42) | 1.25 (0.304) | >2.50 (<0.152) | >2.50 (<0.152) | 0.625 (0.61) | 0.38 |
| EA & DCM | 0.078 (0.641) | 0.156 (0.321) | >2.5 (<0.02) | 1.25 (0.04) | 0.625 (0.08) | 0.625 (0.08) | 0.05 |
| ETOH | 0.625 (0.192) | 0.156 (0.77) | 2.5 (0.048) | 0.625 (0.192) | 0.625 (0.192) | 1.25 (0.096) | 0.12 |
| Gentamicin | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | - | - |
| Amphotericin-B | - | - | - | - | - | 0.02 | - |
| Doxorubicin | - | - | - | - | - | - | 0.01 |
| Isolated Compounds | E. coli | E. faecalis | P. aeruginosa | S. aureus | S. Typhimurium | C. albicans | LC50 |
|---|---|---|---|---|---|---|---|
| 3β-Taraxerol | 250 (>0.8) | >250 (<0.8) | >250 (<0.8) | 250 (>0.8) | 250 (>0.8) | 250 (>0.8) | >200 |
| Dodecyl-p-coumarate | >250 (<0.8) | 62.5 (>3.2) | >250 (<0.8) | >250 (<0.8) | >250 (<0.8) | 250 (>0.8) | >200 |
| Ferulic acid | >250 (<0.8) | >250 (<0.8) | >250 (<0.8) | 250 (>0.8) | >250 (<0.8) | 250 (>0.8) | >200 |
| Scopoletin | 62.5 (>3.2) | 250 (0.8) | >250 (<0.8) | 250 (>0.8) | 125 (>1.6) | 250 (>0.8) | >200 |
| 3,5-Di-O-feruloylquinic acid | >250 (>0.8) | >250 (>0.8) | >250 (>0.8) | >250 (>0.8) | >250 (>0.8) | >250 (>0.8) | >200 |
| Sitosterol-3-O-β-D-glucopyranoside | >250 (<0.8) | 31.25 (>6.4) | >250 (<0.8) | >250 (<0.8) | >250 (<0.8) | 250 (>0.8) | >200 |
| Isofraxidin-7-O-β-D-glucopyranoside | 250 (>0.8) | >250 (<0.8) | >250 (<0.8) | 250 (0.8) | 250 (>0.8) | 250 (>0.8) | >200 |
| Gentamicin | 3.9 | 3.9 | 3.9 | 3.9 | 3.9 | - | - |
| Amphotericin-B | - | - | - | - | - | 3.9 | - |
| Doxorubicin | - | - | - | - | - | - | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molokoane, T.L.; Kemboi, D.; Siwe-Noundou, X.; Famuyide, I.M.; McGaw, L.J.; Tembu, V.J. Extractives from Artemisia afra with Anti-Bacterial and Anti-Fungal Properties. Plants 2023, 12, 3369. https://doi.org/10.3390/plants12193369
Molokoane TL, Kemboi D, Siwe-Noundou X, Famuyide IM, McGaw LJ, Tembu VJ. Extractives from Artemisia afra with Anti-Bacterial and Anti-Fungal Properties. Plants. 2023; 12(19):3369. https://doi.org/10.3390/plants12193369
Chicago/Turabian StyleMolokoane, Tumelo L., Douglas Kemboi, Xavier Siwe-Noundou, Ibukun M. Famuyide, Lyndy J. McGaw, and Vuyelwa J. Tembu. 2023. "Extractives from Artemisia afra with Anti-Bacterial and Anti-Fungal Properties" Plants 12, no. 19: 3369. https://doi.org/10.3390/plants12193369
APA StyleMolokoane, T. L., Kemboi, D., Siwe-Noundou, X., Famuyide, I. M., McGaw, L. J., & Tembu, V. J. (2023). Extractives from Artemisia afra with Anti-Bacterial and Anti-Fungal Properties. Plants, 12(19), 3369. https://doi.org/10.3390/plants12193369