Comparative Study on the Phytochemical Characterization and Biological Activities of Azolla caroliniana and Azolla filiculoides: In Vitro Study
Abstract
1. Introduction
2. Results
2.1. Phytochemical Screening
2.2. Antimicrobial Activity
2.3. Ferric-Reducing Antioxidant Power
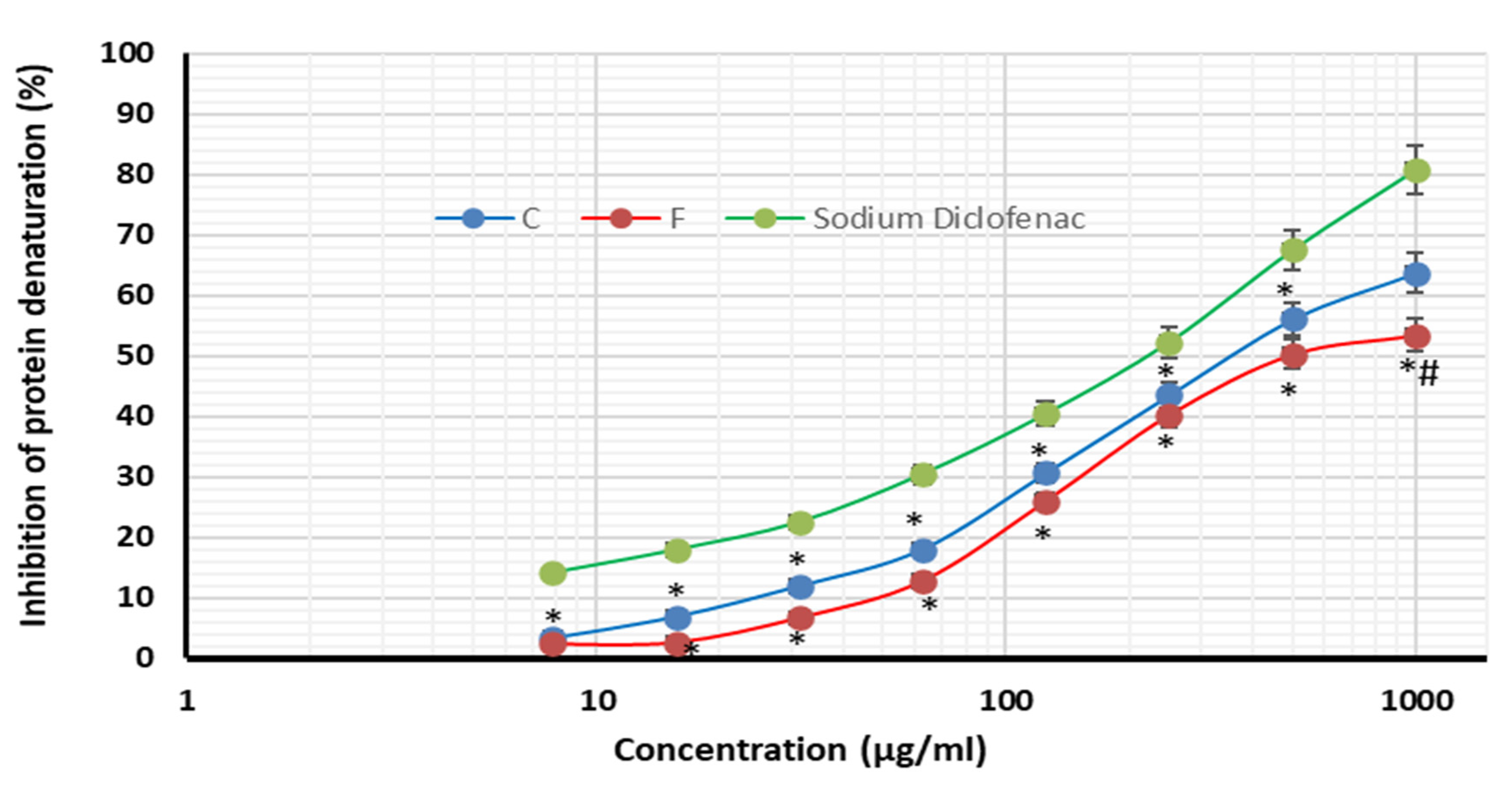
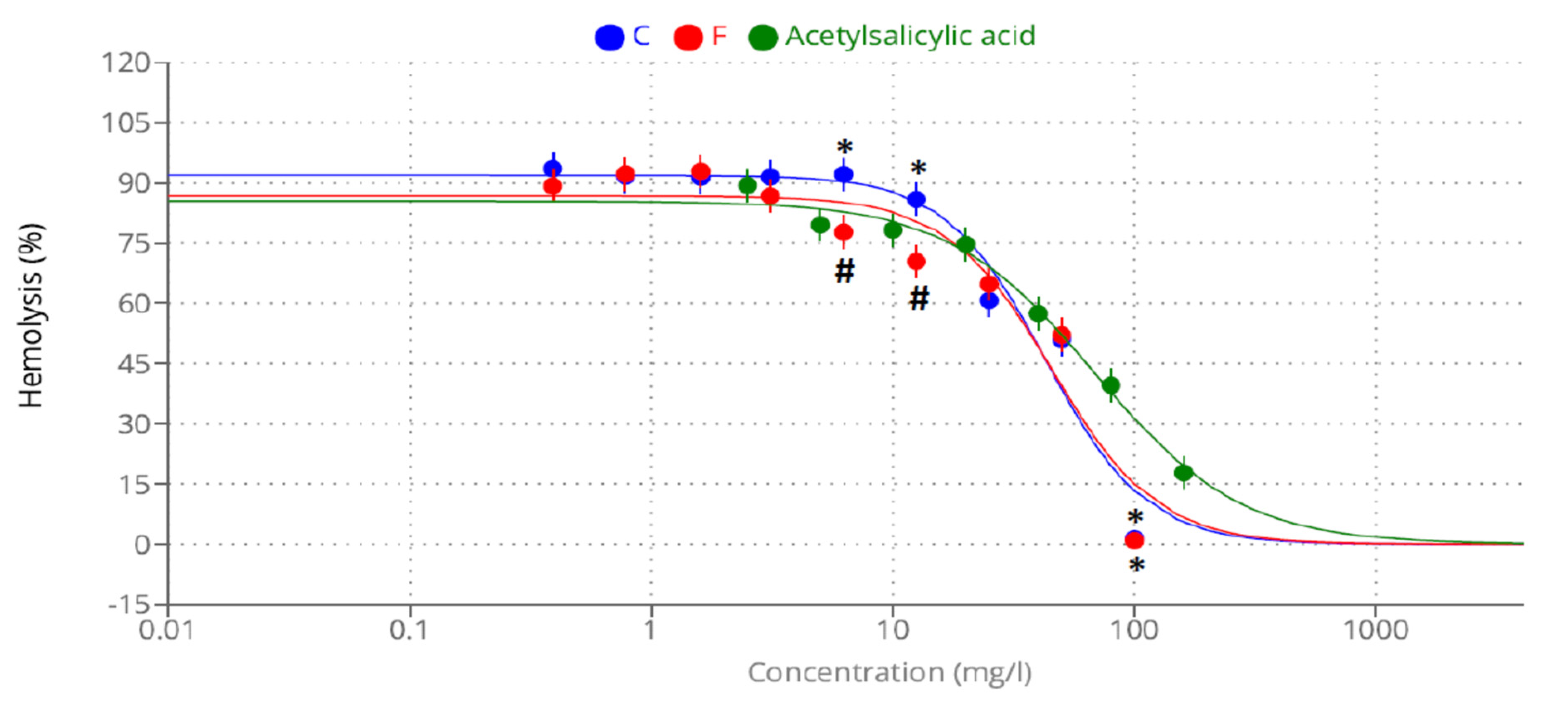
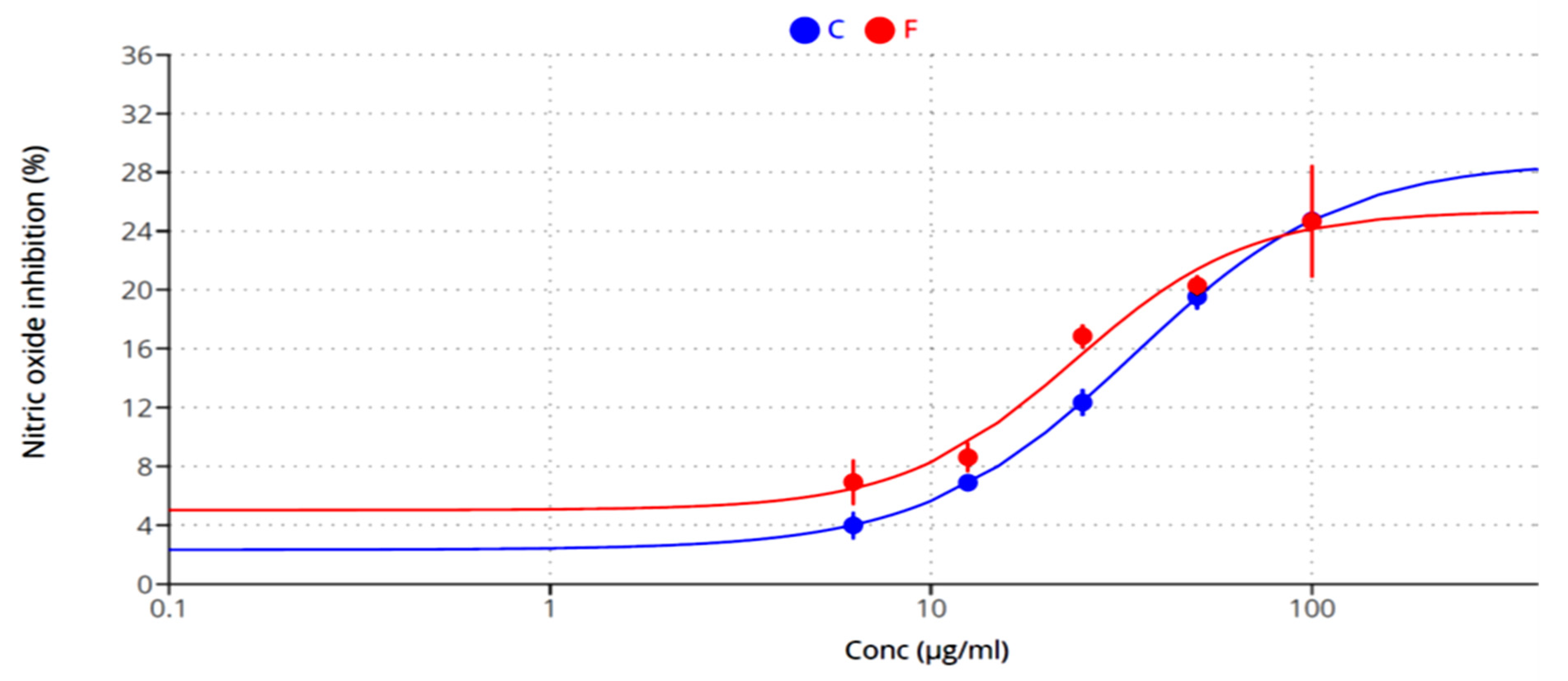
2.4. Anti-Inflammatory Activity
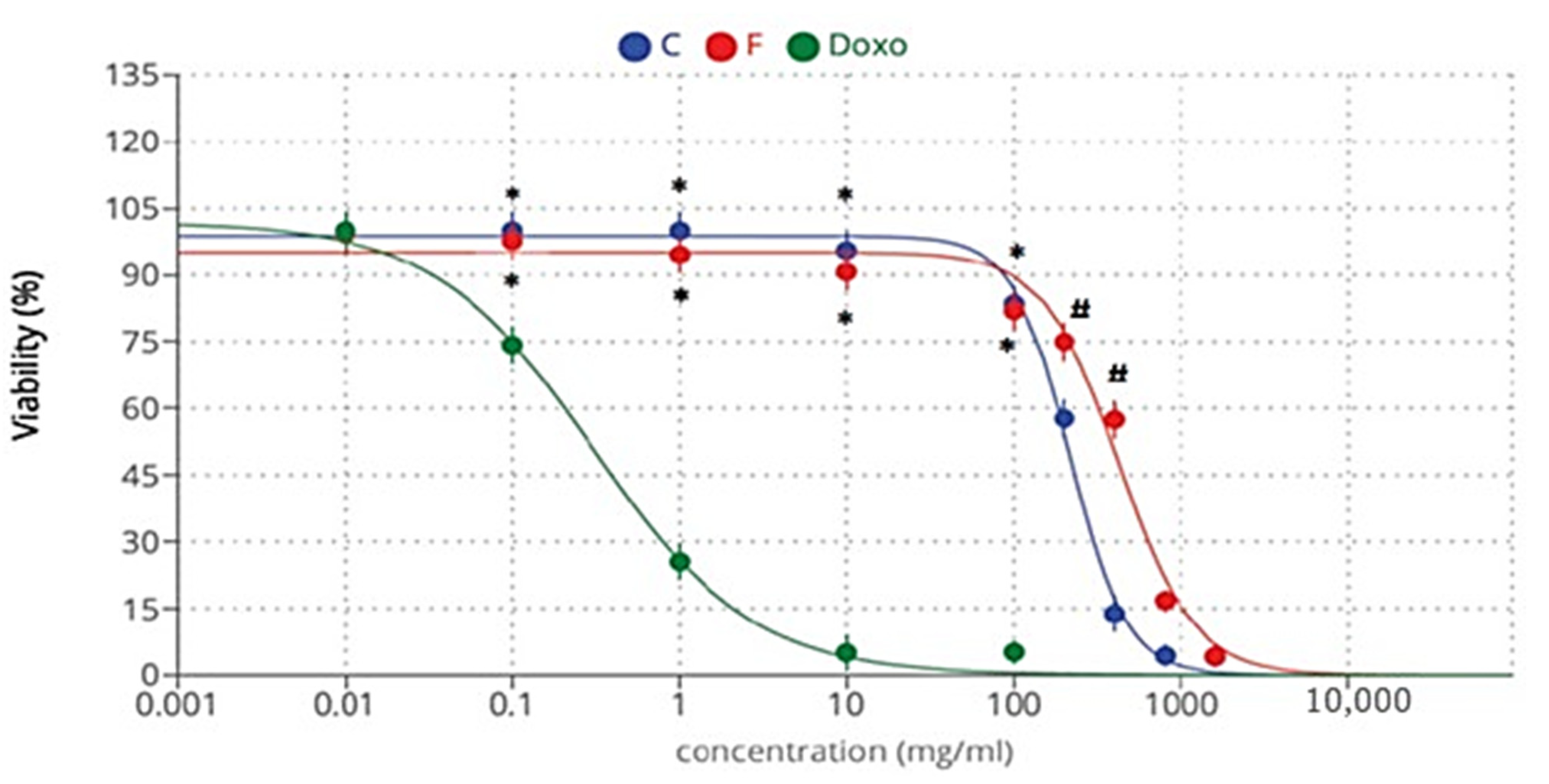
2.5. Cytotoxicity Activity
3. Discussion
4. Materials and Methods
4.1. Chemicals and Supplies
4.2. Plant Material and Growth Conditions
4.3. Phytochemical Screening
4.3.1. Extraction
4.3.2. Gas Chromatography–Mass Spectrometry Analysis (GC-MS)
4.4. Antimicrobial Activity
4.4.1. Preparation of Fungal Inocula
4.4.2. Preparation of Bacterial Inocula
4.5. Antioxidant Activity Using Ferric-Reducing Antioxidant Power (FRAP) Assay
4.6. Anti-Inflammatory Activity
4.6.1. Inhibition of Protein Denaturation
4.6.2. Membrane Stabilization against Heat-Induced Hemolysis
4.6.3. Inhibition of Nitric Oxide Production in the RAW 264.7 Macrophage Cell Line
4.7. Cytotoxicity Activity against the HepG2 Cell Line
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Mosha, S. A review on significance of Azolla meal as a protein plant source in finfish culture. J. Aquac. Res. Dev. 2018, 9, 1000544. [Google Scholar] [CrossRef]
- Yang, G.; Ji, H.; Sheng, J.; Zhang, Y.; Feng, Y.; Guo, Z.; Chen, L. Combining Azolla and urease inhibitor to reduce ammonia volatilization and increase nitrogen use efficiency and grain yield of rice. Sci. Total Environ. 2020, 743, 140799. [Google Scholar] [CrossRef]
- Lumpkin, T.A.; Plucknett, D.L. Azolla: Botany, physiology, and use as a green manure. Econ. Bot. 1980, 34, 111–153. [Google Scholar] [CrossRef]
- Watanabe, I.; Hove, C.V. Phylogenetic, molecular and breeding aspects of Azolla-Anabaena symbiosis. In Pteridology in Perspective; Camus, J.M., Gibby, M., Johns, R.J., Eds.; Royal Botanic Gardens.: Kew, UK, 1996; pp. 611–619. [Google Scholar]
- Sudaryono, A. Use of Azolla (Azolla pinnata) meal as a substitute for defatted soybean meal in diets of juvenile black tiger shrimp (Penaeus monodon). J. Coast. Dev. 2006, 9, 145–154. [Google Scholar]
- Katayama, N.; Yamashita, M.; Kishida, Y.; Liu, C.-C.; Watanabe, I.; Wada, H.; Force, S.A.T. Azolla as a component of the space diet during habitation on Mars. Acta Astronaut. 2008, 63, 1093–1099. [Google Scholar] [CrossRef]
- Al-Mayah, A.; Al-Saadi, S.; Abdulla, J. A new generic record (Azolla, Salviniaceae) to the Aquatic Pteridoflora of Iraq. Int. J. Appl. Rese 2016, 16, 21–23. [Google Scholar]
- Wen, J.; ICKERT-BOND, S.M. Evolution of the Madrean–Tethyan disjunctions and the North and South American amphitropical disjunctions in plants. J. Syst. Evol. 2009, 47, 331–348. [Google Scholar] [CrossRef]
- Elrasoul, A.S.A.; Mousa, A.A.; Orabi, S.H.; Mohamed, M.A.E.-G.; Gad-Allah, S.M.; Almeer, R.; Abdel-Daim, M.M.; Khalifa, S.A.; El-Seedi, H.R.; Eldaim, M.A.A. Antioxidant, anti-inflammatory, and anti-apoptotic effects of Azolla pinnata ethanolic extract against lead-induced hepatotoxicity in rats. Antioxidants 2020, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, R.; Zarkami, R.; Sabetraftar, K.; Van Damme, P. A review of some ecological factors affecting the growth of Azolla spp. CJES 2013, 11, 65–76. Available online: http://research.guilan.ac.ir/cjes (accessed on 20 October 2013).
- Nayak, N.; Padhy, R.N.; Singh, P.K. Evaluation of antibacterial and antioxidant efficacy of the fern Azolla caroliniana symbiotic with the cyanobacterium Anabaena azollae. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2015, 85, 555–569. [Google Scholar] [CrossRef]
- Sathammaipriya, N.; Thamilmaraiselvi, B.; Steffi, P.; Sangeetha, K. Investigation of phytochemical constituents in Azolla microphylla for antibacterial activity. Natl. J. Physiol. Pharm. Pharmacol. 2018, 8, 1500–1504. [Google Scholar] [CrossRef]
- Kadhim, W.; Kadhim, M.; Hameed, I. Antibacterial activity of several plant extracts against Proteus species. Int. J. Pharm. Clin. Res. 2017, 8, 88–94. Available online: http://bjas.bu.edu.eg (accessed on 7 February 2017).
- Chandrasekaran, M.; Kannathasan, K.; Venkatesalu, V. Antimicrobial activity of fatty acid methyl esters of some members of Chenopodiaceae. Z. Naturforsch. 2008, 63, 331–336. [Google Scholar] [CrossRef]
- Badoni, R.; Semwal, D.; Rawat, U. Fatty acid composition and antimicrobial activity of Celtis australis L. fruits. J. Sci. Res. 2010, 2, 397–402. [Google Scholar] [CrossRef]
- Giri, G.F.; Danielli, M.; Marinelli, R.A.; Spanevello, R.A. Cytotoxic effect of levoglucosenone and related derivatives against human hepatocarcinoma cell lines. Bioorg Med. Chem. Lett. 2016, 26, 3955–3957. [Google Scholar] [CrossRef] [PubMed]
- Amado Diago, C.A.; García-Unzueta, M.T.; Fariñas Mdel, C.; Amado, J.A. Calcitriol-modulated human antibiotics: New pathophysiological aspects of vitamin D. Endocrinol. Y Nutr. 2016, 63, 87–94. [Google Scholar] [CrossRef]
- Nithya, T.; Jayanthi, J.; Raghunathan, M. Phytochemical, antibacterial and GC MS analysis of a floating fern Salvinia molesta DS Mitchell (1972). Int. J. Pharm. Technol. Res. 2015, 8, 85–90. [Google Scholar]
- Baluja, S.; Chanda, S.; Nandha, K. Antimicrobial activity of some pyrimidine derivatives in DMF and DMSO. Int. Lett. Chem. Phys. Astron. 2015, 56, 131–141. [Google Scholar] [CrossRef]
- Kumar, A.; Kumari, J.; Kumar, H.; Nath, A.; Singh, J.; Ali, M.; Kumar, R. Hepatoprotective and antioxidant effect of Azolla filiculoides on profenofos induced hepatotoxicity in swiss albino mice. Caribb. J. Sci. Technol. 2014, 2, 372–377. Available online: https://caribjscitech.com/index.php/cjst/article/view/123 (accessed on 22 December 2018).
- Lü, L.; Liu, S.W.; Jiang, S.B.; Wu, S.G. Tannin inhibits HIV-1 entry by targeting gp41. Acta Pharmacol. Sin. 2004, 25, 213–218. [Google Scholar]
- Shahidi, F. Nutraceuticals and functional foods: Whole versus processed foods. Trends Food Sci. Technol. 2009, 20, 376–387. [Google Scholar] [CrossRef]
- Chan, P.; Kao, P.F.; Tomlinson, B. Cardiovascular effects of trilinolein, a natural triglyceride isolated from the herb sanchi (Panax notoginseng). Acta Cardiol. Sin. 2005, 21, 71–76. [Google Scholar]
- Thavasi Alagan, V.; Nakulan Vatsala, R.; Sagadevan, I.; Subbiah, V.; Ragothaman, V. Effect of dietary supplementation of seaweed (Ulva lactuca) and Azolla on growth performance, haematological and serum biochemical parameters of Aseel chicken. BJBAS 2020, 9, 58. [Google Scholar] [CrossRef]
- Kösesakal, T.; Yıldız, M. Growth performance and biochemical profile of Azolla pinnata and Azolla caroliniana grown under greenhouse conditions. Arch. Biol. Sci. 2019, 71, 475–482. [Google Scholar] [CrossRef]
- Aron, A.T.; Gentry, E.C.; McPhail, K.L.; Nothias, L.F.; Nothias-Esposito, M.; Bouslimani, A.; Petras, D.; Gauglitz, J.M.; Sikora, N.; Vargas, F.; et al. Reproducible molecular networking of untargeted mass spectrometry data using GNPS. Nat. Protoc. 2020, 15, 1954–1991. [Google Scholar] [CrossRef] [PubMed]
- Oyedapo, O.O.; Akinpelu, B.A.; Akinwunmi, K.F.; Adeyinka, M.O.; Sipeolu, F.O. Red blood cell membrane stabilizing potentials of extracts of Lantana camara and its fractions. Int. J. Plant Physiol. Biochem. 2010, 2, 46–51. Available online: http://www.academicjournals.org/ij,ppb (accessed on 5 October 2010).
- Kadhim, M.J.; Al-Rubaye, A.F.; Hameed, I.H. Determination of bioactive compounds of methanolic extract of vitis vinifera using GC-MS. Int. J. Toxicol. Pharmacol. Res. 2017, 9, 113–126. [Google Scholar] [CrossRef]
- Peruzzu, D.; Dupuis, M.L.; Pierdominici, M.; Fecchi, K.; Gagliardi, M.C.; Ortona, E.; Pagano, M.T. Anti-Inflammatory Effects of 1,25(OH)2D/Calcitriol in T Cell Immunity: Does Sex Make a Difference? IJMS 2022, 23, 9164. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; Deng, J.S.; Lin, J.G.; Lee, C.Y.; Huang, G.J. Anti-inflammatory effects of trilinolein from Panax notoginseng through the suppression of NF-κB and MAPK expression and proinflammatory cytokine expression. Am. J. Chin. Med. 2014, 42, 1485–1506. [Google Scholar] [CrossRef]
- Guruvayoorappan, C.; Kuttan, G. Beta-carotene inhibits tumor-specific angiogenesis by altering the cytokine profile and inhibits the nuclear translocation of transcription factors in B16F-10 melanoma cells. Integr. Cancer Ther. 2007, 6, 258–270. [Google Scholar] [CrossRef]
- Saeed, N.M.; El-Demerdash, E.; Abdel-Rahman, H.M.; Algandaby, M.M.; Al-Abbasi, F.A.; Abdel-Naim, A.B. Anti-inflammatory activity of methyl palmitate and ethyl palmitate in different experimental rat models. Toxicol. Appl. Pharmacol. 2012, 264, 84–93. [Google Scholar] [CrossRef]
- Dąbrowski, G.; Konopka, I. Update on food sources and biological activity of odd-chain, branched and cyclic fatty acids–A review. Trends Food Sci. Technol. 2022, 119, 514–529. [Google Scholar] [CrossRef]
- Abdoul-Aziz, S.K.A.; Zhang, Y.; Wang, J. Milk Odd and Branched Chain Fatty Acids in Dairy Cows: A Review on Dietary Factors and Its Consequences on Human Health. Animal 2021, 11, 3210. [Google Scholar] [CrossRef] [PubMed]
- Venn-Watson, S.; Lumpkin, R.; Dennis, E.A. Efficacy of dietary odd-chain saturated fatty acid pentadecanoic acid parallels broad associated health benefits in humans: Could it be essential? Sci. Rep. 2020, 10, 8161. [Google Scholar] [CrossRef]
- Park, S.Y.; Seetharaman, R.; Ko, M.J.; Kim, D.Y.; Kim, T.H.; Yoon, M.K.; Kwak, J.H.; Lee, S.J.; Bae, Y.S.; Choi, Y.W. Ethyl linoleate from garlic attenuates lipopolysaccharide-induced pro-inflammatory cytokine production by inducing heme oxygenase-1 in RAW264.7 cells. Int. Immunopharmacol. 2014, 19, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.-C.; Shin, B.-H.; Hong, J.-H.; Lee, J.; Chee, H.-Y.; Song, K.-S.; Lee, K.-B. Isolation of fatty acids with anticancer activity from Protaetia brevitarsis larva. Arch. Pharm. Res. 2007, 30, 361–365. [Google Scholar] [CrossRef]
- Breeta, R.D.I.E.; Grace, V.M.B.; Wilson, D.D. Methyl Palmitate—A suitable adjuvant for Sorafenib therapy to reduce in vivo toxicity and to enhance anti-cancer effects on hepatocellular carcinoma cells. Basic Clin. Pharmacol. Toxicol. 2021, 128, 366–378. [Google Scholar] [CrossRef]
- Elangovan, H.; Chahal, S.; Gunton, J.E. Vitamin D in liver disease: Current evidence and potential directions. Biochim. Biophys. Acta. Mol. Basis Dis. 2017, 1863, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.; Agrawal, D.K. Regulation of TREM1-Mediated Inflammation in Hepatocellular Carcinoma Cells. Reports 2021, 4, 17. [Google Scholar] [CrossRef]
- Upadhyaya, K.R.; Radha, K.S.; Madhyastha, H.K. Cell cycle regulation and induction of apoptosis by beta-carotene in U937 and HL-60 leukemia cells. Int. J. Biochem. Mol. 2007, 40, 1009–1015. [Google Scholar] [CrossRef][Green Version]
- Meyer, F.; Bairati, I.; Jobin, E.; Gélinas, M.; Fortin, A.; Nabid, A.; Têtu, B. Acute adverse effects of radiation therapy and local recurrence in relation to dietary and plasma beta carotene and alpha tocopherol in head and neck cancer patients. Nutr. Cancer 2007, 59, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, R.V.; Peñarreta, J.; Quijano-Avilés, M.; Lucas, A.B.; Chóez-Guaranda, I.; Santana, P.M. Antioxidant activity and GC-MS profile of Conyza bonariensis L. leaves extract and fractions. Rev. Fac. Nac. Agron. Medellin 2020, 73, 9305–9313. [Google Scholar] [CrossRef]
- Hassan, A.; Mohamed, H.E.; Moustafa, E. Nutrient starvation enhances the phenolic compounds and antioxidant activity in Azolla caroliniana plant. Egypt. J. Bot. 2020, 60, 239–247. [Google Scholar] [CrossRef]
- Yao, J.; Moellering, R. Antimicrobial agents. Clin. Microbiol. 1995, 6, 1281–1307. [Google Scholar] [CrossRef]
- Mizushima, Y.; Kobayashi, M. Interaction of anti-inflammatory drugs with serum proteins, especially with some biologically active proteins. J. Pharm. Pharmacol. 1968, 20, 169–173. [Google Scholar] [CrossRef]
- Sakat, S.; Juvekar, A.R.; Gambhire, M.N. In vitro antioxidant and anti-inflammatory activity of methanol extract of Oxalis corniculata Linn. Int. J. Pharm. Pharm. Sci. 2010, 2, 146–155. [Google Scholar] [CrossRef]
- Yoo, M.S.; Shin, J.S.; Choi, H.E.; Cho, Y.W.; Bang, M.H.; Baek, N.I.; Lee, K.T. Fucosterol isolated from Undaria pinnatifida inhibits lipopolysaccharide-induced production of nitric oxide and pro-inflammatory cytokines via the inactivation of nuclear factor-κB and p38 mitogen-activated protein kinase in RAW264.7 macrophages. Food Chem. 2012, 135, 967–975. [Google Scholar] [CrossRef]
- Oliveira, T.; Figueiredo, C.A.; Brito, C.; Stavroullakis, A.; Prakki, A.; Da Silva Velozo, E.; Nogueira-Filho, G. Effect of Allium cepa L. on Lipopolysaccharide-Stimulated Osteoclast Precursor Cell Viability, Count, and Morphology Using 4′,6-Diamidino-2-phenylindole-Staining. Int. J. Cell Biol. 2014, 2014, 535789. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. JNCI 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Allam, R.M.; Al-Abd, A.M.; Khedr, A.; Sharaf, O.A.; Nofal, S.M.; Khalifa, A.E.; Mosli, H.A.; Abdel-Naim, A.B. Fingolimod interrupts the cross talk between estrogen metabolism and sphingolipid metabolism within prostate cancer cells. Toxicol. Lett. 2018, 291, 77–85. [Google Scholar] [CrossRef] [PubMed]
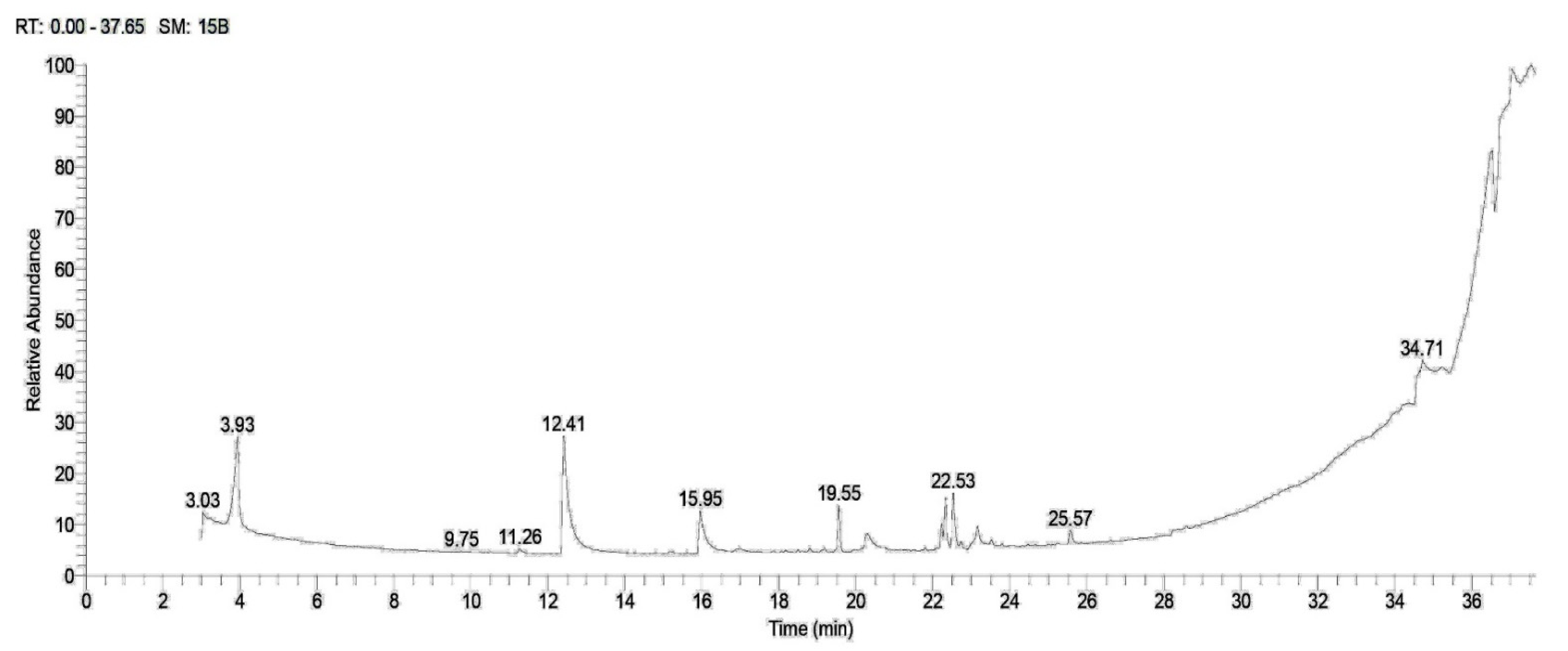
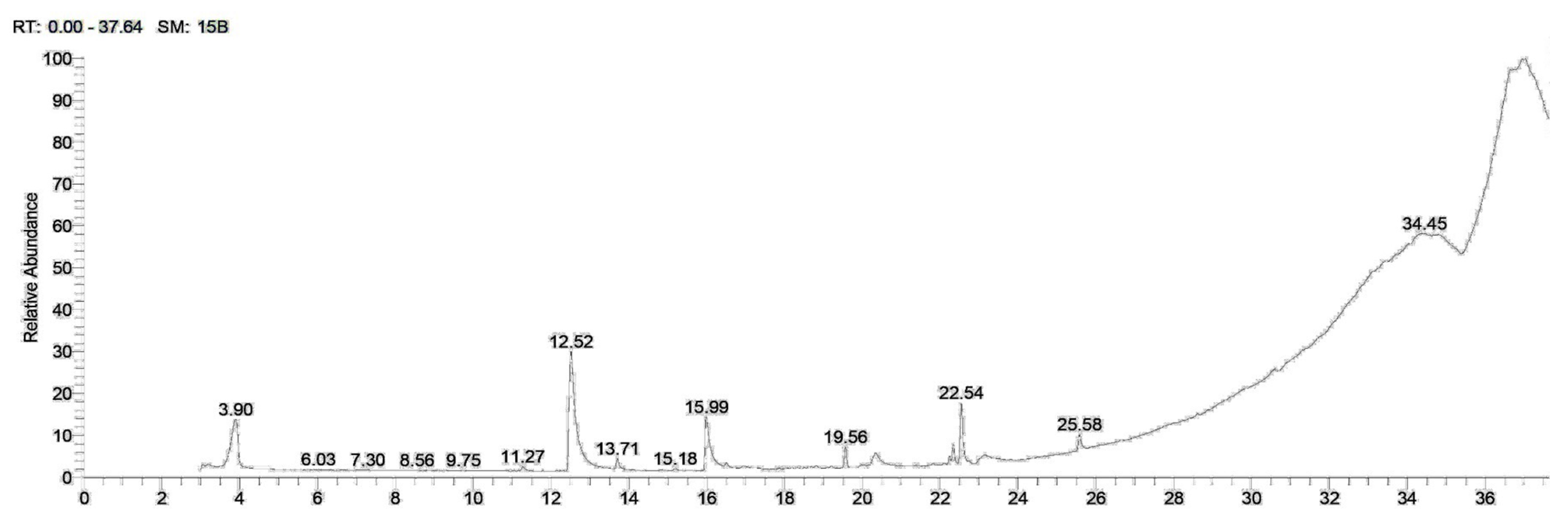


| Rt | Compound | M.F | M.W. | Peak Area (%) | |
|---|---|---|---|---|---|
| C | F | ||||
| 3.03 | 9-Octadecenoic acid, (2-phenyl-1,3-dioxolan-4-yl) methyl ester, cis- | C28H44O4 | 444.6 | 3.03 | 1.34 |
| 11.27 | Isochiapin B | C19H22O6 | 346 | - | 0.74 |
| 12.51 | Dimethyl lauramine | C14H31N | 213 | 13.11 | 23.89 |
| 15.94 | Dimethyl myristamine | C16H35N | 241 | 5.98 | 9.12 |
| 19.56 | Methyl palmitate | C17H34O2 | 270 | 4.57 | 3.14 |
| 20.33 | Pentadecanoic acid | C15H30O2 | 330 | 2.36 | 2. 90 |
| 22.22 | Ethyl linoleate | C20H36O2 | 308 | 2.26 | 1.14 |
| 22.33 | 9,12-Octadecadienoic acid (Z,Z)-, 2-hydroxy-1-(hydroxymethyl)ethyl ester | C21H38O4 | 354 | 5.16 | 3.02 |
| 22.54 | N-Methyl-N-benzyltetradecanamine | C22H39N | 317 | 5.85 | 9.78 |
| 22.73 | Cyclopropanebutanoic acid, 2-[[2-[[2-[(2-pentylcyclopropyl)meth yl]cyclopropyl]methyl]cyclopropyl] methyl]-, methyl ester | C25H42O2 | 374 | 0.53 | - |
| 23.11 | Tricyclo [20.8.0.0(7,16)]triacontane, 1(22),7(16)-diepoxy- | C30H52O2 | 444 | 2.93 | 2.07 |
| 25.57 | Calcitriol | C27H44O3 | 416 | 1.81 | 2.64 |
| 34.27 | Nicotiflorin | C27H30O15 | 594 | 2.92 | 1.41 |
| 34.65 | Trilinolein | C57H98O6 | 878 | 1.28 | 2.10 |
| 36.49 | Rhodopin | C40H58O | 554 | 10.65 | 12.05 |
| Reference Drug | C | F | |
|---|---|---|---|
| Fungi | Ketoconazole (100 μg/mL) | ||
| Fusarium oxysporum (RCMB 001004) | 19 ± 4.00 | NA | NA |
| Trichophyton rubrum (RCMB 025002) | 22 ± 2.31 | NA | NA |
| Candida albicans (RCMB 005003) | 20 ± 2.38 | NA | NA |
| Geotrichum candidum (RCMB 041001) | 26 ± 3.19 | 15 * ± 2.44 | 12 * ± 2.69 |
| Gram-positive bacteria: | Gentamycin (4 μg/mL) | ||
| Staphylococcus aureus ATCC 25923 | 24 ± 3.19 | NA | NA |
| Enterococcus faecalis ATCC 29212 | 26 ± 4.16 | 12 * ± 1.34 | 10 * ± 2.14 |
| Gram-negative bacteria: | Gentamycin (4 μg/mL) | ||
| Enterobacter cloacae ATCC 23355 | 30 ± 2.00 | NA | NA |
| Klebsiella pneumonia ATCC 13883 | 21 ± 1.96 | 11 * ± 1.36 | 13 * ± 2.91 |
| Test Organism | Minimum Inhibitory Concentration (MIC, μg/mL) | |
|---|---|---|
| C | F | |
| Fungi | ||
| Fusarium oxysporum (RCMB 001004) | NA | NA |
| Trichophyton rubrum (RCMB 025002) | NA | NA |
| Candida albicans (RCMB 005003) | NA | NA |
| Geotrichum candidum (RCMB 041001) | 312.5 a ± 7.5 | 625 b ± 4.1 |
| Gram-positive bacteria: | ||
| Staphylococcus aureus ATCC 25923 | NA | NA |
| Enterococcus faecalis ATCC 29212 | 625 a ± 3.2 | 1250 b ± 11.8 |
| Gram-negative bacteria: | ||
| Enterobacter cloacae ATCC 23355 | NA | NA |
| Klebsiella pneumonia ATCC 13883 | 1250 a ± 9.6 | 312.5 b ± 5.8 |
| Biological Activity | Reference Drug | A. caroliniana | A. filiculoides |
|---|---|---|---|
| FRAP | (Ascorbic acid) 416.21 | 55.86 | 92.78 |
| Inhibition of protein denaturation | (Sodium diclofenac) 218.6 | 360.0 | 527.3 |
| Inhibition of heat-induced hemolysis | (Acetyl salicylic acid) 68.25 | 42.78 | 45.85 |
| Inhibition of NO production | - | 33.92 | 23.8 |
| Cytotoxicity against the HepG2 cell line | (Doxorubicin) 0.303 | 216.86 | 421.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, S.M.A.; Kamel, M.A.; Ali, M.A.; Alotaibi, B.S.; Aharthy, O.M.; Shukry, M.; Abd El-Bary, H.M. Comparative Study on the Phytochemical Characterization and Biological Activities of Azolla caroliniana and Azolla filiculoides: In Vitro Study. Plants 2023, 12, 3229. https://doi.org/10.3390/plants12183229
Rahman SMA, Kamel MA, Ali MA, Alotaibi BS, Aharthy OM, Shukry M, Abd El-Bary HM. Comparative Study on the Phytochemical Characterization and Biological Activities of Azolla caroliniana and Azolla filiculoides: In Vitro Study. Plants. 2023; 12(18):3229. https://doi.org/10.3390/plants12183229
Chicago/Turabian StyleRahman, Salwa M. Abdel, Maher A. Kamel, Mennatallah A. Ali, Badriyah S. Alotaibi, Ohud Muslat Aharthy, Mustafa Shukry, and Hala Mohamed Abd El-Bary. 2023. "Comparative Study on the Phytochemical Characterization and Biological Activities of Azolla caroliniana and Azolla filiculoides: In Vitro Study" Plants 12, no. 18: 3229. https://doi.org/10.3390/plants12183229
APA StyleRahman, S. M. A., Kamel, M. A., Ali, M. A., Alotaibi, B. S., Aharthy, O. M., Shukry, M., & Abd El-Bary, H. M. (2023). Comparative Study on the Phytochemical Characterization and Biological Activities of Azolla caroliniana and Azolla filiculoides: In Vitro Study. Plants, 12(18), 3229. https://doi.org/10.3390/plants12183229







