Identification and Expression Analysis of EPSPS and BAR Families in Cotton
Abstract
:1. Introduction
2. Results
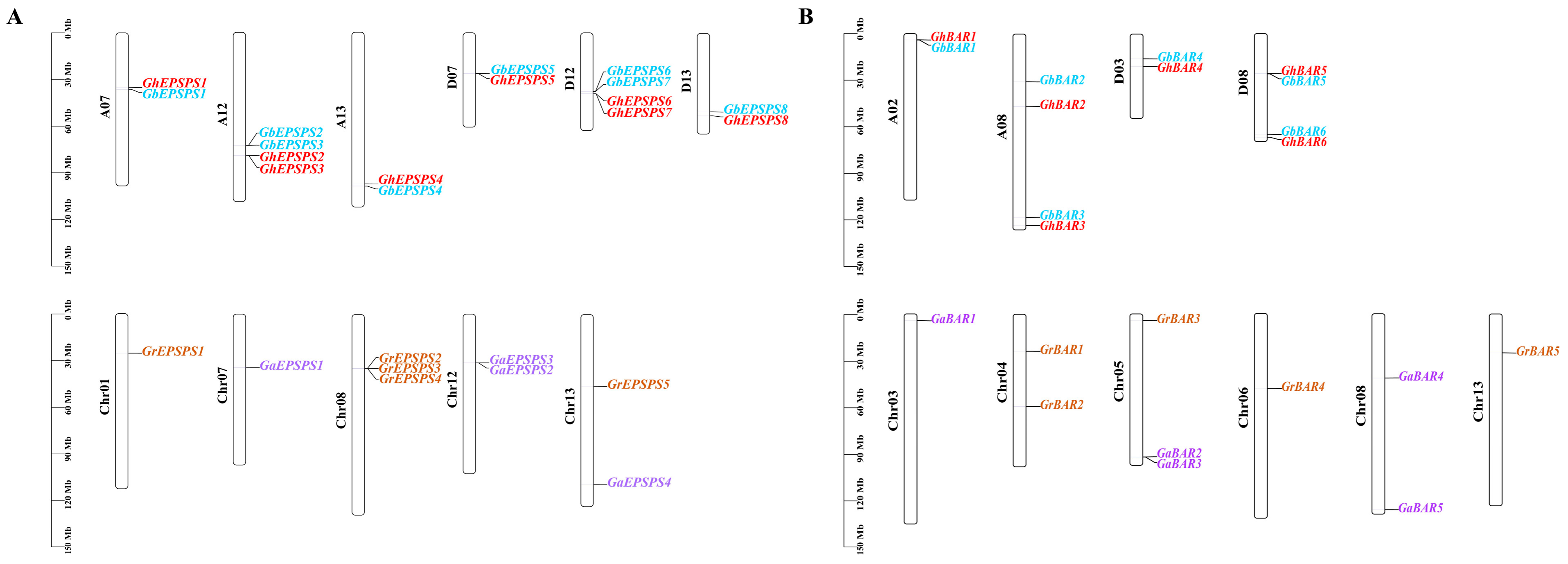
2.1. Identification of EPSPS and BAR Family Gene Members
2.2. Sequence Characterization and Protein Properties of EPSPS and BAR Family Members
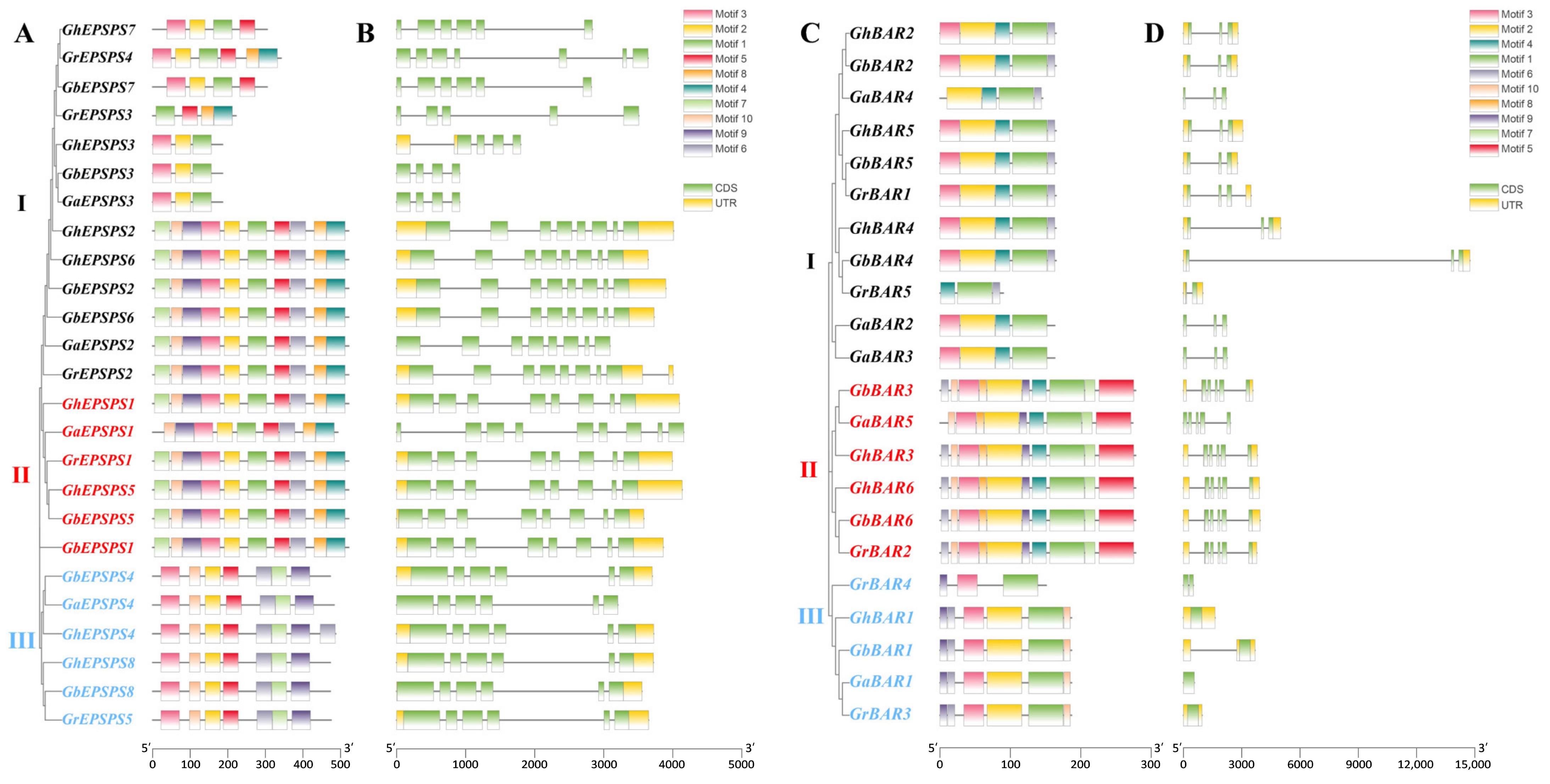
2.3. Analyses of Gene Structures and Protein Motifs of EPSPS and BAR Genes
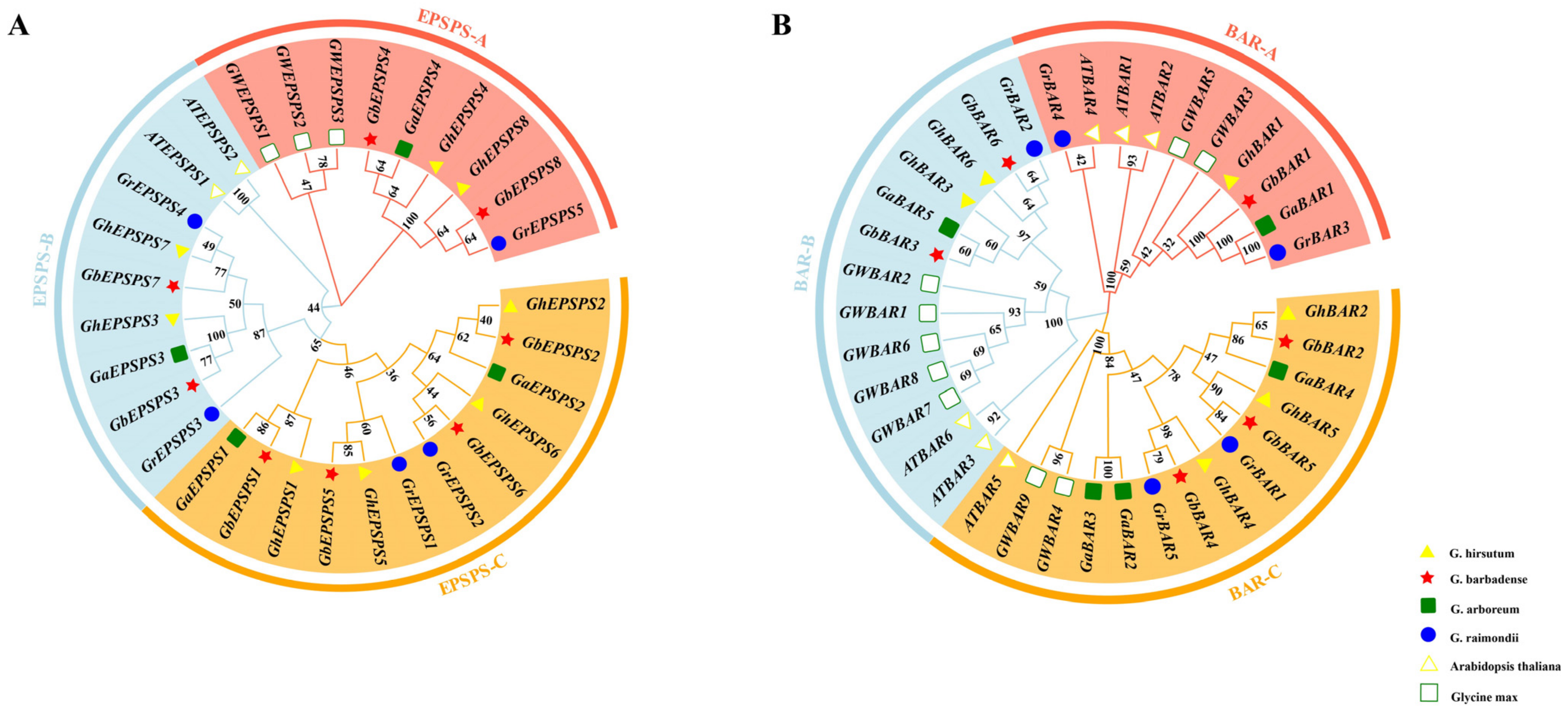
2.4. Phylogenetic Analysis of the EPSPS and BAR Gene Family
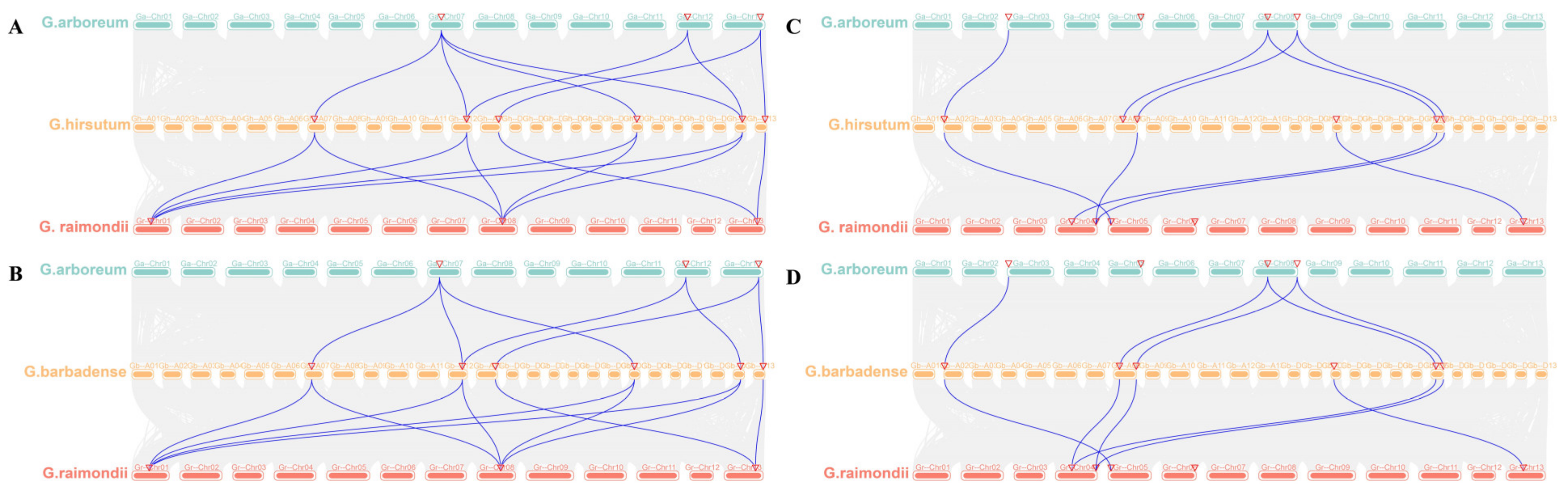
2.5. Collinearity Analysis of EPSPS and BAR Family Members
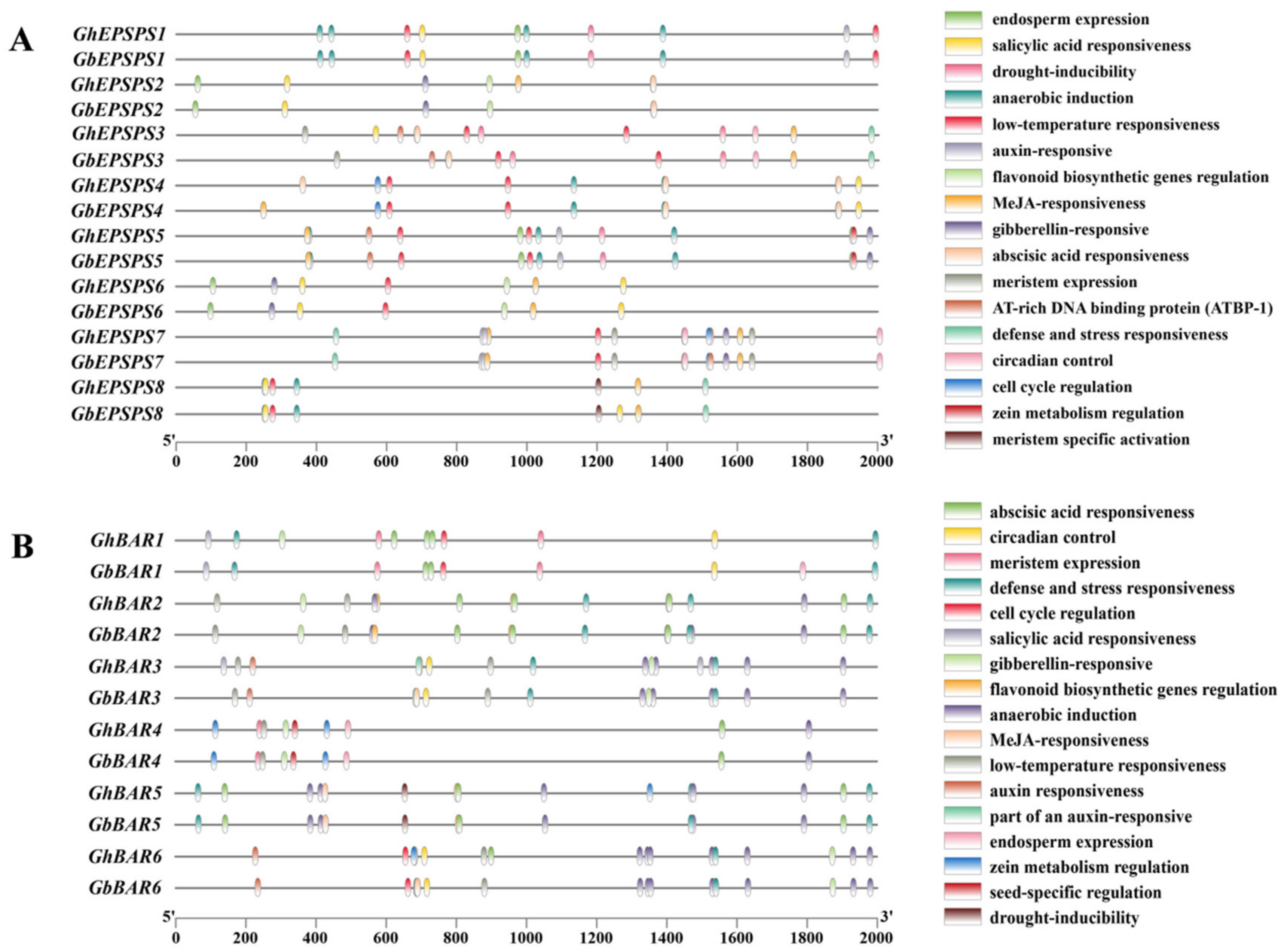
2.6. Analysis of cis-Elements in the Promoter of EPSPS, BAR Family Members
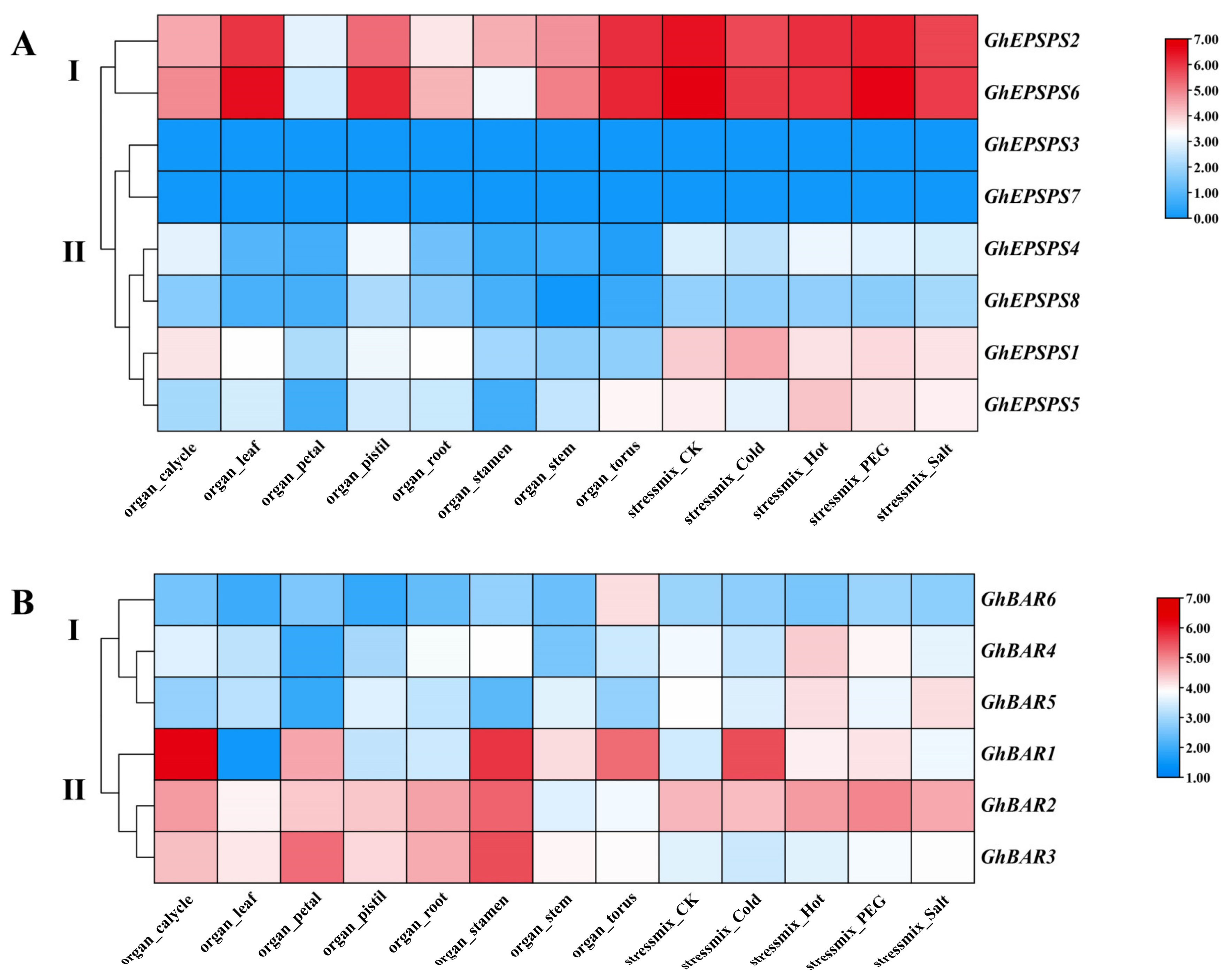
2.7. Expression Analysis of GhEPSPS, GhBAR Family Members
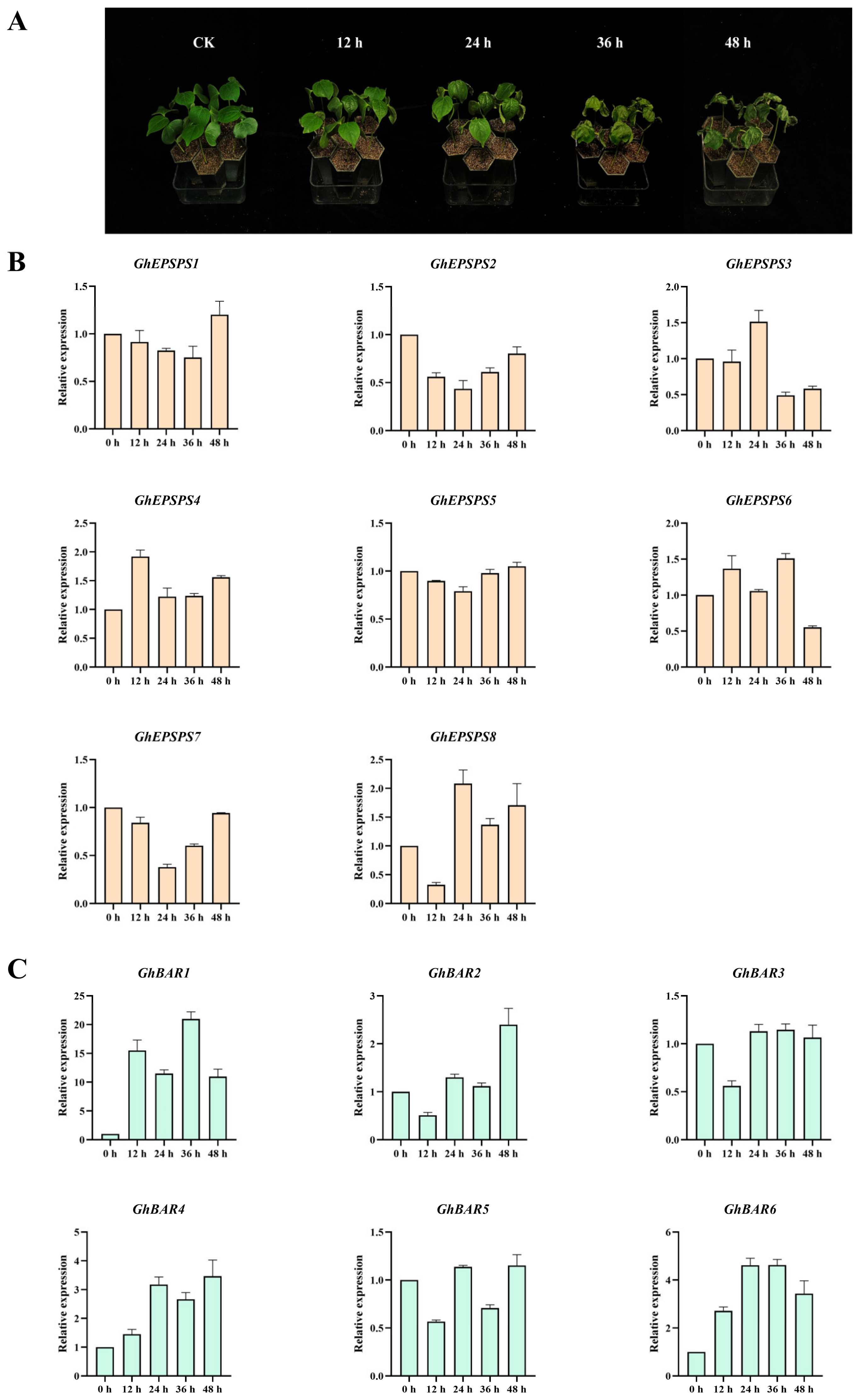
2.8. Expression Levels of EPSPS and BAR Family Members after Herbicide Spraying by qRT-PCR
3. Discussion
4. Materials and Methods
4.1. Identification of EPSPS and BAR Family Members
4.2. Characterization of EPSPS and BAR Family Members
4.3. Conserved Domain and Gene Structure Analysis of EPSPS and BAR Family Members
4.4. Phylogenetic Relationship of EPSPS and BAR Family Members
4.5. Analysis of cis-Elements of Promoters of EPSPS and BAR Family Members
4.6. Collinearity Analysis of EPSPS and BAR Family Members
4.7. Expression Analysis of GhEPSPS and GhBAR Family Members in Different Tissues and under Biotic and Abiotic Stresses
4.8. Gene Expression Analysis of EPSPS and BAR Family Genes in Different Periods after Herbicide Stress
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shahzad, A.; Ullah, S.; Dar, A.A.; Sardar, M.F.; Mehmood, T.; Tufail, M.A.; Shakoor, A.; Haris, M. Nexus on climate change: Agriculture and possible solution to cope future climate change stresses. Environ. Sci. Pollut. Res. 2021, 28, 14211–14232. [Google Scholar] [CrossRef] [PubMed]
- Aslam, S.; Khan, S.H.; Ahmed, A.; Dandekar, A.M. The Tale of Cotton Plant: From Wild Type to Domestication, Leading to Its Improvement by Genetic Transformation. Am. J. Mol. Biol. 2020, 10, 91–127. [Google Scholar] [CrossRef]
- Dong, H.; Huang, Y.; Wang, K. The Development of Herbicide Resistance Crop Plants Using CRISPR/Cas9-Mediated Gene Editing. Genes 2021, 12, 912. [Google Scholar] [CrossRef] [PubMed]
- Kremer, R.J.; Means, N.E. Glyphosate and glyphosate-resistant crop interactions with rhizosphere microorganisms. Eur. J. Agron. 2009, 31, 153–161. [Google Scholar] [CrossRef]
- Duke, S.O. The history and current status of glyphosate. Pest Manag. Sci. 2018, 74, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O.; Powles, S.B. Glyphosate: A once-in-a-century herbicide. Pest Manag. Sci. 2008, 64, 319–325. [Google Scholar] [CrossRef]
- Dill, G.M.; CaJacob, C.A.; Padgette, S.R. Glyphosate-resistant crops: Adoption, use and future considerations. Pest Manag. Sci. 2008, 64, 326–331. [Google Scholar] [CrossRef]
- Green, J.M.; Owen, M.D.K. Herbicide-Resistant Crops: Utilities and Limitations for Herbicide-Resistant Weed Management. J. Agric. Food Chem. 2011, 59, 5819–5829. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, Y.; Luo, R.; Yang, L.; Li, G.; Di, J.; Peng, M.; Li, L.; Wen, Q.; Liang, X. Production of EPSPS and bar gene double-herbicide resistant castor (Ricinus communis L.). Biotechnol. Biotechnol. Equip. 2020, 34, 825–840. [Google Scholar] [CrossRef]
- Schütte, G.; Eckerstorfer, M.; Rastelli, V.; Reichenbecher, W.; Restrepo-Vassalli, S.; Ruohonen-Lehto, M.; Saucy, A.-G.W.; Mertens, M. Herbicide resistance and biodiversity: Agronomic and environmental aspects of genetically modified herbicide-resistant plants. Environ. Sci. Eur. 2017, 29, 5. [Google Scholar] [CrossRef]
- Geiger, D.R.; Fuchs, M.A. Inhibitors of Aromatic Amino Acid Biosynthesis (Glyphosate). In Herbicide Classes in Development: Mode of Action, Targets, Genetic Engineering, Chemistry; Böger, P., Wakabayashi, K., Hirai, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 59–85. [Google Scholar] [CrossRef]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Hernández-Carlos, B.; Villanueva-Cañongo, C. Shikimic acid pathway in biosynthesis of phenolic compounds. Plant Physiol. Asp. Phenolic Compd. 2019, 1, 1–15. [Google Scholar] [CrossRef]
- Achary, V.M.M.; Sheri, V.; Manna, M.; Panditi, V.; Borphukan, B.; Ram, B.; Agarwal, A.; Fartyal, D.; Teotia, D.; Masakapalli, S.K.; et al. Overexpression of improved EPSPS gene results in field level glyphosate tolerance and higher grain yield in rice. Plant Biotechnol. J. 2020, 18, 2504–2519. [Google Scholar] [CrossRef] [PubMed]
- Priestman, M.A.; Healy, M.L.; Funke, T.; Becker, A.; Schönbrunn, E. Molecular basis for the glyphosate-insensitivity of the reaction of 5-enolpyruvylshikimate 3-phosphate synthase with shikimate. FEBS Lett. 2005, 579, 5773–5780. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, A.; Cross, P.J.; Dobson, R.C.; Adams, L.E.; Savka, M.A.; Hudson, A.O. A three-ring circus: Metabolism of the three proteogenic aromatic amino acids and their role in the health of plants and animals. Front. Mol. Biosci. 2018, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zha, W.; Liu, K.; Li, C.; Zhou, L.; He, G.; Xu, H.; Li, P.; Chen, J.; Chen, Z.; et al. Molecular identification and efficacy assessment of a glufosinate-tolerant and brown planthopper-resistant transgenic rice line. J. Plant Physiol. 2022, 273, 153688. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Kuang, Y.; Xu, Z.; Wu, X.; Zhang, D.; Yan, F.; Li, X.; Zhou, X.; Wang, G.; Zhou, H. Three novel alleles of OsGS1 developed by base-editing-mediated artificial evolution confer glufosinate tolerance in rice. Crop J. 2023, 11, 661–665. [Google Scholar] [CrossRef]
- Thompson, C.J.; Movva, N.R.; Tizard, R.; Crameri, R.; Davies, J.E.; Lauwereys, M.; Botterman, J. Characterization of the herbicide-resistance gene bar from Streptomyces hygroscopicus. EMBO J. 1987, 6, 2519–2523. [Google Scholar] [CrossRef]
- Gong, Y.; Guo, S.; Shu, H.; Ni, W.; Maimaiti, P.; Shen, X.; Xu, P.; Zhang, X.; Guo, Q. Analysis of Molecular Evolution and Gene Structure of EPSPS Protein in Plant Shikimate Pathway. Chin. Bull. Bot. 2015, 50, 295–309. [Google Scholar] [CrossRef]
- Aramrak, A.; Kidwell, K.K.; Steber, C.M.; Burke, I.C. Molecular and phylogenetic characterization of the homoeologous EPSP Synthase genes of allohexaploid wheat, Triticum aestivum (L.). BMC Genom. 2015, 16, 844. [Google Scholar] [CrossRef]
- Garg, B.; Vaid, N.; Tuteja, N. In-Silico analysis and expression profiling implicate diverse role of EPSPS family genes in regulating developmental and metabolic processes. BMC Res. Notes 2014, 7, 58. [Google Scholar] [CrossRef]
- Xie, Z.; Sundström, J.F.; Jin, Y.; Liu, C.; Jansson, C.; Sun, C. A selection strategy in plant transformation based on antisense oligodeoxynucleotide inhibition. Plant J. 2014, 77, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Heck, G.R.; Armstrong, C.L.; Astwood, J.D.; Behr, C.F.; Bookout, J.T.; Brown, S.M.; Cavato, T.A.; DeBoer, D.L.; Deng, M.Y.; George, C.; et al. Development and Characterization of a CP4 EPSPS-Based, Glyphosate-Tolerant Corn Event. Crop Sci. 2005, 45, 329–339. [Google Scholar] [CrossRef]
- Chhapekar, S.; Raghavendrarao, S.; Pavan, G.; Ramakrishna, C.; Singh, V.K.; Phanindra, M.L.V.; Dhandapani, G.; Sreevathsa, R.; Ananda Kumar, P. Transgenic rice expressing a codon-modified synthetic CP4-EPSPS confers tolerance to broad-spectrum herbicide, glyphosate. Plant Cell Rep. 2015, 34, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Garg, B.; Gill, S.S.; Biswas, D.K.; Sahoo, R.K.; Kunchge, N.S.; Tuteja, R.; Tuteja, N. Simultaneous Expression of PDH45 with EPSPS Gene Improves Salinity and Herbicide Tolerance in Transgenic Tobacco Plants. Front. Plant Sci. 2017, 8, 364. [Google Scholar] [CrossRef]
- Guo, B.; Guo, Y.; Hong, H.; Jin, L.; Zhang, L.; Chang, R.-Z.; Lu, W.; Lin, M.; Qiu, L.-J. Co-expression of G2-EPSPS and glyphosate acetyltransferase GAT genes conferring high tolerance to glyphosate in soybean. Front. Plant Sci. 2015, 6, 847. [Google Scholar] [CrossRef]
- Takano, H.K.; Dayan, F.E. Glufosinate-ammonium: A review of the current state of knowledge. Pest Manag. Sci. 2020, 76, 3911–3925. [Google Scholar] [CrossRef] [PubMed]
- Zang, N.; Zhai, H.; Gao, S.; Chen, W.; He, S.; Liu, Q. Efficient production of transgenic plants using the bar gene for herbicide resistance in sweetpotato. Sci. Hortic. 2009, 122, 649–653. [Google Scholar] [CrossRef]
- Áy, Z.; Mihály, R.; Cserháti, M.; Kótai, É.; Pauk, J. The Effect of High Concentrations of Glufosinate Ammonium on the Yield Components of Transgenic Spring Wheat (Triticum aestivum L.) Constitutively Expressing the bar Gene. Sci. World J. 2012, 2012, 657945. [Google Scholar] [CrossRef]
- Yu, X.; Sun, Y.; Lin, C.; Wang, P.; Shen, Z.; Zhao, Y. Development of Transgenic Maize Tolerant to Both Glyphosate and Glufosinate. Agronomy 2023, 13, 226. [Google Scholar] [CrossRef]
- Liang, C.; Sun, B.; Meng, Z.; Meng, Z.; Wang, Y.; Sun, G.; Zhu, T.; Lu, W.; Zhang, W.; Malik, W.; et al. Co-expression of GR79 EPSPS and GAT yields herbicide-resistant cotton with low glyphosate residues. Plant Biotechnol. J. 2017, 15, 1622–1629. [Google Scholar] [CrossRef]
- Siddiqui, H.A.; Asad, S.; Naqvi, R.Z.; Asif, M.; Liu, C.; Liu, X.; Farooq, M.; Abro, S.; Rizwan, M.; Arshad, M.; et al. Development and evaluation of triple gene transgenic cotton lines expressing three genes (Cry1Ac-Cry2Ab-EPSPS) for lepidopteran insect pests and herbicide tolerance. Sci. Rep. 2022, 12, 18422. [Google Scholar] [CrossRef]
- Zhang, X.-B.; Tang, Q.-L.; Wang, X.-J.; Wang, Z.-X. Development of glyphosate-tolerant transgenic cotton plants harboring the G2-aroA gene. J. Integr. Agric. 2017, 16, 551–558. [Google Scholar] [CrossRef]
- Keller, G.; Spatola, L.; McCabe, D.; Martinell, B.; Swain, W.; John, M.E. Transgenic cotton resistant to herbicide bialaphos. Transgenic Res. 1997, 6, 385–392. [Google Scholar] [CrossRef]
- Daud, M.; Variath, M.; Ali, S.; Jamil, M.; Khan, M.T.; Shafi, M.; Shuijin, Z. Genetic transformation of bar gene and its inheritance and segregation behavior in the resultant transgenic cotton germplasm (BR001). Pak. J. Bot. 2009, 41, 2167–2178. [Google Scholar] [CrossRef]
- Campbell, B.; Hinze, L. Cotton Production, Processing and Uses of Cotton Raw Material; CABI International: Oxon, UK, 2010; pp. 259–276. [Google Scholar] [CrossRef]
- Dongjun, L.; Rui, Z.; Sandui, G.; Zhigang, M.; Guoqing, S.; Chengshe, W.; Hongji, Z. Research on Expression of epsps Gene in Cotton Cell Line Y18 under Glyphosate Stress. China Biotechnol. 2008, 10, 55–59. [Google Scholar] [CrossRef]
- Yuanyong, G.; Zhenzhen, X.; Shuqiao, G.; Hongmei, S.; Lu, J.; Wanchao, N. Genome-wide Analysis of the EPSPS Genes in Upland Cotton. Acta Agric. Boreali-Sin. 2016, 31, 15–21. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, Y.; Wu, L.; Zhang, G.; Sun, Z.; Li, Z.; Jiang, Y.; Ke, H.; Chen, B.; Liu, Z.; et al. High-quality genome assembly and resequencing of modern cotton cultivars provide resources for crop improvement. Nat. Genet. 2021, 53, 1385–1391. [Google Scholar] [CrossRef]
- Khan, M.A.; Wahid, A.; Ahmad, M.; Tahir, M.T.; Ahmed, M.; Ahmad, S.; Hasanuzzaman, M. World Cotton Production and Consumption: An Overview. In Cotton Production and Uses: Agronomy, Crop Protection, and Postharvest Technologies; Ahmad, S., Hasanuzzaman, M., Eds.; Springer: Singapore, 2020; pp. 1–7. [Google Scholar] [CrossRef]
- Wendel, J.F.; Cronn, R.C. Polyploidy and the evolutionary history of cotton. Adv. Agron. 2003, 78, 139–186. [Google Scholar] [CrossRef]
- Fan, K.; Mao, Z.; Ye, F.; Pan, X.; Li, Z.; Lin, W.; Zhang, Y.; Huang, J.; Lin, W. Genome-wide identification and molecular evolution analysis of the heat shock transcription factor (HSF) gene family in four diploid and two allopolyploid Gossypium species. Genomics 2021, 113, 3112–3127. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Q.; Qiu, L.; Liu, L.L.; Luo, L.; Han, X.P.; Zhai, Y.H.; Wang, W.J.; Ren, M.Z.; Xing, Y.D. Identification and Comprehensive Structural and Functional Analyses of the EXO70 Gene Family in Cotton. Genes 2021, 12, 1594. [Google Scholar] [CrossRef]
- Wang, S.; Chen, J.; Zhang, W.; Hu, Y.; Chang, L.; Fang, L.; Wang, Q.; Lv, F.; Wu, H.; Si, Z.; et al. Sequence-based ultra-dense genetic and physical maps reveal structural variations of allopolyploid cotton genomes. Genome Biol. 2015, 16, 108. [Google Scholar] [CrossRef] [PubMed]
- Pline, W.A.; Wilcut, J.W.; Duke, S.O.; Edmisten, K.L.; Wells, R. Tolerance and Accumulation of Shikimic Acid in Response to Glyphosate Applications in Glyphosate-Resistant and Nonglyphosate-Resistant Cotton (Gossypium hirsutum L.). J. Agric. Food Chem. 2002, 50, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.-T.; Ma, S.-L.; Bai, L.-P.; Zhang, L.; Ma, H.; Jia, P.; Liu, J.; Zhong, M.; Guo, Z.-F. Signal transduction during cold, salt, and drought stresses in plants. Mol. Biol. Rep. 2012, 39, 969–987. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, A.; Zhou, H. Cloning and Expression Analysis of Glyphosate Resistance Gene EPSPS in Sweet Potato. Acta Bot. Boreali-Occident. Sin. 2020, 40, 0035–3342. [Google Scholar] [CrossRef]
- Xuhong, T.; Yuxiang, W.; Shuijins, Z. Cloning of 5-enolpyruvylshikimate-3-phosphate Synthase Genes EPSPS from the Upland Cotton and lts Expression in Different Cotton Tissues. Cotton Sci. 2009, 21, 259–264. [Google Scholar] [CrossRef]
- Huang, Z.F.; Zhang, C.X.; Huang, H.J.; Wei, S.H.; Liu, Y.; Cui, H.L.; Chen, J.C.; Yang, L.; Chen, J.Y. Molecular cloning and characterization of 5-enolpyruvylshikimate-3-phosphate synthase gene from Convolvulus arvensis L. Mol. Biol. Rep. 2014, 41, 2077–2084. [Google Scholar] [CrossRef]
- Barry, G.; Kishore, G.; Padgette, S.; Taylor, M.; Kolacz, K.; Weldon, M.; Re, D.; Eichholtz, D.; Fincher, K.; Hallas, L. Inhibitors of amino acid biosynthesis: Strategies for imparting glyphosate tolerance to crop plants. In Biosynthesis and Molecular Regulation of Amino Acids in Plants; American Society of Plant Physiologists: Rockville, MD, USA, 1992. [Google Scholar]
- He, M.; Yang, Z.-Y.; Nie, Y.-F.; Wang, J.; Xu, P. A new type of class I bacterial 5-enopyruvylshikimate-3-phosphate synthase mutants with enhanced tolerance to glyphosate. Biochim. Biophys. Acta BBA Gen. Subj. 2001, 1568, 1–6. [Google Scholar] [CrossRef]
- Hussain, A.; Ding, X.; Alariqi, M.; Manghwar, H.; Hui, F.; Li, Y.; Cheng, J.; Wu, C.; Cao, J.; Jin, S. Herbicide Resistance: Another Hot Agronomic Trait for Plant Genome Editing. Plants 2021, 10, 621. [Google Scholar] [CrossRef]
- Sun, Y.-C.; Chen, Y.-C.; Tian, Z.-X.; Li, F.-M.; Wang, X.-Y.; Zhang, J.; Xiao, Z.-L.; Lin, M.; Gilmartin, N.; Dowling, D.N.; et al. Novel AroA with High Tolerance to Glyphosate, Encoded by a Gene of Pseudomonas putida 4G-1 Isolated from an Extremely Polluted Environment in China. Appl. Environ. Microbiol. 2005, 71, 4771–4776. [Google Scholar] [CrossRef]
- Gaines, T.A.; Zhang, W.; Wang, D.; Bukun, B.; Chisholm, S.T.; Shaner, D.L.; Nissen, S.J.; Patzoldt, W.L.; Tranel, P.J.; Culpepper, A.S.; et al. Gene amplification confers glyphosate resistance in Amaranthus palmeri. Proc. Natl. Acad. Sci. USA 2010, 107, 1029–1034. [Google Scholar] [CrossRef]
- Kaplan, B.; Davydov, O.; Knight, H.; Galon, Y.; Knight, M.R.; Fluhr, R.; Fromm, H. Rapid Transcriptome Changes Induced by Cytosolic Ca2+ Transients Reveal ABRE-Related Sequences as Ca2+-Responsive cis Elements in Arabidopsis. Plant Cell 2006, 18, 2733–2748. [Google Scholar] [CrossRef] [PubMed]
- Oberdoerfer, R.B.; Shillito, R.D.; De Beuckeleer, M.; Mitten, D.H. Rice (Oryza sativa L.) containing the bar gene is compositionally equivalent to the nontransgenic counterpart. J. Agric. Food Chem. 2005, 53, 1457–1465. [Google Scholar] [CrossRef]
- Xu, C.; Wang, X.; Ma, Z.; Kuai, B. Regulation of bar Gene Expression in the Transgenic Oat T3 Progeny. Physiol. Mol. Biol. Plants 2000, 26, 393–396. [Google Scholar] [CrossRef]
- Wu, C.; Wan, Y.; Xu, J.; Su, J.; Fang, X. Expression Analysis of Rice bar Gene Mediated Matrix Attachment Region(MAR) Sequence Transformed by a Gene Bombardment. J. Agric. Biotechnol. 2002, 10, 5. [Google Scholar] [CrossRef]
- Carbonari, C.A.; Latorre, D.O.; Gomes, G.L.; Velini, E.D.; Owens, D.K.; Pan, Z.; Dayan, F.E. Resistance to glufosinate is proportional to phosphinothricin acetyltransferase expression and activity in LibertyLink® and WideStrike® cotton. Planta 2016, 243, 925–933. [Google Scholar] [CrossRef]
- Taak, P.; Tiwari, S.; Koul, B. Optimization of regeneration and Agrobacterium-mediated transformation of Stevia (Stevia rebaudiana Bertoni): A commercially important natural sweetener plant. Sci. Rep. 2020, 10, 16224. [Google Scholar] [CrossRef]
- Ma, S.; Gong, Q.; Bohnert, H.J. Dissecting salt stress pathways. J. Exp. Bot. 2006, 57, 1097–1107. [Google Scholar] [CrossRef]
- Zhu, J.-K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Petrov, V.; Yun, D.-J.; Gechev, T. Revisiting plant salt tolerance: Novel components of the SOS pathway. Trends Plant Sci. 2023, 28, 1060–1069. [Google Scholar] [CrossRef]
- Ding, S.; Wang, L.; Yang, Z.; Lu, Q.; Wen, X.; Lu, C. Decreased glutathione reductase2 leads to early leaf senescence in Arabidopsis. J. Integr. Plant Biol. 2016, 58, 29–47. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, H.; Chen, F.; Liu, Y. The roles of histone acetylation in seed performance and plant development. Plant Physiol. Biochem. 2014, 84, 125–133. [Google Scholar] [CrossRef]
- Li, F.; Fan, G.; Wang, K.; Sun, F.; Yuan, Y.; Song, G.; Li, Q.; Ma, Z.; Lu, C.; Zou, C.; et al. Genome sequence of the cultivated cotton Gossypium arboreum. Nat. Genet. 2014, 46, 567–572. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Z.; Li, F.; Ye, W.; Wang, J.; Song, G.; Yue, Z.; Cong, L.; Shang, H.; Zhu, S.; et al. The draft genome of a diploid cotton Gossypium raimondii. Nat. Genet. 2012, 44, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Zhang, T.; Hu, Y.; Jiang, W.; Fang, L.; Guan, X.; Chen, J.; Zhang, J.; Saski, C.A.; Scheffler, B.E.; Stelly, D.M.; et al. Sequencing of allotetraploid cotton (Gossypium hirsutum L. acc. TM-1) provides a resource for fiber improvement. Nat. Biotechnol. 2015, 33, 531–537. [Google Scholar] [CrossRef] [PubMed]
| Gene | Gene ID | Chromosome | All Length (bp) | CDS Length (bp) | Number of Exons | Protein Length (aa) | Molecular Weight (kDa) | Isoelectric Point |
|---|---|---|---|---|---|---|---|---|
| GhEPSPS1 | GhM_A07G1799 | A07 | 4095 | 1566 | 8 | 521 | 55.49 | 8.08 |
| GhEPSPS2 | GhM_A12G1349 | A12 | 4011 | 1566 | 8 | 521 | 55.59 | 7.83 |
| GhEPSPS3 | GhM_A12G1350 | A12 | 1797 | 558 | 5 | 185 | 20.56 | 6.49 |
| GhEPSPS4 | GhM_A13G2002 | A13 | 3723 | 1461 | 6 | 486 | 51.93 | 8.43 |
| GhEPSPS5 | GhM_D07G1761 | D07 | 4137 | 1566 | 8 | 521 | 55.37 | 7.92 |
| GhEPSPS6 | GhM_D12G1287 | D12 | 3643 | 1566 | 8 | 521 | 55.6 | 8.38 |
| GhEPSPS7 | GhM_D12G1288 | D12 | 2836 | 915 | 6 | 304 | 32.76 | 6.24 |
| GhEPSPS8 | GhM_D13G1901 | D13 | 3719 | 1419 | 6 | 472 | 50.45 | 8.56 |
| GbEPSPS1 | GbM_A07G1760 | A07 | 3863 | 1566 | 8 | 521 | 55.48 | 8.26 |
| GbEPSPS2 | GbM_A12G1269 | A12 | 3900 | 1566 | 8 | 521 | 55.6 | 7.83 |
| GbEPSPS3 | GbM_A12G1270 | A12 | 915 | 558 | 4 | 185 | 20.56 | 5.67 |
| GbEPSPS4 | GbM_A13G1999 | A13 | 3703 | 1419 | 6 | 472 | 50.23 | 8.14 |
| GbEPSPS5 | GbM_D07G1761 | D07 | 3580 | 1566 | 8 | 521 | 55.45 | 7.92 |
| GbEPSPS6 | GbM_D12G1236 | D12 | 3730 | 1566 | 8 | 521 | 55.6 | 8.38 |
| GbEPSPS7 | GbM_D12G1237 | D12 | 2820 | 915 | 6 | 304 | 32.76 | 5.96 |
| GbEPSPS8 | GbM_D13G1869 | D13 | 3554 | 1419 | 6 | 472 | 50.39 | 8.56 |
| GaEPSPS1 | Ga07G1630 | Chr07 | 4159 | 1479 | 9 | 492 | 52.68 | 7.2 |
| GaEPSPS2 | Ga12G1903 | Chr12 | 3091 | 1566 | 8 | 521 | 55.54 | 7.72 |
| GaEPSPS3 | Ga12G1902 | Chr12 | 915 | 558 | 4 | 185 | 20.62 | 5.68 |
| GaEPSPS4 | Ga13G1869 | Chr13 | 3205 | 1449 | 6 | 482 | 51.46 | 8.3 |
| GrEPSPS1 | Gorai.001G174400 | Chr01 | 3994 | 1566 | 8 | 521 | 55.32 | 7.85 |
| GrEPSPS2 | Gorai.008G113600 | Chr08 | 4053 | 1566 | 9 | 521 | 55.52 | 8.73 |
| GrEPSPS3 | Gorai.008G113700 | Chr08 | 3508 | 666 | 5 | 221 | 24.12 | 6.87 |
| GrEPSPS4 | Gorai.008G113800 | Chr08 | 3642 | 1026 | 7 | 341 | 37.52 | 7.73 |
| GrEPSPS5 | Gorai.013G173300 | Chr13 | 3731 | 1425 | 6 | 474 | 50.69 | 8.62 |
| Gene | Gene ID | Chromosome | All Length (bp) | CDS Length (bp) | Number of Exons | Protein Length (aa) | Molecular Weight (kDa) | Isoelectric Point |
|---|---|---|---|---|---|---|---|---|
| GhBAR1 | GhM_A02G0363 | A02 | 1627 | 561 | 1 | 186 | 21.23 | 6.47 |
| GhBAR2 | GhM_A08G1199 | A08 | 2810 | 495 | 3 | 164 | 18.4 | 8.43 |
| GhBAR3 | GhM_A08G2862 | A08 | 3804 | 834 | 6 | 277 | 31.75 | 9.03 |
| GhBAR4 | GhM_D03G0774 | D03 | 5007 | 495 | 3 | 164 | 18.51 | 8.43 |
| GhBAR5 | GhM_D08G1150 | D08 | 3053 | 495 | 3 | 164 | 18.43 | 8.99 |
| GhBAR6 | GhM_D08G2805 | D08 | 3909 | 834 | 6 | 277 | 31.72 | 9.04 |
| GbBAR1 | GbM_A02G0373 | A02 | 3684 | 561 | 2 | 186 | 21.23 | 6.47 |
| GbBAR2 | GbM_A08G1080 | A08 | 2763 | 495 | 3 | 164 | 18.4 | 8.43 |
| GbBAR3 | GbM_A08G2815 | A08 | 3570 | 834 | 6 | 277 | 31.73 | 9.18 |
| GbBAR4 | GbM_D03G0750 | D03 | 14,746 | 495 | 3 | 164 | 18.51 | 8.43 |
| GbBAR5 | GbM_D08G1153 | D08 | 2778 | 495 | 3 | 164 | 18.43 | 8.99 |
| GbBAR6 | GbM_D08G2789 | D08 | 3941 | 834 | 6 | 277 | 31.72 | 9.04 |
| GaBAR1 | Ga03G0387 | Chr03 | 561 | 561 | 1 | 186 | 21.23 | 6.52 |
| GaBAR2 | Ga05G4007 | Chr05 | 2224 | 489 | 3 | 162 | 18.37 | 9.51 |
| GaBAR3 | Ga05G4011 | Chr05 | 2250 | 489 | 3 | 162 | 18.35 | 9.37 |
| GaBAR4 | Ga08G1028 | Chr08 | 2202 | 438 | 3 | 145 | 16.43 | 8.82 |
| GaBAR5 | Ga08G2611 | Chr08 | 2415 | 822 | 5 | 273 | 31.01 | 8.77 |
| GrBAR1 | Gorai.004G110900 | Chr04 | 3619 | 495 | 4 | 164 | 18.43 | 9.05 |
| GrBAR2 | Gorai.004G256900 | Chr04 | 3777 | 834 | 6 | 277 | 31.75 | 9.08 |
| GrBAR3 | Gorai.005G044300 | Chr05 | 957 | 561 | 1 | 186 | 21.17 | 5.85 |
| GrBAR4 | Gorai.006G233400 | Chr06 | 508 | 453 | 2 | 150 | 17.23 | 9.64 |
| GrBAR5 | Gorai.013G110600 | Chr13 | 992 | 270 | 2 | 89 | 10.23 | 8.22 |
| Primers | Sequence (5′–3′) | Primers | Sequence (5′–3′) |
|---|---|---|---|
| GhEPSPS1-qF | GAAATCCCTCTGGAAGGAAACA | GhBAR1-qF | GAACAAGGTTGTGCCTCACCCT |
| GhEPSPS1-qR | GCAGTAGGACCATCAGCA | GhBAR1-qR | TGCCTGAATTTGCACTCACCGA |
| GhEPSPS2-qF | TGATGGGTGCCAAAGTCACCTG | GhBAR2-qF | AAGATGCCATCAACTTCTATC |
| GhEPSPS2-qR | AAGAGTCATAGCAACGTCCGGC | GhBAR2-qR | GTAAGAACAAAGCAGTCGGGAG |
| GhEPSPS3-qF | ACGAGCCGTCCTCAAAGGTTAC | GhBAR3-qF | CAAAGATGTCGTTCAATTGCG |
| GhEPSPS3-qR | CATATTGCGGTTCCAACATTCC | GhBAR3-qR | GACCCCGGGTTTCGTTACC |
| GhEPSPS4-qF | AGGAGTCCGTGTTTGACAACCG | GhBAR4-qF | CGGGTTTGAAATCACCGAGACA |
| GhEPSPS4-qR | TAGTGCATTGCTCCCGCTAACC | GhBAR4-qR | TTTGTTCGCTTGAGATGTAGTG |
| GhEPSPS5-qF | CCGGACCACCAAGAAATCCCTC | GhBAR5-qF | CCATCCGTGTGTACATCATGACA |
| GhEPSPS5-qR | CTGCTGTCACATTTGGTTCGCC | GhBAR5-qR | TAGAAGTTGATGGCATCTTCA |
| GhEPSPS6-qF | ATCACGGGTGGGACTGTCACG | GhBAR6-qF | GCTTACGCGTTCGATGCAGGTA |
| GhEPSPS6-qR | CCGCATCCTTCTACCGTGACAG | GhBAR6-qR | ATCGAGTTCGGATCGAGGCAAG |
| GhEPSPS7-qF | ATAAACGGAAAGGGTGGTCTTC | UBQ14-F | CAACGCTCCATCTTGTCCTT |
| GhEPSPS7-qR | AGCTAAATGAGCTGCCATGAGT | UBQ14-R | TGATCGTCTTTCCCGTAAGC |
| GhEPSPS8-qF | CTTCTCCACAACCTTCCCAATG | ||
| GhEPSPS8-qR | AATCAACCTCCACTTTGCCAACC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zhang, Z.; Liu, Y.; Ma, Y.; Lv, X.; Zhang, D.; Gu, Q.; Ke, H.; Wu, L.; Zhang, G.; et al. Identification and Expression Analysis of EPSPS and BAR Families in Cotton. Plants 2023, 12, 3366. https://doi.org/10.3390/plants12193366
Li Z, Zhang Z, Liu Y, Ma Y, Lv X, Zhang D, Gu Q, Ke H, Wu L, Zhang G, et al. Identification and Expression Analysis of EPSPS and BAR Families in Cotton. Plants. 2023; 12(19):3366. https://doi.org/10.3390/plants12193366
Chicago/Turabian StyleLi, Zhao, Zhen Zhang, Yinbo Liu, Yuanqi Ma, Xing Lv, Dongmei Zhang, Qishen Gu, Huifeng Ke, Liqiang Wu, Guiyin Zhang, and et al. 2023. "Identification and Expression Analysis of EPSPS and BAR Families in Cotton" Plants 12, no. 19: 3366. https://doi.org/10.3390/plants12193366
APA StyleLi, Z., Zhang, Z., Liu, Y., Ma, Y., Lv, X., Zhang, D., Gu, Q., Ke, H., Wu, L., Zhang, G., Ma, Z., Wang, X., & Sun, Z. (2023). Identification and Expression Analysis of EPSPS and BAR Families in Cotton. Plants, 12(19), 3366. https://doi.org/10.3390/plants12193366






