Plant Growth in LED-Sourced Biophilic Environments Is Improved by the Biochar Amendment of Low-Fertility Soil, the Reflection of Low-Intensity Light, and a Continuous Photoperiod
Abstract
:1. Introduction
2. Results
2.1. Characteristics of the Growing Media
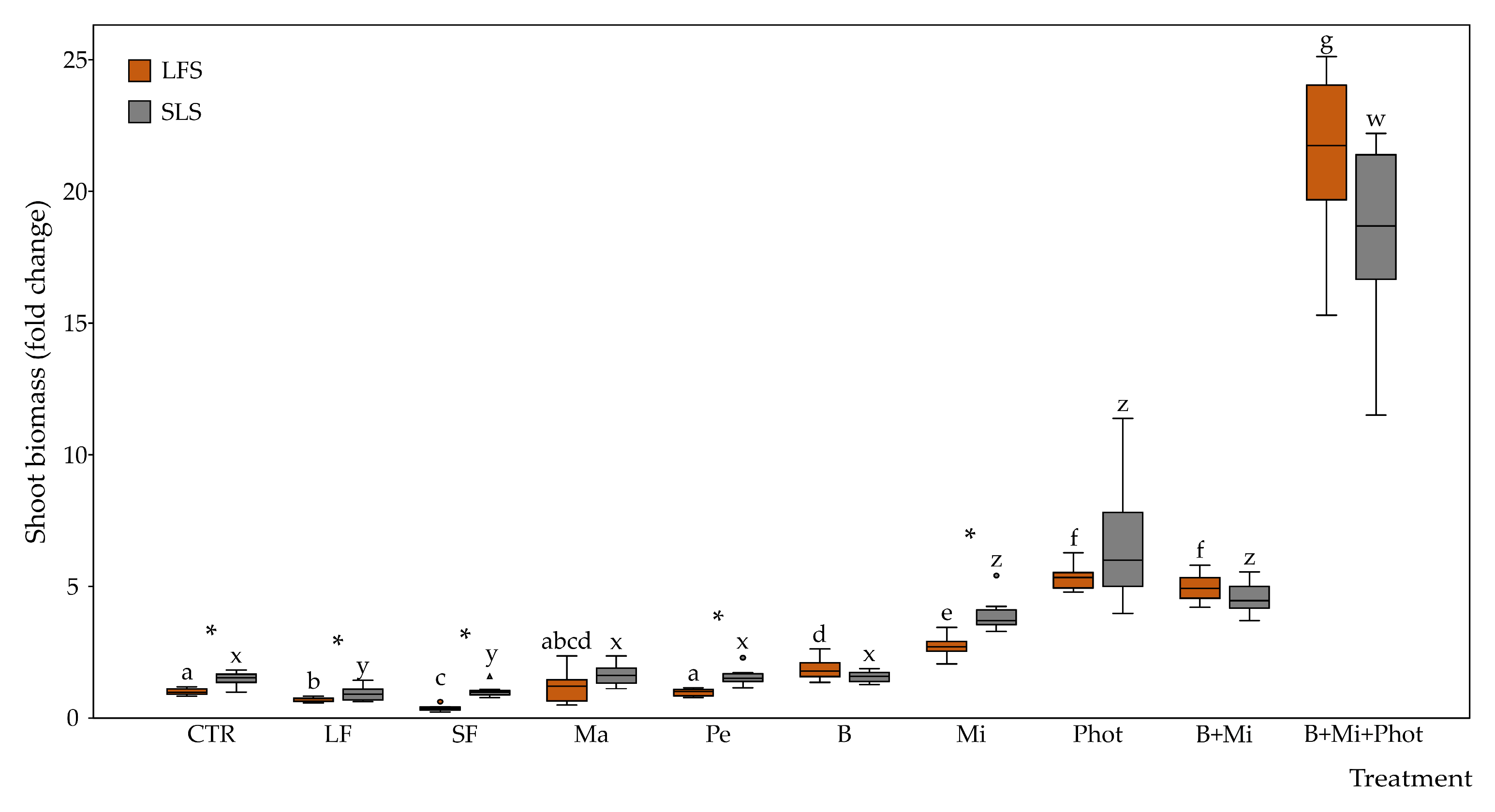
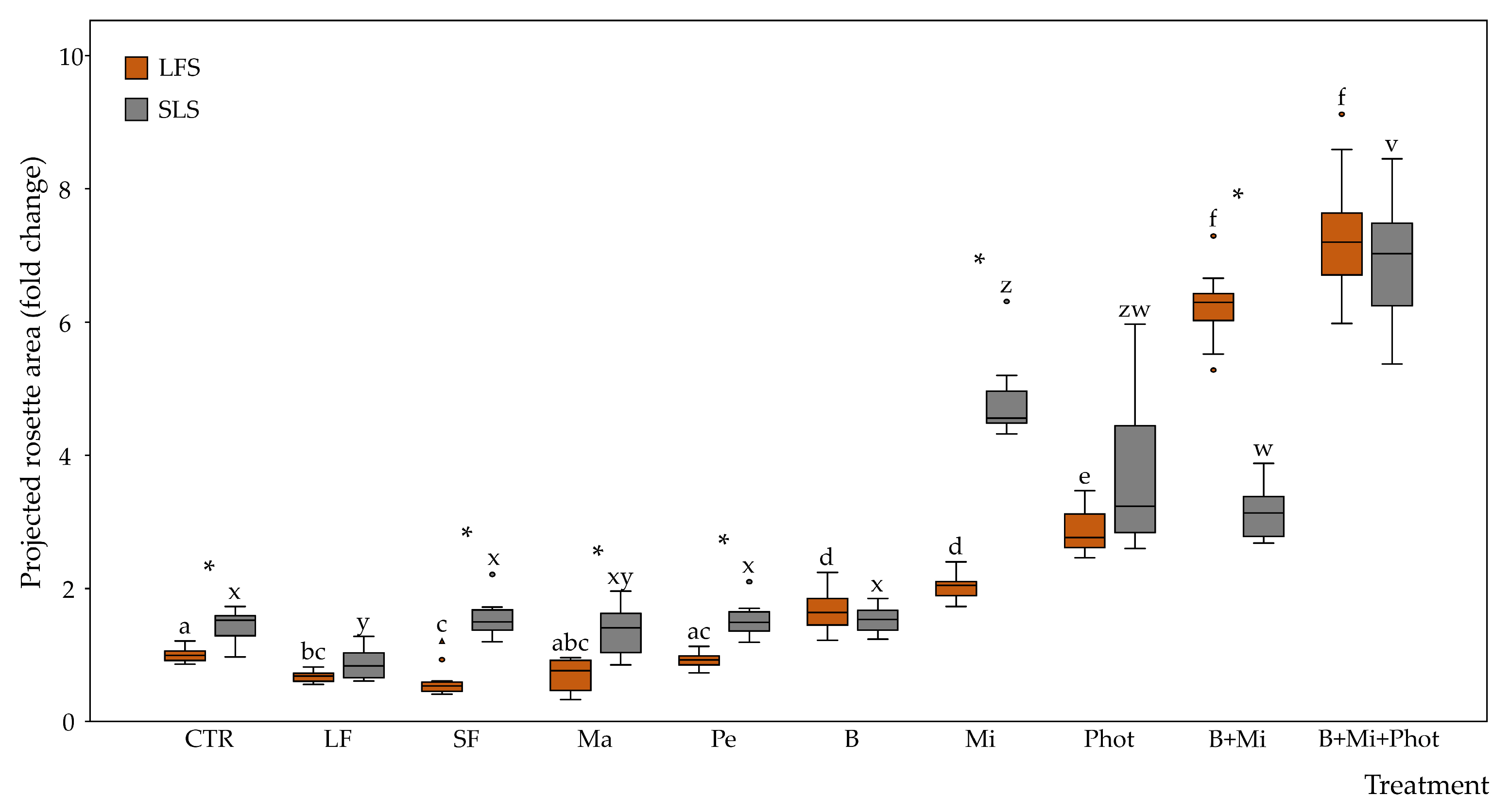
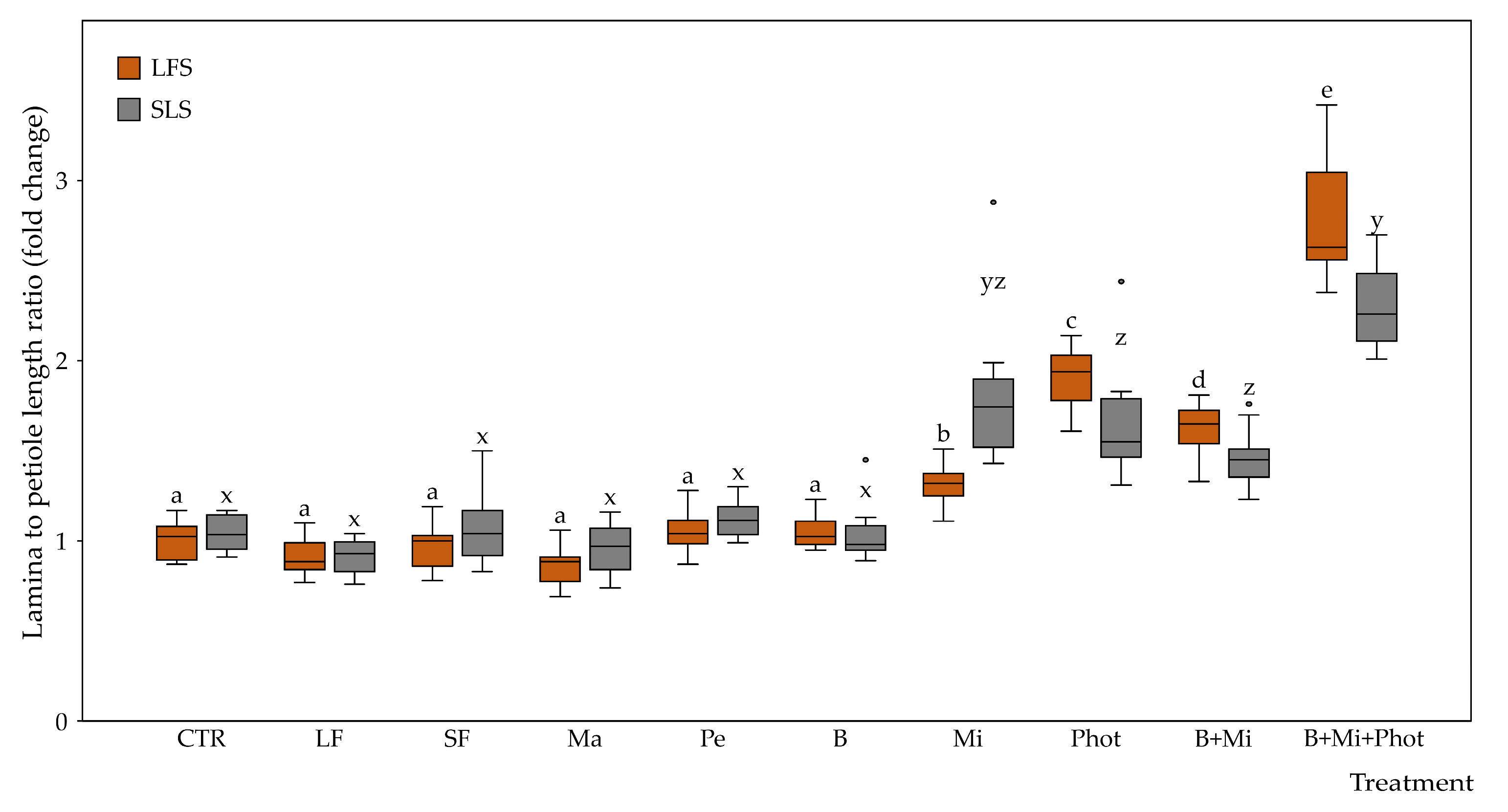
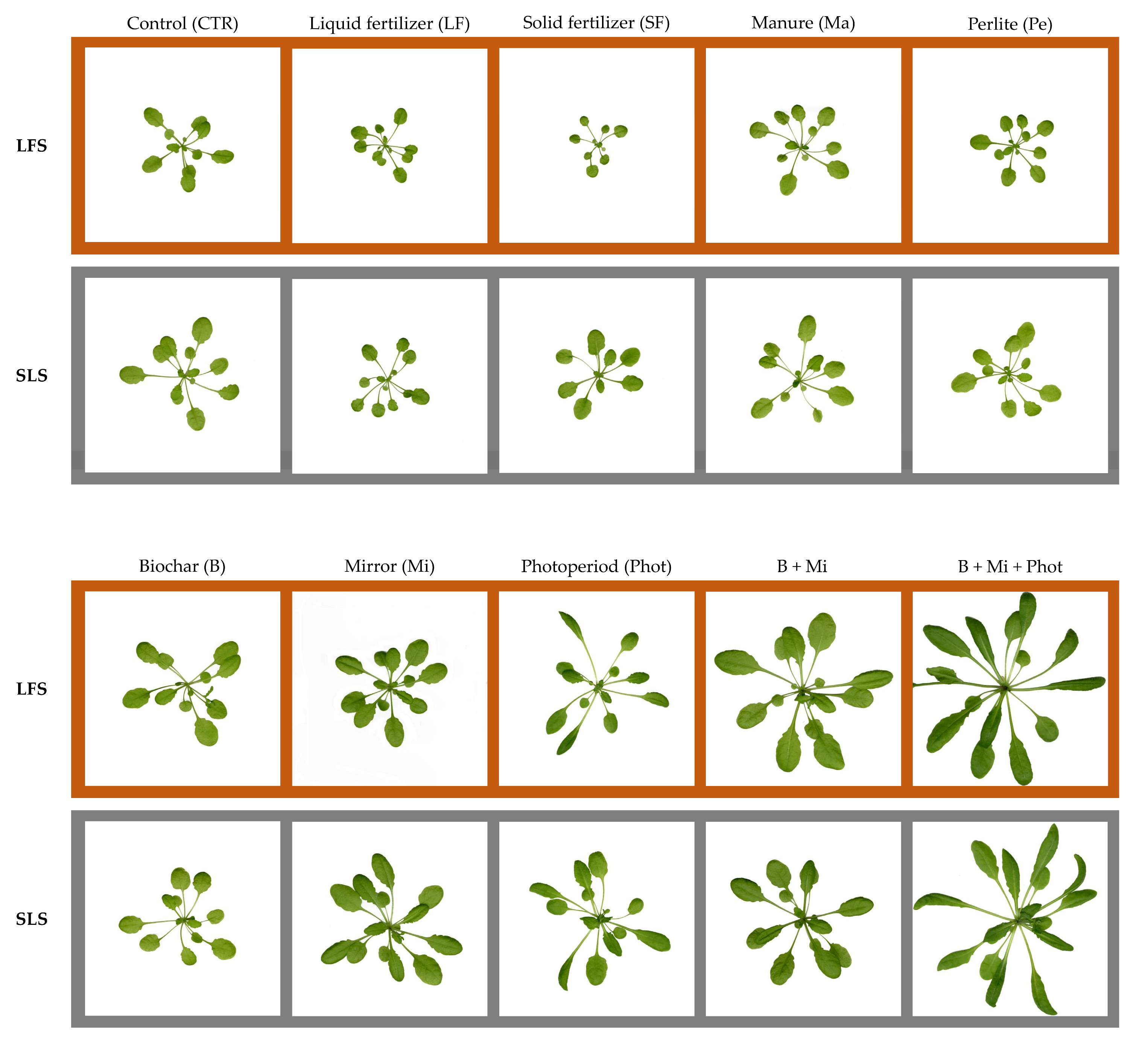
2.2. Morphological Traits of the Plants
3. Discussion
3.1. Specific Amendments Can Improve Plant Growth under Biophilic Lighting
3.2. Fertilization Showed No Positive Effects on Plants Growing under Limited Light Conditions
3.3. Mirror Reflection and a Continuous Light Photoperiod Can Boost Plant Growth under Biophilic Lighting
3.4. Combined Treatments Can Lead to Even Better Growth Performance
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Growing Media and Imposed Treatments
- ➢
- Liquid fertilizer (LF): 1 mL of liquid fertilizer (Concime per piante verdi—Compo—Italy), with a NPK ratio of 7.5:3:6, was diluted in 1 L of tap water and supplied weekly to the plants’ tray. The first application was provided 10 days after sowing;
- ➢
- Solid fertilizer (SF): 0.1 g of solid fertilizer (Blu concime universale—Compo—Italy) with a NPK ratio of 12:12:17 was applied directly on the growing media surface 10 days after sowing;
- ➢
- Manure (Ma): Commercial bovine and equine manure pellets (Stallatico micro pellettato—Vigorplant®—Italy) were crushed with a mortar and pestle and sieved with a mesh of 2 mm. A quantity of 10 mL of manure was thoroughly mixed with 990 mL of growing media to obtain a 1% v/v concentration of manure;
- ➢
- Perlite (Pe): 500 mL of agricultural perlite (Agrilit® 3—Perlite Italiana srl; pH 6.5–7.5) was thoroughly mixed with 500 mL of growing media to obtain a 50% v/v concentration;
- ➢
- Biochar (B): The biochar used in this study was produced by Romagna Carbone s.n.c. (Italy) from orchard pruning biomass through a slow pyrolysis process with an average residence time of 3 h at 500 °C [12]. The raw biochar was crushed with a mortar and pestle and sieved with a mesh of 2 mm. A quantity of 200 mL of biochar was thoroughly mixed with 800 mL of growing media to obtain a 20% v/v concentration of biochar;
- ➢
- Mirror (Mi): A mirror was placed behind the tray to reflect part of the artificial sunlight that would not reach the plants. With this setup, the light intensity ranged between 40 and 55 μmol m−2s−1, with an average DLI of 2.4 mol m−2d−1. Spectrum measurements every 1 nm in the range between 380 and 780 nm were taken on a horizontal white reflector using the Spectraval 1511 instrument (JETI Technische Instrumente GmbH—Germany), both with and without the mirror’s presence, to assess that no spectral variations were introduced by the mirror’s application (Figure 6). To allow a comparison, photon counts measurements were normalized on the luminance of the respective spectrum;
- ➢
- Photoperiod (Phot): A 24 h light photoperiod was applied with an average DLI of 2.6 mol m−2d−1;
- ➢
- Biochar and mirror (B + Mi): Both B and Mi treatments were applied;
- ➢
- Biochar, mirror, and photoperiod (B + Mi + Phot): The B, Mi, and Phot treatments were applied. The average DLI was 4.1 mol m−2d−1.
4.3. Analysis of Growing Media
4.4. Plant Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hähn, N.; Essah, E.; Blanusa, T. Biophilic design and office planting: A case study of effects on perceived health, well-being and performance metrics in the workplace. Intell. Build. Int. 2021, 13, 241–260. [Google Scholar] [CrossRef]
- Kellert, S.R.; Calabrese, E.F. The Practice of Biophilic Design. 2015. Available online: https://www.biophilic-design.com (accessed on 10 September 2023).
- Chang, C.Y.; Chen, P.K. Human response to window views and indoor plants in the workplace. HortScience 2005, 40, 1354–1359. [Google Scholar] [CrossRef]
- Keller, S.R.; Heerwagen, J.H.; Mador, M.L. Biophilic Design: The Theory, Science, and Practice of Bringing Buildings to Life; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; ISBN 9788490225370. [Google Scholar]
- Di Trapani, P.; Magatti, D. Artificial Illumination Device. U.S. Patent 9,709,245 B2, 18 July 2017. [Google Scholar]
- Di Trapani, P.; Magatti, D. Artificial Lighting System for Simulating Natural Lighting. U.S. Patent 2014/0133125 A1, 15 May 2014. [Google Scholar]
- Canazei, M.; Laner, M.; Staggl, S.; Pohl, W.; Ragazzi, P.; Magatti, D.; Martinelli, E.; Di Trapani, P. Room- and illumination-related effects of an artificial skylight. Light. Res. Technol. 2016, 48, 539–558. [Google Scholar] [CrossRef]
- Beatrice, P.; Terzaghi, M.; Chiatante, D.; Scippa, G.S.; Montagnoli, A. Morpho-Physiological Responses of Arabidopsis thaliana L. to the LED-Sourced CoeLux® System. Plants 2021, 10, 1310. [Google Scholar] [CrossRef]
- Beatrice, P.; Chiatante, D.; Scippa, G.S.; Montagnoli, A. Photoreceptors’ gene expression of Arabidopsis thaliana grown with biophilic LED-sourced lighting systems. PLoS ONE 2022, 17, e0269868. [Google Scholar] [CrossRef]
- Beatrice, P.; Saviano, G.; Reguzzoni, M.; Divino, F.; Fantasma, F.; Chiatante, D.; Montagnoli, A. Light spectra of biophilic LED-sourced system modify essential oils composition and plant morphology of Mentha piperita L. and Ocimum basilicum L. Front. Plant Sci. 2023, 14, 1093883. [Google Scholar] [CrossRef] [PubMed]
- Sharanappa. Soil Fertility and Nutrient Management—Principles and Practices; CRC Press-Taylor & Francis Group: Abingdon, UK, 2023; ISBN 9781032429243. [Google Scholar]
- Montagnoli, A.; Baronti, S.; Alberto, D.; Chiatante, D.; Scippa, G.S.; Terzaghi, M. Pioneer and fibrous root seasonal dynamics of Vitis vinifera L. are affected by biochar application to a low fertility soil: A rhizobox approach. Sci. Total Environ. 2021, 751, 141455. [Google Scholar] [CrossRef]
- Steiner, C.; Harttung, T. Biochar as a growing media additive and peat substitute. Solid Earth 2014, 5, 995–999. [Google Scholar] [CrossRef]
- Verhoeven, J.T.A. Wetlands in Europe: Perspectives for restoration of a lost paradise. Ecol. Eng. 2014, 66, 6–9. [Google Scholar] [CrossRef]
- Taparia, T.; Hendrix, E.; Nijhuis, E.; de Boer, W.; van der Wolf, J. Circular alternatives to peat in growing media: A microbiome perspective. J. Clean. Prod. 2021, 327, 129375. [Google Scholar] [CrossRef]
- Joosten, H.; Sirin, A.; Couwenberg, J.; Laine, J.; Smith, P. The role of peatlands in climate regulation. In Peatland Restoration and Ecosystem Services: Science, Policy and Practice; Cambridge University Press: Cambridge, UK, 2016; pp. 63–76. ISBN 9781139177788. [Google Scholar]
- Caron, J.; Rochefort, L. Use of peat in growing media: State of the art on industrial and scientific efforts envisioning sustainability. Acta Hortic. 2013, 982, 15–22. [Google Scholar] [CrossRef]
- Simiele, M.; Argentino, O.; Baronti, S.; Scippa, G.S.; Chiatante, D.; Terzaghi, M.; Montagnoli, A. Biochar Enhances Plant Growth, Fruit Yield, and Antioxidant Content of Cherry Tomato (Solanum lycopersicum L.) in a Soilless Substrate. Agriculture 2022, 12, 1135. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to improve soil fertility. A review. Agron. Sustain. Dev. 2016, 36, 36. [Google Scholar] [CrossRef]
- Bird, M.I.; Wurster, C.M.; de Paula Silva, P.H.; Paul, N.A.; de Nys, R. Algal biochar: Effects and applications. GCB Bioenergy 2012, 4, 61–69. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Laird, D.A. The charcoal vision: A win-win-win scenario for simultaneously producing bioenergy, permanently sequestering carbon, while improving soil and water quality. Agron. J. 2008, 100, 178–181. [Google Scholar] [CrossRef]
- Galinato, S.P.; Yoder, J.K.; Granatstein, D. The economic value of biochar in crop production and carbon sequestration. Energy Policy 2011, 39, 6344–6350. [Google Scholar] [CrossRef]
- El-Naggar, A.; Lee, S.S.; Rinklebe, J.; Farooq, M.; Song, H.; Sarmah, A.K.; Zimmerman, A.R.; Ahmad, M.; Shaheen, S.M.; Ok, Y.S. Biochar application to low fertility soils: A review of current status, and future prospects. Geoderma 2019, 337, 536–554. [Google Scholar] [CrossRef]
- Polzella, A.; Terzaghi, M.; Trupiano, D.; Baronti, S.; Scippa, G.S.; Chiatante, D.; Montagnoli, A. Morpho-physiological responses of Pisum sativum L. to different light-emitting diode (led) light spectra in combination with biochar amendment. Agronomy 2020, 10, 398. [Google Scholar] [CrossRef]
- Deng, B.; Shang, X.; Fang, S.; Li, Q.; Fu, X.; Su, J. Integrated effects of light intensity and fertilization on growth and flavonoid accumulation in Cyclocarya Paliurus. J. Agric. Food Chem. 2012, 60, 6286–6292. [Google Scholar] [CrossRef]
- Grubb, P.J.; Lee, W.G.; Kollmann, J.; Wilson, J.B. Interaction of Irradiance and Soil Nutrient Supply on Growth of Seedlings of Ten European Tall-Shrub Species and Fagus Sylvatica. J. Ecol. 1996, 84, 827–840. [Google Scholar] [CrossRef]
- Keller, M.M.; Jaillais, Y.; Pedmale, U.V.; Moreno, J.E.; Chory, J.; Ballaré, C.L. Cryptochrome 1 and phytochrome B control shade-avoidance responses in Arabidopsis via partially independent hormonal cascades. Plant J. 2011, 67, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Kami, C.; Lorrain, S.; Hornitschek, P.; Fankhauser, C. Light-regulated plant growth and development. Curr. Top. Dev. Biol. 2010, 91, 29–66. [Google Scholar] [CrossRef] [PubMed]
- Shirley, H.L. The Influence of Light Intensity and Light Quality Upon the Growth of Plants. Am. J. Bot. 1929, 16, 354–390. [Google Scholar] [CrossRef]
- Sulpice, R.; Flis, A.; Ivakov, A.A.; Apelt, F.; Krohn, N.; Encke, B.; Abel, C.; Feil, R.; Lunn, J.E.; Stitt, M. Arabidopsis coordinates the diurnal regulation of carbon allocation and growth across a wide range of Photoperiods. Mol. Plant 2014, 7, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Minotta, G.; Pinzauti, S. Effects of light and soil fertility on growth, leaf chlorophyll content and nutrient use efficiency of beech (Fagus sylvatica L.) seedlings. For. Ecol. Manag. 1996, 86, 61–71. [Google Scholar] [CrossRef]
- Seehausen, M.L.; Gale, N.V.; Dranga, S.; Hudson, V.; Liu, N.; Michener, J.; Thurston, E.; Williams, C.; Smith, S.M.; Thomas, S.C. Is there a positive synergistic effect of biochar and compost soil amendments on plant growth and physiological performance? Agronomy 2017, 7, 13. [Google Scholar] [CrossRef]
- Miyagi, A.; Uchimiya, H.; Kawai-Yamada, M. Synergistic effects of light quality, carbon dioxide and nutrients on metabolite compositions of head lettuce under artificial growth conditions mimicking a plant factory. Food Chem. 2017, 218, 561–568. [Google Scholar] [CrossRef]
- Zea, L.; Oliva, G.; Vigliotta, G.; Terzaghi, M.; Guarino, F.; Cicatelli, A.; Montagnoli, A.; Castiglione, S. Counteracting action of Bacillus stratosphericus and Staphylococcus succinus strains against deleterious salt effects on Zea mays L. Front. Microbiol. 2023, 14, 1171980. [Google Scholar]
- Redondo-Gómez, S.; García-López, J.V.; Mesa-Marín, J.; Pajuelo, E.; Rodriguez-Llorente, I.D.; Mateos-Naranjo, E. Synergistic Effect of Plant-Growth-Promoting Rhizobacteria Improves Strawberry Growth and Flowering with Soil Salinization and Increased Atmospheric CO2 Levels and Temperature Conditions. Agronomy 2022, 12, 2082. [Google Scholar] [CrossRef]
- Sagar, A.; Rathore, P.; Ramteke, P.W.; Ramakrishna, W.; Reddy, M.S.; Pecoraro, L. Plant growth promoting rhizobacteria, arbuscular mycorrhizal fungi and their synergistic interactions to counteract the negative effects of saline soil on agriculture: Key macromolecules and mechanisms. Microorganisms 2021, 9, 1491. [Google Scholar] [CrossRef] [PubMed]
- Cresswell, H.P.; Green, T.W.; McKenzie, N.J. The Adequacy of Pressure Plate Apparatus for Determining Soil Water Retention. Soil Sci. Soc. Am. J. 2008, 72, 41–49. [Google Scholar] [CrossRef]
- Liao, P.B.; Kramer, S.S.L. Ion exchange systems for water recirculation. J. World Maric. Soc. 1981, 2, 32–39. [Google Scholar] [CrossRef]
- Bowman, R.A. A Rapid Method to Determine Total Phosphorus in Soils. Soil Sci. Soc. Am. J. 1988, 52, 1301–1304. [Google Scholar] [CrossRef]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction With Sodium Bicarbonate; US Department of Agriculture: Washington, DC, USA, 1954.
- US-EPA. Method 3052—Microwave assisted acid digestion of siliceous and organically based matrices. Tests Methods Eval. Solid Waste Phys. Methods SW 846—US Gov. Print. Off. 1996, 20. Available online: https://www.epa.gov/sites/default/files/2015-12/documents/3052.pdf (accessed on 10 September 2023).

| Growing Media | Treatment | pH | AWC (m3 m−3) | CEC (cmol kg−1) | EC (dS m−1) | ||||||||
| Low-fertility soil | CTR | 5.32 | ±0.05 | a * | 0.088 | ±0.004 | a | 12.58 | ±0.13 | a * | 1.23 | ±0.08 | a |
| Pe | 6.03 | ±0.05 | b * | 0.089 | ±0.009 | a | 12.43 | ±0.40 | a * | 1.30 | ±0.30 | ab | |
| B | 6.46 | ±0.09 | c | 0.085 | ±0.006 | a * | 14.40 | ±0.20 | b | 1.77 | ±0.20 | ab | |
| Ma | 5.04 | ±0.08 | d * | 0.114 | ±0.014 | a | 11.73 | ±0.80 | a * | 1.73 | ±0.10 | b | |
| SF | 5.77 | ±0.02 | e * | 0.097 | ±0.010 | a * | 11.97 | ±0.40 | a * | 1.83 | ±0.20 | ab | |
| LF | 5.92 | ±0.03 | b * | 0.100 | ±0.006 | a * | 12.93 | ±0.30 | a * | 1.83 | ±0.10 | b | |
| Soil-less substrate | CTR | 6.52 | ±0.03 | x * | 0.167 | ±0.032 | x | 16.50 | ±0.30 | x * | 1.43 | ±0.30 | xy |
| Pe | 6.67 | ±0.06 | x * | 0.157 | ±0.051 | x | 16.57 | ±0.10 | x * | 1.37 | ±0.20 | xy | |
| B | 6.62 | ±0.10 | xy | 0.174 | ±0.007 | x * | 17.23 | ±1.00 | x | 1.37 | ±0.10 | x | |
| Ma | 6.28 | ±0.07 | zw * | 0.150 | ±0.062 | x | 16.40 | ±0.50 | x * | 1.63 | ±0.10 | y | |
| SF | 6.36 | ±0.03 | yz * | 0.186 | ±0.021 | x * | 17.23 | ±0.70 | x * | 1.77 | ±0.30 | xy | |
| LF | 6.21 | ±0.03 | w * | 0.171 | ±0.011 | x * | 17.33 | ±1.00 | x * | 1.77 | ±0.10 | y | |
| Growing media | Treatment | Ctot (%) | Corg (%) | Ntot (%) | Norg (%) | ||||||||
| Low-fertility soil | CTR | 1.80 | ±0.01 | a * | 1.66 | ±0.37 | a | 0.16 | ±0.00 | ab * | 0.15 | ±0.03 | ab |
| Pe | 2.02 | ±0.01 | b * | 2.48 | ±0.69 | ab | 0.18 | ±0.00 | b * | 0.12 | ±0.00 | a | |
| B | 3.23 | ±0.01 | c * | 2.44 | ±0.49 | a * | 0.18 | ±0.00 | ab * | 0.15 | ±0.01 | b | |
| Ma | 2.09 | ±0.02 | d * | 2.63 | ±0.76 | a * | 0.20 | ±0.00 | c * | 0.14 | ±0.01 | ab * | |
| SF | 1.86 | ±0.01 | e * | 1.33 | ±0.07 | ab * | 0.18 | ±0.00 | a * | 0.13 | ±0.02 | ab | |
| LF | 1.96 | ±0.07 | abde * | 0.54 | ±0.42 | b | 0.19 | ±0.00 | c | 0.14 | ±0.06 | ab | |
| Soil-less substrate | CTR | 9.06 | ±0.01 | x * | 2.39 | ±0.14 | x | 0.23 | ±0.01 | xz * | 0.21 | ±0.03 | xy |
| Pe | 8.62 | ±0.03 | y * | 2.90 | ±0.06 | y | 0.27 | ±0.01 | xyz * | 0.25 | ±0.07 | xy | |
| B | 11.87 | ±0.07 | z * | 9.45 | ±0.21 | z * | 0.27 | ±0.00 | y * | 0.23 | ±0.06 | xy | |
| Ma | 8.32 | ±0.01 | w * | 6.60 | ±0.09 | w * | 0.25 | ±0.01 | z * | 0.23 | ±0.01 | x * | |
| SF | 8.21 | ±0.02 | v * | 2.46 | ±0.21 | xy * | 0.27 | ±0.00 | yz * | 0.22 | ±0.04 | xy | |
| LF | 6.22 | ±0.00 | u * | 1.60 | ±0.50 | xy | 0.19 | ±0.01 | w | 0.16 | ±0.01 | y | |
| Growing media | Treatment | Ptot (ppm) | Pav (ppm) | Ktot (ppm) | Kav (ppm) | ||||||||
| Low-fertility soil | CTR | 574.5 | ±0.53 | a * | 0.38 | ±0.01 | a * | 1129.3 | ±5.70 | a * | 19.4 | ±0.08 | a |
| Pe | 579.8 | ±2.82 | a * | 0.46 | ±0.03 | a * | 1267.7 | ±0.47 | b * | 22.5 | ±0.25 | b * | |
| B | 706.2 | ±0.59 | b * | 0.56 | ±0.01 | b * | 1283.8 | ±23.67 | b * | 23.3 | ±1.62 | abc | |
| Ma | 605.5 | ±0.89 | c * | 0.41 | ±0.01 | a * | 1777.7 | ±3.32 | c * | 25.7 | ±0.44 | c * | |
| SF | 590.7 | ±0.54 | d * | 0.41 | ±0.01 | a * | 1893.8 | ±5.09 | d * | 292.6 | ±3.41 | d * | |
| LF | 603.3 | ±2.75 | c * | 1.23 | ±0.07 | c * | 1778.8 | ±0.61 | c * | 314.5 | ±10.45 | d | |
| Soil-less substrate | CTR | 878.8 | ±0.66 | x * | 0.98 | ±0.01 | x * | 1677.5 | ±3.13 | x * | 20.3 | ±0.32 | x |
| Pe | 868.4 | ±8.44 | x * | 0.96 | ±0.09 | xz * | 1679.0 | ±0.02 | x * | 19.6 | ±0.44 | x * | |
| B | 577.4 | ±6.02 | y * | 0.39 | ±0.01 | y * | 1787.1 | ±3.91 | y * | 26.0 | ±1.15 | y | |
| Ma | 880.9 | ±1.11 | x * | 0.90 | ±0.01 | z * | 2739.7 | ±0.99 | z * | 103.4 | ±2.22 | z * | |
| SF | 998.1 | ±2.54 | z * | 1.03 | ±0.04 | xz * | 1784.9 | ±1.35 | y * | 314.3 | ±0.89 | w * | |
| LF | 1005.7 | ±2.11 | w * | 1.89 | ±0.04 | w * | 2949.3 | ±27.90 | w * | 320.9 | ±8.17 | w | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beatrice, P.; Miali, A.; Baronti, S.; Chiatante, D.; Montagnoli, A. Plant Growth in LED-Sourced Biophilic Environments Is Improved by the Biochar Amendment of Low-Fertility Soil, the Reflection of Low-Intensity Light, and a Continuous Photoperiod. Plants 2023, 12, 3319. https://doi.org/10.3390/plants12183319
Beatrice P, Miali A, Baronti S, Chiatante D, Montagnoli A. Plant Growth in LED-Sourced Biophilic Environments Is Improved by the Biochar Amendment of Low-Fertility Soil, the Reflection of Low-Intensity Light, and a Continuous Photoperiod. Plants. 2023; 12(18):3319. https://doi.org/10.3390/plants12183319
Chicago/Turabian StyleBeatrice, Peter, Alessio Miali, Silvia Baronti, Donato Chiatante, and Antonio Montagnoli. 2023. "Plant Growth in LED-Sourced Biophilic Environments Is Improved by the Biochar Amendment of Low-Fertility Soil, the Reflection of Low-Intensity Light, and a Continuous Photoperiod" Plants 12, no. 18: 3319. https://doi.org/10.3390/plants12183319
APA StyleBeatrice, P., Miali, A., Baronti, S., Chiatante, D., & Montagnoli, A. (2023). Plant Growth in LED-Sourced Biophilic Environments Is Improved by the Biochar Amendment of Low-Fertility Soil, the Reflection of Low-Intensity Light, and a Continuous Photoperiod. Plants, 12(18), 3319. https://doi.org/10.3390/plants12183319








