Divergence in Glyphosate Susceptibility between Steinchisma laxum Populations Involves a Pro106Ser Mutation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Glyphosate Dose–Response Experiments
2.3. Quantification of Accumulated Shikimic Acid
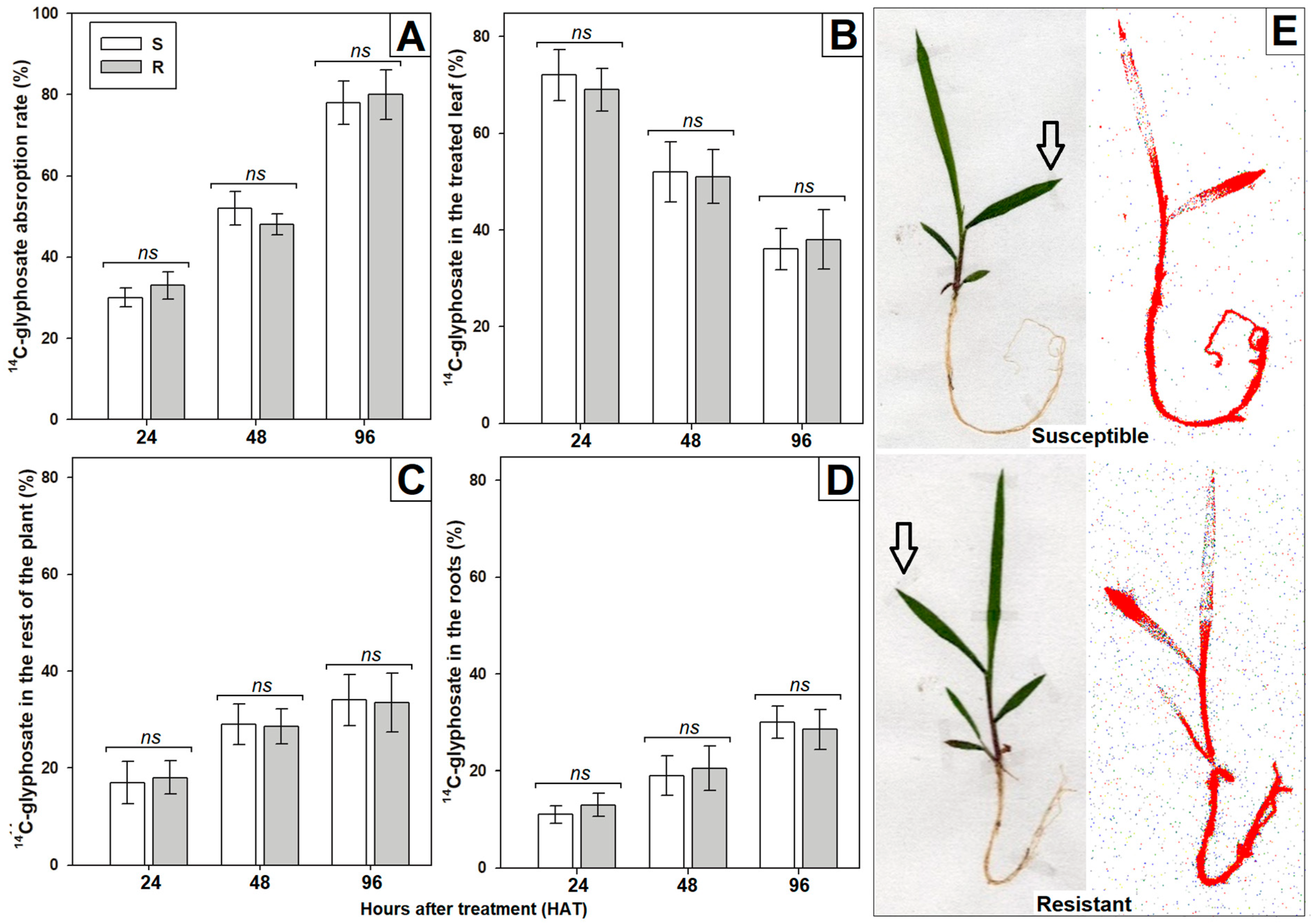
2.4. Determination of Absorption and Translocation Rates with 14C-Radilabeled Glyphosate
2.5. Quantification of Glyphosate and Potential Metabolites Using CE
2.6. Enzymatic Interaction of Glyphosate with Its Target Site
2.7. EPSPS Gene Sequencing
2.8. Statistics
3. Results
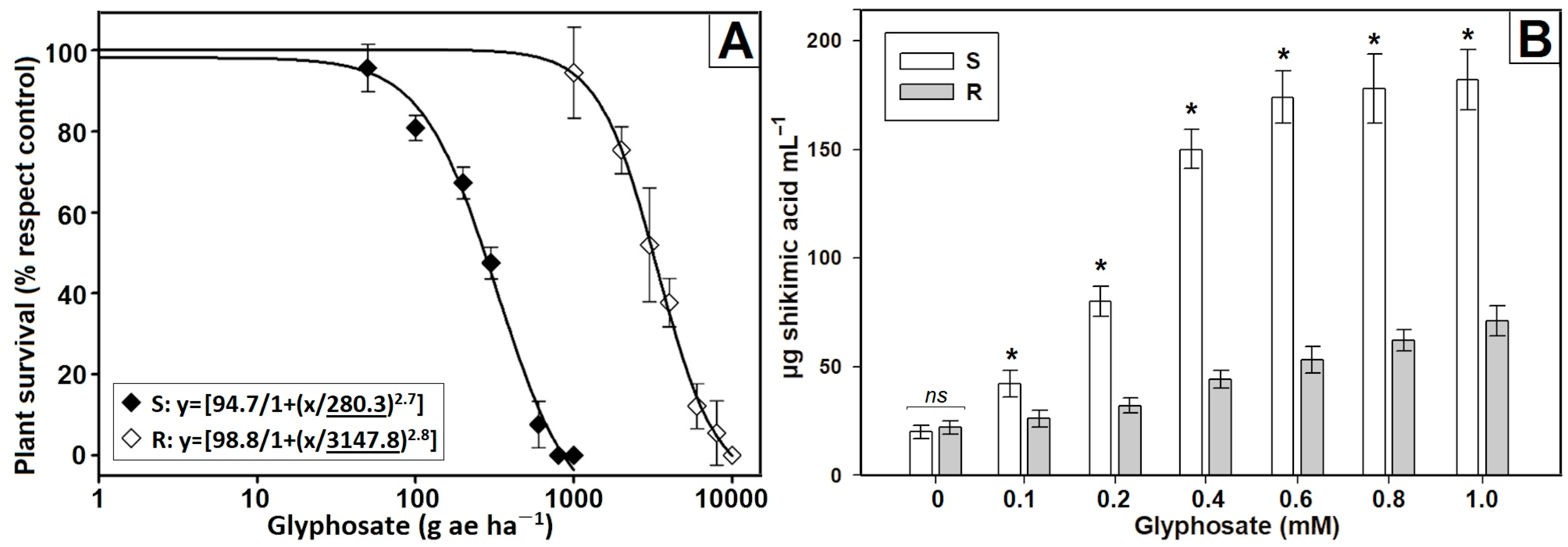
3.1. Confirmation of Resistance
3.2. Characterization of Non-Target Site Mechanisms
3.3. Characterization of Target Site Mechanisms
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prasad, R.; Shivay, Y.S.; Kumar, D. Current status, challenges, and opportunities in rice production. In Rice Production Worldwide; Chauhan, B.S., Jabran, K., Mahajan, G., Eds.; Springer: Cham, Switzerland, 2017; Volume 1, pp. 1–32. [Google Scholar]
- Chica, L.J.; Tirado, O.Y.C.; Barreto, O.J.M. Competitive indicators from Colombia and U.S. rice production. Rev. Cienc. Agríc. 2016, 33, 16–31. [Google Scholar]
- Parra-Peña, R.I.; Flórez, S.; Rodríguez, D. La Competitividad de la Cadena del Arroz en Colombia: Un Compromiso con el Bienestar del Agricultor; Fedesarrollo: Bogotá, Colombia, 2022; Available online: https://www.repository.fedesarrollo.org.co/handle/11445/4237 (accessed on 31 May 2023).
- Ramirez, J.G.; Plaza, G. Effect of post-emergence herbicide applications on rice crop weed communities in Tolima, Colombia. Planta Daninha 2015, 33, 499–508. [Google Scholar] [CrossRef]
- Singh, V.; Zhou, S.; Ganie, Z.; Valverde, B.; Avila, L.; Marchesan, E.; Merotto, A.; Zorrilla, G.; Burgos, N.; Norsworthy, J.; et al. Rice production in the Americas. In Rice Production Worldwide; Chauhan, B.S., Jabran, K., Mahajan, G., Eds.; Springer: Cham, Switzerland, 2017; Volume 1, pp. 137–168. [Google Scholar]
- Calha, I.; Oliveira, M.F.; Reis, P. Weed management challenges in rice cultivation in the context of pesticide use reduction: A survey approach. Sustainability 2023, 15, 244. [Google Scholar] [CrossRef]
- Renton, M.; Busi, R.; Neve, P.; Thornby, D.; Vila-Aiub, M. Herbicide resistance modelling: Past, present and future. Pest Manag. Sci. 2014, 70, 1394–1404. [Google Scholar] [CrossRef] [PubMed]
- Hulme, P.E. Global drivers of herbicide-resistant weed richness in major cereal crops worldwide. Pest Manag. Sci. 2022, 78, 1824–1832. [Google Scholar] [CrossRef]
- Plaza, G.; Hoyos, V.; Vázquez-García, J.G.; Alcántara-de la Cruz, R.; De Prado, R. First case of multiple resistance to EPSPS and PSI in Eleusine indica (L.) Gaertn. collected in rice and herbicide-resistant crops in Colombia. Agronomy 2021, 11, 96. [Google Scholar] [CrossRef]
- Hoyos, V.; Plaza, G.; Vázquez-Garcia, J.G.; Palma-Bautista, C.; Rojano-Delgado, A.M.; De Prado, R. Confirmation of multiple resistant Chloris radiata population, harvested in Colombian rice fields. Agronomy 2021, 11, 496. [Google Scholar] [CrossRef]
- Vázquez-García, J.G.; Hoyos, V.; Plaza, G.; Palma-Bautista, C.; Alcántara-de la Cruz, R.; De Prado, R. Glyphosate resistance in Chloris radiata from Colombian rice fields involves one target-site mechanism. Chemosphere 2021, 281, 130888. [Google Scholar] [CrossRef]
- Giraldo-Cañas, D. Distribution and invasion of C3 and C4 grasses (Poaceae) along an altitudinal gradient in the Andes of Colombia. Caldasia 2010, 32, 65–86. [Google Scholar]
- GBIF Secretariat. Steinchisma laxum (Sw.) Zuloaga. GBIF Backbone Taxonomy. Available online: https://www.gbif.org/species/4933007 (accessed on 3 June 2023).
- Heap, I. The International Herbicide-Resistant Weed Database. Available online: https://www.weedscience.com (accessed on 28 May 2023).
- Travlos, I.; De Prado, R.; Chachalis, D.; Bilalis, D.J. Herbicide resistance in weeds: Early detection, mechanisms, dispersal, new insights and management issues. Front. Ecol. Evol. 2020, 8, 213. [Google Scholar] [CrossRef]
- Heap, I.; Duke, S.O. Overview of glyphosate-resistant weeds worldwide. Pest Manag. Sci. 2018, 74, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O. The history and current status of glyphosate. Pest Manag. Sci. 2018, 74, 1027–1034. [Google Scholar] [CrossRef]
- Orcaray, L.; Zulet, A.; Zabalza, A.; Royuela, M. Impairment of carbon metabolism induced by the herbicide glyphosate. J. Plant Physiol. 2012, 169, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Fedearroz. Manejo Integrado del Cultivo del Arroz. Produmedios. Available online: https://fedearroz.com.co/es/noticias/2012/01/17/manejo-integrado-del-cultivo-del-arroz/ (accessed on 3 June 2023).
- Hoyos, V.; Plaza, G.; Caicedo, A.L. Characterization of the phenotypic variability in Colombian weedy rice (Oryza spp.). Weed Sci. 2019, 67, 441–452. [Google Scholar] [CrossRef]
- de María, N.; Becerril, J.M.; García-Plazaola, J.I.; Hernández, A.; De Felipe, M.R.; Fernández-Pascual, M. New insights on glyphosate mode of action in nodular metabolism: Role of shikimate accumulation. J. Agric. Food Chem. 2006, 54, 2621–2628. [Google Scholar] [CrossRef]
- Rojano-Delgado, A.M.; Ruiz-Jiménez, J.; De Castro, M.D.L.; De Prado, R. Determination of glyphosate and its metabolites in plant material by reversed-polarity CE with indirect absorptiometric detection. Electrophoresis 2010, 31, 1423–1430. [Google Scholar] [CrossRef]
- Dayan, F.E.; Owens, D.K.; Corniani, N.; Silva, F.M.L.; Watson, S.B.; Howell, J.; Shaner, D.L. Biochemical markers and enzyme assays for herbicide mode of action and resistance studies. Weed Sci. 2015, 63, 23–63. [Google Scholar] [CrossRef]
- Rio, D.C.; Ares, M.; Hannon, G.J.; Nilsen, T.W. Purification of RNA using TRIzol (TRI Reagent). Cold Spring Harb. Protoc. 2010, 5, pdb.prot5439. [Google Scholar] [CrossRef]
- Alcántara-de la Cruz, R.; Fernández-Moreno, P.T.; Ozuna, C.V.; Rojano-Delgado, A.M.; Cruz-Hipolito, H.E.; Domínguez-Valenzuela, J.A.; Barro, F.; De Prado, R. Target and non-target site mechanisms developed by glyphosate-resistant hairy beggarticks (Bidens pilosa L.) populations from Mexico. Front. Plant Sci. 2016, 7, 1492. [Google Scholar] [CrossRef]
- Keshtkar, E.; Kudsk, P.; Mesgaran, M.B. Common errors in dose–response analysis and how to avoid them. Pest Manag. Sci. 2021, 77, 2599–2608. [Google Scholar] [CrossRef]
- Seefeldt, S.S.; Jensen, J.E.; Fuerst, E.P. Log-logistic analysis of herbicide dose-response relationships. Weed Technol. 1995, 9, 218–227. [Google Scholar] [CrossRef]
- Nielsen, O.K.; Ritz, C.; Streibig, J.C. Nonlinear mixed-model regression to analyze herbicide dose–response relationships. Weed Technol. 2004, 18, 30–37. [Google Scholar] [CrossRef]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Hernández-Carlos, B.; Villanueva-Cañongo, C.; Santos-Sánchez, N.F. Shikimic acid pathway in biosynthesis of phenolic compounds. In Plant Physiological Aspects of Phenolic Compounds.; Soto-Hernández, M., García-Mateos, R., Palma-Tenango, M., Eds.; IntechOpen: London, UK, 2019; Volume 1, pp. 35–50. [Google Scholar]
- Baek, Y.; Bobadilla, L.K.; Giacomini, D.A.; Montgomery, J.S.; Murphy, B.P.; Tranel, P.J. Evolution of glyphosate-resistant weeds. Rev. Environ. Contam. Toxicol. 2021, 255, 93–128. [Google Scholar] [PubMed]
- Nandula, V.K.; Wright, A.A.; Van Horn, C.R.; Molin, W.T.; Westra, P.; Reddy, K.N. Glyphosate resistance in giant ragweed (Ambrosia trifida L.) from mississippi is partly due to reduced translocation. Am. J. Plant Sci. 2015, 6, 2104–2113. [Google Scholar] [CrossRef]
- Palma-Bautista, C.; Torra, J.; Garcia, M.J.; Bracamonte, E.; Rojano-Delgado, A.M.; Alcántara-De La Cruz, R.; De Prado, R. Reduced absorption and impaired translocation endows glyphosate resistance in Amaranthus palmeri harvested in glyphosate-resistant soybean from Argentina. J. Agric. Food Chem. 2019, 67, 1052–1060. [Google Scholar] [CrossRef]
- Duke, S.O. Enhanced metabolic degradation: The last evolved glyphosate resistance mechanism of weeds? Plant Physiol. 2019, 181, 1401–1403. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.J.; Peter, F.; Norsworthy, J.K.; Beffa, R. Uptake, translocation, and metabolism of glyphosate, glufosinate, and dicamba mixtures in Echinochloa crus-galli and Amaranthus palmeri. Pest Manag. Sci. 2020, 76, 3078–3087. [Google Scholar] [CrossRef]
- Gaines, T.A.; Patterson, E.L.; Neve, P. Molecular mechanisms of adaptive evolution revealed by global selection for glyphosate resistance. New Phytol. 2019, 223, 1770–1775. [Google Scholar] [CrossRef]
- Yanniccari, M.; Vázquez-García, J.G.; Gigón, R.; Palma-Bautista, C.; Vila-Aiub, M.; De Prado, R. A novel EPSPS Pro-106-His mutation confers the first case of glyphosate resistance in Digitaria sanguinalis. Pest Mang. Sci. 2022, 78, 3135–3143. [Google Scholar] [CrossRef]
- Han, H.; Yu, Q.; Widderick, M.J.; Powles, S.B. Target-site EPSPS Pro-106 mutations: Sufficient to endow glyphosate resistance in polyploid Echinochloa colona? Pest Mang. Sci. 2016, 72, 264–271. [Google Scholar] [CrossRef]
- Fonseca, E.C.M.; Da Costa, K.S.; Lameira, J.; Alves, C.N.; Lima, A.H. Investigation of the target-site resistance of EPSPS synthase mutants P106T and T102I/P106S against glyphosate. RSC Adv. 2020, 10, 44352–44360. [Google Scholar] [CrossRef] [PubMed]
- Sammons, R.D.; Gaines, T.A. Glyphosate resistance: State of knowledge. Pest Mang. Sci. 2014, 70, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- McElroy, J.S.; Hall, N.D. Echinochloa colona with reported resistance to glyphosate conferred by aldo-keto reductase also contains a Pro-106-Thr EPSPS target site mutation. Plant Physiol. 2020, 183, 447–450. [Google Scholar] [CrossRef]
- Yanniccari, M.; Palma-Bautista, C.; Vázquez-García, J.G.; Gigón, R.; Mallory-Smith, C.A.; De Prado, R. Constitutive overexpression of EPSPS by gene duplication is involved in glyphosate resistance in Salsola tragus. Pest Mang. Sci. 2023, 79, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Barroso, A.A.M.; Michelon, T.B.; Alves, P.L.C.A.; Han, H.; Yu, Q.; Powles, S.B.; Vila-Aiub, M.M. Challenging glyphosate resistance EPSPS P106S and TIPS mutations with soybean competition and glyphosate: Implications for management. Pest Mang. Sci. 2022, 78, 4764–4773. [Google Scholar] [CrossRef]
- Mathioni, S.M.; Oliveira, C.D.; Lemes, L.N.; Ozório, E.G.; Rosa, D.D. PCR-based assay to detect the EPSPS TAP-IVS substitution in Amaranthus hybridus. Adv. Weed Sci. 2022, 40, e20210048. [Google Scholar] [CrossRef]
- Takano, H.K.; Fernandes, V.N.; Adegas, F.S.; Oliveira, R.S., Jr.; Westra, P.; Gaines, T.A.; Dayan, F.E. A novel TIPT double mutation in EPSPS conferring glyphosate resistance in tetraploid Bidens subalternans. Pest Mang. Sci. 2020, 76, 95–102. [Google Scholar] [CrossRef]
- Beres, Z.T.; Giese, L.A.; Mackey, D.M.; Owen, M.D.K.; Page, E.R.; Snow, A.A. Target-site EPSPS Pro-106-Ser mutation in Conyza canadensis biotypes with extreme resistance to glyphosate in Ohio and Iowa, USA. Sci. Rep. 2020, 10, 7577. [Google Scholar] [CrossRef]
- Pan, L.; Yu, Q.; Wang, J.; Han, H.; Mao, L.; Nyporko, A.; Maguza, A.; Fan, L.; Bai, L.; Powles, S. An ABCC-type transporter endowing glyphosate resistance in plants. Proc. Natl. Acad. Sci. USA 2021, 118, e2100136118. [Google Scholar] [CrossRef]


| Compound | Population | |
|---|---|---|
| S | R | |
| Glyphosate ns | 96.2 ± 2.7 | 97.4 ± 3.6 |
| AMPA ns | 3.8 ± 2.2 | 2.6 ± 1.8 |
| Glyoxylate | nd | nd |
| Sarcosine | nd | nd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoyos, V.; Plaza, G.; Palma-Bautista, C.; Vázquez-García, J.G.; Dominguez-Valenzuela, J.A.; Alcántara-de la Cruz, R.; De Prado, R. Divergence in Glyphosate Susceptibility between Steinchisma laxum Populations Involves a Pro106Ser Mutation. Plants 2023, 12, 3315. https://doi.org/10.3390/plants12183315
Hoyos V, Plaza G, Palma-Bautista C, Vázquez-García JG, Dominguez-Valenzuela JA, Alcántara-de la Cruz R, De Prado R. Divergence in Glyphosate Susceptibility between Steinchisma laxum Populations Involves a Pro106Ser Mutation. Plants. 2023; 12(18):3315. https://doi.org/10.3390/plants12183315
Chicago/Turabian StyleHoyos, Veronica, Guido Plaza, Candelario Palma-Bautista, Jose G. Vázquez-García, José Alfredo Dominguez-Valenzuela, Ricardo Alcántara-de la Cruz, and Rafael De Prado. 2023. "Divergence in Glyphosate Susceptibility between Steinchisma laxum Populations Involves a Pro106Ser Mutation" Plants 12, no. 18: 3315. https://doi.org/10.3390/plants12183315
APA StyleHoyos, V., Plaza, G., Palma-Bautista, C., Vázquez-García, J. G., Dominguez-Valenzuela, J. A., Alcántara-de la Cruz, R., & De Prado, R. (2023). Divergence in Glyphosate Susceptibility between Steinchisma laxum Populations Involves a Pro106Ser Mutation. Plants, 12(18), 3315. https://doi.org/10.3390/plants12183315









