Maturity Assessment of Different Table Grape Cultivars Grown at Six Different Altitudes in Lebanon
Abstract
1. Introduction
2. Material and Method
2.1. Plant Materials and Vineyard Locations
2.2. Physiochemical Parameters
2.3. Phytochemical Analyses
2.4. FRAP Assay
2.5. Statistical Analyses
3. Results
3.1. Evaluation of Maturity Indices during Maturation
3.2. Physiochemical Characteristics of Grapes at Harvest
3.3. Location Effect on Clusters
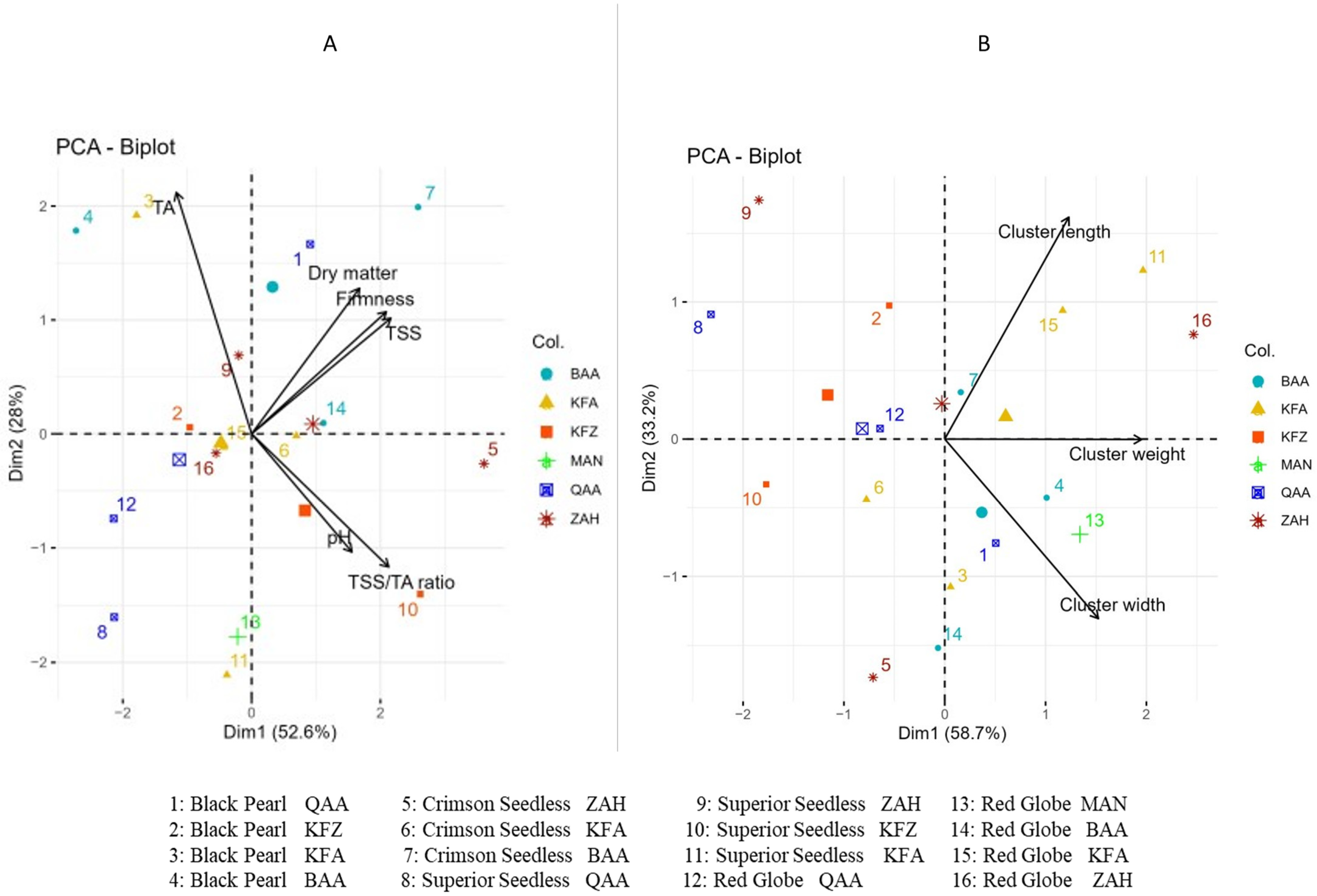
3.4. Principal Component Analysis (PCA)
3.5. Phytochemicals and Antioxidant Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. The Food and Agriculture Organization Corporate Statistical Database. Available online: https://www.fao.org/faostat/ (accessed on 8 November 2022).
- OIV. International Organisation of Vine and Wine. 2019 Statistical Report on World Viticulture. Available online: http://oiv.int/public/medias/6782/oiv-2019-statistical-report-on-world-vitiviniculture.pdf (accessed on 15 November 2022).
- Ghedira, K. Les flavonoïdes: Structure, propriétés biologiques, rôle prophylactique et emplois en thérapeutique. Phytothérapie 2005, 3, 162–169. [Google Scholar] [CrossRef]
- Brat, P.; Georgé, S.; Bellamy, A.; Chaffaut, L.D.; Scalbert, A.; Mennen, L.; Arnault, N.; Amiot, M.J. Daily polyphenol intake in France from fruit and vegetables. J. Nutr. 2006, 136, 2368–2373. [Google Scholar] [CrossRef]
- Tenore, G.C.; Calabrese, G.; Stiuso, P.; Ritieni, A.; Giannetti, D.; Novellino, E. Effects of Annurca apple polyphenols on lipid metabolism in HepG2 cell lines: A source of nutraceuticals potentially indicated for the metabolic syndrome. Food Res. Int. 2014, 63, 252–257. [Google Scholar] [CrossRef]
- Zohary, D. The domestication of the grapevine Vitis vinifera L. in the Near East. In The Origins and Ancient History of Wine; Routledge: London, UK, 2003; pp. 44–51. [Google Scholar]
- Chalak, L.; Touma, S.; Rahme, S.; Azzi, R.; Guiberteau, F.; Touma, J.-A. Assessment of the Lebanese grapevine germplasm reveals a substantial diversity and a high potential for selection. In Proceedings of the BIO Web of Conferences, Bento Gonçalves, Brazil, 24–28 October 2016; p. 01020. [Google Scholar] [CrossRef]
- Tabaja, N.; Siblini, M.; Chalak, L. Current status of registered grapevine saplings in Lebanon. In Proceedings of the International Horticultural Congress IHC2018: International Symposium on Viticulture: Primary Production and Processing, Istanbul, Turkey, 12–16 August 2018; pp. 265–270. [Google Scholar]
- Habib, W.; Khalil, J.; Mincuzzi, A.; Saab, C.; Gerges, E.; Tsouvalakis, H.C.; Ippolito, A.; Sanzani, S.M. Fungal pathogens associated with harvested table grapes in Lebanon, and characterization of the mycotoxigenic genera. Phytopathol. Mediterr. 2021, 60, 427–439. [Google Scholar] [CrossRef]
- Kontoudakis, N.; Esteruelas, M.; Fort, F.; Canals, J.M.; De Freitas, V.; Zamora, F. Influence of the heterogeneity of grape phenolic maturity on wine composition and quality. Food Chem. 2011, 124, 767–774. [Google Scholar] [CrossRef]
- Segade, S.R.; Giacosa, S.; de Palma, L.; Novello, V.; Torchio, F.; Gerbi, V.; Rolle, L. Effect of the cluster heterogeneity on mechanical properties, chromatic indices and chemical composition of Italia table grape berries (Vitis vinifera L.) sorted by flotation. Int. J. Food Sci. Technol. 2013, 48, 103–113. [Google Scholar] [CrossRef]
- Palou, L.; Serrano, M.; Martínez-Romero, D.; Valero, D. New approaches for postharvest quality retention of table grapes. Fresh Prod. 2010, 4, 103–110. [Google Scholar]
- Hamie, N.; Tarricone, L.; Verrastro, V.; Natrella, G.; Faccia, M.; Gambacorta, G. Assessment of “Sugranineteen” Table Grape Maturation Using Destructive and Auto-Fluorescence Methods. Foods 2022, 11, 663. [Google Scholar] [CrossRef]
- Piazzolla, F.; Amodio, M.L.; Colelli, G. Spectra evolution over on-vine holding of Italia table grapes: Prediction of maturity and discrimination for harvest times using a Vis-NIR hyperspectral device. J. Agric. Eng. 2017, 48, 109–116. [Google Scholar] [CrossRef][Green Version]
- Gross, K.; Wang, C.Y.; Saltveit, M. The Commercial Storage of Fruits, Vegetables, and Florist and Nursery Stocks; United States Department of Agriculture, Agricultural Research Service: Davis, CA, USA, 2016; Volume 66, pp. 344–348. Available online: https://www.ars.usda.gov/is/np/CommercialStorage/CommercialStorage.pdf (accessed on 8 November 2022).
- Ferrer-Gallego, R.; Hernández-Hierro, J.M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Influence of climatic conditions on the phenolic composition of Vitis vinifera L. cv. Graciano. Anal. Chem. Acta 2012, 732, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Nogales-Bueno, J.; Hernández-Hierro, J.M.; Rodríguez-Pulido, F.J.; Heredia, F.J. Determination of technological maturity of grapes and total phenolic compounds of grape skins in red and white cultivars during ripening by near infrared hyperspectral image: A preliminary approach. Food Chem. 2014, 152, 586–591. [Google Scholar] [CrossRef]
- Meinert, L.D. The science of terroir. Elements 2018, 14, 153–158. [Google Scholar] [CrossRef]
- Cureau, N.; Threlfall, R.; Savin, M.; Marasini, D.; Lavefve, L.; Carbonero, F. Year, location, and variety impact on grape-, soil-, and leaf-associated fungal microbiota of arkansas-grown table grapes. Microb. Ecol. 2021, 82, 73–86. [Google Scholar] [CrossRef]
- Zhang, X.; Kontoudakis, N.; Šuklje, K.; Antalick, G.; Blackman, J.W.; Rutledge, D.N.; Schmidtke, L.M.; Clark, A.C. Changes in red wine composition during bottle aging: Impacts of grape variety, vineyard location, maturity, and oxygen availability during aging. J. Agric. Food Chem. 2020, 68, 13331–13343. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Regules, A.; Romero-Cascales, I.; López-Roca, J.M.; Ros-García, J.M.; Gómez-Plaza, E. Anthocyanin fingerprint of grapes: Environmental and genetic variations. J. Sci. Food Agric. 2006, 86, 1460–1467. [Google Scholar] [CrossRef]
- Raspor, P.; Milek, D.M.; Polanc, J.; Možina, S.S.; Čadež, N. Yeasts isolated from three varieties of grapes cultivated in different locations of the Dolenjska vine-growing region, Slovenia. Int. J. Food Microbiol. 2006, 109, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Pajovic, R.; Raicevic, D.; Popovic, T.; Sivilotti, P.; Lisjak, K.; Vanzo, A. Polyphenolic characterisation of Vranac, Kratosija and Cabernet Sauvignon (Vitis vinifera L. cv.) grapes and wines from different vineyard locations in Montenegro. S. Afr. J. Enol. Vitic. 2014, 35, 139–148. [Google Scholar] [CrossRef]
- Folin, O.; Denis, W. A colorimetric method for the determination of phenols (and phenol derivatives) in urine. J. Biol. Chem. 1915, 22, 305–308. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, Y.; Cao, L.; Lu, J. Phenolic compounds and antioxidant properties of different grape cultivars grown in China. Food Chem. 2010, 119, 1557–1565. [Google Scholar] [CrossRef]
- Kelebek, H.; Canbas, A.; Jourdes, M.; Teissedre, P.L. HPLC-DAD-MS determination of colored and colorless phenolic compounds in Kalecik karasi wines: Effect of different vineyard locations. Anal. Lett. 2011, 44, 991–1008. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Moreno-Simunovic, Y. Location effects on ripening and grape phenolic composition of eight ‘Carignan’vineyards from Maule Valley (Chile). Chil. J. Agric. Res. 2018, 78, 139–149. [Google Scholar] [CrossRef]
- Barriga-Sanchez, M.; Rosales-Hartshorn, M. Effects of subcritical water extraction and cultivar geographical location on the phenolic compounds and antioxidant capacity of Quebranta (Vitis vinifera) grape seeds from the Peruvian pisco industry by-product. Food Sci. Technol. 2022, 42, e107321. [Google Scholar] [CrossRef]
- Mansour, G.; Ghanem, C.; Mercenaro, L.; Nassif, N.; Hassoun, G.; Del Caro, A. Effects of altitude on the chemical composition of grapes and wine: A review. OENO One 2022, 56, 227–239. [Google Scholar] [CrossRef]
- Vial, P.; Crisosto, C.; Crisosto, G. Early harvest delays berry skin browning of ‘Princess’ table grapes. Calif. Agric. 2005, 59, 103–108. [Google Scholar] [CrossRef][Green Version]
- Arias, L.A.; Berli, F.; Fontana, A.; Bottini, R.; Piccoli, P. Climate change effects on grapevine physiology and biochemistry: Benefits and challenges of high altitude as an adaptation strategy. Front. Plant Sci. 2022, 13, 835425. [Google Scholar] [CrossRef] [PubMed]
- Conde, C.; Silva, P.; Fontes, N.; Dias, A.C.P.; Tavares, R.M.; Sousa, M.J.; Agasse, A.; Delrot, S.; Gerós, H. Biochemical changes throughout grape berry development and fruit and wine quality. Food 2007, 1, 1–22. [Google Scholar]
- Segade, S.R.; Giacosa, S.; Torchio, F.; de Palma, L.; Novello, V.; Gerbi, V.; Rolle, L. Impact of different advanced ripening stages on berry texture properties of ‘Red Globe’and ‘Crimson Seedless’ table grape cultivars (Vitis vinifera L.). Sci. Hortic. 2013, 160, 313–319. [Google Scholar] [CrossRef]
- Brummell, D.A. Cell wall disassembly in ripening fruit. Funct. Plant Biol. 2006, 33, 103–119. [Google Scholar] [CrossRef]
- Goulao, L.F. Pectin de-esterification and fruit softening: Revisiting a classical hypothesis. Stew. Postharvest Rev. 2010, 6, 1–12. [Google Scholar] [CrossRef]
- Balic, I.; Ejsmentewicz, T.; Sanhueza, D.; Silva, C.; Peredo, T.; Olmedo, P.; Barros, M.; Verdonk, J.C.; Paredes, R.; Meneses, C. Biochemical and physiological study of the firmness of table grape berries. Postharvest Biol. Technol. 2014, 93, 15–23. [Google Scholar] [CrossRef]
- Commission, E. Commission Implementing Regulation (EU) No 543/2011 of 7 June 2011, laying down detailed rules for the application of council regulation (EC) No 1234/2007 in respect of the fruit and vegetables and processed fruit and vegetables sectors. Off J. Eur. Union. 2011, 157, 1–163. [Google Scholar]
- Crisosto, C.; Smilanick, J. Table Grapes Postharvest Quality Maintenance Guidelines; Online publication; The University of California Davis: Davis, CA, USA, 2007; Available online: http://kare.ucanr.edu/files/123831.pdf (accessed on 10 December 2021).
- Dami, I. Determining Grape Maturity and Fruit Sampling; Ohio State University: Columbos, OH, USA, 2014; Available online: https://ohioline.osu.edu/factsheet/HYG-1436 (accessed on 15 November 2022).
- Nicolosi, E.; Ferlito, F.; Amenta, M.; Russo, T.; Rapisarda, P. Changes in the quality and antioxidant components of minimally processed table grapes during storage. Sci. Hortic. 2018, 232, 175–183. [Google Scholar] [CrossRef]
- Admane, N.; Genovese, F.; Altieri, G.; Tauriello, A.; Trani, A.; Gambacorta, G.; Verrastro, V.; Di Renzo, G.C. Effect of ozone or carbon dioxide pre-treatment during long-term storage of organic table grapes with modified atmosphere packaging. LWT 2018, 98, 170–178. [Google Scholar] [CrossRef]
- Cantos, E.; Espin, J.C.; Tomás-Barberán, F.A.J. Varietal differences among the polyphenol profiles of seven table grape cultivars studied by LC−DAD−MS−MS. J. Agric. Food Chem. 2002, 50, 5691–5696. [Google Scholar] [CrossRef]
- de Palma, L.; Tarricone, L.; Limosani, P.; De Michele, M.; Novello, V. Skin phenols in seedless grapes (Vitis vinifera L.) as influenced by genotype and irrigation. In Proceedings of the Actes de Congrès: 35Ème Congrès de la Vigne ET du Vin, Izmir, Turkey, 18–22 June 2012; pp. 1–8. Available online: https://iris.unito.it/handle/2318/138867 (accessed on 8 November 2022).
- Callaghan, C.M.; Leggett, R.E.; Levin, R.M. A comparison of total antioxidant capacities of concord, purple, red, and green grapes using the CUPRAC assay. Antioxidants 2013, 2, 257–264. [Google Scholar] [CrossRef]
- Negro, C.; Tommasi, L.; Miceli, A. Phenolic compounds and antioxidant activity from red grape marc extracts. Bioresour. Technol. 2003, 87, 41–44. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jeong, S.M.; Park, W.P.; Nam, K.; Ahn, D.; Lee, S.C. Effect of heating conditions of grape seeds on the antioxidant activity of grape seed extracts. Food Chem. 2006, 97, 472–479. [Google Scholar] [CrossRef]
- Wu, Y.; Duan, S.; Zhao, L.; Gao, Z.; Luo, M.; Song, S.; Xu, W.; Zhang, C.; Ma, C.; Wang, S. Aroma Characterization Based on Aromatic Series Analysis in Table Grapes. Sci. Rep. 2016, 6, 31116. [Google Scholar] [CrossRef] [PubMed]
- Nacouzi, D.; Masry, R.; El Kayal, W. Quality and Phytochemical Composition of Sweet Cherry Cultivars Can Be Influenced by Altitude. Plants 2023, 12, 2254. [Google Scholar] [CrossRef]
- Salameh, M.; Nacouzi, D.; Lahoud, G.; Riachy, I.; El Kayal, W. Evaluation of Postharvest Maturity Indices of Commercial Avocado Varieties Grown at Various Elevations Along Lebanon’s Coast. Front. Plant Sci. 2022, 13, 895964. [Google Scholar] [CrossRef] [PubMed]

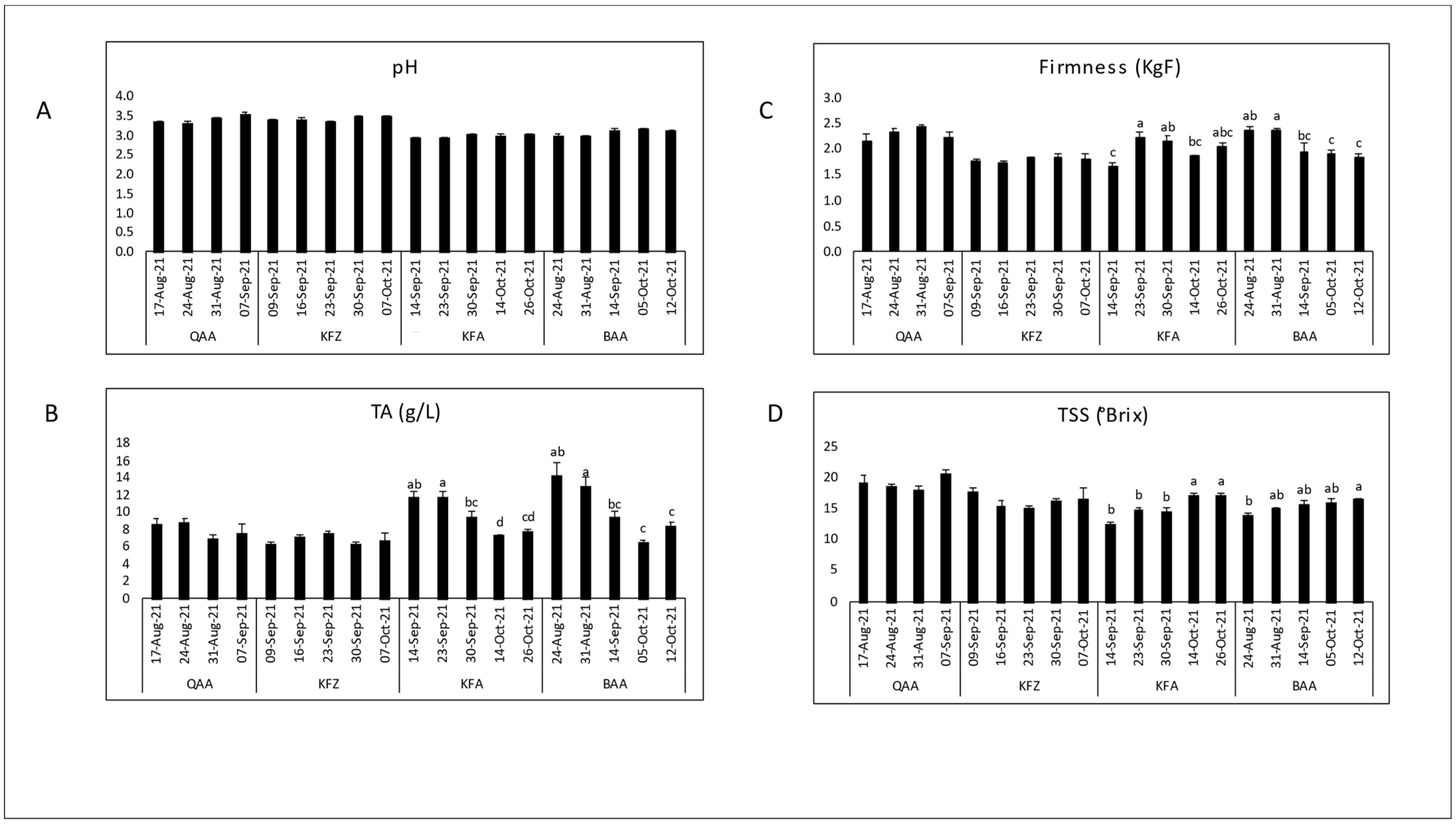
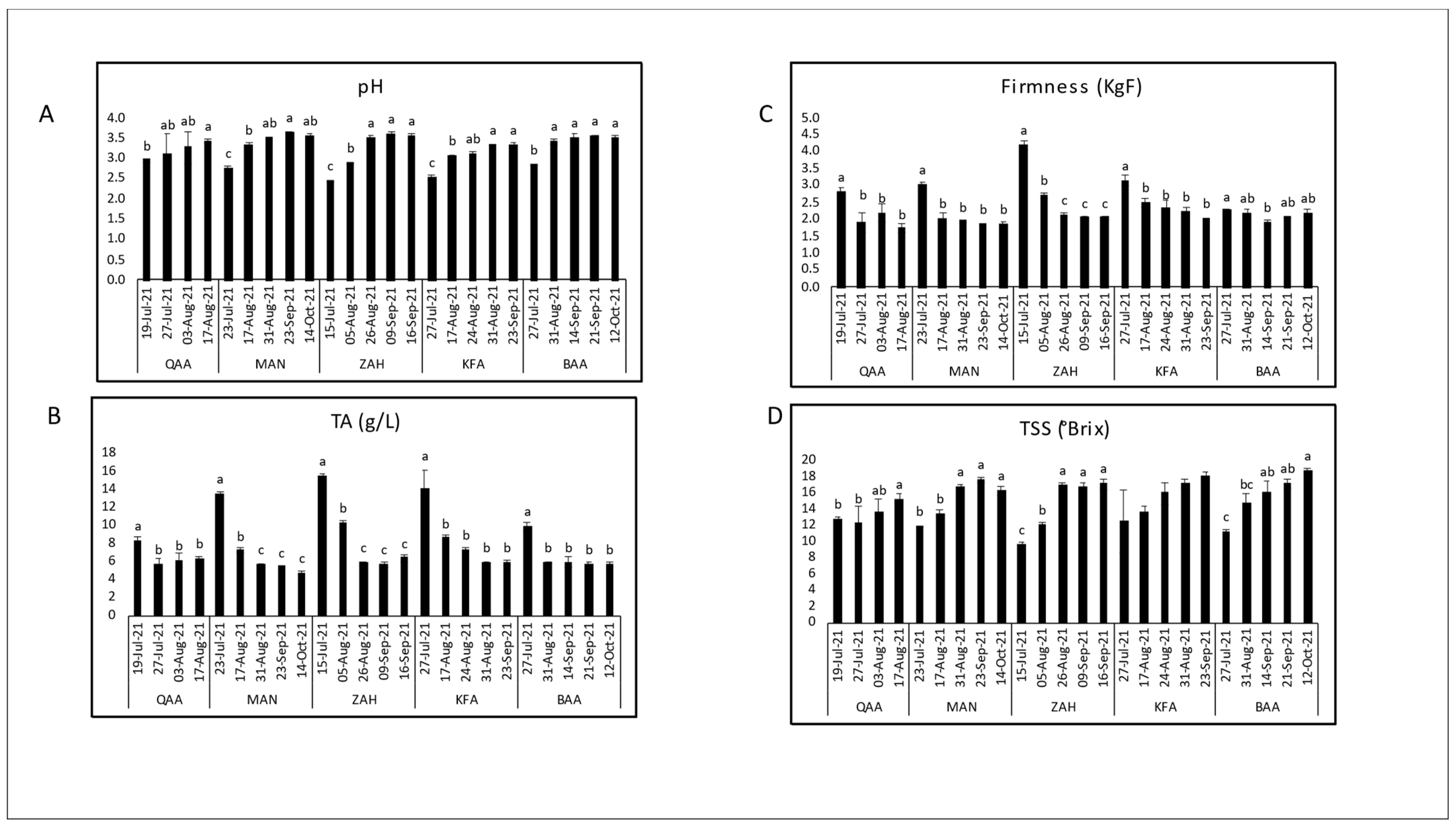
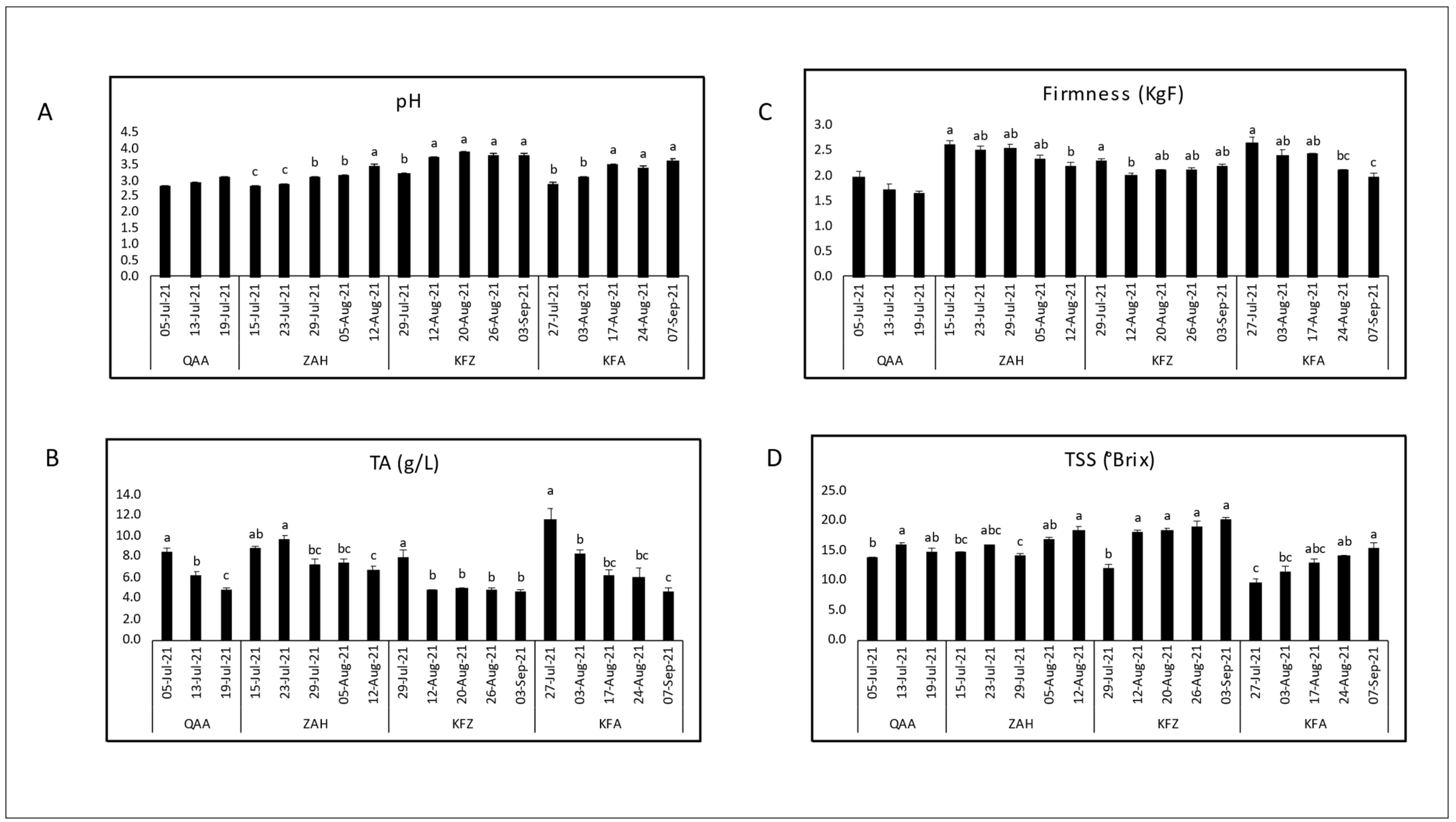
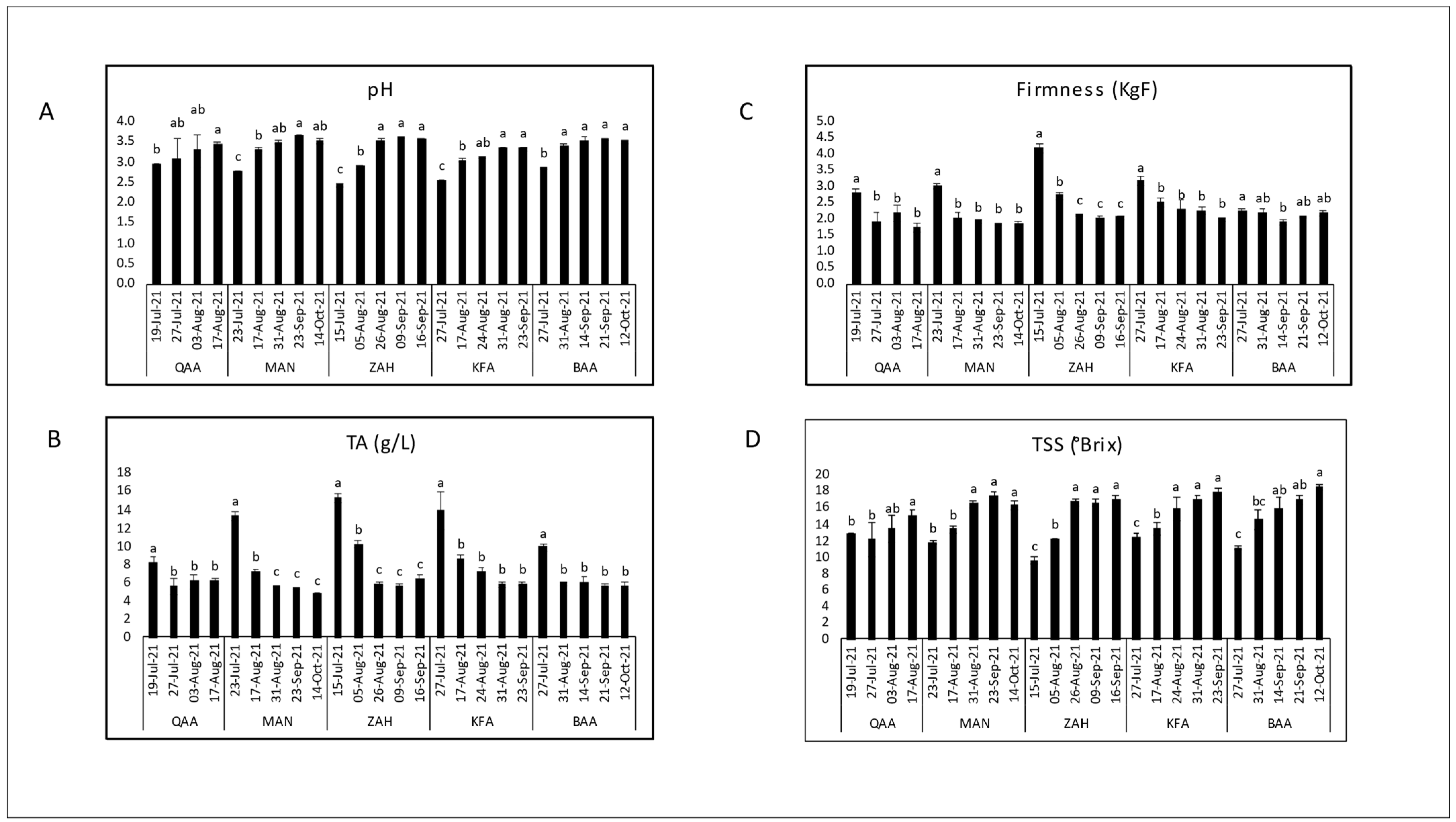
| Cultivar | Location | Elevation (m asl) | pH | TSS (°Brix) | TA (g/L) | TSS/TA Ratio | Dry Matter (%) | Firmness (KgF) |
|---|---|---|---|---|---|---|---|---|
| Black Pearl | QAA | 650 | 3.56 ± 0.03 a | 20.4 ± 0.7 a | 7.45 ± 1.26 | 27.4 ± 2.7 | 20.54 ± 0.89 a | 2.2 ± 0.1 a |
| KFZ | 1000 | 3.48 ± 0.04 a | 18.4 ± 0.6 ab | 6.77 ± 0.75 | 27.2 ± 4.1 | 17.64 ± 1.72 b | 1.8 ± 0.1 b | |
| KFA | 1100 | 3.05 ± 0.02 c | 16.9 ± 0.5 b | 7.67 ± 0.20 | 20.9 ± 1.7 | 19.01 ± 0.68 ab | 2.1 ± 0.1 ab | |
| BAA | 1150 | 3.11 ± 0.05 bc | 16.3 ± 0.4 b | 8.36 ± 0.55 | 19.5 ± 1.5 | 17.75 ± 0.61 b | 1.8 ± 0.1 b | |
| Crimson Seedless | ZAH | 950 | 3.55 ± 0.05 a | 21.4 ± 0.3 | 4.64 ± 0.26 b | 46.1 ± 3.4 a | 22.27 ± 1.05 a | 2.4 ± 0.1 |
| KFA | 1100 | 3.22 ± 0.02 b | 20.6 ± 0.4 | 5.58 ± 0.24 ab | 36.9 ± 2.1 b | 14.33 ± 0.31 b | 2.4 ± 0.1 | |
| BAA | 1150 | 3.39 ± 0.02 a | 20.7 ± 0.7 | 6.12 ± 0.35 a | 33.8 ± 1.5 b | 24.06 ± 0.33 a | 2.6 ± 0.1 | |
| Superior Seedless | QAA | 650 | 3.11 ± 0.03 b | 14.5 ± 0.8 c | 4.81 ± 0.26 b | 30.1 ± 2.9 b | 15.85 ± 0.79 b | 1.6 ± 0.1 b |
| ZAH | 950 | 3.42 ± 0.06 b | 18.3 ± 0.8 ab | 6.70 ± 0.42 a | 27.3 ± 2.1 b | 17.90 ± 0.7 ab | 2.1 ± 0.1 a | |
| KFZ | 1000 | 3.78 ± 0.05 a | 20.2 ± 0.4 a | 4.63 ± 0.16 b | 43.6 ± 1.7 a | 19.20 ± 0.39 a | 2.1 ± 0.1 a | |
| KFA | 1100 | 3.59 ± 0.06 b | 15.1 ± 1.0 bc | 4.59 ± 0.38 b | 32.9 ± 4.1 ab | 15.90 ± 1.13 b | 1.9 ± 0.1 b | |
| Red Globe | QAA | 650 | 3.43 ± 0.06 ab | 15.1 ± 0.8 c | 6.20 ± 0.28 a | 24.2 ± 1.9 b | 16.19 ± 0.74 b | 1.7 ± 0.1 b |
| MAN | 900 | 3.52 ± 0.06 ab | 16.2 ± 0.5 bc | 4.67 ± 0.18 b | 34.7 ± 1.4 a | 17.35 ± 0.89 b | 1.8 ± 0.1 b | |
| ZAH | 950 | 3.53 ± 0.06 a | 16.9 ± 0.6 abc | 6.31 ± 0.47 a | 26.8 ± 2.1 ab | 17.32 ± 0.61 b | 2.1 ± 0.1 ab | |
| KFA | 1100 | 3.32 ± 0.04 b | 17.8 ± 0.6 ab | 5.82 ± 0.21 a | 30.6 ± 1.9 ab | 17.75 ± 0.62 b | 1.9 ± 0.1 ab | |
| BAA | 1150 | 3.51 ± 0.04 ab | 18.6 ± 0.3 a | 5.60 ± 0.39 ab | 33.2 ± 1.9 a | 20.67 ± 0.74 a | 2.1 ± 0.1 a | |
| Cultivar ∗ Location | *** | *** | *** | *** | *** | *** | ||
| Cultivar | Location | Elevation (m asl) | Cluster Weight (g) | Cluster Length (cm) | Cluster Width (cm) |
|---|---|---|---|---|---|
| Black Pearl | QAA | 650 | 609.8 ± 67.5 | 18.4 ± 1.1 | 13.7 ± 0.8 |
| KFZ | 1000 | 472.9 ± 41.7 | 20.1 ± 0.6 | 10.9 ± 1.5 | |
| KFA | 1100 | 459.1 ± 61.1 | 18.2 ± 1.1 | 14.3 ± 0.7 | |
| BAA | 1150 | 542.1 ± 47.9 | 20.1 ± 1.1 | 14.8 ± 0.8 | |
| Crimson Seedless | ZAH | 950 | 405.5 ± 52.4 | 16.4 ± 1.4 b | 14.1 ± 1.1 |
| KFA | 1100 | 374.8 ± 34.1 | 18.2 ± 0.6 ab | 12.7 ± 1.1 | |
| BAA | 1150 | 365.1 ± 45.1 | 20.9 ± 1.3 a | 13.7 ± 0.9 | |
| Superior Seedless | QAA | 650 | 199.9 ± 15.4 c | 18.7 ± 1.1 ab | 9.6 ± 0.2 b |
| ZAH | 950 | 292.1 ± 17.8 bc | 20.1 ± 0.8 ab | 8.9 ± 0.7 b | |
| KFZ | 1000 | 320.6 ± 37.6 b | 17.1 ± 0.9 b | 11.1 ± 0.3 ab | |
| KFA | 1100 | 716.8 ± 24.9 a | 22.8 ± 1.1 a | 13.5 ± 0.5 a | |
| Red Globe | QAA | 650 | 474.5 ± 49.1 b | 18.6 ± 1.1 | 11.6 ± 1.4 |
| MAN | 900 | 735.1 ± 71.7 ab | 19.1 ± 1.5 | 14.3 ± 0.8 | |
| ZAH | 950 | 909.5 ± 87.1 a | 21.8 ± 0.8 | 13.6 ± 1.4 | |
| KFA | 1100 | 689.5 ± 32.1 ab | 21.3 ± 1.5 | 12.6 ± 0.5 | |
| BAA | 1150 | 572.2 ± 68.2 b | 16.7 ± 1.7 | 13.7 ± 0.9 | |
| Cultivar ∗ Location | *** | *** | * | ||
| Cultivar | Color | Total Polyphenols (mg GAE/kg) | Total Anthocyanins (mg/kg) | FRAP (μM Fe2+/kg) | |||
|---|---|---|---|---|---|---|---|
| Pulp | Seeds | Pulp | Seeds | Pulp | Seeds | ||
| Black Pearl | Black/purple | 946.9 ± 17.7 a | 1696.3 ± 70.7 A | 1799.3 ± 15.4 a | 64.6 ± 3.1 B | 3082.0 | 6799.6 |
| Crimson Seedless | Red | 588.9 ± 77.6 b | - | 187.1 ± 21.3 c | - | 1878.9 | - |
| Superior Seedless | Bright yellow/greenish | 672.2 ± 98.3 b | - | 6.7 ± 7.1 d | - | 2053.6 | - |
| Red Globe | Red | 493.7 ± 18.1 b | 1490.7 ± 141.4 A | 280.5 ± 4.7 b | 60.7 ± 1.5 B | 1613.5 | 6764.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamie, N.; Nacouzi, D.; Choker, M.; Salameh, M.; Darwiche, L.; El Kayal, W. Maturity Assessment of Different Table Grape Cultivars Grown at Six Different Altitudes in Lebanon. Plants 2023, 12, 3237. https://doi.org/10.3390/plants12183237
Hamie N, Nacouzi D, Choker M, Salameh M, Darwiche L, El Kayal W. Maturity Assessment of Different Table Grape Cultivars Grown at Six Different Altitudes in Lebanon. Plants. 2023; 12(18):3237. https://doi.org/10.3390/plants12183237
Chicago/Turabian StyleHamie, Najwane, Diana Nacouzi, Mariam Choker, Maya Salameh, Linda Darwiche, and Walid El Kayal. 2023. "Maturity Assessment of Different Table Grape Cultivars Grown at Six Different Altitudes in Lebanon" Plants 12, no. 18: 3237. https://doi.org/10.3390/plants12183237
APA StyleHamie, N., Nacouzi, D., Choker, M., Salameh, M., Darwiche, L., & El Kayal, W. (2023). Maturity Assessment of Different Table Grape Cultivars Grown at Six Different Altitudes in Lebanon. Plants, 12(18), 3237. https://doi.org/10.3390/plants12183237






