Chromium Recovery from Chromium-Loaded Cupressus lusitanica Bark in Two-Stage Desorption Processes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Selection of Eluent Solutions for Chromium Desorption from Chromium-Loaded CLB
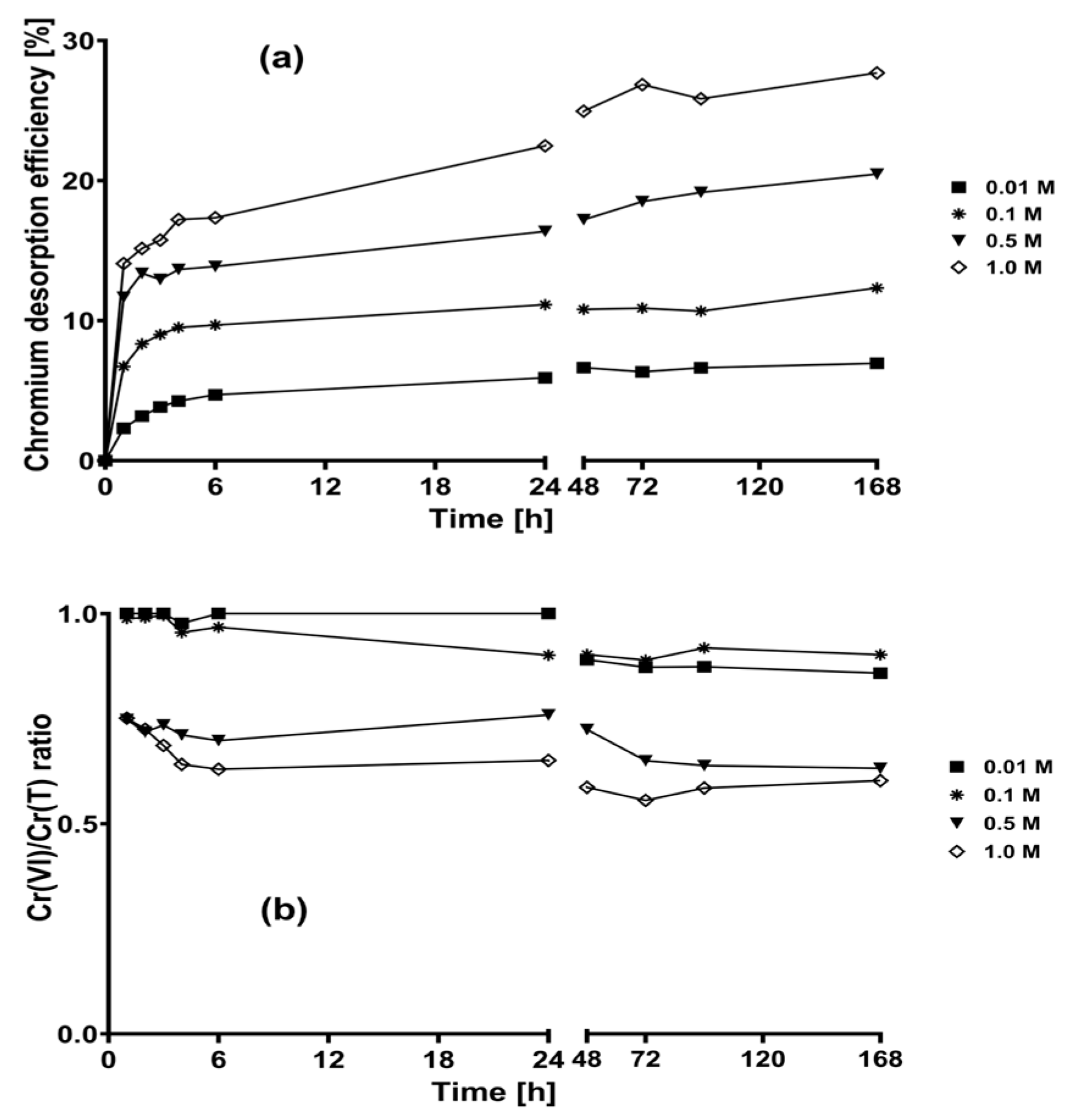
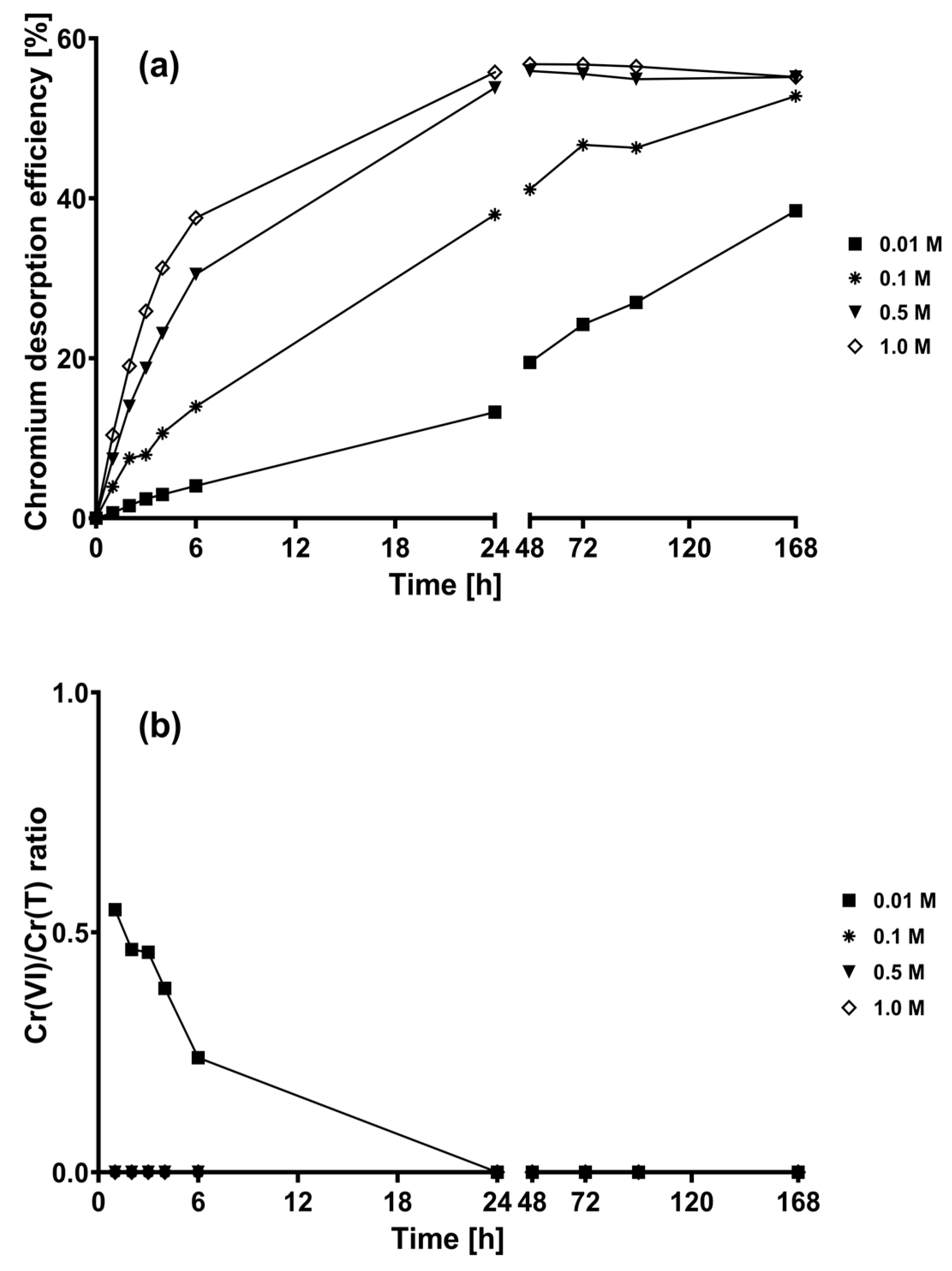
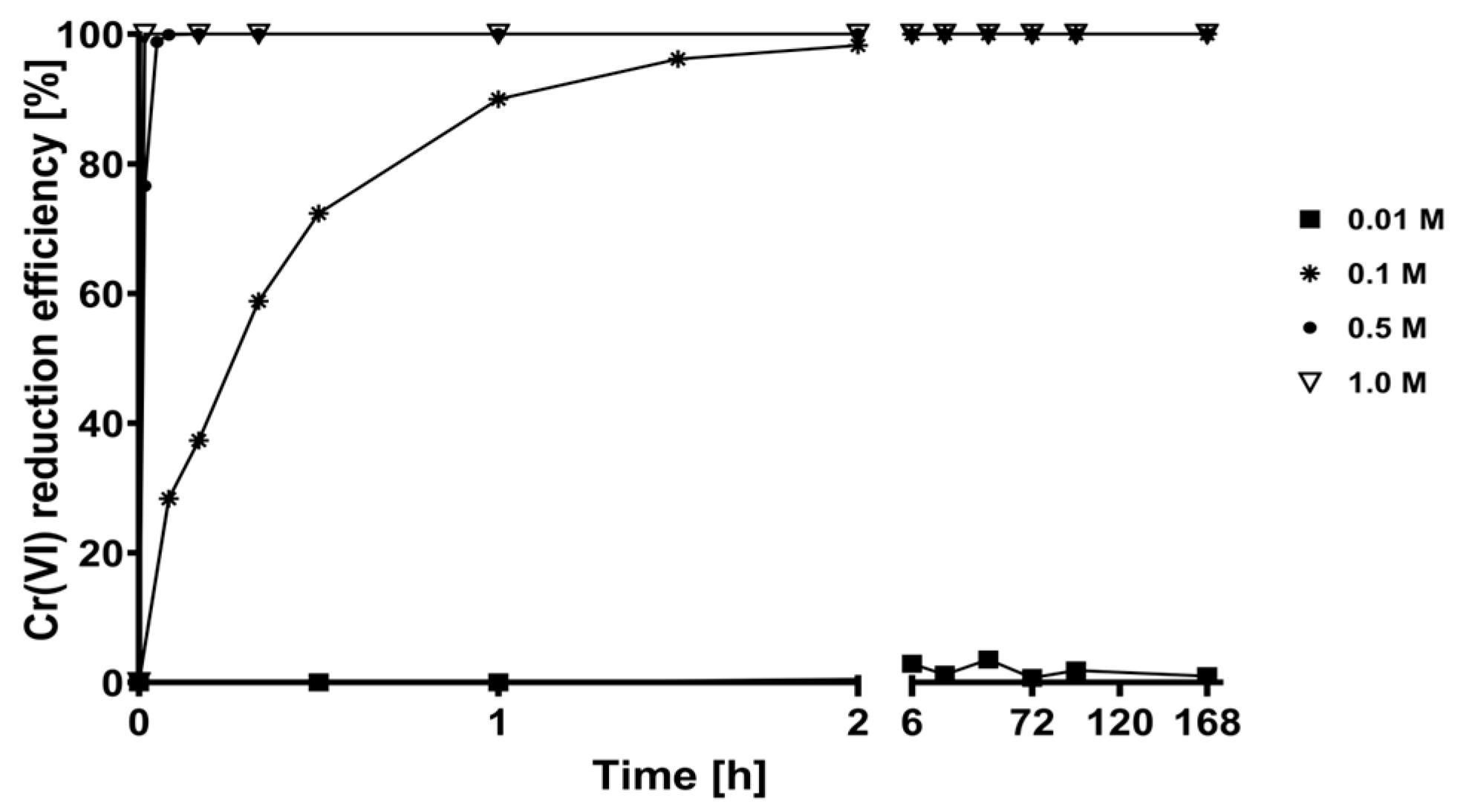
2.2. Desorption Kinetics of the Chromium Biosorbed on CLB at Different Concentrations of the Eluent Solutions
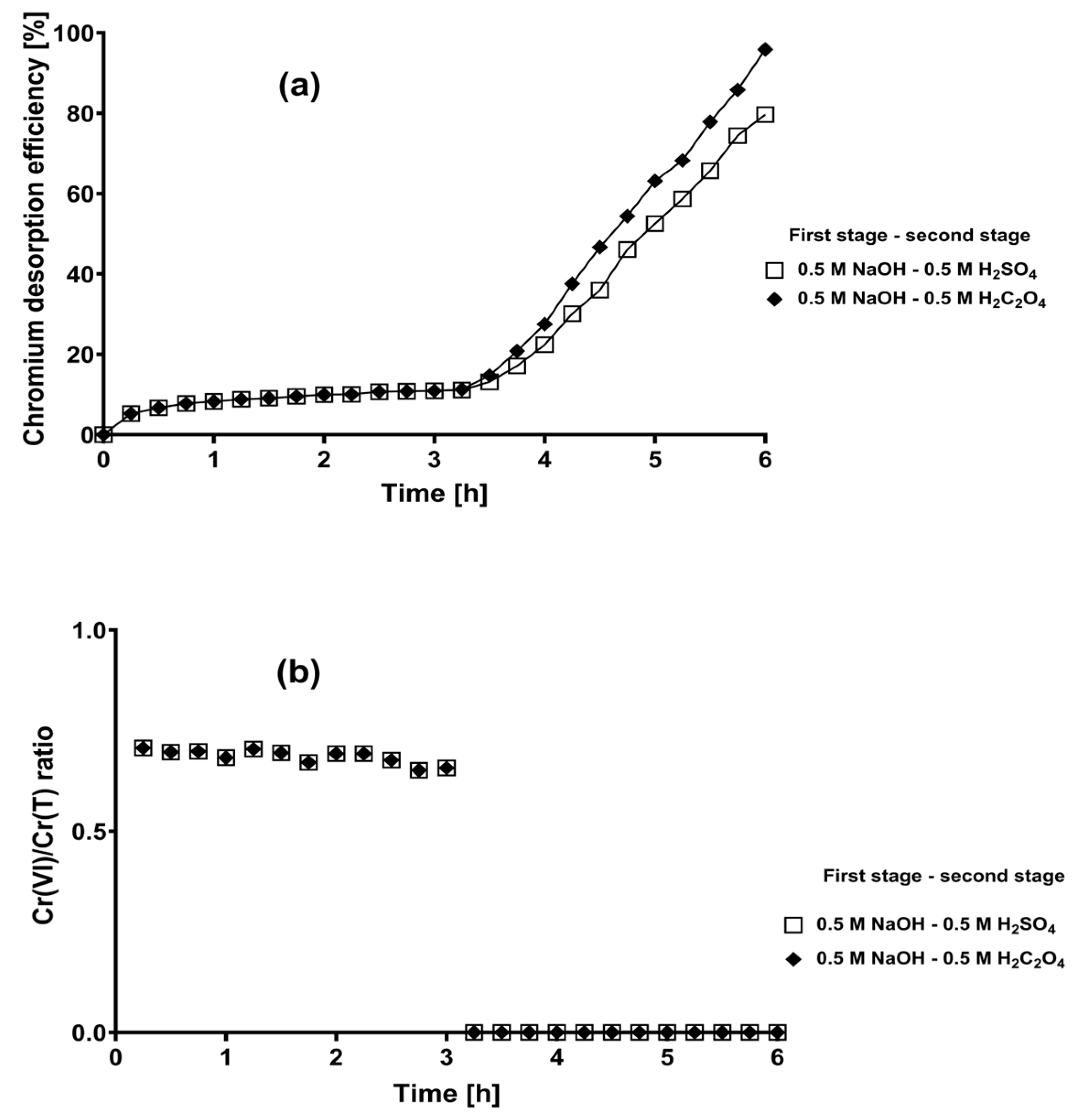
2.3. Two-Stage Desorption Kinetics of Total Chromium from Loaded CLB
3. Materials and Methods
3.1. Chemical Reagents
3.2. Biomaterial
3.3. Chromium Desorption Studies
3.3.1. Selection of Eluting Solutions
3.3.2. Chromium Desorption Kinetic Studies in Single and Two Stages
3.3.3. Cr(III) Oxidation Assays by CLB in NaOH Solutions
3.3.4. Cr(VI) Reduction Assays by Oxalic Acid (H2C2O4) at Different Concentrations
3.4. Analytical Methods
3.4.1. Determination of Cr(VI), Cr(III), and Total Chromium Concentrations in Solution
3.4.2. Determination of Total Chromium Concentration in Chromium-Loaded Biosorbent
3.4.3. Estimation of Chromium Desorption Efficiency
3.4.4. Predominant Oxidation State of Recovered Chromium in the Eluting Solution
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aranda-García, E.; Cristiani-Urbina, E. Hexavalent chromium removal and total chromium biosorption from aqueous solution by Quercus crassipes acorn shell in a continuous up-flow fixed-bed column: Influencing parameters, kinetics, and mechanism. PLoS ONE 2020, 15, e0227953. [Google Scholar] [CrossRef]
- Arishi, A.; Mashhour, I. Microbial mechanisms for remediation of hexavalent chromium and their large-scale applications; current research and future directions. J. Pure Appl. Microbiol. 2021, 15, 53–67. [Google Scholar] [CrossRef]
- Tang, X.; Huang, Y.; Li, Y.; Wang, L.; Pei, X.; Zhou, D.; He, P.; Hughes, S.S. Study on detoxification and removal mechanisms of hexavalent chromium by microorganisms. Ecotoxicol. Environ. Saf. 2021, 208, 111699. [Google Scholar] [CrossRef] [PubMed]
- Kalsoom, A.; Batool, R. Biological and nonbiological approaches for treatment of Cr(VI) in tannery effluent. In Emerging Eco-friendly Green Technologies for Wastewater Treatment. Microorganisms for Sustainability 18; Bharagava, R.N., Ed.; Springer Nature: Singapore, 2020; pp. 147–170. [Google Scholar] [CrossRef]
- Singh, S.; Kumar Naik, T.S.S.; Chauhan, V.; Shehata, N.; Kaur, H.; Dhanjal, D.S.; Marcelino, L.A.; Bhati, S.; Subramanian, S.; Singh, J.; et al. Ecological effects, remediation, distribution, and sensing techniques of chromium. Chemosphere 2022, 307, 135804. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Song, Z.; Jeyakumar, P.; Shaheen, S.M.; Rinklebe, J.; Ok, Y.S.; Bolan, N.; Wang, H. A critical review on bioremediation technologies for Cr(VI)-contaminated soils and wastewater. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1027–1078. [Google Scholar] [CrossRef]
- Orooji, Y.; Nezafat, Z.; Nasrollahzadeh, M.; Kamali, T.A. Polysaccharide-based (Nano)materials for Cr(VI) removal. Int. J. Biol. Macromol. 2021, 188, 950–973. [Google Scholar] [CrossRef]
- Zhang, R.; Tian, Y. Characteristics of natural biopolymers and their derivative as sorbents for chromium adsorption: A review. J. Leather Sci. Eng. 2020, 2, 24. [Google Scholar] [CrossRef]
- Bayuo, J. An extensive review on chromium (vi) removal using natural and agricultural wastes materials as alternative biosorbents. J. Environ. Health Sci. Eng. 2021, 19, 1193–1207. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Adeniyi, A.G. Adsorption of pollutants by plant bark derived adsorbents: An empirical review. J. Water Process Eng. 2020, 35, 101228. [Google Scholar] [CrossRef]
- Jobby, R.; Jha, P.; Yadav, A.K.; Desai, N. Biosorption and biotransformation of hexavalent chromium [Cr(VI)]: A comprehensive review. Chemosphere 2018, 207, 255–266. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Selvasembian, R.; Ashiq, A.; Gunarathne, V.; Ekanayake, A.; Perera, V.O.; Wijesekera, H.; Mia, S.; Ahmad, M.; Vithanage, M.; et al. A systematic review on adsorptive removal of hexavalent chromium from aqueous solutions: Recent advances. Sci. Total Environ. 2022, 809, 152055. [Google Scholar] [CrossRef]
- Agarwal, A.; Upadhyay, U.; Sreedhar, I.; Singh, S.A.; Patel, C.M. A review on valorization of biomass in heavy metal removal from wastewater. J. Water Process Eng. 2020, 38, 101602. [Google Scholar] [CrossRef]
- Beni, A.A.; Esmaeili, A. Biosorption, an efficient method for removing heavy metals from industrial effluents: A review. Environ. Technol. Innov. 2020, 17, 100503. [Google Scholar] [CrossRef]
- Londono-Zuluaga, C.; Jameel, H.; Gonzalez, R.W.; Lucia, L. Crustacean shell-based biosorption water remediation platforms: Status and perspectives. J. Environ. Manag. 2019, 231, 757–762. [Google Scholar] [CrossRef]
- Bessaha, F.; Marouf-Khelifa, K.; Batonneau-Gener, I.; Khelifa, A. Characterization and application of heat-treated and acid-leached halloysites in the removal of malachite green: Adsorption, desorption, and regeneration studies. Desalin. Water Treat. 2016, 57, 14609–14621. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, A.E.; Reyes-Ledezma, J.L.; Chávez-Camarillo, G.M.; Cristiani-Urbina, E.; Morales-Barrera, L. Cyclic biosorption and desorption of acid red 27 onto Eichhornia crassipes leaves. Rev. Mex. Ing. Quim. 2018, 17, 1121–1134. [Google Scholar] [CrossRef]
- Gautam, R.K.; Mudhoo, A.; Lofrano, G.; Chattopadhyaya, M.C. Biomass-derived biosorbents for metal ions sequestration: Adsorbent modification and activation methods and adsorbent regeneration. J. Environ. Chem. Eng. 2014, 2, 239–259. [Google Scholar] [CrossRef]
- Hamad, H.N.; Idrus, S. Recent developments in the application of bio-waste-derived adsorbents for the removal of methylene blue from wastewater: A review. Polymers 2022, 14, 783. [Google Scholar] [CrossRef]
- Volesky, B. Sorption and Biosorption; BV Sorbex, Inc.: Montreal-St-Lambert, QC, Canada, 2003. [Google Scholar]
- Aoyama, M.; Kishino, M.; Jo, T.-S. Biosorption of Cr(VI) on Japanese cedar bark. Sep. Sci. Technol. 2005, 39, 1149–1162. [Google Scholar] [CrossRef]
- Kumar, P.A.; Chakraborty, S.; Ray, M. Removal and recovery of chromium from wastewater using short chain polyaniline synthesized on jute fiber. Chem. Eng. J. 2008, 141, 130–140. [Google Scholar] [CrossRef]
- Agarwal, G.S.; Bhuptawat, H.K.; Chaudhari, S. Biosorption of aqueous chromium(VI) by Tamarindus indica seeds. Bioresour. Technol. 2006, 97, 949–956. [Google Scholar] [CrossRef]
- Cabatingan, L.K.; Agapay, R.C.; Rakels, J.L.L.; Ottens, M.; Van der Wielen, L.A.M. Potential of biosorption for the recovery of chromate in industrial wastewaters. Ind. Eng. Chem. Res. 2001, 40, 2302–2309. [Google Scholar] [CrossRef]
- Kratochvil, D.; Pimentel, P.; Volesky, B. Removal of trivalent and hexavalent chromium by seaweed biosorbent. Environ. Sci. Technol. 1998, 32, 2693–2698. [Google Scholar] [CrossRef]
- Suksabye, P.; Thiravetyan, P.; Nakbanpote, W.; Chayabutra, S. Chromium removal from electroplating wastewater by coir pith. J. Hazard. Mater. 2007, 141, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Aranda-García, E.; Cristiani-Urbina, E. Effect of pH on hexavalent and total chromium removal from aqueous solutions by avocado shell using batch and continuous systems. Environ. Sci. Pollut. Res. 2019, 26, 3157–3173. [Google Scholar] [CrossRef]
- Dupont, L.; Guillon, E. Removal of hexavalent chromium with a lignocellulosic substrate extracted from wheat bran. Environ. Sci. Technol. 2003, 37, 4235–4241. [Google Scholar] [CrossRef]
- Park, D.; Yun, Y.S.; Jo, J.H.; Park, J.M. Mechanism of hexavalent chromium removal by dead fungal biomass of Aspergillus niger. Water Res. 2005, 39, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Yun, Y.S.; Park, J.M. XAS and XPS studies on chromium-binding groups of biomaterial during Cr(VI) biosorption. J. Colloid Interface Sci. 2008, 317, 54–61. [Google Scholar] [CrossRef]
- Suksabye, P.; Thiravetyan, P. Cr(VI) adsorption from electroplating wastewater by chemically modified coir pith. J. Environ. Manag. 2012, 102, 1–8. [Google Scholar] [CrossRef]
- Suksabye, P.; Nakajima, A.; Thiravetyan, P.; Baba, Y.; Nakbanpote, W. Mechanism of Cr(VI) adsorption by coir pith studied by ESR and adsorption kinetic. J. Hazard. Mater. 2009, 161, 1103–1108. [Google Scholar] [CrossRef]
- Deng, S.; Ting, Y.P. Polyethylenimine-modified fungal biomass as a high-capacity biosorbent for Cr(VI) anions: Sorption capacity and uptake mechanisms. Environ. Sci. Technol. 2005, 39, 8490–8496. [Google Scholar] [CrossRef] [PubMed]
- Netzahuatl-Muñoz, A.R.; Guillén-Jiménez, F.D.M.; Chávez-Gómez, B.; Villegas-Garrido, T.L.; Cristiani-Urbina, E. Kinetic study of the effect of pH on hexavalent and trivalent chromium removal from aqueous solution by Cupressus lusitanica bark. Water Air Soil Pollut. 2012, 223, 625–641. [Google Scholar] [CrossRef]
- Netzahuatl-Muñoz, A.R.; Cristiani-Urbina, M.D.C.; Cristiani-Urbina, E. Chromium biosorption from Cr(VI) aqueous solutions by Cupressus lusitanica bark: Kinetics, equilibrium and thermodynamic studies. PLoS ONE 2015, 10, e0137086. [Google Scholar] [CrossRef]
- Ansari, M.I.; Masood, F.; Malik, A. Bacterial biosorption: A technique for remediation of heavy metals. In Microbes and Microbial Technology; Springer: New York, NY, USA, 2011; pp. 283–319. [Google Scholar]
- Vijayaraghavan, K.; Balasubramanian, R. Is biosorption suitable for decontamination of metal-bearing wastewaters? A critical review on the state-of-the-art of biosorption processes and future directions. J. Environ. Manag. 2015, 160, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Njikam, E.; Schiewer, S. Optimization and kinetic modeling of cadmium desorption from citrus peels: A process for biosorbent regeneration. J. Hazard. Mater. 2012, 213–214, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Fiol, N.; Villaescusa, I.; Martinez, M.; Miralles, N.; Poch, J.; Serarols, J. Biosorption of Cr(VI) using low cost sorbents. Environ. Chem. Lett. 2003, 1, 135–139. [Google Scholar] [CrossRef]
- Aoyama, M.; Saito, S.; Tagami, M. Sorption of Cr(VI) on the wood of Japanese larch treated with concentrated sulfuric acid. J. Wood Sci. 2007, 53, 545–549. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U. Activated carbons and low cost adsorbents for remediation of tri-and hexavalent chromium from water. J. Hazard. Mater. 2006, 137, 762–811. [Google Scholar] [CrossRef]
- Arıca, M.Y.; Tüzün, İ.; Yalçın, E.; İnce, Ö.; Bayramoğlu, G. Utilisation of native, heat and acid-treated microalgae Chlamydomonas reinhardtii preparations for biosorption of Cr(VI) ions. Process Biochem. 2005, 40, 2351–2358. [Google Scholar] [CrossRef]
- Arıca, M.Y.; Bayramoğlu, G. Cr(VI) biosorption from aqueous solutions using free and immobilized biomass of Lentinus sajor-caju: Preparation and kinetic characterization. Colloids Surf. A Physicochem. Eng. Aspects 2005, 253, 203–211. [Google Scholar] [CrossRef]
- Bai, R.S.; Abraham, T.E. Studies on chromium(VI) adsorption–desorption using immobilized fungal biomass. Bioresour. Technol. 2003, 87, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Tewari, N.; Vasudevan, P.; Guha, B.K. Study on biosorption of Cr(VI) by Mucor hiemalis. Biochem. Eng. J. 2005, 23, 185–192. [Google Scholar] [CrossRef]
- Unnithan, M.R.; Vinod, V.P.; Anirudhan, T.S. Synthesis, characterization, and application as chromium(VI) adsorbent of amine-modified polyacrylamide-grafted coconut coir pith. Ind. Eng. Chem. Res. 2004, 43, 2247–2255. [Google Scholar] [CrossRef]
- Tunali, S.; Kiran, I.; Akar, T. Chromium(VI) biosorption characteristics of Neurospora crassa fungal biomass. Miner. Eng. 2005, 18, 681–689. [Google Scholar] [CrossRef]
- Dubbin, W.E. Influence of organic ligands on Cr desorption from hydroxy-Cr intercalated montmorillonite. Chemosphere 2004, 54, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- García Rodenas, L.A.; Iglesias, A.M.; Weisz, A.D.; Morando, P.J.; Blesa, M.A. Surface complexation description of the dissolution of chromium(III) hydrous oxides by oxalic acid. Inorg. Chem. 1997, 36, 6423–6430. [Google Scholar] [CrossRef]
- Kakitani, T.; Hata, T.; Kajimoto, T.; Imamura, Y. Effect of pyrolysis on solvent extractability of toxic metals from chromated copper arsenate (CCA)-treated wood. J. Hazard. Mater. 2004, 109, 53–57. [Google Scholar] [CrossRef]
- Ribeiro, A.B.; Rodríguez-Maroto, J.M.; Mateus, E.P.; Velizarova, E.; Ottosen, L.M. Modeling of electrodialytic and dialytic removal of Cr, Cu and As from CCA-treated wood chips. Chemosphere 2007, 66, 1716–1726. [Google Scholar] [CrossRef]
- Janin, A.; Blais, J.F.; Mercier, G.; Drogui, P. Optimization of a chemical leaching process for decontamination of CCA-treated wood. J. Hazard. Mater. 2009, 169, 136–145. [Google Scholar] [CrossRef]
- Humar, M.; Pohleven, F.; Šentjurc, M. Effect of oxalic, acetic acid, and ammonia on leaching of Cr and Cu from preserved wood. Wood Sci. Technol. 2004, 37, 463–473. [Google Scholar] [CrossRef]
- Hasan, F.; Roček, J. Three-electron oxidations. II. Chromium(VI) oxidation of oxalic acid. J. Am. Chem. Soc. 1972, 94, 9073–9081. [Google Scholar] [CrossRef]
- Hug, S.J.; Laubscher, H.U.; James, B.R. Iron(III) catalyzed photochemical reduction of chromium(VI) by oxalate and citrate in aqueous solutions. Environ. Sci. Technol. 1997, 31, 160–170. [Google Scholar] [CrossRef]
- Mytych, P.; Cieślan, P.; Stasicka, Z. Photoredox processes in the Cr(VI)-Cr (III)-oxalate system and their environmental relevance. Appl. Catal. B 2005, 59, 161–170. [Google Scholar] [CrossRef]
- Farrell, R.P.; Lay, P.A.; Levina, A.; Maxwell, I.A.; Bramley, R.; Brumby, S.; Ji, J.Y. An EPR spectroscopic study of chromium (V) oxalate complexes in aqueous solutions. Mechanism of the chromium(VI) oxidation of oxalic acid. Inorg. Chem. 1998, 37, 3159–3166. [Google Scholar] [CrossRef]
- Hach Company. Hach Water Analysis Handbook, 5th ed.; Hach Company: Loveland, CO, USA, 2008. [Google Scholar]
- Aljohani, M.M.; Al-Qahtani, S.D.; Alshareef, M.; El-Desouky, M.G.; El-Bindary, A.A.; El-Metwaly, N.M.; El-Bindary, M.A. Highly efficient adsorption and removal bio-staining dye from industrial wastewater onto mesoporous Ag-MOFs. Process Saf. Environ. Protect. 2023, 172, 395–407. [Google Scholar] [CrossRef]



| Eluent Solution | Chromium Desorption Efficiency (%) | Cr(VI)/Cr(T) Ratio |
|---|---|---|
| Deionized water | 0.96 | 0.927 |
| EDTA (0.1 M) | 1.57 | 0.932 |
| Sodium sulfate (0.1 M) | 1.07 | 0.966 |
| Potassium sulfate (0.1 M) | 1.67 | 0.965 |
| Sodium nitrate (0.1 M) | 1.65 | 0.904 |
| Potassium chloride (0.1 M) | 1.38 | 0.917 |
| Sodium chloride (0.1 M) | 1.30 | 0.907 |
| Calcium chloride (0.1 M) | 1.69 | 0.891 |
| Sodium oxalate (0.1 M) | 1.90 | 0.929 |
| Sodium citrate (0.1 M) | 1.59 | 0.835 |
| Phosphate buffer (pH 7.0) | 2.08 | 0.942 |
| Phtalate buffer (pH 4.0) | 1.92 | 0.925 |
| Tartaric acid (0.1 M) | 2.31 | 0.000 |
| Oxalic acid (0.1 M) | 10.10 | 0.000 |
| Citric acid (0.1 M) | 1.66 | 0.494 |
| Boric acid (0.1 M) | 1.19 | 0.870 |
| Hydrochloric acid (0.1 M) | 2.75 | 0.772 |
| Sulfuric acid (0.1 M) | 7.04 | 0.623 |
| Nitric acid (0.1 M) | 3.02 | 0.713 |
| Acetic acid (0.1 M) | 1.00 | 0.886 |
| Potassium hydroxide (0.01 M) | 5.32 | 0.962 |
| Potassium hydroxide (0.1 M) | 12.60 | 0.932 |
| Sodium hydroxide (0.01 M) | 5.32 | 0.905 |
| Sodium hydroxide (0.1 M) | 13.00 | 0.863 |
| Potassium carbonate (0.01 M) | 3.66 | 0.919 |
| Potassium carbonate (0.1 M) | 6.63 | 0.860 |
| Sodium carbonate (0.01 M) | 4.15 | 0.944 |
| Sodium carbonate (0.1 M) | 6.36 | 0.930 |
| Sodium bicarbonate (0.01 M) Sodium bicarbonate (0.1 M) | 2.18 3.05 | 0.925 0.938 |
| First Desorption Stage (Contact Time: 168 h) | Second Desorption Stage (Contact Time: 168 h) | Overall Desorption Process | ||||
|---|---|---|---|---|---|---|
| Eluent Solution | Chromium Desorption Efficiency (%) | Cr(VI)/Cr(T) Ratio | Eluent Solution | Chromium Desorption Efficiency (%) | Cr(VI)/Cr(T) Ratio | Chromium Desorption Efficiency (%) |
| Oxalic acid (0.01 M) | 38.4 | 0.00 | Sodium hydroxide (0.5 M) | 15.8 | 0.540 | 54.2 |
| Oxalic acid (0.1 M) | 52.8 | 0.00 | Sodium hydroxide (0.5 M) | 11.3 | 0.811 | 64.1 |
| Oxalic acid (0.5 M) | 55.2 | 0.00 | Sodium hydroxide (0.5 M) | 11.1 | 0.799 | 66.3 |
| Oxalic acid (1.0 M) | 55.2 | 0.00 | Sodium hydroxide (0.5 M) | 9.17 | 0.699 | 64.4 |
| Sulfuric acid (0.01 M) | 15.3 | 0.00 | Sodium hydroxide (0.5 M) | 14.6 | 0.435 | 29.9 |
| Sulfuric acid (0.1 M) | 40.0 | 0.00 | Sodium hydroxide (0.5 M) | 14.9 | 0.473 | 54.9 |
| Sulfuric acid (0.5 M) | 50.0 | 0.00 | Sodium hydroxide (0.5 M) | 11.3 | 0.683 | 61.3 |
| Sulfuric acid (1 M) | 52.9 | 0.00 | Sodium hydroxide (0.5 M) | 11.1 | 0.605 | 64.0 |
| Sodium hydroxide (0.01 M) | 6.96 | 0.858 | Oxalic acid (0.5 M) | 72.4 | 0 | 79.4 |
| Sodium hydroxide (0.1 M) | 12.3 | 0.902 | Oxalic acid (0.5 M) | 81.2 | 0 | 93.5 |
| Sodium hydroxide (0.5 M) | 20.5 | 0.632 | Oxalic acid (0.5 M) | 75.6 | 0 | 96.1 |
| Sodium hydroxide (0.01 M) | 6.96 | 0.858 | Sulfuric acid (0.5 M) | 52.1 | 0 | 59.1 |
| Sodium hydroxide (0.1 M) | 12.3 | 0.902 | Sulfuric acid (0.5 M) | 66.2 | 0 | 78.5 |
| Sodium hydroxide (0.5 M) | 20.5 | 0.632 | Sulfuric acid (0.5 M) | 66.9 | 0 | 87.4 |
| First desorption stage (contact time: 3 h) | Second desorption stage (contact time: 3 h) | Overall desorption process | ||||
| Eluent Solution | Chromium Desorption Efficiency (%) | Cr(VI)/Cr(T) Ratio | Eluent Solution | Chromium Desorption Efficiency (%) | Cr(VI)/Cr(T) Ratio | Chromium Desorption Efficiency (%) |
| Sodium hydroxide (0.5 M) | 10.9 | 0.657 | Oxalic acid (0.5 M) | 85.0 | 0 | 95.9 |
| Sodium hydroxide (0.5 M) | 10.9 | 0.657 | Sulfuric acid (0.5 M) | 68.7 | 0 | 79.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Netzahuatl-Muñoz, A.R.; Aranda-García, E.; Cristiani-Urbina, E. Chromium Recovery from Chromium-Loaded Cupressus lusitanica Bark in Two-Stage Desorption Processes. Plants 2023, 12, 3222. https://doi.org/10.3390/plants12183222
Netzahuatl-Muñoz AR, Aranda-García E, Cristiani-Urbina E. Chromium Recovery from Chromium-Loaded Cupressus lusitanica Bark in Two-Stage Desorption Processes. Plants. 2023; 12(18):3222. https://doi.org/10.3390/plants12183222
Chicago/Turabian StyleNetzahuatl-Muñoz, Alma Rosa, Erick Aranda-García, and Eliseo Cristiani-Urbina. 2023. "Chromium Recovery from Chromium-Loaded Cupressus lusitanica Bark in Two-Stage Desorption Processes" Plants 12, no. 18: 3222. https://doi.org/10.3390/plants12183222
APA StyleNetzahuatl-Muñoz, A. R., Aranda-García, E., & Cristiani-Urbina, E. (2023). Chromium Recovery from Chromium-Loaded Cupressus lusitanica Bark in Two-Stage Desorption Processes. Plants, 12(18), 3222. https://doi.org/10.3390/plants12183222








