The Interplay of Physiological and Biochemical Response to Short-Term Drought Exposure in Garlic (Allium sativum L.)
Abstract
1. Introduction
2. Results
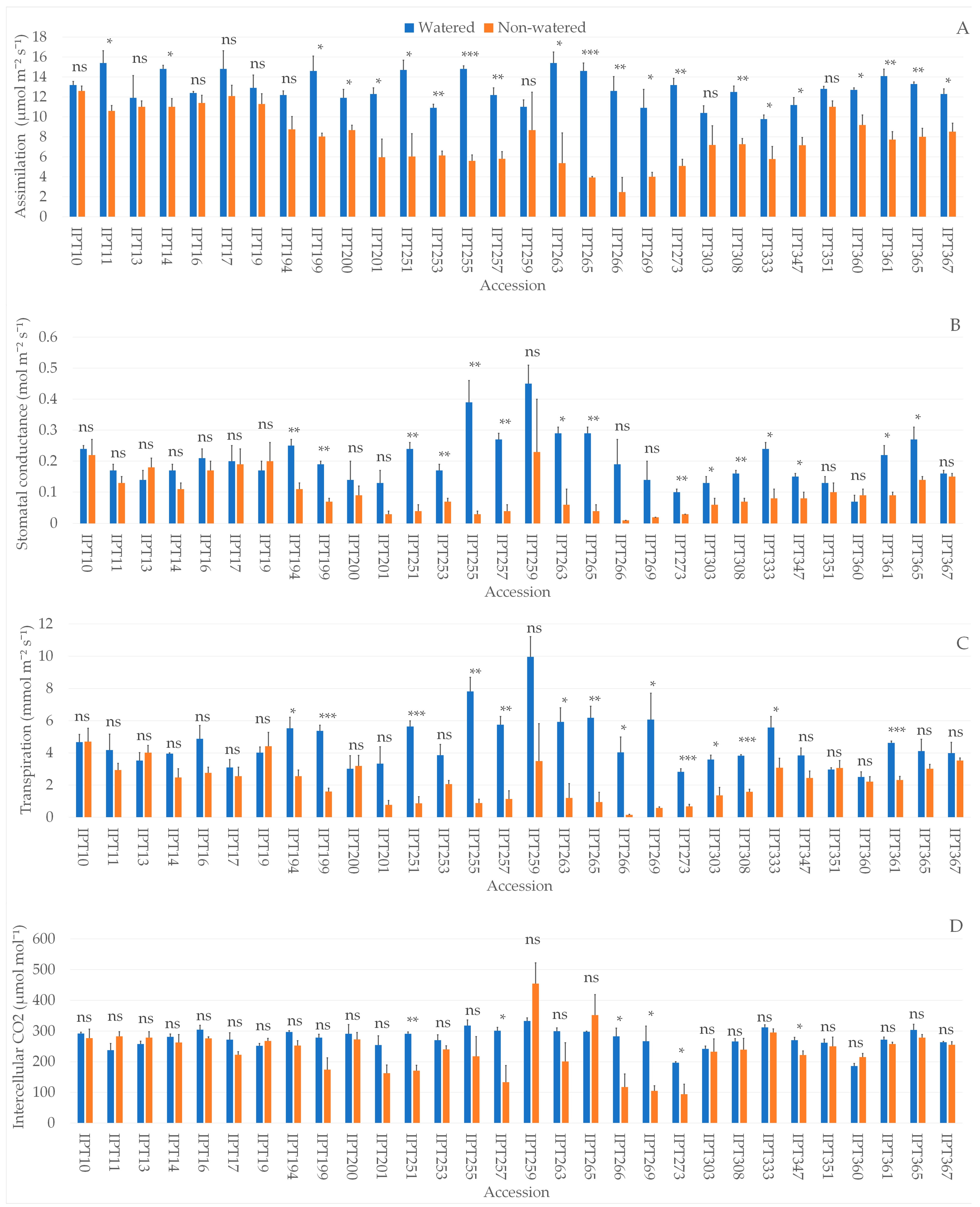
2.1. Photosynthetic Parameters
2.2. Digital Morphological Parameters
2.3. Spectral Parameters and Spectral Vegetation Indices
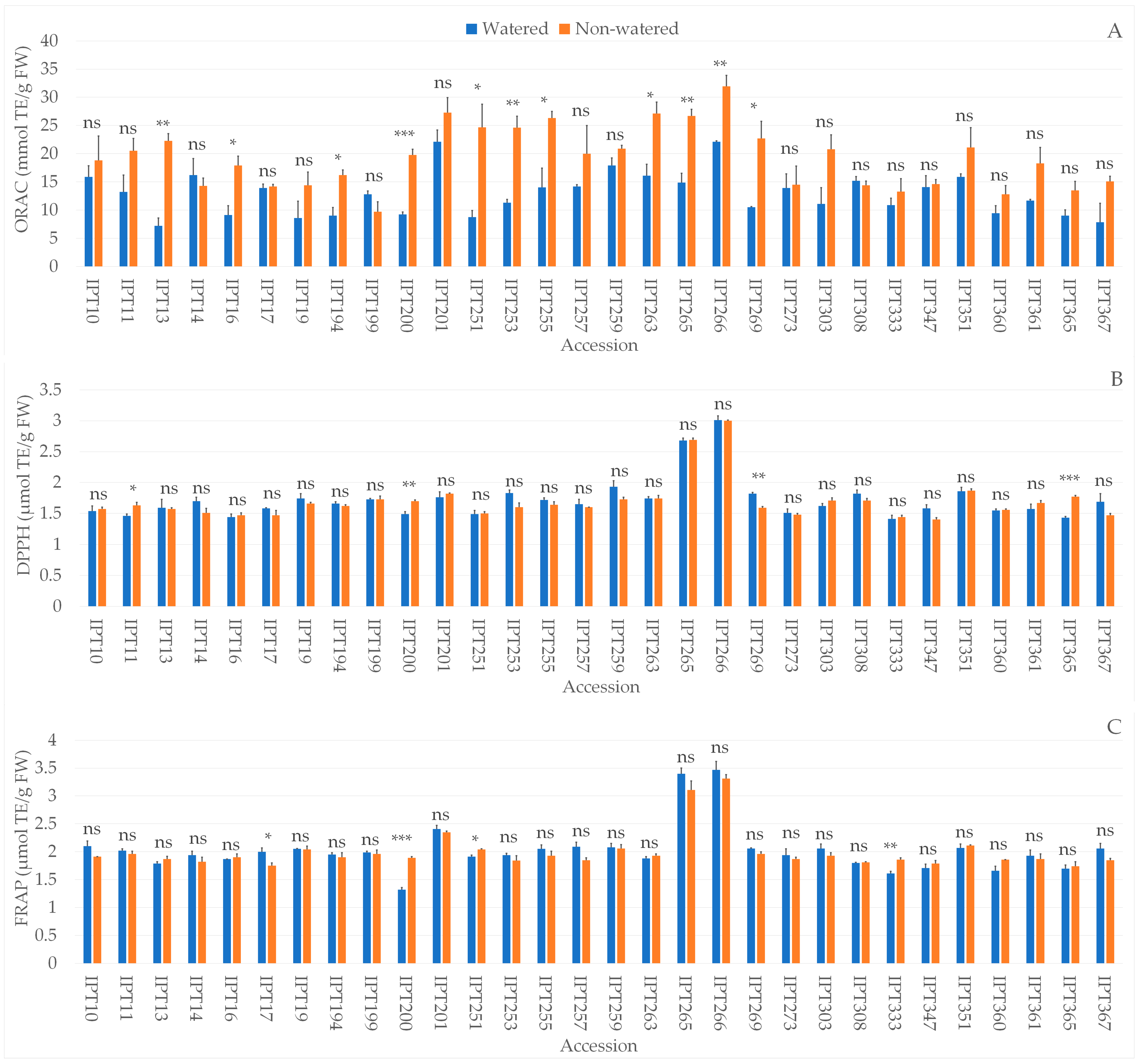
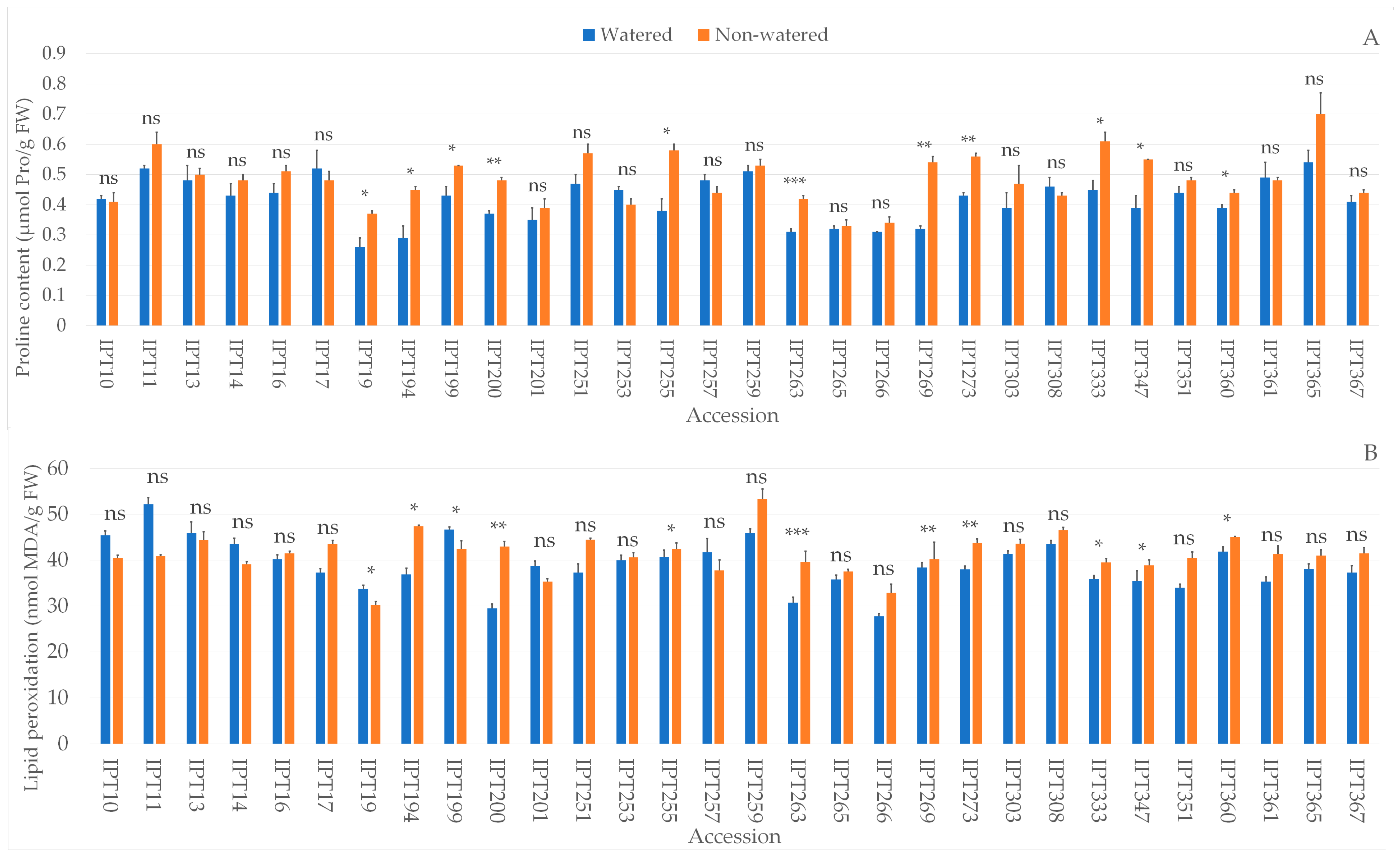
2.4. Biochemical Parameters
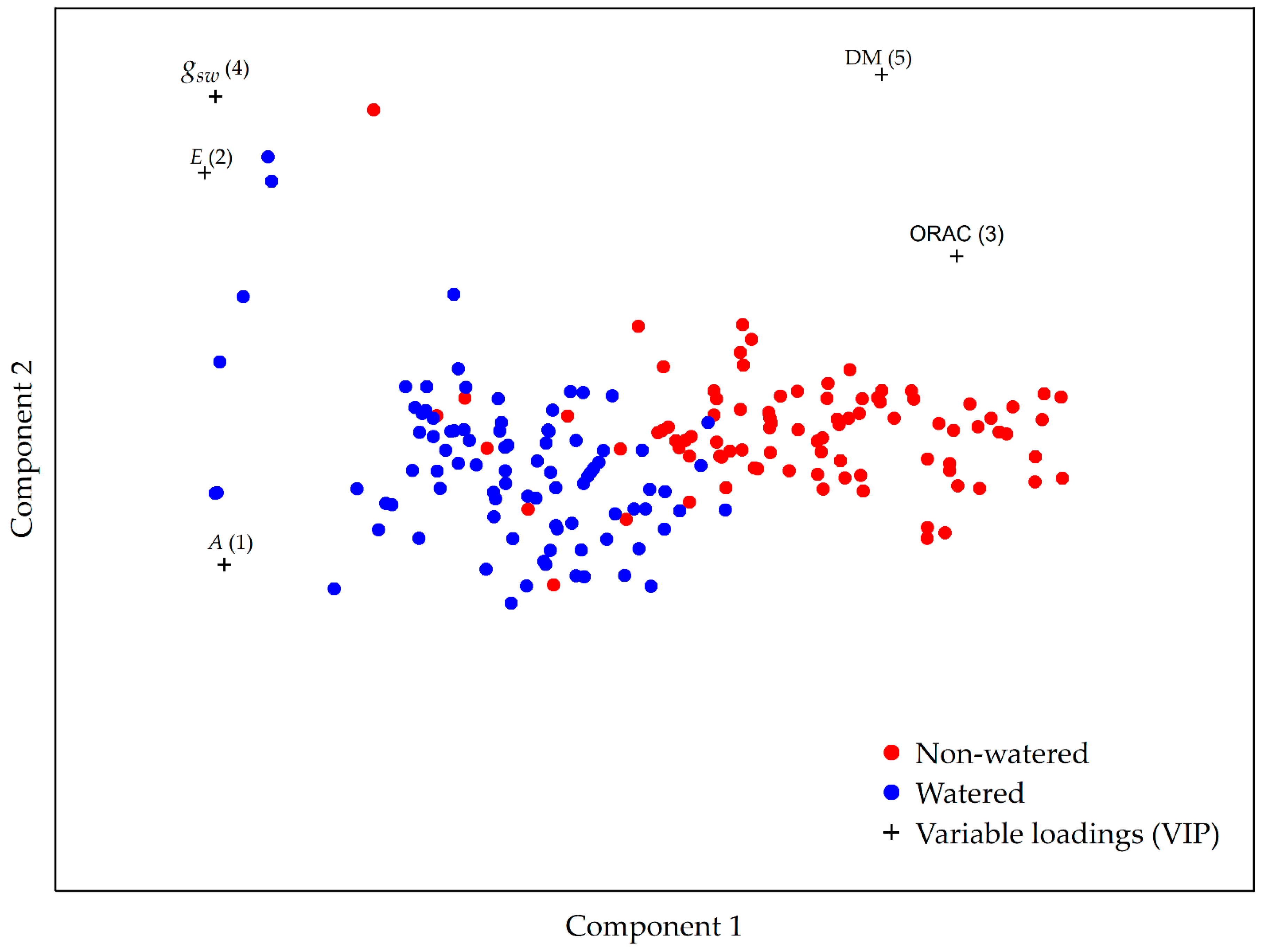
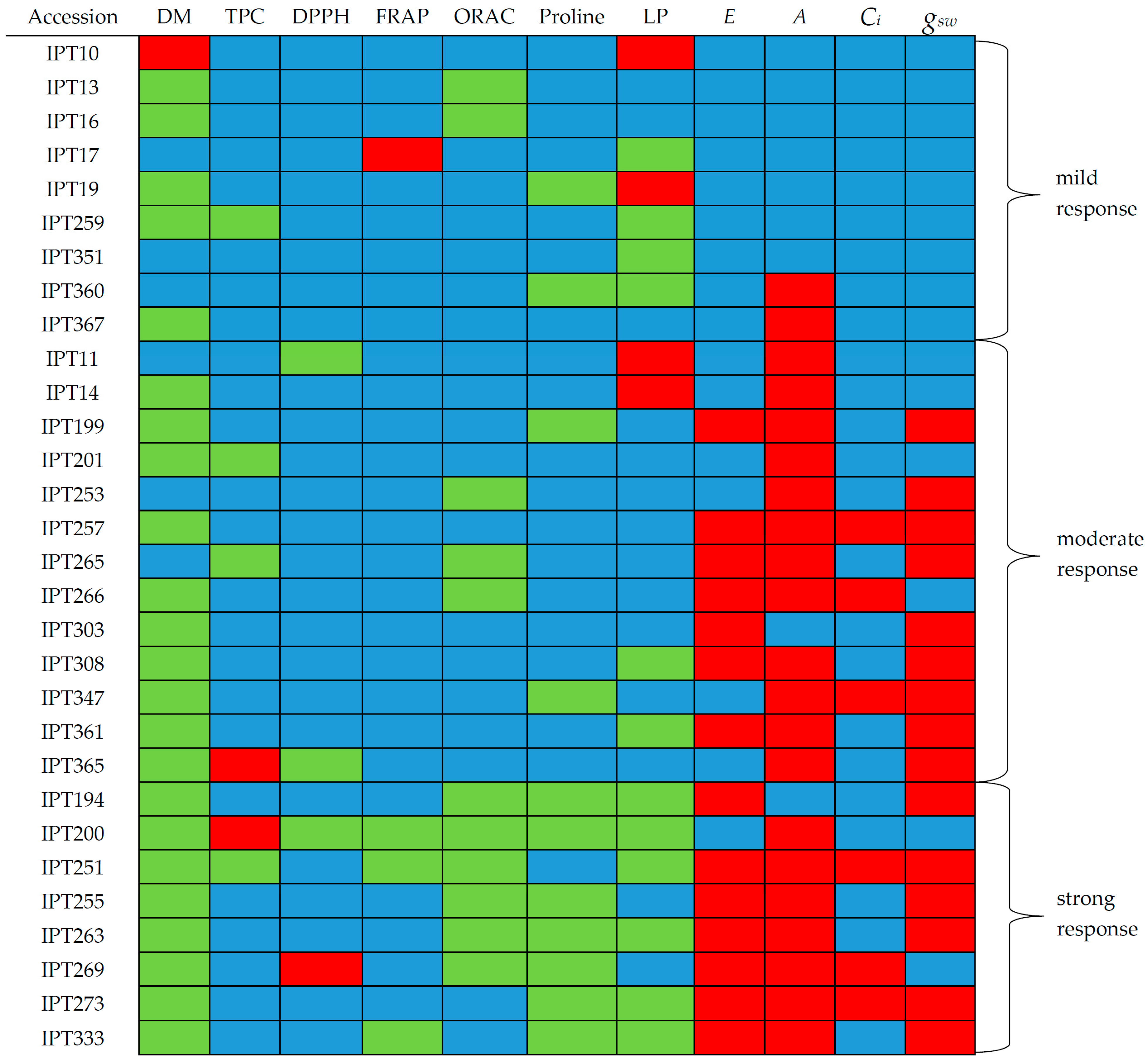
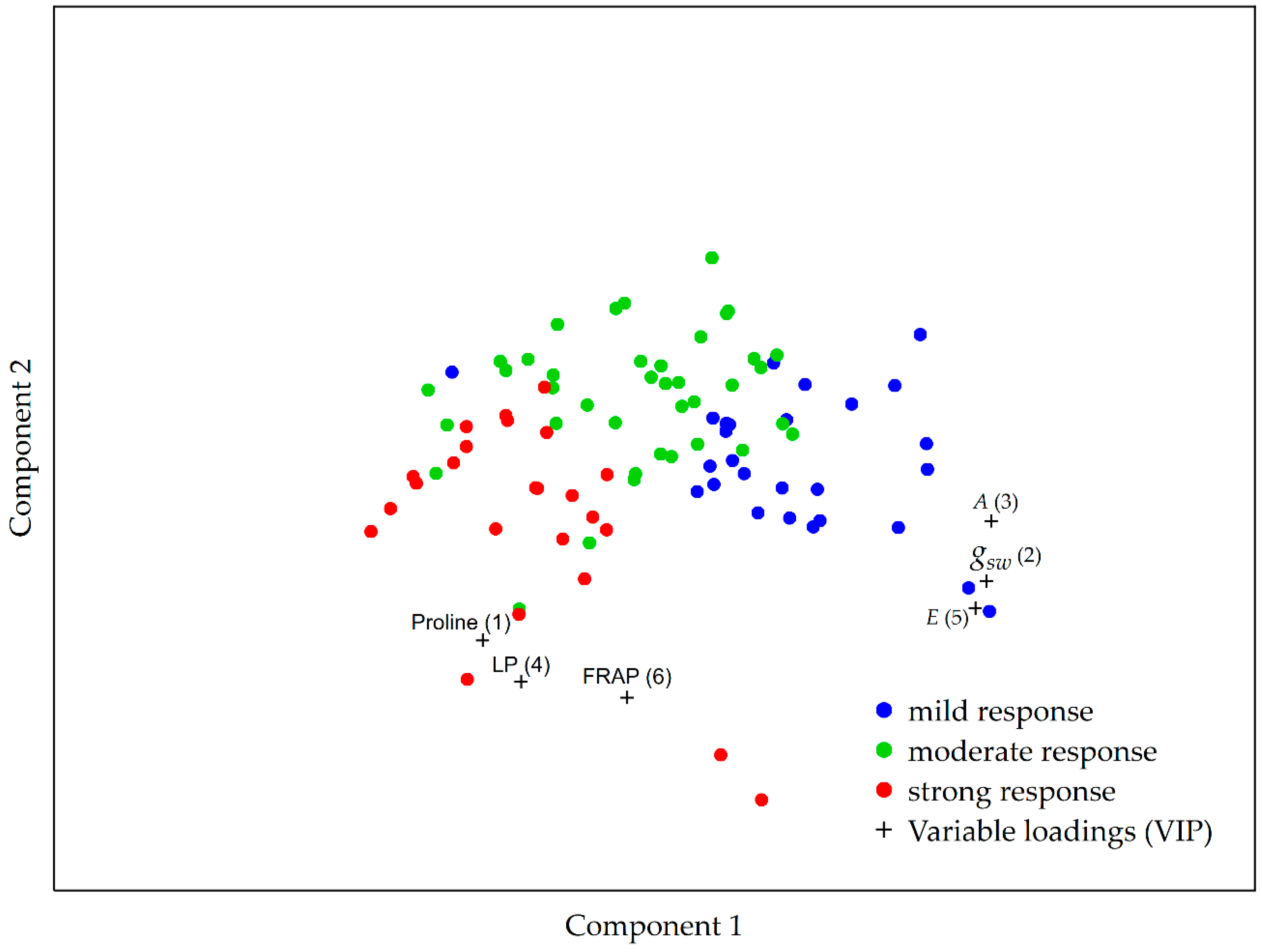
2.5. A Multivariate Approach to Garlic Drought Response Analysis Based on Photosynthetic and Biochemical Markers
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Experimental Setup and Plant Material
4.3. Drought Monitoring
4.4. Measurement of Photosynthetic Parameters
4.5. Measurements of Morphological and Spectral Parameters and Spectral Vegetation Indices
4.6. Dry Matter Content
4.7. Phytochemical Compound Extraction
4.8. Total Phenolic Content and Antioxidant Capacity
4.9. Proline Determination
4.10. Lipid Peroxidation
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, S.; Shen, X.; Cheng, S.; Li, P.; Du, J.; Chang, Y.; Meng, H. Evaluation of Garlic Cultivars for Polyphenolic Content and Antioxidant Properties. PLoS ONE 2013, 8, e79730. [Google Scholar] [CrossRef]
- Badran, A.E. Comparative Analysis of Some Garlic Varieties under Drought Stress Conditions. J. Agric. Sci. 2015, 7, 271. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. S.D. FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 2 March 2023).
- Baghalian, K.; Ziai, S.A.; Naghavi, M.R.; Badi, H.N.; Khalighi, A. Evaluation of Allicin Content and Botanical Traits in Iranian Garlic (Allium sativum L.) Ecotypes. Sci. Hortic. 2005, 103, 155–166. [Google Scholar] [CrossRef]
- Beato, V.M.; Orgaz, F.; Mansilla, F.; Montaño, A. Changes in Phenolic Compounds in Garlic (Allium sativum L.) Owing to the Cultivar and Location of Growth. Plant Foods Hum. Nutr. 2011, 66, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, X.; Shen, D.; Oiu, Y.; Song, J. Diversity Evaluation of Morphological Traits and Allicin Content in Garlic (Allium sativum L.) from China. Euphytica 2014, 198, 243–254. [Google Scholar] [CrossRef]
- Poljuha, D.; Franic, M.; Kralj, I.; Weber, T.; Šatovic, Z.; Ban, D.; Toth, N.; Dumičic, G.; Kereša, S.; Da Cunha, C.P.; et al. Genetic Diversity and Structure Analysis of Croatian Garlic Collection Assessed by SSR Markers. Folia Hortic. 2021, 33, 157–171. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Giorgi, F.; Lionello, P. Climate Change Projections for the Mediterranean Region. Glob. Planet. Change 2008, 63, 90–104. [Google Scholar] [CrossRef]
- Shanker, A.K.; Maheswari, M.; Yadav, S.K.; Desai, S.; Bhanu, D.; Attal, N.B.; Venkateswarlu, B. Drought Stress Responses in Crops. Funct. Integr. Genomics 2014, 14, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.A.; Xie, X.; Wang, L.c.; Saleem, M.F.; Man, C.; Lei, W. Morphological, Physiological and Biochemical Responses of Plants to Drought Stress. African J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar] [CrossRef]
- Abdelaal, K.; Attia, K.A.; Niedbała, G.; Wojciechowski, T.; Hafez, Y.; Alamery, S.; Alateeq, T.K.; Arafa, S.A. Mitigation of Drought Damages by Exogenous Chitosan and Yeast Extract with Modulating the Photosynthetic Pigments, Antioxidant Defense System and Improving the Productivity of Garlic Plants. Horticulturae 2021, 7, 510. [Google Scholar] [CrossRef]
- Oguz, M.C.; Aycan, M.; Oguz, E.; Poyraz, I.; Yildiz, M. Drought Stress Tolerance in Plants: Interplay of Molecular, Biochemical and Physiological Responses in Important Development Stages. Physiologia 2022, 2, 180–197. [Google Scholar] [CrossRef]
- Papaioannou, C.; Fassou, G.; Petropoulos, S.A.; Lamari, F.N.; Bebeli, P.J.; Papasotiropoulos, V. Evaluation of the Genetic Diversity of Greek Garlic (Allium sativum L.) Accessions Using DNA Markers and Association with Phenotypic and Chemical Variation. Agriculture 2023, 13, 1408. [Google Scholar] [CrossRef]
- Sánchez-Virosta, A.; Sánchez-Gómez, D. Inter-Cultivar Variability in the Functional and Biomass Response of Garlic (Allium sativum L.) to Water Availability. Sci. Hortic. 2019, 252, 243–251. [Google Scholar] [CrossRef]
- Sánchez-Virosta, Á.; Sadras, V.O.; Sánchez-Gómez, D. Phenotypic Plasticity in Relation to Inter-Cultivar Variation of Garlic (Allium sativum L.) Functional Performance and Yield-Stability in Response to Water Availability. Sci. Hortic. 2021, 285, 110128. [Google Scholar] [CrossRef]
- Sánchez-Virosta, A.; Léllis, B.C.; Pardo, J.J.; Martínez-Romero, A.; Sánchez-Gómez, D.; Domínguez, A. Functional Response of Garlic to Optimized Regulated Deficit Irrigation (ORDI) across Crop Stages and Years: Is Physiological Performance Impaired at the Most Sensitive Stages to Water Deficit? Agric. Water Manag. 2020, 228, 105886. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant Drought Stress: Effects, Mechanisms and Management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Kaiser, W.M. Effects of Water Deficit on Photosynthetic Capacity. Physiol. Plant. 1987, 71, 142–149. [Google Scholar] [CrossRef]
- Tominaga, J.; Shimada, H.; Kawamitsu, Y. Direct Measurement of Intercellular CO2 Concentration in a Gas-Exchange System Resolves Overestimation Using the Standard Method. J. Exp. Bot. 2018, 69, 1981–1991. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Condori-Apfata, J.A.; Liu, X.; Condori-Pacsi, S.J.; Valencia, M.V.; Zhang, C. Transcriptomic Changes Induced by Drought Stress in Hardneck Garlic during the Bolting/Bulbing Stage. Agronomy 2021, 11, 246. [Google Scholar] [CrossRef]
- Edris, A.E.; Fadel, H.M. Investigation of the Volatile Aroma Components of Garlic Leaves Essential Oil. Possibility of Utilization to Enrich Garlic Bulb Oil. Eur. Food Res. Technol. 2002, 214, 105–107. [Google Scholar] [CrossRef]
- Panthee, D.R.; KC, R.B.; Regmi, H.N.; Subedi, P.P.; Bhattarai, S.; Dhakal, J. Diversity Analysis of Garlic (Allium sativum L.) Germplasms Available in Nepal Based on Morphological Characters. Genet. Resour. Crop Evol. 2006, 53, 205–212. [Google Scholar] [CrossRef]
- Reichle, D.E. Energy Relationships between Organisms and Their Environment. In The Global Carbon Cycle and Climate Change; Elsevier: Amsterdam, The Netherlands, 2020; pp. 15–41. [Google Scholar] [CrossRef]
- Konrad, W.; Katul, G.; Roth-Nebelsick, A. Leaf Temperature and Its Dependence on Atmospheric CO2 and Leaf Size. Geol. J. 2021, 56, 866–885. [Google Scholar] [CrossRef]
- Kjaer, K.H.; Ottosen, C.O. 3D Laser Triangulation for Plant Phenotyping in Challenging Environments. Sensors 2015, 15, 13533–13547. [Google Scholar] [CrossRef]
- Gedam, P.A.; Thangasamy, A.; Shirsat, D.V.; Ghosh, S.; Bhagat, K.P.; Sogam, O.A.; Gupta, A.J.; Mahajan, V.; Soumia, P.S.; Salunkhe, V.N.; et al. Screening of Onion (Allium cepa L.) Genotypes for Drought Tolerance Using Physiological and Yield Based Indices Through Multivariate Analysis. Front. Plant Sci. 2021, 12, 122. [Google Scholar] [CrossRef]
- Lazarević, B.; Kontek, M.; Carović-Stanko, K.; Clifton-Brown, J.; Al Hassan, M.; Trindade, L.M.; Jurišić, V. Multispectral Image Analysis Detects Differences in Drought Responses in Novel Seeded Miscanthus Sinensis Hybrids. GCB Bioenergy 2022, 14, 1219–1234. [Google Scholar] [CrossRef]
- Zin, T.T. Morphological and Anatomical Studies on Allium sativum L. Cv. Yatsauk Hmwar Phyu. Ph.D. Thesis, University of Mandalay, Mandalay, Myanmar, 2017. [Google Scholar]
- Nemeskéri, E.; Molnár, K.; Helyes, L. Relationships of Spectral Traits with Yield and Nutritional Quality of Snap Beans (Phaseolus vulgaris L.) in Dry Seasons. Arch. Agron. Soil Sci. 2017, 64, 1222–1239. [Google Scholar] [CrossRef]
- Anderegg, J.; Yu, K.; Aasen, H.; Walter, A.; Liebisch, F.; Hund, A. Spectral Vegetation Indices to Track Senescence Dynamics in Diverse Wheat Germplasm. Front. Plant Sci. 2020, 10, 466315. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, J.L.; Prueger, J.H. Value of Using Different Vegetative Indices to Quantify Agricultural Crop Characteristics at Different Growth Stages under Varying Management Practices. Remote Sens. 2010, 2, 562–578. [Google Scholar] [CrossRef]
- Kandil, A.; Attia, A.; Sharief, A.; Leilh, A. Response of Onion (Allium cepa L.) Yield to Water Stress and Mineral Biofertilization. Acta Agron. Hungarica 2011, 59, 361–370. [Google Scholar] [CrossRef]
- Marostica, T.F.; Cazarolli, L.H.; Moura, G.S.; Luz, V.C.D.; Guimarães, E.A.C.M.; Cargnelutti, D. Does Allium sativum L. Tolerate Water Deficit. Sci. Electron. Arch. 2019, 12, 43. [Google Scholar] [CrossRef][Green Version]
- Akbari, S.; Kafi, M.; Rezvan Beidokhti, S. The Effects of Drought Stress on Yield, Yield Components and Anti-Oxidant of Two Garlic (Allium sativum L.) Ecotypes with Different Planting Densities. J. Agroecol. 2016, 8, 95–106. [Google Scholar] [CrossRef]
- Akbari, S.; Kafi, M.; Rezvan Beidokhti, S. Effect of Drought Stress on Growth and Morphological Characteristics of Two Garlic (Allium sativum L.) Ecotypes in Different Planting Densities. J. Agroecol. 2017, 9, 559–574. [Google Scholar] [CrossRef]
- Taha, N.; Res, S.A.-E.-M.E.J.A. Improving Yield and Quality of Garlic (Allium sativum L.) under Water Stress Conditions. 2019. Available online: Curresweb.com (accessed on 15 June 2023.).
- Csiszár, J.; Lantos, E.; Tari, I.; Madosa, E.; Wodala, B.; Vashegyi, Á.; Horváth, F.; Pécsváradi, A.; Szabó, M.; Bartha, B.; et al. Antioxidant Enzyme Activities in Allium Species and Their Cultivars under Water Stress. Plant Soil Environ. 2007, 53, 517. [Google Scholar] [CrossRef]
- Thangasamy, A.; Khade, Y. Physiological and Biochemical Responses in Onion Crop to Drought Stress Integrated Water and Nutrient Management and Physiological Manipulation for Improving Productivity of Onion and Garlic View Project Okra Breeing View Project Pranjali Harischandra Gho. Artic. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2054–2062. [Google Scholar] [CrossRef]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of Antioxidant Activities of Common Vegetables Employing Oxygen Radical Absorbance Capacity (ORAC) and Ferric Reducing Antioxidant Power (FRAP) Assays: A Comparative Study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-Throughput Assay of Oxygen Radical Absorbance Capacity (ORAC) Using a Multichannel Liquid Handling System Coupled with a Microplate Fluorescence Reader in 96-Well Format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Habuš Jerčić, I.; Bošnjak Mihovilović, A.; Matković Stanković, A.; Lazarević, B.; Goreta Ban, S.; Ban, D.; Major, N.; Tomaz, I.; Banjavčić, Z.; Kereša, S. Garlic Ecotypes Utilise Different Morphological, Physiological and Biochemical Mechanisms to Cope with Drought Stress. Plants 2023, 12, 1824. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Pérez-Gregorio, M.R.; García-Falcón, M.S.; Simal-Gándara, J.; Almeida, D.P.F. Effect of Meteorological Conditions on Antioxidant Flavonoids in Portuguese Cultivars of White and Red Onions. Food Chem. 2011, 124, 303–308. [Google Scholar] [CrossRef]
- Najjaa, H.; Boubakri, A.; Ben Arfa, A.; Zouari, N.; Neffati, M. Salinity and Drought Stresses Improve Antioxidant Potential of Allium roseum L., an Edible Medicinal Plant. Acta Physiol. Plant. 2018, 40, 195. [Google Scholar] [CrossRef]
- Rana, V.; Ram, S.; Nehra, K. Review Proline Biosynthesis and Its Role in Abiotic Stress. Int. J. Agric. Innov. Res. 2017, 6, 2319–2473. [Google Scholar]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of Proline under Changing Environments. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Anwar Hossain, M.; Hoque, M.A.; Burritt, D.J.; Fujita, M. Proline Protects Plants Against Abiotic Oxidative Stress: Biochemical and Molecular Mechanisms. In Oxidative Damage to Plants Antioxid. Networks Signal; Elsevier: Amsterdam, The Netherlands, 2014; pp. 477–522. [Google Scholar] [CrossRef]
- Dar, M.I.; Naikoo, M.I.; Rehman, F.; Naushin, F.; Khan, F.A. Proline Accumulation in Plants: Roles in Stress Tolerance and Plant Development. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies; Iqbal, N., Nazar, R.A., Khan, N., Eds.; Springer: New Delhi, India, 2015; pp. 155–166. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Gill, S.S.; Fujita, M. Drought Stress Responses in Plants, Oxidative Stress, and Antioxidant Defense. In Climate Change and Plant Abiotic Stress Tolerance; Wiley: Hoboken, NJ, USA, 2013; pp. 209–250. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Troll, W.; Lindsey, J. A Photometric Method for the Determination of Proline. J. Biol. Chem. 1955, 215, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Gaebler, D.M.; Jones, S.J.; Mandigo, R.W. Adaptation of Microplate Reader for Measuring Oxidative Rancidity in Meat Products. Meat Sci. 2002, 62, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Meier, U. A Note on the Power of Fisher’s Least Significant Difference Procedure. Pharm. Stat. 2006, 5, 253–263. [Google Scholar] [CrossRef]

| Digital Biomass | Height | Height Max | Leaf Angle | Leaf Area | Leaf Area Index | Leaf Area (Projected) | Leaf Inclination | Light Penetration Depth | |
|---|---|---|---|---|---|---|---|---|---|
| (dm3) | (mm) | (mm) | (°) | (dm2) | (cm2/cm2) | (m2) | (cm2/cm2) | (mm) | |
| Treatment | |||||||||
| Watered | 49.0 ± 1.5 | 335 ± 6 | 542 ± 7.7 | 54.6 ± 0.6 | 14.4 ± 0.4 | 0.47 ± 0.1 | 11.8 ± 0.3 | 1.25 ± 0.1 | 289 ± 6 |
| Non-watered | 49.1 ± 0.6 | 320 ± 5 | 564 ± 0.3 | 57.7 ± 0.1 | 15.3 ± 0.1 | 0.49 ± 0.1 | 12.9 ± 0.1 | 1.18 ± 0.1 | 276 ± 5 |
| p-value | ns | ** | ** | *** | * | * | *** | *** | *** |
| Accession | |||||||||
| IPT10 | 49.5 ± 2.42 | 320 ± 18 d–j 1 | 565 ± 0.2 | 57.5 ± 0.3 | 15.3 ± 0.1 a–c | 0.49 ± 0.1 a–d | 12.8 ± 0.1 a–d | 1.19 ± 0.1 | 268 ± 19 e–i |
| IPT11 | 41.8 ± 8.05 | 310 ± 21 e–j | 517 ± 48 | 54.6 ± 3.1 | 13.0 ± 2.3 b–f | 0.42 ± 0.1 b–g | 10.9 ± 2.0 b–f | 1.25 ± 0.1 | 260 ± 26 f–i |
| IPT13 | 46.9 ± 2.05 | 297 ± 13 g–j | 565 ± 0.2 | 57.2 ± 0.4 | 15.7 ± 0.1 ab | 0.51 ± 0.1 ab | 13.2 ± 0.1 a | 1.19 ± 0.1 | 252 ± 13 g–i |
| IPT14 | 45.6 ± 1.04 | 281 ± 6 j | 564 ± 0.3 | 57.5 ± 0.5 | 16.2 ± 0.1 a | 0.52 ± 0.1 a | 13.6 ± 0.1 a | 1.19 ± 0.1 | 236 ± 6 i |
| IPT16 | 46.8 ± 1.32 | 300 ± 12 g–j | 565 ± 0.2 | 57.5 ± 0.5 | 15.0 ± 0.2 a–e | 0.49 ± 0.1 a–d | 12.8 ± 0.3 a–c | 1.19 ± 0.1 | 255 ± 12 f–i |
| IPT17 | 47.5 ± 1.89 | 305 ± 13 f–j | 556 ± 5.5 | 57.7 ± 0.6 | 15.8 ± 0.1 ab | 0.50 ± 0.1 a–c | 12.9 ± 0.1 a–c | 1.18 ± 0.1 | 260 ± 13 f–i |
| IPT19 | 54.0 ± 2.37 | 349 ± 17 a–f | 565 ± 0.2 | 56.8 ± 0.3 | 15.3 ± 0.1 a–c | 0.49 ± 0.1 a–e | 12.6 ± 0.2 a–d | 1.20 ± 0.1 | 304 ± 17 a–f |
| IPT194 | 47.0 ± 1.95 | 294 ± 11 h–j | 564 ± 0.2 | 56.6 ± 0.6 | 16.0 ± 0.2 a | 0.52 ± 0.1 a | 13.3 ± 0.1 a | 1.20 ± 0.1 | 255 ± 10 g–i |
| IPT199 | 45.7 ± 0.91 | 293 ± 7 h–j | 565 ± 0.2 | 57.2 ± 0.2 | 15.7 ± 0.2 ab | 0.51 ± 0.1 ab | 13.3 ± 0.1 a | 1.18 ± 0.1 | 248 ± 8 hi |
| IPT200 | 46.8 ± 0.74 | 280 ± 7 j | 559 ± 5.1 | 57.4 ± 0.3 | 16.3 ± 0.3 a | 0.53 ± 0.1 a | 13.6 ± 0.2 a | 1.19 ± 0.1 | 242 ± 9 i |
| IPT201 | 50.4 ± 9.24 | 394 ± 29 a | 516 ± 48 | 53.9 ± 3.7 | 12.3 ± 2.1 ef | 0.40 ± 0.1 fg | 10.1 ± 1.8 f | 1.28 ± 0.1 | 350 ± 29 a |
| IPT251 | 48.0 ± 1.72 | 302 ± 11 g–j | 564 ± 0.2 | 57.1 ± 0.4 | 15.9 ± 0.2 a | 0.51 ± 0.1 ab | 13.3 ± 0.1 a | 1.19 ± 0.1 | 258 ± 11 f–i |
| IPT253 | 47.1 ± 2.07 | 297 ± 13 g–j | 564 ± 0.5 | 57.2 ± 0.4 | 16.0 ± 0.2 a | 0.52 ± 0.1 a | 13.3 ± 0.1 a | 1.19 ± 0.1 | 252 ± 13 g–i |
| IPT255 | 53.1 ± 2.98 | 354 ± 19 a–e | 565 ± 0.2 | 56.7 ± 0.6 | 15.3 ± 0.1 a–c | 0.49 ± 0.1 a–d | 12.5 ± 0.1 a–e | 1.20 ± 0.1 | 310 ± 19 a–e |
| IPT257 | 49.6 ± 8.88 | 367 ± 29 a–c | 517 ± 48 | 52.7 ± 4.0 | 13.1 ± 2.1 b–f | 0.41 ± 0.1 d–g | 10.7 ± 1.9 c–f | 1.30 ± 0.1 | 312 ± 38 a–e |
| IPT259 | 37.3 ± 6.91 | 283 ± 13 ij | 517 ± 49 | 53.4 ± 3.9 | 12.8 ± 2.3 c–f | 0.42 ± 0.1 c–g | 10.6 ± 2.0 c–f | 1.28 ± 0.1 | 238 ± 13 i |
| IPT263 | 57.9 ± 3.27 | 356 ± 24 a–d | 565 ± 0.2 | 56.0 ± 0.4 | 15.7 ± 0.2 ab | 0.51 ± 0.1 ab | 12.9 ± 0.2 a–c | 1.21 ± 0.1 | 330 ± 21 a–d |
| IPT265 | 59.0 ± 1.41 | 395 ± 10 a | 564 ± 0.2 | 56.2 ± 0.3 | 14.9 ± 0.2 a–e | 0.48 ± 0.1 a–f | 12.3 ± 0.2 a–f | 1.20 ± 0.1 | 350 ± 10 a |
| IPT266 | 58.2 ± 1.77 | 387 ± 13 ab | 565 ± 0.2 | 57.3 ± 0.7 | 14.9 ± 0.2 a–e | 0.48 ± 0.1 a–f | 12.5 ± 0.2 a–d | 1.19 ± 0.1 | 343 ± 13 a–c |
| IPT269 | 46.4 ± 8.75 | 351 ± 20 a–e | 517 ± 48 | 52.6 ± 3.7 | 12.5 ± 2.3 d–f | 0.40 ± 0.1 e–g | 10.5 ± 2 d–f | 1.30 ± 0.1 | 299 ± 27 b–g |
| IPT273 | 54.5 ± 3.09 | 372 ± 10 a–c | 549 ± 16 | 54.7 ± 1.6 | 14.1 ± 0.8 a–f | 0.45 ± 0.1 a–g | 11.5 ± 0.7 a–f | 1.26 ± 0.1 | 336 ± 11 a–c |
| IPT303 | 56.1 ± 3.77 | 391 ± 22 a | 548 ± 16 | 55.3 ± 1.5 | 14.1 ± 0.7 a–f | 0.46 ± 0.1 a–g | 11.6 ± 0.6 a–f | 1.24 ± 0.1 | 345 ± 22 ab |
| IPT308 | 51.2 ± 1.73 | 328 ± 10 c–i | 565 ± 0.2 | 56.5 ± 0.3 | 15.6 ± 0.1 ab | 0.50 ± 0.1 a–c | 13.0 ± 0.1 ab | 1.19 ± 0.1 | 283 ± 10 d–i |
| IPT333 | 47.2 ± 2.41 | 316 ± 17 d–j | 565 ± 0.2 | 57.3 ± 0.4 | 15.1 ± 0.2 a–d | 0.49 ± 0.1 a–e | 12.6 ± 0.2 a–d | 1.19 ± 0.1 | 271 ± 17 e–i |
| IPT347 | 44.2 ± 8.60 | 342 ± 19 b–g | 516 ± 48 | 52.9 ± 4.3 | 12.1 ± 2.3 f | 0.39 ± 0.1 g | 10.2 ± 2.0 ef | 1.31 ± 0.1 | 299 ± 18 b–g |
| IPT351 | 48.4 ± 3.82 | 330 ± 25 c–h | 565 ± 0.1 | 57.7 ± 0.5 | 14.5 ± 0.1 a–f | 0.47 ± 0.1 a–g | 12.2 ± 0.1 a–f | 1.19 ± 0.1 | 285 ± 25 d–i |
| IPT360 | 50.9 ± 2.86 | 353 ± 18 a–e | 564 ± 0.2 | 56.2 ± 0.3 | 15.0 ± 0.2 a–d | 0.49 ± 0.1 a–f | 12.5 ± 0.2 a–e | 1.20 ± 0.1 | 295 ± 22 c–h |
| IPT361 | 46.7 ± 1.17 | 287 ± 3 h–j | 562 ± 2.4 | 56.7 ± 0.3 | 15.8 ± 0.1 a | 0.51 ± 0.1 a | 13.2 ± 0.1 a | 1.20 ± 0.1 | 242 ± 3 i |
| IPT365 | 47.0 ± 2.59 | 300 ± 17 g–j | 565 ± 0.2 | 57.4 ± 0.8 | 15.5 ± 0.1 ab | 0.51 ± 0.1 ab | 13.1 ± 0.1 ab | 1.19 ± 0.1 | 255 ± 17 f–i |
| IPT367 | 46.5 ± 1.25 | 289 ± 8 h–j | 565 ± 0.2 | 57.3 ± 0.5 | 15.8 ± 0.2 a | 0.51 ± 0.1 a | 13.2 ± 0.1 a | 1.19 ± 0.1 | 245 ± 8 i |
| p–value | ns | *** | ns | ns | * | * | * | ns | *** |
| Treatment x Accession | |||||||||
| p–value | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Greenness | Hue | NDVI | NPCI | PSRI | |
|---|---|---|---|---|---|
| Treatment | |||||
| Watered | 0.00 ± 0.1 | 27.7 ± 2.3 | 0.15 ± 0.1 | 0.20 ± 0.1 | 0.22 ± 0.1 |
| Non-watered | −0.01 ± 0.1 | 20.3 ± 0.4 | 0.13 ± 0.1 | 0.21 ± 0.1 | 0.24 ± 0.1 |
| p-value | *** | *** | ** | *** | *** |
| Accession | |||||
| IPT10 | −0.01 ± 0.1 | 21.1 ± 1.1 b–d 1 | 0.14 ± 0.1 | 0.21 ± 0.1 | 0.24 ± 0.1 |
| IPT11 | 0.02 ± 0.1 | 35.8 ± 14 a–c | 0.19 ± 0.1 | 0.19 ± 0.1 | 0.22 ± 0.1 |
| IPT13 | −0.02 ± 0.1 | 17.4 ± 0.9 d | 0.12 ± 0.1 | 0.21 ± 0.1 | 0.24 ± 0.1 |
| IPT14 | −0.02 ± 0.1 | 17.4 ± 0.8 d | 0.12 ± 0.1 | 0.22 ± 0.1 | 0.24 ± 0.1 |
| IPT16 | −0.02 ± 0.1 | 17.3 ± 1.6 d | 0.13 ± 0.1 | 0.21 ± 0.1 | 0.23 ± 0.1 |
| IPT17 | −0.01 ± 0.1 | 19.2 ± 1.1 cd | 0.13 ± 0.1 | 0.21 ± 0.1 | 0.23 ± 0.1 |
| IPT19 | −0.01 ± 0.1 | 22.1 ± 0.9 b–d | 0.14 ± 0.1 | 0.20 ± 0.1 | 0.23 ± 0.1 |
| IPT194 | −0.02 ± 0.1 | 17.3 ± 0.6 d | 0.13 ± 0.1 | 0.21 ± 0.1 | 0.23 ± 0.1 |
| IPT199 | −0.01 ± 0.1 | 18.5 ± 0.7 d | 0.13 ± 0.1 | 0.22 ± 0.1 | 0.24 ± 0.1 |
| IPT200 | −0.02 ± 0.1 | 17.3 ± 1.8 d | 0.12 ± 0.1 | 0.21 ± 0.1 | 0.24 ± 0.1 |
| IPT201 | 0.03 ± 0.1 | 42.0 ± 13 a–c | 0.20 ± 0.1 | 0.18 ± 0.1 | 0.21 ± 0.1 |
| IPT251 | −0.01 ± 0.1 | 19.1 ± 0.7 cd | 0.13 ± 0.1 | 0.21 ± 0.1 | 0.24 ± 0.1 |
| IPT253 | −0.01 ± 0.1 | 19.3 ± 1.1 cd | 0.12 ± 0.1 | 0.21 ± 0.1 | 0.24 ± 0.1 |
| IPT255 | −0.01 ± 0.1 | 23.3 ± 2.0 b–d | 0.14 ± 0.1 | 0.20 ± 0.1 | 0.23 ± 0.1 |
| IPT257 | 0.03 ± 0.1 | 38.1 ± 13 ab | 0.19 ± 0.1 | 0.19 ± 0.1 | 0.21 ± 0.1 |
| IPT259 | 0.02 ± 0.1 | 33.7 ± 15 a–d | 0.18 ± 0.1 | 0.18 ± 0.1 | 0.21 ± 0.1 |
| IPT263 | 0.00 ± 0.1 | 25.9 ± 2.3 a–d | 0.15 ± 0.1 | 0.20 ± 0.1 | 0.23 ± 0.1 |
| IPT265 | −0.01 ± 0.1 | 22.4 ± 0.6 b–d | 0.14 ± 0.1 | 0.20 ± 0.1 | 0.22 ± 0.1 |
| IPT266 | −0.01 ± 0.1 | 24.5 ± 1.3 b–d | 0.14 ± 0.1 | 0.21 ± 0.1 | 0.23 ± 0.1 |
| IPT269 | 0.02 ± 0.1 | 35.9 ± 14 a–c | 0.18 ± 0.1 | 0.19 ± 0.1 | 0.22 ± 0.1 |
| IPT273 | 0.01 ± 0.1 | 32.0 ± 6.2 a–d | 0.16 ± 0.1 | 0.20 ± 0.1 | 0.22 ± 0.1 |
| IPT303 | 0.01 ± 0.1 | 32.1 ± 6.0 a–d | 0.16 ± 0.1 | 0.19 ± 0.1 | 0.21 ± 0.1 |
| IPT308 | −0.01 ± 0.1 | 21.3 ± 1.0 b–d | 0.13 ± 0.1 | 0.21 ± 0.1 | 0.24 ± 0.1 |
| IPT333 | −0.01 ± 0.1 | 19.5 ± 0.9 cd | 0.12 ± 0.1 | 0.22 ± 0.1 | 0.24 ± 0.1 |
| IPT347 | 0.02 ± 0.1 | 33.3 ± 13 a–d | 0.17 ± 0.1 | 0.20 ± 0.1 | 0.24 ± 0.1 |
| IPT351 | −0.02 ± 0.1 | 19.4 ± 1.0 cd | 0.13 ± 0.1 | 0.21 ± 0.1 | 0.24 ± 0.1 |
| IPT360 | −0.01 ± 0.1 | 22.3 ± 1.1 b–d | 0.14 ± 0.1 | 0.20 ± 0.1 | 0.23 ± 0.1 |
| IPT361 | −0.01 ± 0.1 | 18.2 ± 1.1 d | 0.13 ± 0.1 | 0.22 ± 0.1 | 0.24 ± 0.1 |
| IPT365 | −0.02 ± 0.1 | 17.4 ± 0.7 d | 0.12 ± 0.1 | 0.21 ± 0.1 | 0.24 ± 0.1 |
| IPT367 | −0.02 ± 0.1 | 17.4 ± 1.2 d | 0.12 ± 0.1 | 0.21 ± 0.1 | 0.24 ± 0.1 |
| p-value | ns | * | ns | ns | ns |
| Treatment X Accession | |||||
| p-value | ns | ns | ns | ns | ns |
| Assimilation Rate (µmol m−2 s−1) | Date | |||
|---|---|---|---|---|
| 15 March | 22 March | 24 March | 26 March | |
| Watered | 15.2 | 12.9 | 12.3 | 12.1 |
| Non-watered | 13.2 | 12.3 | 10.5 | 7.7 |
| Percentage decrease in non-watered | −13.5% | −5.2% | −15.1% | −36.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovačević, T.K.; Išić, N.; Major, N.; Krpan, M.; Ban, D.; Franić, M.; Goreta Ban, S. The Interplay of Physiological and Biochemical Response to Short-Term Drought Exposure in Garlic (Allium sativum L.). Plants 2023, 12, 3215. https://doi.org/10.3390/plants12183215
Kovačević TK, Išić N, Major N, Krpan M, Ban D, Franić M, Goreta Ban S. The Interplay of Physiological and Biochemical Response to Short-Term Drought Exposure in Garlic (Allium sativum L.). Plants. 2023; 12(18):3215. https://doi.org/10.3390/plants12183215
Chicago/Turabian StyleKovačević, Tvrtko Karlo, Nina Išić, Nikola Major, Marina Krpan, Dean Ban, Mario Franić, and Smiljana Goreta Ban. 2023. "The Interplay of Physiological and Biochemical Response to Short-Term Drought Exposure in Garlic (Allium sativum L.)" Plants 12, no. 18: 3215. https://doi.org/10.3390/plants12183215
APA StyleKovačević, T. K., Išić, N., Major, N., Krpan, M., Ban, D., Franić, M., & Goreta Ban, S. (2023). The Interplay of Physiological and Biochemical Response to Short-Term Drought Exposure in Garlic (Allium sativum L.). Plants, 12(18), 3215. https://doi.org/10.3390/plants12183215









