Coated Hematite Nanoparticles Alleviate Iron Deficiency in Cucumber in Acidic Nutrient Solution and as Foliar Spray
Abstract
:1. Introduction
2. Results
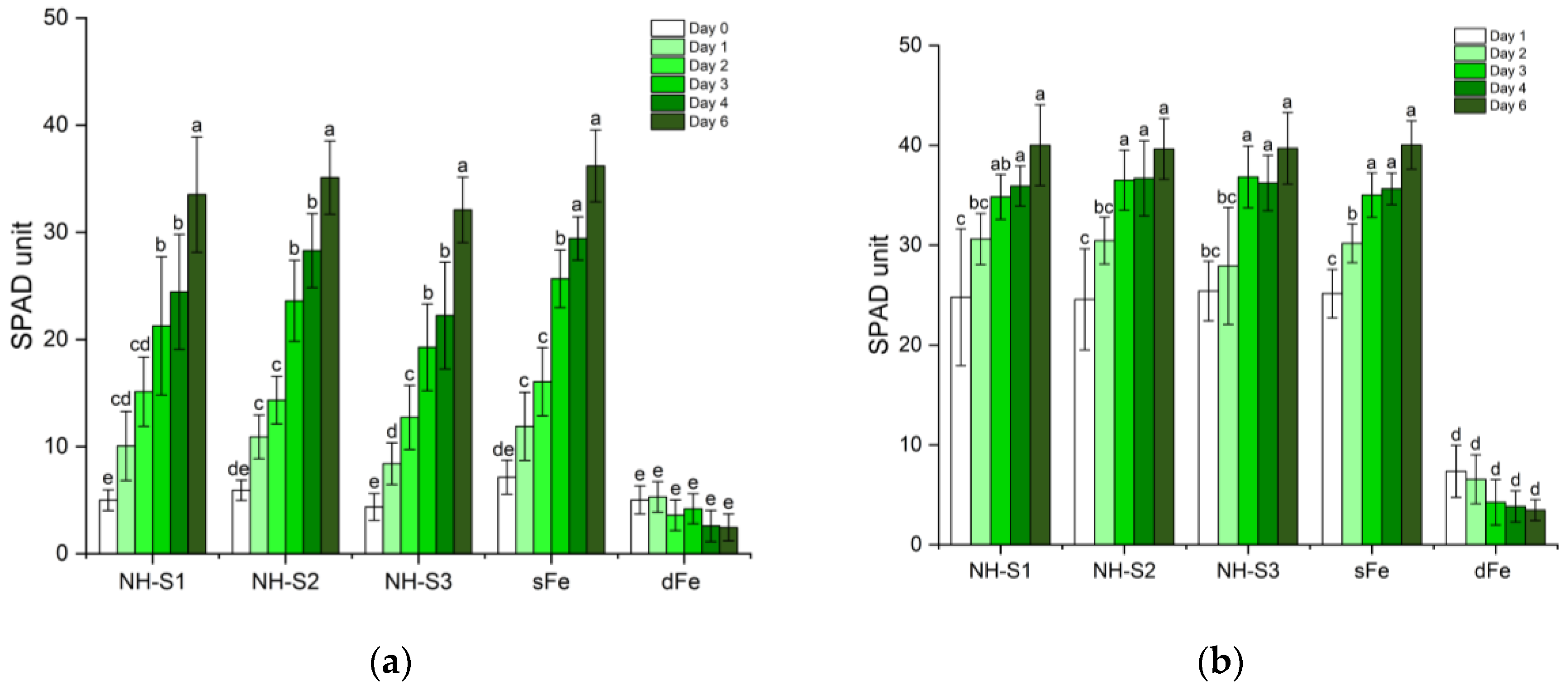
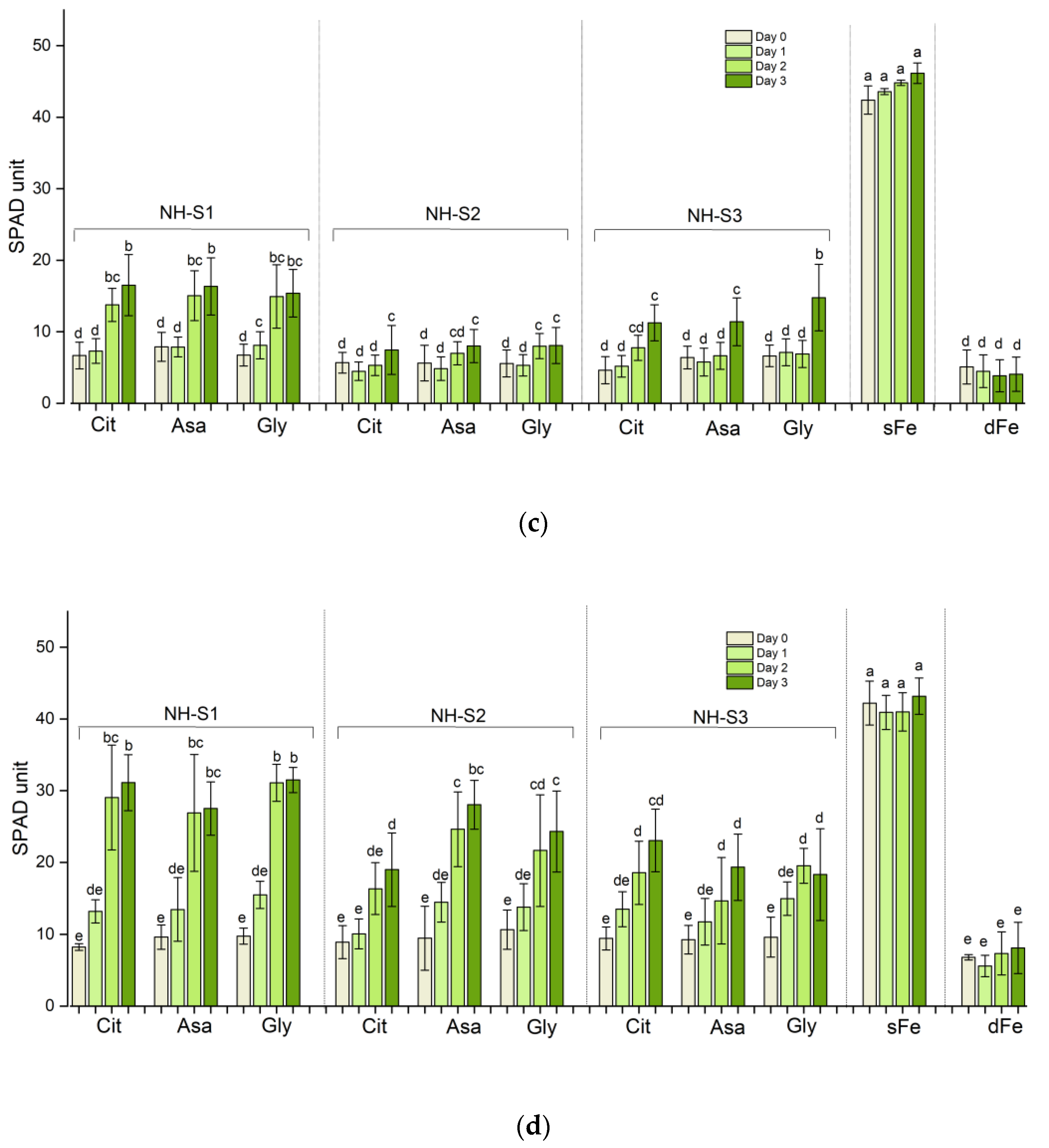
2.1. Greening and Chlorophyll Concentration
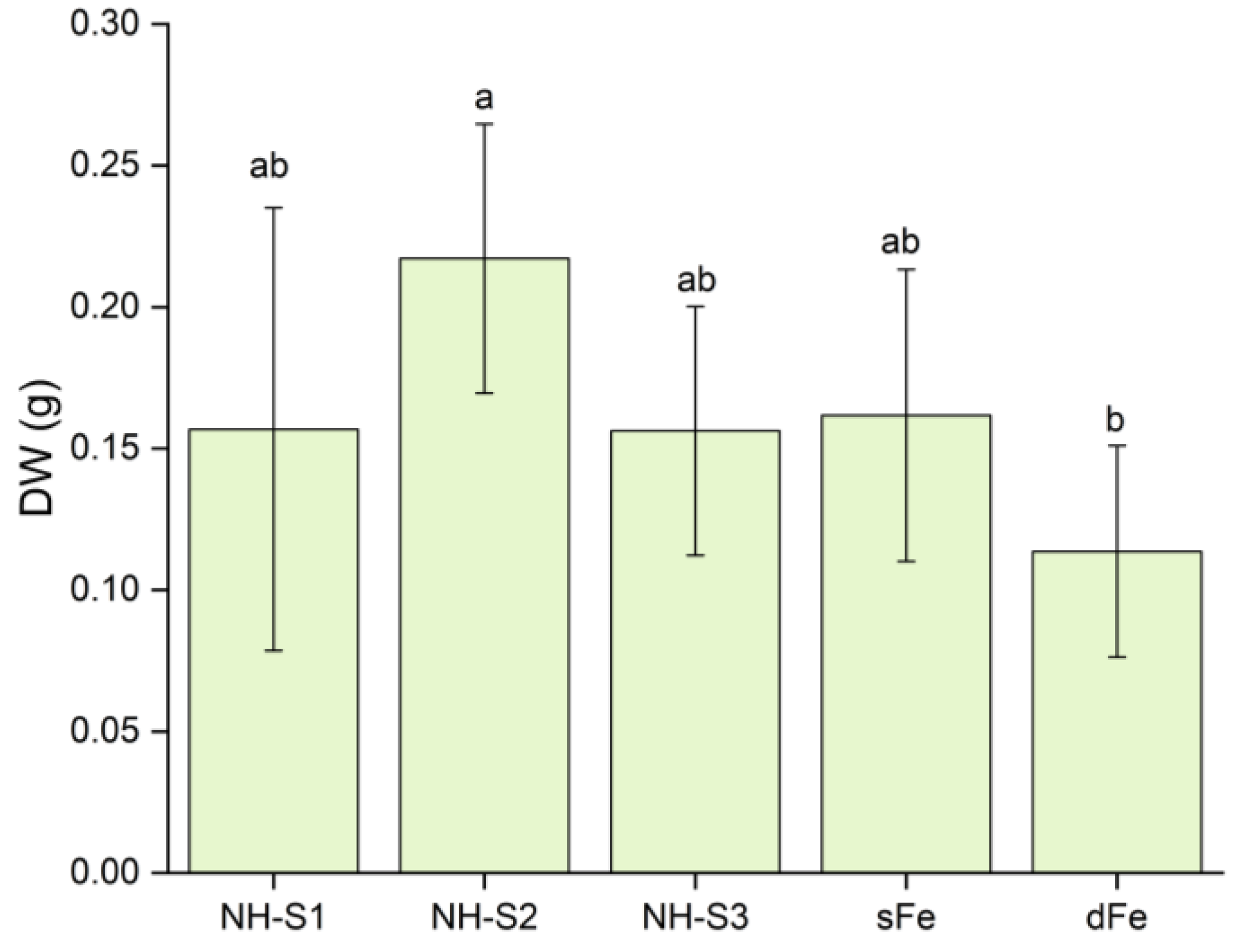
2.2. Biomass and pH
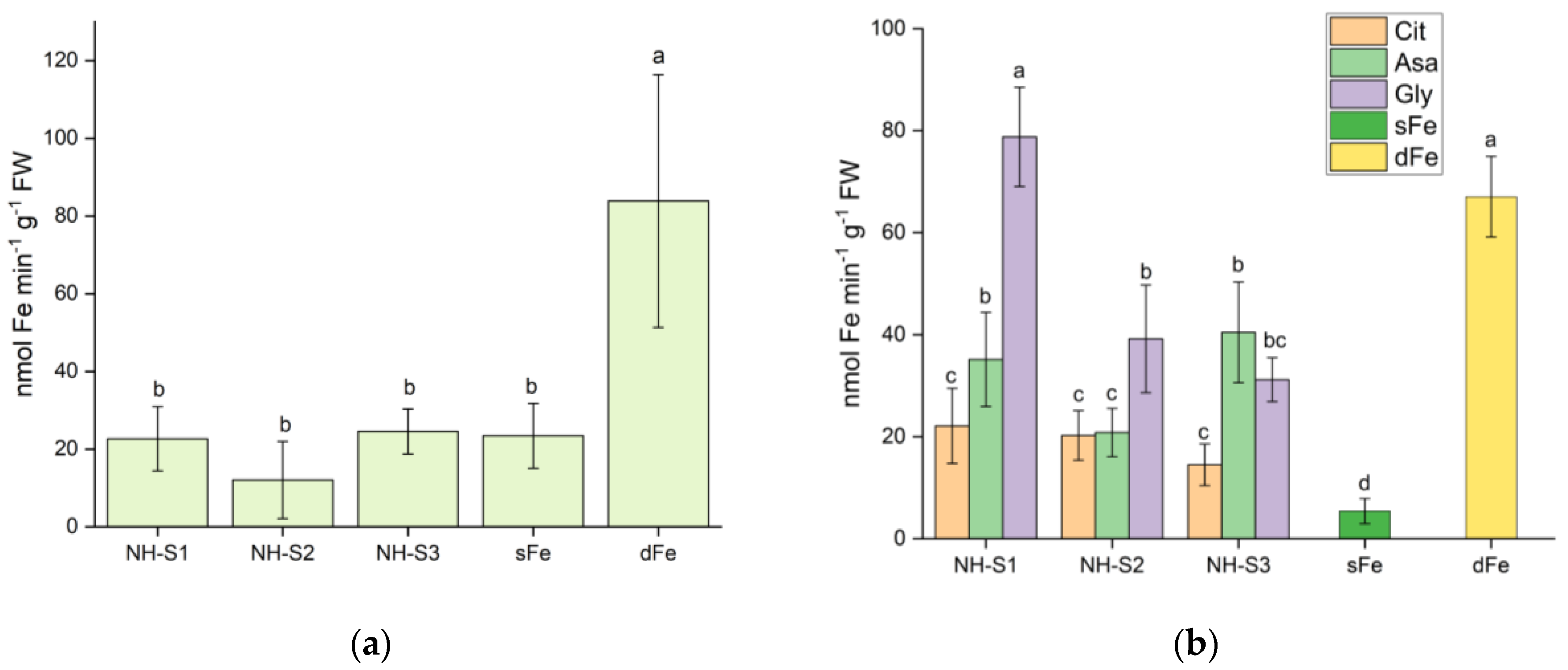
2.3. Ferric Chelate Reductase Activity
2.4. Photosynthetic Efficiency
2.5. Element Analysis
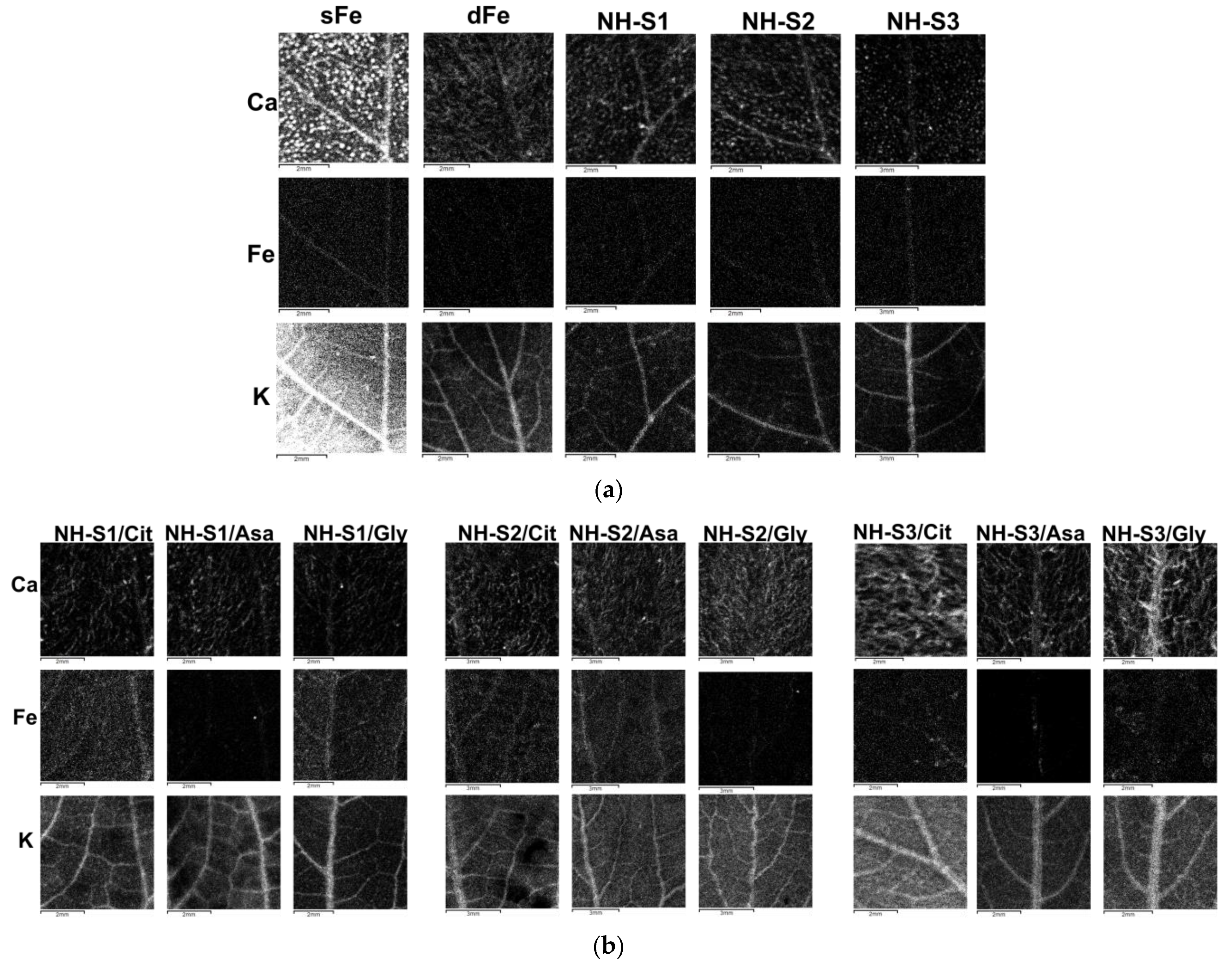
2.6. X-ray Fluorescence Mapping of the Leaves
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Treatment with Nanomaterials
4.3. Physiological Parameters
4.4. X-ray Fluorescence Imaging
4.5. Ferric Chelate Reductase Assay
4.6. Element Analysis
4.7. Statistical Treatment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aung, M.S.; Kobayashi, T.; Masuda, H.; Nishizawa, N.K. Rice HRZ Ubiquitin Ligases Are Crucial for the Response to Excess Iron. Physiol. Plant. 2018, 163, 282–296. [Google Scholar] [CrossRef]
- Lemanceau, P.; Bauer, P.; Kraemer, S.; Briat, J.-F. Iron Dynamics in the Rhizosphere as a Case Study for Analyzing Interactions between Soils, Plants and Microbes. Plant Soil 2009, 321, 513–535. [Google Scholar] [CrossRef]
- Valentinuzzi, F.; Pii, Y.; Carlo, P.; Roberto, T.; Fontanella, M.C.; Beone, G.M.; Astolfi, S.; Mimmo, T.; Cesco, S. Root-Shoot-Root Fe Translocation in Cucumber Plants Grown in a Heterogeneous Fe Provision. Plant Sci. 2020, 293, 110431. [Google Scholar] [CrossRef]
- Zuo, Y.; Zhang, F. Soil and Crop Management Strategies to Prevent Iron Deficiency in Crops. Plant Soil 2011, 339, 83–95. [Google Scholar] [CrossRef]
- Abadía, J.; Vázquez, S.; Rellán-Álvarez, R.; El-Jendoubi, H.; Abadía, A.; Álvarez-Fernández, A.; López-Millán, A.F. Towards a knowledge-based correction of iron chlorosis. Plant Physiol. Biochem. 2011, 49, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Sárvári, É.; Mihailova, G.; Solti, Á.; Keresztes, Á.; Velitchkova, M.; Georgieva, K. Comparison of Thylakoid Structure and Organization in Sun and Shade Haberlea Rhodopensis Populations under Desiccation and Rehydration. J. Plant Physiol. 2014, 171, 1591–1600. [Google Scholar] [CrossRef]
- Kroh, G.E.; Pilon, M. Regulation of Iron Homeostasis and Use in Chloroplasts. Int. J. Mol. Sci. 2020, 21, 3395. [Google Scholar] [CrossRef] [PubMed]
- Hindt, M.N.; Akmakjian, G.Z.; Pivarski, K.L.; Punshon, T.; Baxter, I.; Salt, D.E.; Guerinot, M. Lou BRUTUS and Its Paralogs, BTS LIKE1 and BTS LIKE2, Encode Important Negative Regulators of the Iron Deficiency Response in Arabidopsis Thaliana. Metallomics 2017, 9, 876–890. [Google Scholar] [CrossRef]
- Nozoye, T.; Nagasaka, S.; Kobayashi, T.; Takahashi, M.; Sato, Y.; Sato, Y.; Uozumi, N.; Nakanishi, H.; Nishizawa, N.K. Phytosiderophore Efflux Transporters Are Crucial for Iron Acquisition in Graminaceous Plants. J. Biol. Chem. 2011, 286, 5446–5454. [Google Scholar] [CrossRef]
- Kurt, F.; Filiz, E. Genome-Wide and Comparative Analysis of BHLH38, BHLH39, BHLH100 and BHLH101 Genes in Arabidopsis, Tomato, Rice, Soybean and Maize: Insights into Iron (Fe) Homeostasis. Biometals 2018, 31, 489–504. [Google Scholar] [CrossRef]
- Selote, D.; Samira, R.; Matthiadis, A.; Gillikin, J.W.; Long, T.A. Iron-Binding E3 Ligase Mediates Iron Response in Plants by Targeting Basic Helix-Loop-Helix Transcription Factors1. Plant Physiol. 2015, 167, 273–286. [Google Scholar] [CrossRef]
- Rajaie, M.; Tavakoly, A.R. Iron and/or Acid Foliar Spray versus Soil Application of Fe-EDDHA for Prevention of Iron Deficiency in Valencia Orange Grown on a Calcareous Soil. J. Plant Nutr. 2018, 41, 150–158. [Google Scholar] [CrossRef]
- Zuluaga, M.Y.A.; Cardarelli, M.; Rouphael, Y.; Cesco, S.; Pii, Y.; Colla, G. Iron Nutrition in Agriculture: From Synthetic Chelates to Biochelates. Sci. Hortic. 2023, 312, 111833. [Google Scholar] [CrossRef]
- Hochmuth, G. Iron (Fe) Nutrition of Plants Function of Fe in the Plant; Department of Soil and Water Sciences, UF/IFAS Extension; Institute of Food and Agricultural Sciences, University of Florida: Gainesville, FL, USA, 2011; pp. 1–8. [Google Scholar]
- Cieschi, M.T.; Polyakov, A.Y.; Lebedev, V.A.; Volkov, D.S.; Pankratov, D.A.; Veligzhanin, A.A.; Perminova, I.V.; Lucena, J.J. Eco-Friendly Iron-Humic Nanofertilizers Synthesis for the Prevention of Iron Chlorosis in Soybean (Glycine Max) Grown in Calcareous Soil. Front. Plant Sci. 2019, 10, 413. [Google Scholar] [CrossRef] [PubMed]
- Raliya, R.; Saharan, V.; Dimkpa, C.; Biswas, P. Nanofertilizer for Precision and Sustainable Agriculture: Current State and Future Perspectives. J. Agric. Food Chem. 2018, 66, 6487–6503. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Singh, J.; Yogalakshmi, K.N. Laccase Immobilized Magnetic Iron Nanoparticles: Fabrication and Its Performance Evaluation in Chlorpyrifos Degradation. Int. Biodeterior. Biodegrad. 2017, 117, 183–189. [Google Scholar] [CrossRef]
- Boutchuen, A.; Zimmerman, D.; Aich, N.; Masud, A.M.; Arabshahi, A.; Palchoudhury, S. Increased Plant Growth with Hematite Nanoparticle Fertilizer Drop and Determining Nanoparticle Uptake in Plants Using Multimodal Approach. J. Nanomater. 2019, 2019, 6890572. [Google Scholar] [CrossRef]
- Tombuloglu, H.; Slimani, Y.; Tombuloglu, G.; Almessiere, M.; Baykal, A. Uptake and Translocation of Magnetite (Fe3O4) Nanoparticles and Its Impact on Photosynthetic Genes in Barley (Hordeum Vulgare L.). Chemosphere 2019, 226, 110–122. [Google Scholar] [CrossRef]
- Pariona, N.; Martinez, A.I.; Hdz-García, H.M.; Cruz, L.A.; Hernandez-Valdes, A. Effects of Hematite and Ferrihydrite Nanoparticles on Germination and Growth of Maize Seedlings. Saudi J. Biol. Sci. 2017, 24, 1547–1554. [Google Scholar] [CrossRef]
- Claudio, C.; Iorio, E.D.; Liu, Q.; Jiang, Z.; Barrón, V. Iron oxide nanoparticles in soils: Environmental and agronomic importance. J. Nanosci. Nanotechnol. 2017, 17, 4449–4460. [Google Scholar] [CrossRef]
- Kraemer, S.M. Iron Oxide Dissolution and Solubility in the Presence of Siderophores. Aquat. Sci. 2004, 66, 3–18. [Google Scholar] [CrossRef]
- Shimizu, K.; Tschulik, K.; Compton, R.G. Exploring the Mineral-Water Interface: Reduction and Reaction Kinetics of Single Hematite (α-Fe2O3) Nanoparticles. Chem. Sci. 2016, 7, 1408–1414. [Google Scholar] [CrossRef]
- Colombo, C.; Palumbo, G.; He, J.Z.; Pinton, R.; Cesco, S. Review on Iron Availability in Soil: Interaction of Fe Minerals, Plants, and Microbes. J. Soils Sediments 2014, 14, 538–548. [Google Scholar] [CrossRef]
- Mazeina, L.; Navrotsky, A. Enthalpy of Water Adsorption and Surface Enthalpy of Goethite (α-FeOOH) and Hematite (α-Fe2O3). Chem. Mater. 2007, 19, 825–833. [Google Scholar] [CrossRef]
- Gracheva, M.; Klencsár, Z.; Kis, V.K.; Béres, K.A.; May, Z.; Halasy, V.; Singh, A.; Fodor, F.; Solti, Á.; Kiss, L.F.; et al. Iron Nanoparticles for Plant Nutrition: Synthesis, Transformation, and Utilization by the Roots of Cucumis Sativus. J. Mater. Res. 2022, 38, 1035–1047. [Google Scholar] [CrossRef]
- Martín-Barranco, A.; Spielmann, J.; Dubeaux, G.; Vert, G.; Zelazny, E. Dynamic Control of the High-Affinity Iron Uptake Complex in Root Epidermal Cells. Plant Physiol. 2020, 184, 1236–1250. [Google Scholar] [CrossRef] [PubMed]
- Ndou, N.; Rakgotho, T.; Nkuna, M.; Doumbia, I.Z.; Mulaudzi, T.; Ajayi, R.F. Green Synthesis of Iron Oxide (Hematite) Nanoparticles and Their Influence on Sorghum Bicolor Growth under Drought Stress. Plants 2023, 12, 1425. [Google Scholar] [CrossRef] [PubMed]
- Rath, K.; Ranganathan, P.; Vasappa, R.K.; Balasundaram, S.T. Superparamagnetic Hematite Nanoparticle: Cytogenetic Impact on Onion Roots and Seed Germination Response of Major Crop Plants. IET Nanobiotechnology 2020, 14, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Zargar Shooshtari, F.; Souri, M.K.; Hasandokht, M.R.; Jari, S.K. Glycine Mitigates Fertilizer Requirements of Agricultural Crops: Case Study with Cucumber as a High Fertilizer Demanding Crop. Chem. Biol. Technol. Agric. 2020, 7, 19. [Google Scholar] [CrossRef]
- Mosa, W.F.A.; Ali, H.M.; Abdelsalam, N.R. The Utilization of Tryptophan and Glycine Amino Acids as Safe Alternatives to Chemical Fertilizers in Apple Orchards. Environ. Sci. Pollut. Res. 2021, 28, 1983–1991. [Google Scholar] [CrossRef]
- Sh Sadak, M.; Abdelhamid, M.T.; Schmidhalter, U. Effect of Foliar Application of Amino acids On Plant Yield And Some Physiological Parameters In Bean Plants Irrigated With Seawater. Acta Biológica Colomb. 2015, 20, 141–152. [Google Scholar]
- Rasp, H. Control of Grape Chlorosis through Nutrient Application on Leaves. In Foliar Fertilization, Proceedings of the First International Symposium on Foliar Fertilization, Berlin, Germany, 14–16 March 1985; Springer Science & Business Media: Dordrecht, The Netherlands, 1986; Volume 22, p. 242. [Google Scholar]
- Tejada, M.; Gonzalez, J.L. Influence of Foliar Fertilization with Amino Acids and Humic Acids on Productivity and Quality of Asparagus. Biol. Agric. Hortic. 2003, 21, 277–291. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, A.; Sánchez-Andreu, J.; Juárez, M.; Jordá, J.; Bermúdez, D. Humic Substances and Amino Acids Improve Effectiveness of Chelate Fe-EDDHA in Lemon Trees. J. Plant Nutr. 2002, 25, 2433–2442. [Google Scholar] [CrossRef]
- Sánchez, A.S.; Juárez, M.; Sánchez-Andreu, J.; Jordá, J.; Bermúdez, D. Use of Humic Substances and Amino Acids to Enhance Iron Availability for Tomato Plants from Applications of the Chelate FeEDDHA. J. Plant Nutr. 2005, 28, 1877–1886. [Google Scholar] [CrossRef]
- Marschner, H.; Römheld, V.; Kissel, M. Different Strategies in Higher Plants in Mobilization and Uptake of Iron. J. Plant Nutr. 1986, 9, 695–713. [Google Scholar] [CrossRef]
- Durrett, T.P.; Gassmann, W.; Rogers, E.E. The FRD3-Mediated Efflux of Citrate into the Root Vasculature Is Necessary for Efficient Iron Translocation. Plant Physiol. 2007, 144, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Rellán-Álvarez, R.; Andaluz, S.; Rodríguez-Celma, J.; Wohlgemuth, G.; Zocchi, G.; Álvarez-Fernández, A.; Fiehn, O.; López-Millán, A.F.; Abadía, J. Changes in the Proteomic and Metabolic Profiles of Beta Vulgaris Root Tips in Response to Iron Deficiency and Resupply. BMC Plant Biol. 2010, 10, 120. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive Oxygen Species (ROS) and Response of Antioxidants as ROS-Scavengers during Environmental Stress in Plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Kapoor, D.; Singh, S.; Kumar, V.; Romero, R.; Prasad, R.; Singh, J. Antioxidant Enzymes Regulation in Plants in Reference to Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS). Plant Gene 2019, 19, 100182. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Ascorbate and Glutathione: The Heart of the Redox Hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.S.; Kodanko, J.J. Iron-Binding and Mobilization from Ferritin by Polypyridyl Ligands. Metallomics 2010, 2, 407–411. [Google Scholar] [CrossRef]
- Atanassova, B.D.; Tzatchev, K.N. Ascorbic acid-important for iron metabolism. Folia Medica 2008, 50, 11. [Google Scholar] [PubMed]
- Shokr, M.M.B.; Abdelhamid, M.T. Using Some Antioxidant Substances for Enhancing Thermotolerance and Improving Productivity of Pea (Pisum Sativum L.) Plants Under Local Environment of Early Summer Season. Agric. Res. J. 2009, 9, 69–76. [Google Scholar]
- Shafeek, M.; Helmy, Y.; Marzauk, N.M.; Magda, A.S.; Nadia, M.O. Effect of foliar application of some antioxidants on growth, yield and chemical composition of Lettuce plants (Lactuca Sativa L.) under plastic house condition. Middle East J. Appl. Sci. 2013, 3, 70–75. [Google Scholar]
- Mansour, M.M. Response of soybean plants to exogenously applied with ascorbic acid, zinc sulphate and paclobutrazol. Rep. Opin. 2014, 6, 17–25. [Google Scholar]
- Ramírez, L.; Bartoli, C.G.; Lamattina, L. Glutathione and Ascorbic Acid Protect Arabidopsis Plants against Detrimental Effects of Iron Deficiency. J. Exp. Bot. 2013, 64, 3169–3178. [Google Scholar] [CrossRef]
- Khan, S.; Yu, H.; Li, Q.; Gao, Y.; Sallam, B.N.; Wang, H.; Liu, P.; Jiang, W. Exogenous Application of Amino Acids Improves the Growth and Yield of Lettuce by Enhancing Photosynthetic Assimilation and Nutrient Availability. Agronomy 2019, 9, 266. [Google Scholar] [CrossRef]
- Souri, M.K.; Naiji, M.; Aslani, M. Effect of Fe-Glycine Aminochelate on Pod Quality and Iron Concentrations of Bean (Phaseolus Vulgaris L.) Under Lime Soil Conditions. Commun. Soil Sci. Plant Anal. 2018, 49, 215–224. [Google Scholar] [CrossRef]
- Mohammadipour, N.; Souri, M.K. Beneficial Effects of Glycine on Growth and Leaf Nutrient Concentrations of Coriander (Coriandrum Sativum) Plants. J. Plant Nutr. 2019, 42, 1637–1644. [Google Scholar] [CrossRef]
- Noroozlo, Y.A.; Souri, M.K.; Delshad, M. Stimulation Effects of Foliar Applied Glycine and Glutamine Amino Acids on Lettuce Growth. Open Agric. 2019, 4, 164–172. [Google Scholar] [CrossRef]
- Xu, M.; Du, L.; Liu, M.; Zhou, J.; Pan, W.; Fu, H.; Zhang, X.; Ma, Q.; Wu, L. Glycine-Chelated Zinc Rather than Glycine-Mixed Zinc Has Lower Foliar Phytotoxicity than Zinc Sulfate and Enhances Zinc Biofortification in Waxy Corn. Food Chem. 2022, 370, 131031. [Google Scholar] [CrossRef] [PubMed]
- Mijovilovich, A.; Morina, F.; Bokhari, S.N.; Wolff, T.; Küpper, H.; Küpper, H. Analysis of Trace Metal Distribution in Plants with Lab-Based Microscopic X-Ray Fluorescence Imaging. Plant Methods 2020, 16, 82. [Google Scholar] [CrossRef]
- Terzano, R.; Alfeld, M.; Janssens, K.; Vekemans, B.; Schoonjans, T.; Vincze, L.; Tomasi, N.; Pinton, R.; Cesco, S. Spatially Resolved (Semi)Quantitative Determination of Iron (Fe) in Plants by Means of Synchrotron Micro X-Ray Fluorescence. Anal. Bioanal. Chem. 2013, 405, 3341–3350. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of Accurate Extinction Coefficients and Simultaneous Equations for Assaying Chlorophylls a and b Extracted with Four Different Solvents: Verification of the Concentration of Chlorophyll Standards by Atomic Absorption Spectroscopy. Biochim. Biophys. Acta-Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Kovács, K.; Kuzmann, E.; Tatár, E.; Vértes, A.; Fodor, F. Investigation of Iron Pools in Cucumber Roots by Mössbauer Spectroscopy: Direct Evidence for the Strategy I Iron Uptake Mechanism. Planta 2009, 229, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.F.; McCurdy, W.H.; Diehl, H. The Colorimetric Determination of Iron in Raw and Treated Municipal Water Supplies by Use of 4:7-Diphenyl-1:10-Phenanthroline. Analyst 1952, 77, 418–422. [Google Scholar] [CrossRef]

| Treatment | Chl a+b (µg/g FW) | Chl a/b | ||
|---|---|---|---|---|
| Acidic pH | Alkaline pH | Acidic pH | Alkaline pH | |
| NH-S1 | 1924.8 ± 287.3 a | 171.9 ± 28.99 b | 3.0 ± 0.05 b | 3.0 ± 0.22 |
| NH-S2 | 2063.9 ± 76.3 a | 173.8 ± 28.30 b | 3.0 ± 0.09 b | 3.0 ± 0.57 |
| NH-S3 | 2092.9 ± 171.8 a | 168.2 ± 39.80 b | 2.9 ± 0.03 b | 2.7 ± 0.28 |
| sFe | 2555.28 ± 191.76 a | 2273.06 ± 246.92 a | 2.4 ± 0.14 b | 2.8 ± 0.03 |
| dFe | 92.2 ± 27.7 b | 162.6 ± 70.97 b | 4.9 ± 1.03 a | 2.8 ± 0.54 |
| Treatment | Root Supply | Foliar Supply | ||
|---|---|---|---|---|
| Cit | Asa | Gly | ||
| NH-S1 | 4.27 ± 0.20 B | 4.57 ± 0.03 ab | 4.36 ± 0.19 b | 4.04 ± 0.16 b |
| NH-S2 | 4.39 ± 0.25 AB | 4.46 ± 0.17 b | 4.13 ± 0.13 b | 3.84 ± 0.15 b |
| NH-S3 | 4.37 ± 0.26 AB | 4.60 ± 0.22 ab | 4.89 ± 0.05 ab | 4.18 ± 0.35 b |
| sFe | 6.29 ± 0.08 A | 5.97 ± 0.20 a | ||
| dFe | 3.88 ± 0.17 C | 4.07 ± 0.36 b | ||
| Root Supply | Assimilation (Anet) (µmol CO2 m−2 s−1) | Fv/Fm | Fv’/Fm’ | ΦPSII | ΦCO2 (μmol CO2 μmol−1 Photons) | NPQ |
|---|---|---|---|---|---|---|
| NH-S1 | 12.901 ± 2.526 a | 0.798 ± 0.005 a | 0.634 ± 0.034 a | 0.478 ± 0.031 a | 0.033 ± 0.006 A | 0.914 ± 0.242 a |
| NH-S2 | 14.840 ± 1.426 a | 0.798 ± 0.001 a | 0.641 ± 0.025 a | 0.488 ± 0.029 a | 0.036 ± 0.003 A | 0.832 ± 0.173 a |
| NH-S3 | 14.251 ± 0.560 a | 0.799 ± 0.004 a | 0.637 ± 0.001 a | 0.487 ± 0.010 a | 0.033 ± 0.002 A | 0.921 ± 0.124 a |
| sFe | 14.156 ± 3.421 a | 0.806 ± 0.004 a | 0.650 ± 0.027 a | 0.491 ± 0.046 a | 0.033 ± 0.007 A | 0.845 ± 0.130 a |
| dFe | −0.506 ± 0.123 b | 0.570 ± 0.047 b | 0.389 ± 0.125 b | 0.090 ± 0.009 b | 0.003 ± 0.003 b | 0.574 ± 0.130 b |
| Foliar supply | ||||||
| NH-S1 | ||||||
| Cit | 9.596 ± 2.374 ab | 0.805 ± 0.002 a | 0.568 ± 0.007 a | 0.388 ± 0.015 ab | 0.025 ± 0.004 a | 1.351 ± 0.105 a |
| Asa | 10.725 ± 3.810 ab | 0.807 ± 0.006 a | 0.587 ± 0.045 a | 0.426 ± 0.041 ab | 0.028 ± 0.006 a | 1.309 ± 0.317 a |
| Gly | 11.193 ±3.895 ab | 0.809 ± 0.002 a | 0.593 ± 0.015 a | 0.426 ± 0.031 ab | 0.029 ± 0.008 a | 1.204 ± 0.128 a |
| NH-S2 | ||||||
| Cit | 6.423 ± 0.356 b | 0.772 ± 0.002 a | 0.532 ± 0.042 a | 0.324 ± 0.046 b | 0.024 ± 0.004 a | 1.111 ± 0.199 a |
| Asa | 9.618 ± 2.917 ab | 0.777 ± 0.008 a | 0.586 ± 0.047 a | 0.401 ± 0.089 ab | 0.026 ± 0.004 a | 1.196 ± 0.447 a |
| Gly | 10.241 ± 1.368 ab | 0.776 ± 0.008 a | 0.611 ± 0.026 a | 0.434 ± 0.017 a | 0.035 ± 0.011 a | 0.867 ± 0.079 a |
| NH-S3 | ||||||
| Cit | 4.835 ± 1.478 b | 0.790 ± 0.009 a | 0.579 ± 0.038 a | 0.286 ± 0.047 b | 0.020 ± 0.005 a | 1.082 ± 0.387 a |
| Asa | 6.308 ± 1.163 b | 0.777 ± 0.009 a | 0.596 ± 0.062 a | 0.319 ± 0.033 b | 0.023 ± 0.003 a | 0.941 ± 0.501 a |
| Gly | 5.999 ± 1.689 b | 0.776 ± 0.013 a | 0.588 ± 0.036 a | 0.322 ± 0.046 b | 0.022 ± 0.005 a | 0.972 ± 0.320 a |
| sFe | 14.770 ± 3.799 a | 0.797 ± 0.006 a | 0.637 ± 0.031 a | 0.463 ± 0.051 a | 0.040 ± 0.006 a | 0.821 ± 0.186 a |
| dFe | −1.950 ± 0.656 c | 0.473 ± 0.051 b | 0.313 ± 0.076 b | 0.043 ± 0.006 c | 0.004 ± 0.001 b | 0.462 ± 0.187 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.; Pankaczi, F.; Rana, D.; May, Z.; Tolnai, G.; Fodor, F. Coated Hematite Nanoparticles Alleviate Iron Deficiency in Cucumber in Acidic Nutrient Solution and as Foliar Spray. Plants 2023, 12, 3104. https://doi.org/10.3390/plants12173104
Singh A, Pankaczi F, Rana D, May Z, Tolnai G, Fodor F. Coated Hematite Nanoparticles Alleviate Iron Deficiency in Cucumber in Acidic Nutrient Solution and as Foliar Spray. Plants. 2023; 12(17):3104. https://doi.org/10.3390/plants12173104
Chicago/Turabian StyleSingh, Amarjeet, Fruzsina Pankaczi, Deepali Rana, Zoltán May, Gyula Tolnai, and Ferenc Fodor. 2023. "Coated Hematite Nanoparticles Alleviate Iron Deficiency in Cucumber in Acidic Nutrient Solution and as Foliar Spray" Plants 12, no. 17: 3104. https://doi.org/10.3390/plants12173104
APA StyleSingh, A., Pankaczi, F., Rana, D., May, Z., Tolnai, G., & Fodor, F. (2023). Coated Hematite Nanoparticles Alleviate Iron Deficiency in Cucumber in Acidic Nutrient Solution and as Foliar Spray. Plants, 12(17), 3104. https://doi.org/10.3390/plants12173104







