Identifying and Managing Areas under Threat in the Iberian Peninsula: An Invasion Risk Atlas for Non-Native Aquatic Plant Species as a Potential Tool
Abstract
:1. Introduction
2. Results
2.1. Occurrence Data
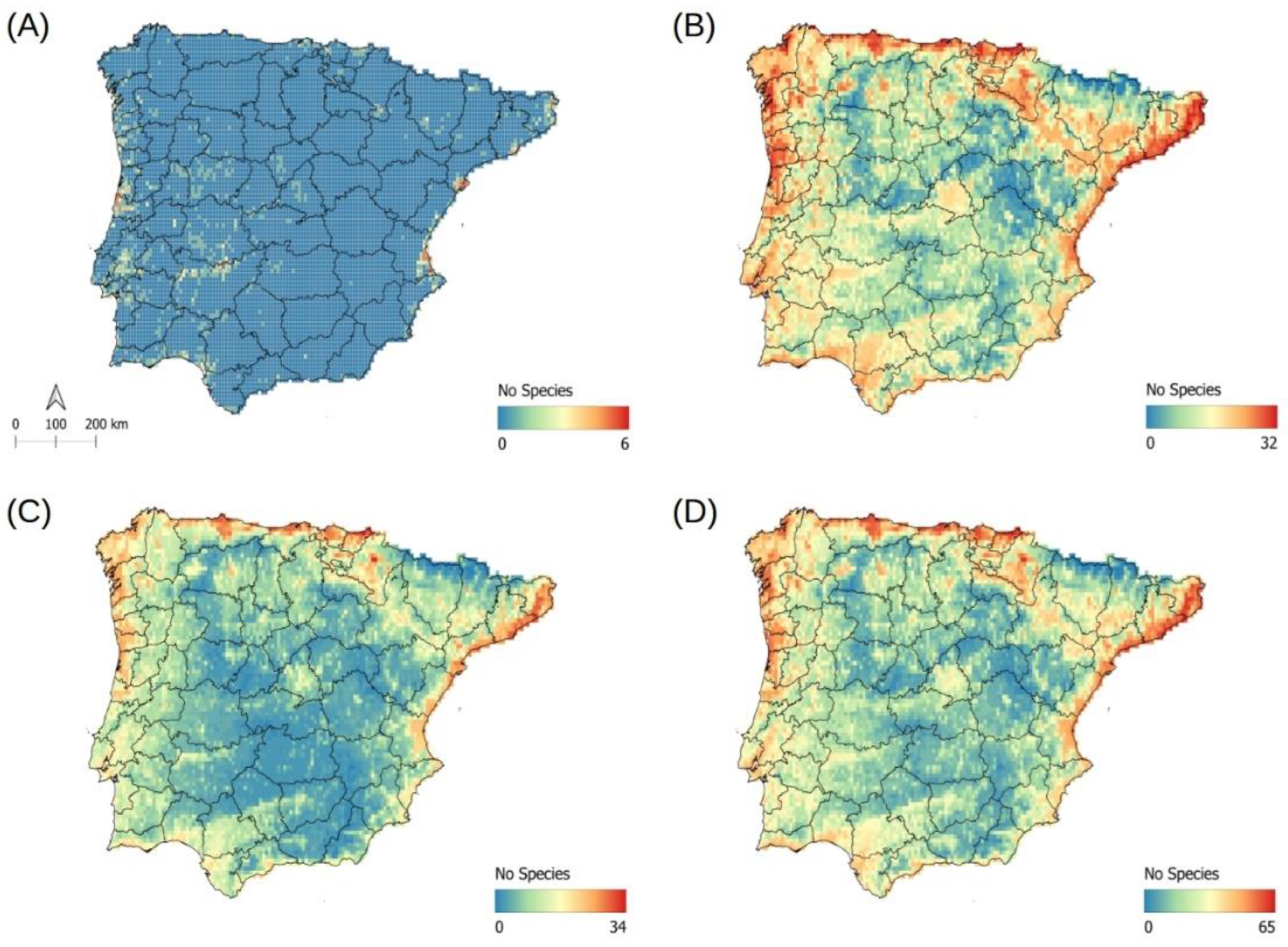
2.2. Distribution of Currently Established Non-Native Aquatic Plant Species
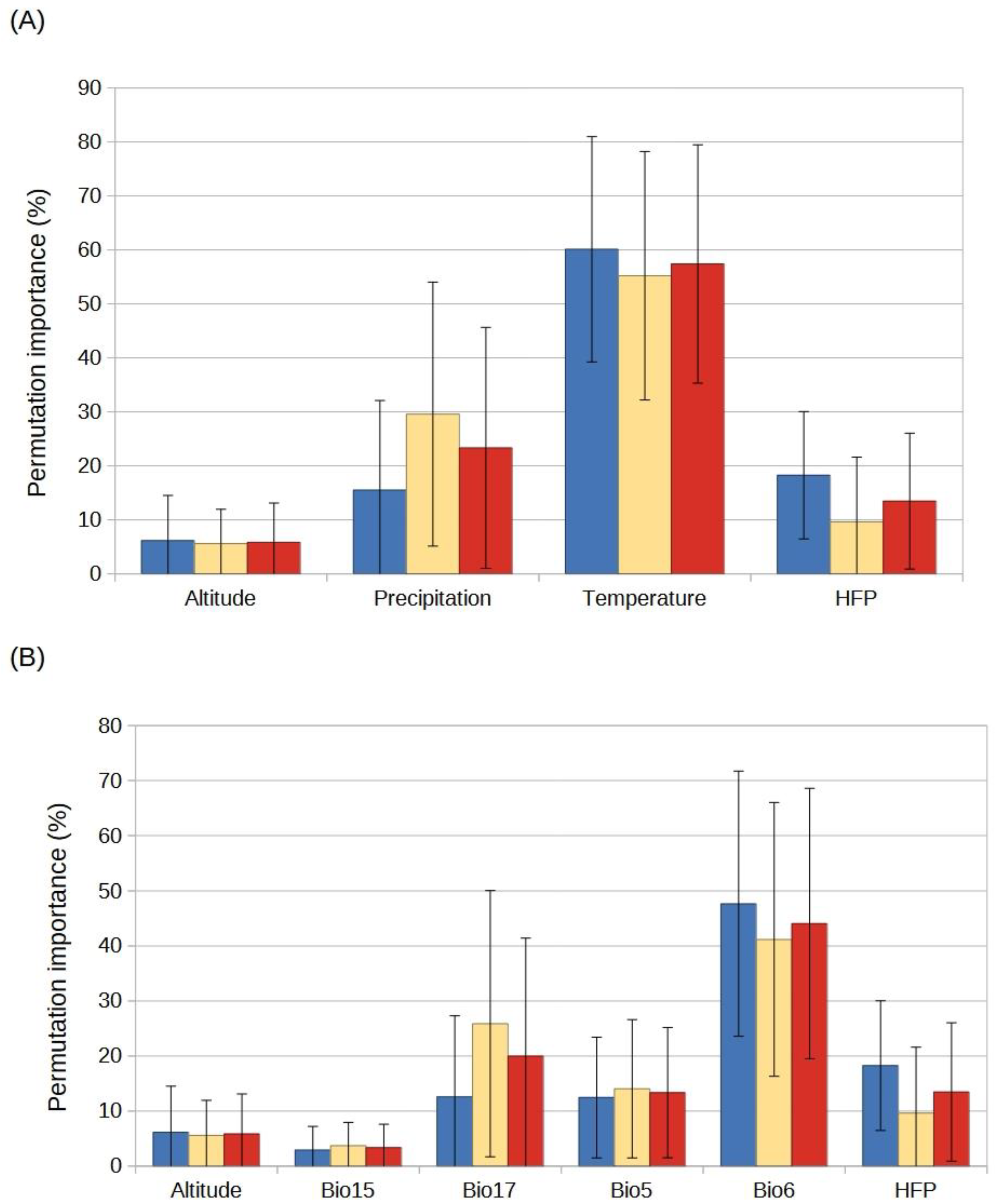
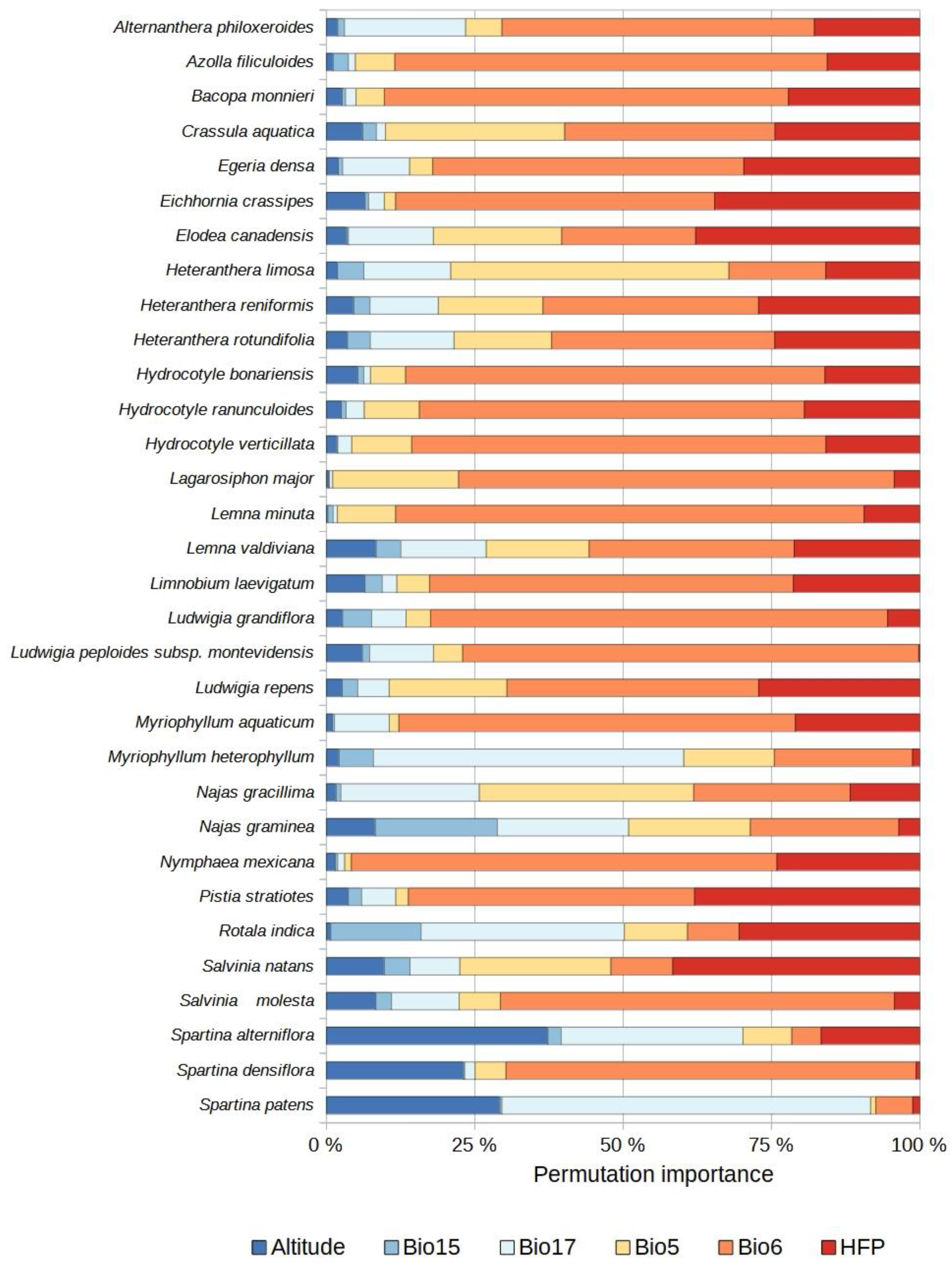
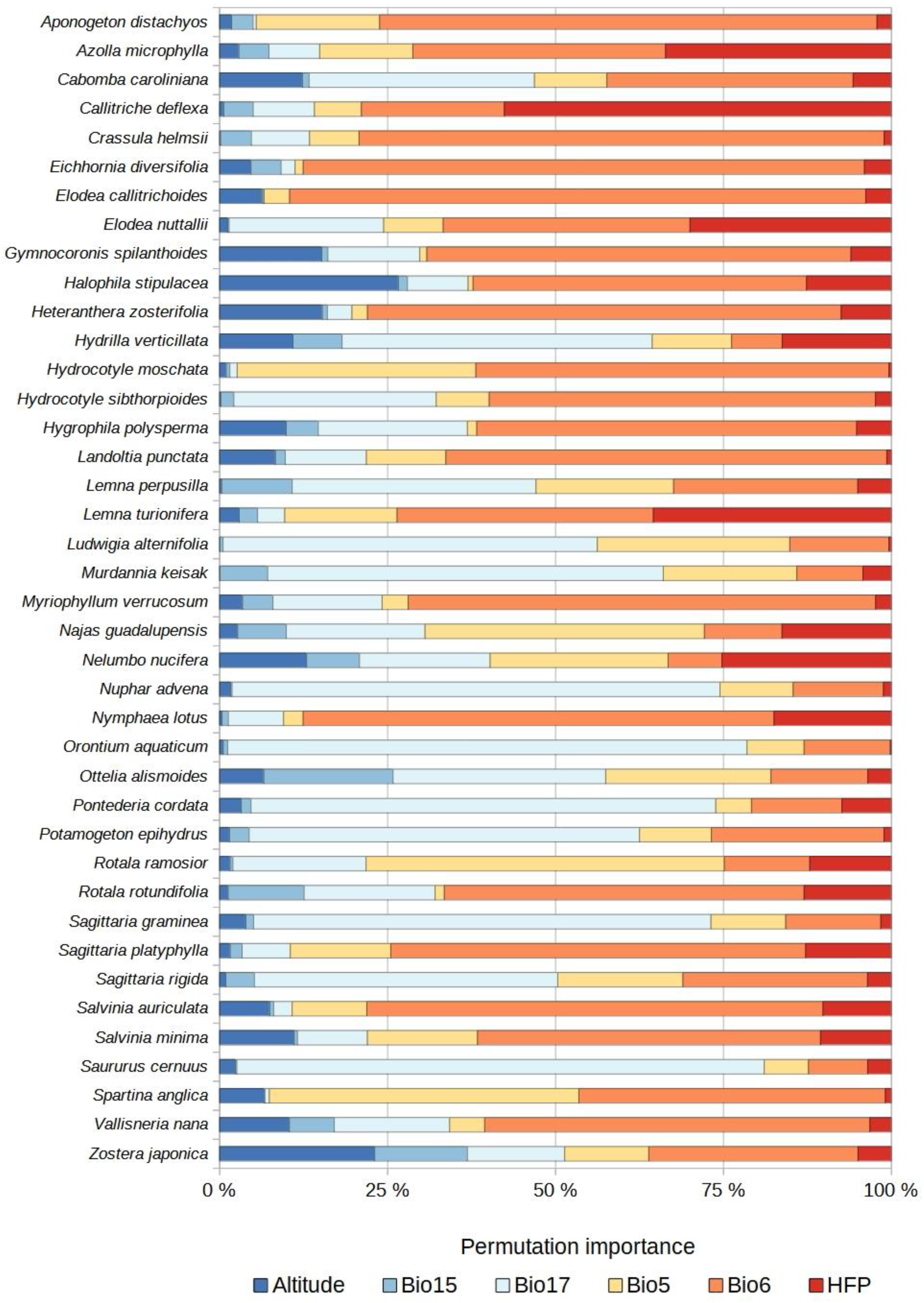
2.3. Species Distribution Models
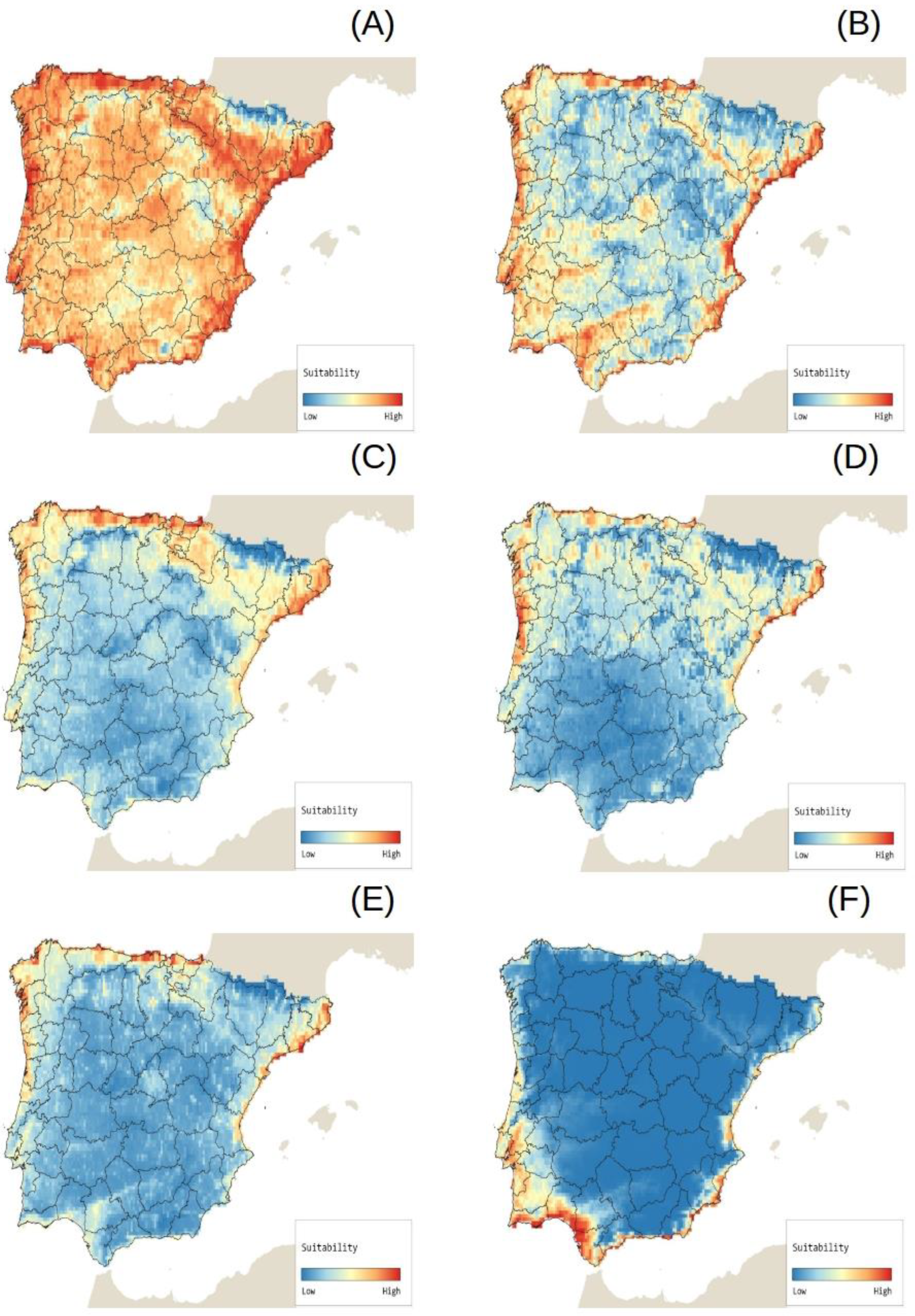
2.4. Potential Species Distributions (Potential Invasion Risk)
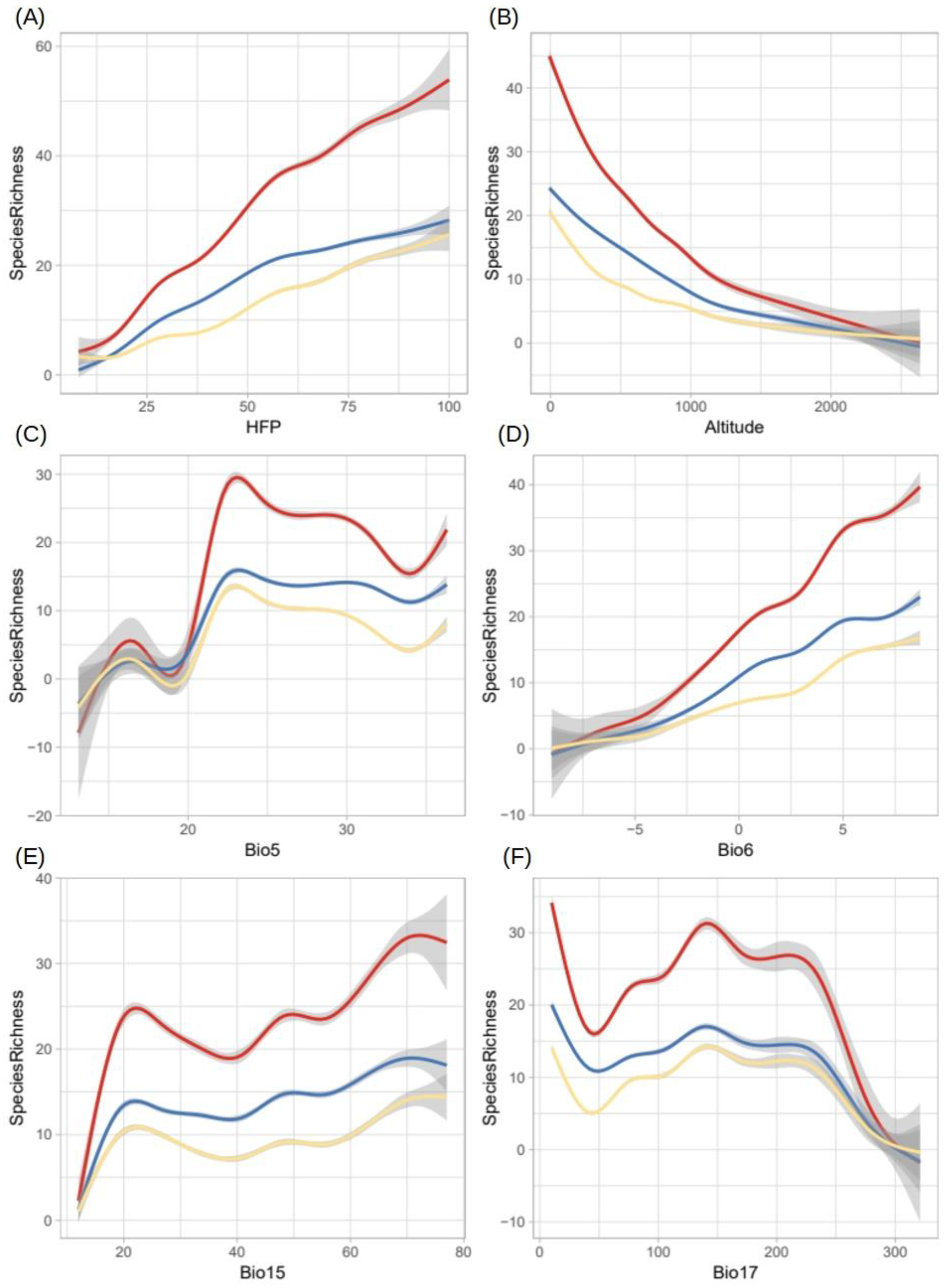
2.5. Potential Richness of Non-Native Aquatic Plant Species
3. Discussion
4. Materials and Methods
4.1. Study Area
4.2. Species Occurrence Records
4.3. Selection of Predictor Variables
4.4. Species Distribution Models
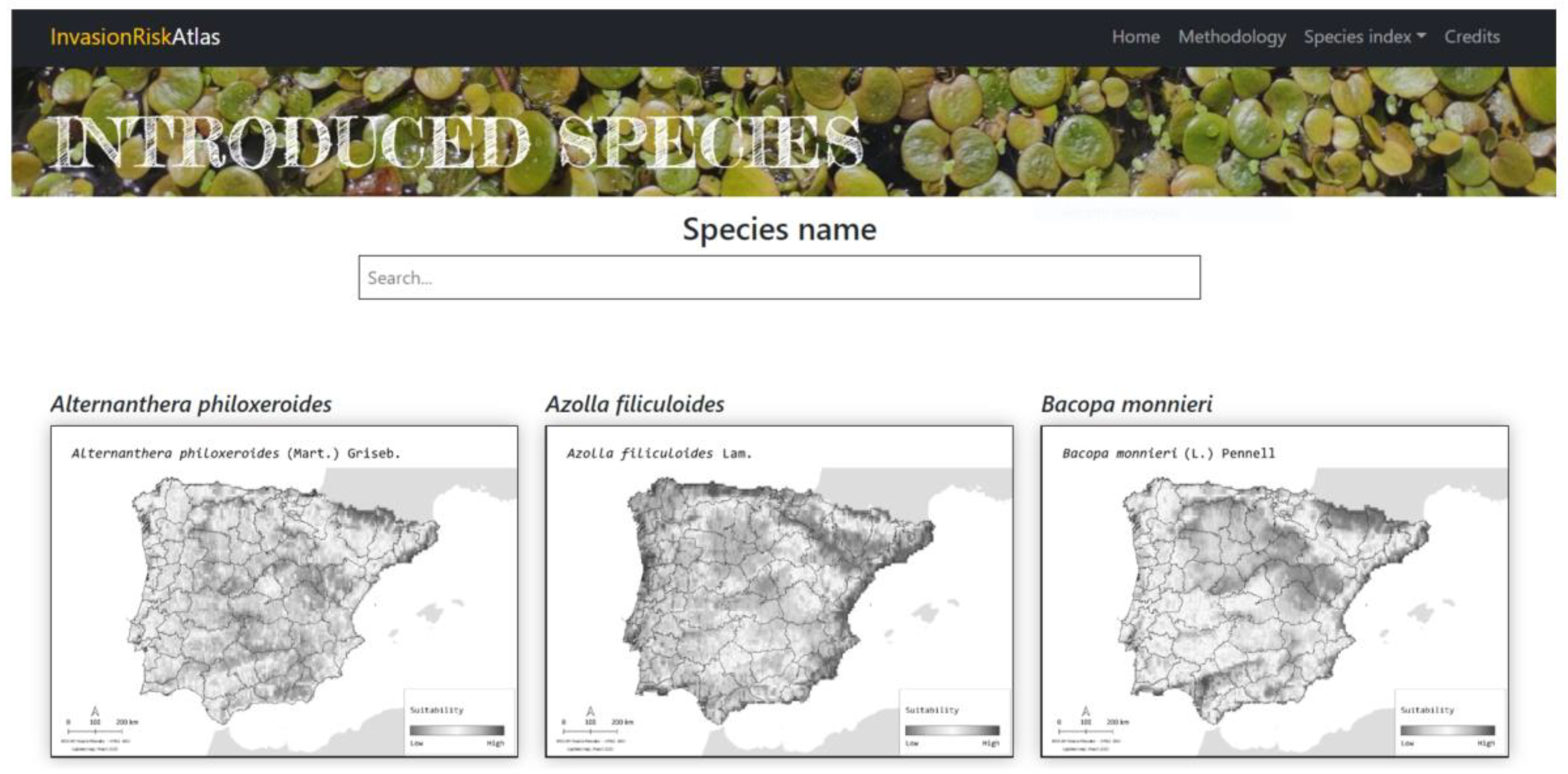
4.5. Online Invasion Risk Atlas
4.6. Potential Richness of Non-Native Aquatic Plant Species
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cuthbert, R.N.; Pattison, Z.; Taylor, N.G.; Verbrugge, L.; Diagne, C.; Ahmed, D.A.; Leroy, B.; Angulo, E.; Briski, E.; Capinha, C.; et al. Global Economic Costs of Aquatic Invasive Alien Species. Sci. Total Environ. 2021, 775, 145238. [Google Scholar] [CrossRef]
- Cuthbert, R.N.; Diagne, C.; Hudgins, E.J.; Turbelin, A.; Ahmed, D.A.; Albert, C.; Bodey, T.W.; Briski, E.; Essl, F.; Haubrock, P.J.; et al. Biological Invasion Costs Reveal Insufficient Proactive Management Worldwide. Sci. Total Environ. 2022, 819, 153404. [Google Scholar] [CrossRef] [PubMed]
- Diagne, C.; Leroy, B.; Vaissière, A.-C.; Gozlan, R.E.; Roiz, D.; Jarić, I.; Salles, J.-M.; Bradshaw, C.J.A.; Courchamp, F. High and Rising Economic Costs of Biological Invasions Worldwide. Nature 2021, 592, 571–576. [Google Scholar] [CrossRef]
- Bellard, C.; Cassey, P.; Blackburn, T.M. Alien Species as a Driver of Recent Extinctions. Biol. Lett. 2016, 12, 20150623. [Google Scholar] [CrossRef]
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ Warning on Invasive Alien Species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef]
- Seebens, H.; Essl, F.; Dawson, W.; Fuentes, N.; Moser, D.; Pergl, J.; Pyšek, P.; van Kleunen, M.; Weber, E.; Winter, M.; et al. Global Trade Will Accelerate Plant Invasions in Emerging Economies under Climate Change. Glob. Change Biol. 2015, 21, 4128–4140. [Google Scholar] [CrossRef]
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; Winter, M.; Arianoutsou, M.; et al. No Saturation in the Accumulation of Alien Species Worldwide. Nat. Commun. 2017, 8, 14435. [Google Scholar] [CrossRef] [PubMed]
- Seebens, H.; Bacher, S.; Blackburn, T.M.; Capinha, C.; Dawson, W.; Dullinger, S.; Genovesi, P.; Hulme, P.E.; van Kleunen, M.; Kühn, I.; et al. Projecting the Continental Accumulation of Alien Species through to 2050. Glob. Change Biol. 2021, 27, 970–982. [Google Scholar] [CrossRef]
- Crafton, R.E. Modeling Invasion Risk for Coastal Marine Species Utilizing Environmental and Transport Vector Data. Hydrobiologia 2015, 746, 349–362. [Google Scholar] [CrossRef]
- Roy, H.E.; Peyton, J.; Aldridge, D.C.; Bantock, T.; Blackburn, T.M.; Britton, R.; Clark, P.; Cook, E.; Dehnen-Schmutz, K.; Dines, T.; et al. Horizon Scanning for Invasive Alien Species with the Potential to Threaten Biodiversity in Great Britain. Glob. Change Biol. 2014, 20, 3859–3871. [Google Scholar] [CrossRef]
- Fletcher, D.H.; Gillingham, P.K.; Britton, J.R.; Blanchet, S.; Gozlan, R.E. Predicting Global Invasion Risks: A Management Tool to Prevent Future Introductions. Sci. Rep. 2016, 6, 26316. [Google Scholar] [CrossRef] [PubMed]
- Ryo, M.; Angelov, B.; Mammola, S.; Kass, J.M.; Benito, B.M.; Hartig, F. Explainable Artificial Intelligence Enhances the Ecological Interpretability of Black-Box Species Distribution Models. Ecography 2021, 44, 199–205. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W. Predicting Species Distribution: Offering More than Simple Habitat Models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef] [PubMed]
- Thuiller, W.; Richardson, D.M.; Pyšek, P.; Midgley, G.F.; Hughes, G.O.; Rouget, M. Niche-Based Modelling as a Tool for Predicting the Risk of Alien Plant Invasions at a Global Scale. Glob. Change Biol. 2005, 11, 2234–2250. [Google Scholar] [CrossRef]
- Gozlan, R.E.; Britton, J.R.; Cowx, I.; Copp, G.H. Current Knowledge on Non-Native Freshwater Fish Introductions. J. Fish Biol. 2010, 76, 751–786. [Google Scholar] [CrossRef]
- Strayer, D.L. Alien Species in Fresh Waters: Ecological Effects, Interactions with Other Stressors, and Prospects for the Future. Freshw. Biol. 2010, 55, 152–174. [Google Scholar]
- Moorhouse, T.P.; Macdonald, D.W. Are Invasives Worse in Freshwater than Terrestrial Ecosystems? WIREs Water 2015, 2, 1–8. [Google Scholar] [CrossRef]
- Gallardo, B.; Clavero, M.; Sánchez, M.I.; Vilà, M. Global Ecological Impacts of Invasive Species in Aquatic Ecosystems. Glob. Change Biol. 2016, 22, 151–163. [Google Scholar] [CrossRef]
- Rodríguez Merino, A.; Fernández Zamudio MD, R.; García Murillo, P. An Invasion Risk Map for Non-Native Aquatic Macrophytes of the Iberian Peninsula. An. Jard. Bot. Madr. 2017, 74, e055. [Google Scholar] [CrossRef]
- Rodríguez-Merino, A.; García-Murillo, P.; Cirujano, S.; Fernández-Zamudio, R. Predicting the Risk of Aquatic Plant Invasions in Europe: How Climatic Factors and Anthropogenic Activity Influence Potential Species Distributions. J. Nat. Conserv. 2018, 45, 58–71. [Google Scholar] [CrossRef]
- Araújo, M.B.; Lobo, J.M.; Moreno, J.C. The Effectiveness of Iberian Protected Areas in Conserving Terrestrial Biodiversity. Conserv. Biol. J. Soc. Conserv. Biol. 2007, 21, 1423–1432. [Google Scholar] [CrossRef]
- Rodríguez-Merino, A. Detección de la distribución potencial de plantas acuáticas emergentes invasoras en la península Ibérica, un reto para la conservación. In Bosque Mediterráneo y Humedales: Paisaje, Evolución y Conservación: Aportaciones Desde la Biogeografía; Gosálvez Rey, R.U., Díaz Sanz, M.C., García Rayego, J.L., Serrano de la Cruz Santos-Olmo, M.A., Jerez García, O., Eds.; Almud, Ediciones de Castilla-La Mancha: Ciudad Real, Spain, 2018; Volume 1, pp. 512–522. ISBN 978-84-948075-6-5. [Google Scholar]
- Oficialdegui, F.J.; Zamora-Marín, J.M.; Guareschi, S.; Anastácio, P.M.; García-Murillo, P.; Ribeiro, F.; Miranda, R.; Cobo, F.; Gallardo, B.; García-Berthou, E.; et al. A Horizon Scan Exercise for Aquatic Invasive Alien Species in Iberian Inland Waters. Sci. Total Environ. 2023, 869, 161798. [Google Scholar] [CrossRef]
- Rodríguez-Merino, A. Azolla filiculoides Lam. Invasion Risk Atlas for Alien Aquatic Plants in the Iberian Peninsula. 2023. Available online: https://InvasionRiskAtlas.github.io/ (accessed on 11 July 2023).
- Rodríguez-Merino, A. Eichhornia crassipes (Mart.) Solms. Invasion Risk Atlas for Alien Aquatic Plants in the Iberian Peninsula. 2023. Available online: https://InvasionRiskAtlas.github.io/ (accessed on 11 July 2023).
- Rodríguez-Merino, A. Ludwigia grandiflora (Michx.) Greuter & Burdet. Invasion Risk Atlas for Alien Aquatic Plants in the Iberian Peninsula. 2023. Available online: https://InvasionRiskAtlas.github.io/ (accessed on 11 July 2023).
- Rodríguez-Merino, A. Salvinia natans (L.) All. Risk Atlas for Alien Aquatic Plants in the Iberian Peninsula. 2023. Available online: https://InvasionRiskAtlas.github.io/ (accessed on 11 July 2023).
- Rodríguez-Merino, A. Salvinia molesta D.S. Mitch. Invasion Risk Atlas for Alien Aquatic Plants in the Iberian Peninsula. 2023. Available online: https://InvasionRiskAtlas.github.io/ (accessed on 11 July 2023).
- Rodríguez-Merino, A. Spartina densiflora Brongn. Invasion Risk Atlas for Alien Aquatic Plants in the Iberian Peninsula. 2023. Available online: https://InvasionRiskAtlas.github.io/ (accessed on 11 July 2023).
- Chappuis, E.; Ballesteros, E.; Gacia, E. Distribution and Richness of Aquatic Plants across Europe and Mediterranean Countries: Patterns, Environmental Driving Factors and Comparison with Total Plant Richness. J. Veg. Sci. 2012, 23, 985–997. [Google Scholar] [CrossRef]
- Rodríguez-Merino, A.; Fernández-Zamudio, R.; García-Murillo, P. Identifying Areas of Aquatic Plant Richness in a Mediterranean Hotspot to Improve the Conservation of Freshwater Ecosystems. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 589–602. [Google Scholar] [CrossRef]
- Sanz Elorza, M.; Sobrino Vesperinas, E.; Dana Sánchez, E.D. Atlas de las Plantas Alóctonas Invasoras en España; Ministerio para la Transición Ecológica y el Reto Demográfico: Madrid, Spain, 2004; ISBN 978-84-8014-575-6. [Google Scholar]
- Gassó, N.; Sol, D.; Pino, J.; Dana, E.D.; Lloret, F.; Sanz-Elorza, M.; Sobrino, E.; Vilà, M. Exploring Species Attributes and Site Characteristics to Assess Plant Invasions in Spain. Divers. Distrib. 2009, 15, 50–58. [Google Scholar] [CrossRef]
- Rodríguez-Merino, A.; Fernández Zamudio, R.; García Murillo, P. Determinación de las zonas con mayor riesgo de invasión por macrófitos acuáticos exóticos en la Península Ibérica. In Teledetección: Humedales y Espacios Protegidos; Bustamante, J., Díaz-Delgado, R., Aragonés, D., Afán, I., García, D., Eds.; XVI Congreso de la Asociación Española de Teledetección; Asociación Española de Teledetección: Sevilla, Spain, 2015; pp. 338–341. [Google Scholar]
- Rodríguez-Merino, A. Invasion Risk Atlas for Alien Aquatic Plants in the Iberian Peninsula. 2023. Available online: https://InvasionRiskAtlas.github.io/ (accessed on 11 July 2023).
- Gallardo, B.; Zieritz, A.; Aldridge, D.C. The Importance of the Human Footprint in Shaping the Global Distribution of Terrestrial, Freshwater and Marine Invaders. PLoS ONE 2015, 10, e0125801. [Google Scholar] [CrossRef] [PubMed]
- Cano-Barbacil, C.; Radinger, J.; García-Berthou, E. The Importance of Seawater Tolerance and Native Status in Mediating the Distribution of Inland Fishes. J. Biogeogr. 2022, 49, 2037–2049. [Google Scholar] [CrossRef]
- Bartomeus, I.; Sol, D.; Pino, J.; Vicente, P.; Font, X. Deconstructing the Native–Exotic Richness Relationship in Plants. Glob. Ecol. Biogeogr. 2012, 21, 524–533. [Google Scholar] [CrossRef]
- Bellard, C.; Leroy, B.; Thuiller, W.; Rysman, J.-F.; Courchamp, F. Major Drivers of Invasion Risks throughout the World. Ecosphere 2016, 7, e01241. [Google Scholar] [CrossRef]
- Pearson, R.G.; Dawson, T.P. Predicting the Impacts of Climate Change on the Distribution of Species: Are Bioclimate Envelope Models Useful? Glob. Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef]
- Kelly, R.; Leach, K.; Cameron, A.; Maggs, C.A.; Reid, N. Combining Global Climate and Regional Landscape Models to Improve Prediction of Invasion Risk. Divers. Distrib. 2014, 20, 884–894. [Google Scholar] [CrossRef]
- Gillard, M.; Thiébaut, G.; Deleu, C.; Leroy, B. Present and Future Distribution of Three Aquatic Plants Taxa across the World: Decrease in Native and Increase in Invasive Ranges. Biol. Invasions 2017, 19, 2159–2170. [Google Scholar] [CrossRef]
- Gallardo, B.; Aldridge, D.C.; González-Moreno, P.; Pergl, J.; Pizarro, M.; Pyšek, P.; Thuiller, W.; Yesson, C.; Vilà, M. Protected Areas Offer Refuge from Invasive Species Spreading under Climate Change. Glob. Change Biol. 2017, 23, 5331–5343. [Google Scholar] [CrossRef] [PubMed]
- Evans, K.L.; Warren, P.H.; Gaston, K.J. Species-Energy Relationships at the Macroecological Scale: A Review of the Mechanisms. Biol. Rev. 2005, 80, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Reshetnikov, A.N.; Ficetola, G.F. Potential Range of the Invasive Fish Rotan (Perccottus glenii) in the Holarctic. Biol. Invasions 2011, 13, 2967–2980. [Google Scholar] [CrossRef]
- Soomers, H.; Karssenberg, D.; Soons, M.B.; Verweij, P.A.; Verhoeven, J.T.A.; Wassen, M.J. Wind and Water Dispersal of Wetland Plants Across Fragmented Landscapes. Ecosystems 2013, 16, 434–451. [Google Scholar] [CrossRef]
- Rodríguez-Merino, A.; Fernández-Zamudio, R.; García-Murillo, P.; Muñoz, J. Climatic Niche Shift during Azolla filiculoides Invasion and Its Potential Distribution under Future Scenarios. Plants 2019, 8, 424. [Google Scholar] [CrossRef]
- Rodríguez-Merino, A. Efecto del clima en la distribución futura de plantas acuáticas exóticas en Europa. In Bosque Mediterráneo y Humedales: PAISAJE, Evolución y Conservación: Aportaciones Desde la Biogeografía; Gosálvez Rey, R.U., Díaz Sanz, M.C., García Rayego, J.L., Serrano de la Cruz Santos-Olmo, M.A., Jerez García, O., Eds.; Almud, Ediciones de Castilla-La Mancha: Ciudad Real, Spain, 2018; Volume 1, pp. 523–531. ISBN 978-84-948075-6-5. [Google Scholar]
- Hof, A.R.; Jansson, R.; Nilsson, C. The Usefulness of Elevation as a Predictor Variable in Species Distribution Modelling. Ecol. Model. 2012, 246, 86–90. [Google Scholar] [CrossRef]
- Gallardo, B.; Castro-Díez, P.; Saldaña-López, A.; Alonso, Á. Integrating Climate, Water Chemistry and Propagule Pressure Indicators into Aquatic Species Distribution Models. Ecol. Indic. 2020, 112, 106060. [Google Scholar] [CrossRef]
- Gillard, M.B.; Aroviita, J.; Alahuhta, J. Same Species, Same Habitat Preferences? The Distribution of Aquatic Plants Is Not Explained by the Same Predictors in Lakes and Streams. Freshw. Biol. 2020, 65, 878–892. [Google Scholar] [CrossRef]
- Gallardo, B.; Vila, L. La influencia humana, clave para entender la biogeografía de especies invasoras en el Antropoceno. Cuad. Investig. Geogr. 2019, 45, 61–86. [Google Scholar] [CrossRef]
- Kalkhoff, S.J.; Hubbard, L.E.; Tomer, M.D.; James, D.E. Effect of Variable Annual Precipitation and Nutrient Input on Nitrogen and Phosphorus Transport from Two Midwestern Agricultural Watersheds. Sci. Total Environ. 2016, 559, 53–62. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander Jr, J.A. A Practical Guide to MaxEnt for Modeling Species’ Distributions: What It Does, and Why Inputs and Settings Matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- García-Roselló, E.; Guisande, C.; Manjarrés-Hernández, A.; González-Dacosta, J.; Heine, J.; Pelayo-Villamil, P.; González-Vilas, L.; Vari, R.P.; Vaamonde, A.; Granado-Lorencio, C.; et al. Can We Derive Macroecological Patterns from Primary Global Biodiversity Information Facility Data? Glob. Ecol. Biogeogr. 2015, 24, 335–347. [Google Scholar] [CrossRef]
- Pérez, G.; Vilà, M.; Gallardo, B. Potential Impact of Four Invasive Alien Plants on the Provision of Ecosystem Services in Europe under Present and Future Climatic Scenarios. Ecosyst. Serv. 2022, 56, 101459. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Fady, B.; Conord, C. Macroecological Patterns of Species and Genetic Diversity in Vascular Plants of the Mediterranean Basin. Divers. Distrib. 2010, 16, 53–64. [Google Scholar] [CrossRef]
- Oliva-Paterna, F.J.; Ribeiro, F.; Miranda, R.; Anastácio, P.; García-Murillo, P.; Cobo, F.; Gallardo, B.; García-Berthou, E.; Boix, D.; Medina, L.; et al. List of Aquatic Alien Species of the Iberian peninsula (2020). Updated List of Aquatic Alien Species Introduced and Established in Iberian Inland Waters; Technical Report prepared by LIFE INVASAQUA (LIFE17 GIE/ES/000515); 2021; ISBN 978-84-12-35001-2. Available online: http://dspace.uevora.pt/rdpc/handle/10174/34424 (accessed on 11 April 2023).
- Oliva-Paterna, F.J.; Ribeiro, F.; Miranda, R.; Anastácio, P.; García-Murillo, P.; Cobo, F.; Gallardo, B.; García-Berthou, E.; Boix, D.; Medina, L.; et al. List of Potential Aquatic Alien Species of the Iberian Peninsula (2020). Updated List of the Potential Aquatic Alien Species with High Risk of Invasion in Iberian Inland Waters; Technical Report prepared by LIFE INVASAQUA (LIFE17 GIE/ES/000515); 2021; ISBN 978-84-12-35004-3. Available online: https://lifeinvasaqua.com/wp-content/uploads/2021/05/Lista-especies-acu%C3%A1ticas-potencialmente-invasoras-libro-2-ingles-PDF-INTERACTIVO.pdf (accessed on 11 April 2023).
- Oliva-Paterna, F.J.; Oficialdegui, F.J.; Anastácio, P.; García-Murillo, P.; Zamora-Marín, J.M.; Ribeiro, F.; Miranda, R.; Cobo, F.; Gallardo, B.; García-Berthou, E.; et al. Black List and Alert List of The Aquatic Invasive Alien Species of the Iberian Peninsula—Horizon Scanning Exercise Focused on the High-Risk Aquatic Invasive Alien Species for the Iberian Inland Waters; Technical Report prepared by LIFE INVASAQUA (LIFE17 GIE/ES/000515); 2022; ISBN 978-84-12-35008-1. Available online: https://lifeinvasaqua.com/wp-content/uploads/2023/01/TechRepp_3_INVASAQUA_Complet_ING.pdf (accessed on 11 April 2023).
- Broennimann, O.; Guisan, A. Predicting Current and Future Biological Invasions: Both Native and Invaded Ranges Matter. Biol. Lett. 2008, 4, 585–589. [Google Scholar] [CrossRef]
- Wisz, M.S.; Hijmans, R.J.; Li, J.; Peterson, A.T.; Graham, C.H.; Guisan, A.; Group, N.P.S.D.W. Effects of Sample Size on the Performance of Species Distribution Models. Divers. Distrib. 2008, 14, 763–773. [Google Scholar] [CrossRef]
- Zizka, A.; Silvestro, D.; Andermann, T.; Azevedo, J.; Duarte Ritter, C.; Edler, D.; Farooq, H.; Herdean, A.; Ariza, M.; Scharn, R.; et al. CoordinateCleaner: Standardized Cleaning of Occurrence Records from Biological Collection Databases. Methods Ecol. Evol. 2019, 10, 744–751. [Google Scholar] [CrossRef]
- Boria, R.A.; Olson, L.E.; Goodman, S.M.; Anderson, R.P. Spatial Filtering to Reduce Sampling Bias Can Improve the Performance of Ecological Niche Models. Ecol. Model. 2014, 275, 73–77. [Google Scholar] [CrossRef]
- Pereira, P.F.; Barbosa, A.M.; Godinho, C.; Salgueiro, P.A.; Silva, R.R.; Lourenço, R. The Spread of the Red-Billed Leiothrix (Leiothrix lutea) in Europe: The Conquest by an Overlooked Invader? Biol. Invasions 2020, 22, 709–722. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Papeş, M.; Gaubert, P. Modelling Ecological Niches from Low Numbers of Occurrences: Assessment of the Conservation Status of Poorly Known Viverrids (Mammalia, Carnivora) across Two Continents. Divers. Distrib. 2007, 13, 890–902. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-Km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Venter, O.; Sanderson, E.W.; Magrach, A.; Allan, J.R.; Beher, J.; Jones, K.R.; Possingham, H.P.; Laurance, W.F.; Wood, P.; Fekete, B.M.; et al. Last of the Wild Project, Version 3 (LWP-3): 2009 Human Footprint, 2018 Release; NASA Socioeconomic Data and Applications Center (SEDAC): Palisades, NY, USA, 2018. [Google Scholar]
- Heiberger, R.M. HH: Statistical Analysis and Data Display: Heiberger and Holland 2022. R Package Version 3.1-49. Available online: https://CRAN.R-project.org/package=HH (accessed on 11 April 2023).
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel Methods Improve Prediction of Species’ Distributions from Occurrence Data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Graham, C.H.; Elith, J.; Hijmans, R.J.; Guisan, A.; Townsend Peterson, A.; Loiselle, B.A.; Group, T.N.P.S.D.W. The Influence of Spatial Errors in Species Occurrence Data Used in Distribution Models. J. Appl. Ecol. 2008, 45, 239–247. [Google Scholar] [CrossRef]
- Kaky, E.; Nolan, V.; Alatawi, A.; Gilbert, F. A Comparison between Ensemble and MaxEnt Species Distribution Modelling Approaches for Conservation: A Case Study with Egyptian Medicinal Plants. Ecol. Inform. 2020, 60, 101150. [Google Scholar] [CrossRef]
- Fois, M.; Cuena-Lombraña, A.; Fenu, G.; Bacchetta, G. Using Species Distribution Models at Local Scale to Guide the Search of Poorly Known Species: Review, Methodological Issues and Future Directions. Ecol. Model. 2018, 385, 124–132. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of Species Distributions with Maxent: New Extensions and a Comprehensive Evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A Statistical Explanation of MaxEnt for Ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Pearce, J.; Ferrier, S. Evaluating the Predictive Performance of Habitat Models Developed Using Logistic Regression. Ecol. Model. 2000, 133, 225–245. [Google Scholar] [CrossRef]
- Barnes, M.A.; Jerde, C.L.; Wittmann, M.E.; Chadderton, W.L.; Ding, J.; Zhang, J.; Purcell, M.; Budhathoki, M.; Lodge, D.M. Geographic Selection Bias of Occurrence Data Influences Transferability of Invasive Hydrilla verticillata Distribution Models. Ecol. Evol. 2014, 4, 2584–2593. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting Pseudo-Absences for Species Distribution Models: How, Where and How Many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D.; Posit, P.B.C. Ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics 2023. R Package Version 3.4.3. Available online: https://CRAN.R-project.org/web/packages/ggplot2 (accessed on 11 April 2023).
- Wood, S.N. Fast Stable Direct Fitting and Smoothness Selection for Generalized Additive Models. J. R. Stat. Soc. Ser. B Stat. Methodol. 2008, 70, 495–518. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Merino, A. Identifying and Managing Areas under Threat in the Iberian Peninsula: An Invasion Risk Atlas for Non-Native Aquatic Plant Species as a Potential Tool. Plants 2023, 12, 3069. https://doi.org/10.3390/plants12173069
Rodríguez-Merino A. Identifying and Managing Areas under Threat in the Iberian Peninsula: An Invasion Risk Atlas for Non-Native Aquatic Plant Species as a Potential Tool. Plants. 2023; 12(17):3069. https://doi.org/10.3390/plants12173069
Chicago/Turabian StyleRodríguez-Merino, Argantonio. 2023. "Identifying and Managing Areas under Threat in the Iberian Peninsula: An Invasion Risk Atlas for Non-Native Aquatic Plant Species as a Potential Tool" Plants 12, no. 17: 3069. https://doi.org/10.3390/plants12173069
APA StyleRodríguez-Merino, A. (2023). Identifying and Managing Areas under Threat in the Iberian Peninsula: An Invasion Risk Atlas for Non-Native Aquatic Plant Species as a Potential Tool. Plants, 12(17), 3069. https://doi.org/10.3390/plants12173069






