Allantoin: A Potential Compound for the Mitigation of Adverse Effects of Abiotic Stresses in Plants
Abstract
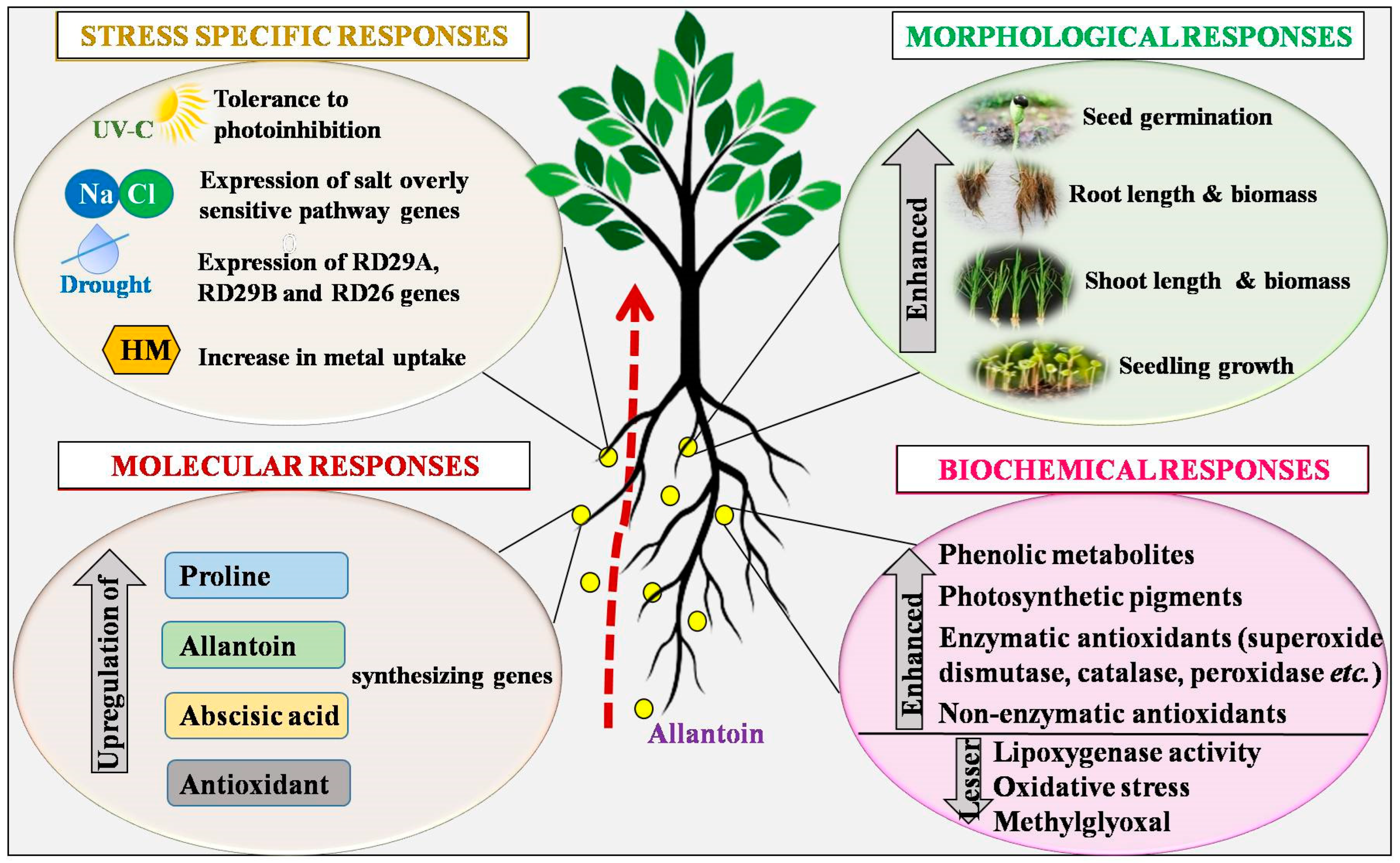
1. Introduction
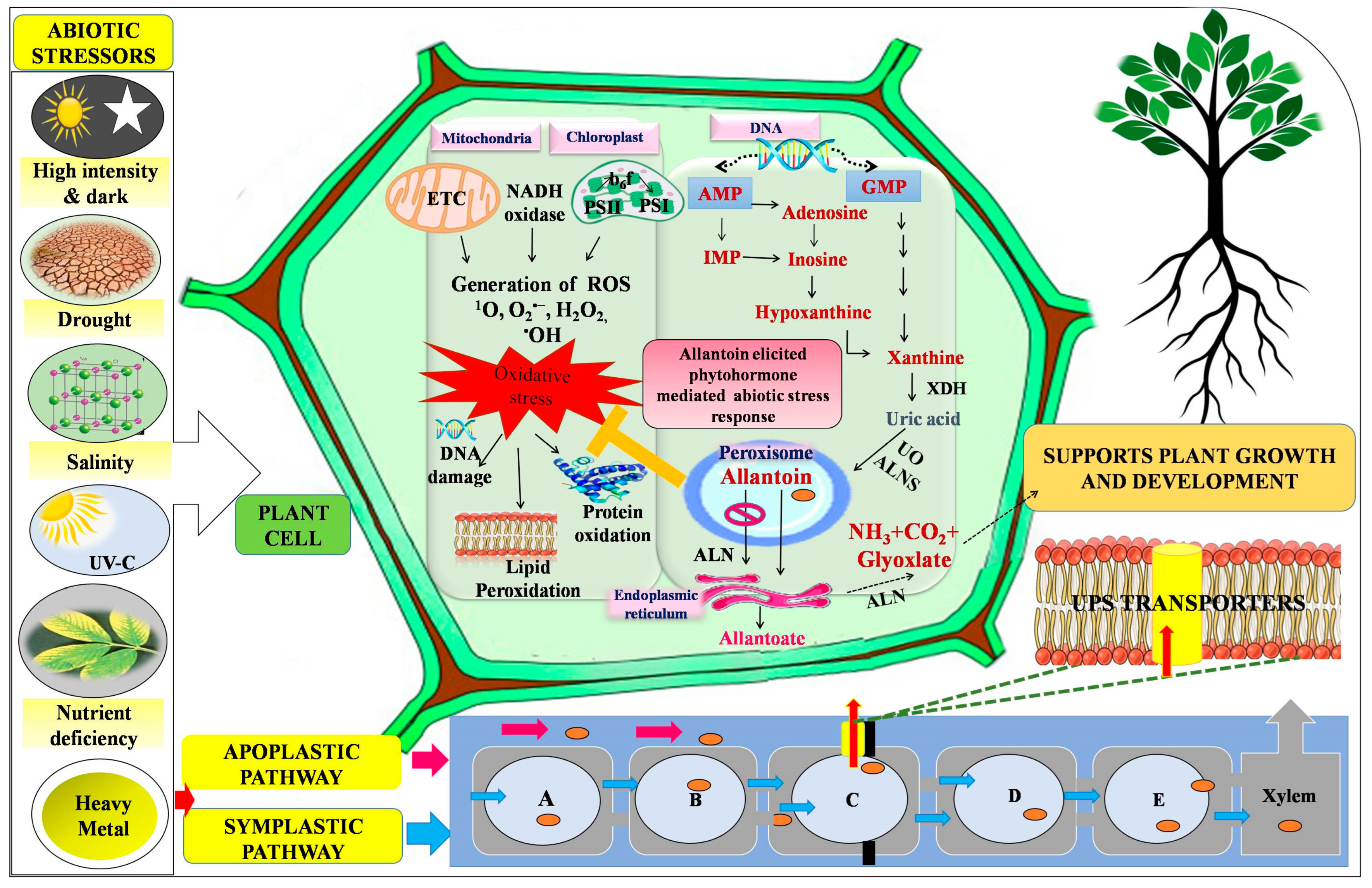
2. Synthesis and Transportation of Allantoin
3. Defensive Strategies of Allantoin against Abiotic Stressors
3.1. Salinity Stress
3.2. Drought Stress
3.3. Heavy Metals
3.3.1. Cadmium
| S. No. | Stress Factors | Plant Species | Mutants Developed | Inference | Parameters Studied | References |
|---|---|---|---|---|---|---|
| Heavy metal | ||||||
| 1. | Cadmium | Arabidopsis thaliana | ALN-negative (aln-3) | Shoot allantoin↑, UO activity↑, ALN↓, Proline content↑, ROS levels↓, Antioxidant levels↑ |  | Nourimand and Todd [50] |
| 2. | Cadmium | Arabidopsis thaliana | ALN-negative (aln-3) | Root Cd level↑, Root biomass↑, UO transcript level↑, ROS levels↓, Antioxidant levels↑ |  | Nourimand and Todd [51] |
| 3. | Zinc/Lead | Echium vulgare | - | Allantoin levels↑, Metal accumulation in roots↑ |  | Dresler et al. [57] |
| 4. | Zinc/Lead | Echium vulgare | - | Allantoin levels↓, Rosmarinic acid↓ |  | Dresler et al. [58] |
| 5. | Zinc, Lead, and Cadmium | Echium vulgare | - | Plant biomass↑, Allantoin levels↑, Chlorogenicandrosmarinic acids↑, Total phenolicsand flavonoids↑, Malate and citrate content↑ |  | Dresler et al. [59] |
| 6. | Strontium | Glycine max | - | ↑Allantoin levels in roots |  | Dresler et al. [60] |
| 7. | Cadmium | Arabidopsis thaliana | ALNox, abi mutants | Seedling growth↑, Root elongation↑, Antioxidant levels↑ |  | Nourimand and Todd [55] |
| 8. | Cadmium | Cucumis sativus | - | Shoot biomass↑, Leaf area↑, Citric acid↑, Phytochelatins↓, ROS levels↓, Glutathione and ascorbic acid↑, Photosynthetic pigments↑ |  | Dresler et al. [54] |
| 9. | Strontium | Glycine max | - | ↑Allantoin levels in roots |  | Hanaka et al. [61] |
| Nutrient deficiency | ||||||
| 10. | Sulphur deficiency | Arabidopsis thaliana | - | Ureides content↑ |  | Nikiforova et al. [62] |
| 11. | Nitrogen deficiency | Arabidopsis thaliana | Atxdh1, Ataln, Ataah | Ureides content↑ |  | Soltabayeva et al. [29] |
| UV-C Stress | ||||||
| 12. | - | Solanum lycopersicum | - | Allantoin and allantoate content↑, PAL↑, Antioxidants↑, Chlorophyll↑, Soluble protein and carbohydrate↑ |  | Dawood et al. [63] |
| 13. | UV-C+ Wounding | Arabidopsis thaliana | Atxdh1 | Allantoin↓, ROS levels↑, MDA↑, Shoot fresh weight↓, EL↑, Chlorophyll↓, Senescence gene expression↑, Autophagy↑ |  | Soltabayeva et al. [64] |
3.3.2. Other Heavy Metals
3.4. High Irradiance
3.5. Dark Stress
3.6. Nutrient Deficiency
3.7. Ultraviolet-C
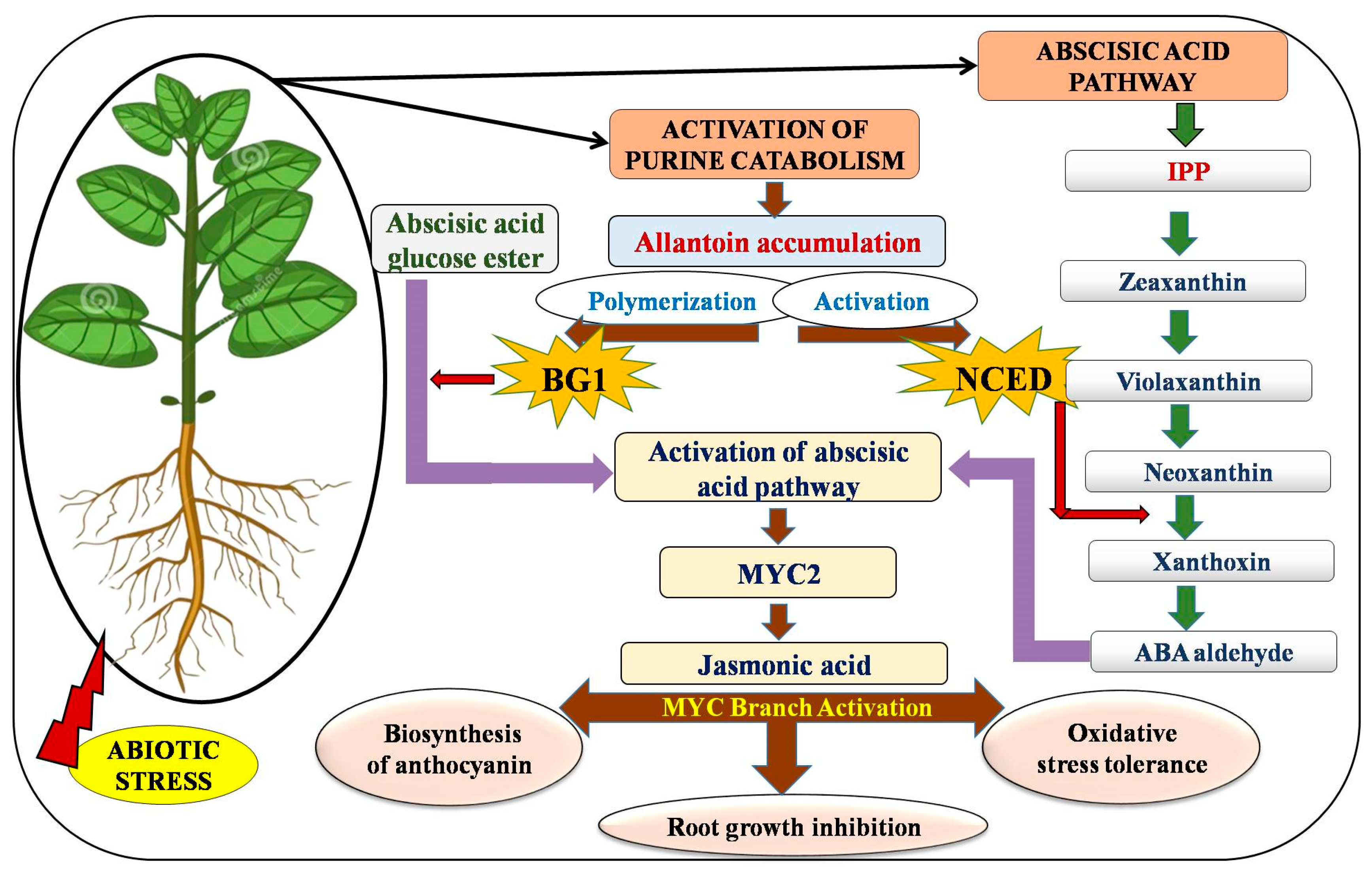
4. Hormonal Regulation of Allantoin Activation
5. Conclusions and Future Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Usman, B.; Derakhshani, B.; Jung, K.H. Recent molecular aspects and integrated omics strategies for understanding the abiotic stress tolerance of rice. Plants 2023, 12, 2019. [Google Scholar] [CrossRef]
- Nahar, K.; Rhaman, M.S.; Parvin, K.; Bardhan, K.; Marques, D.N.; Garcia-Caparros, P.; Hasanuzzaman, M. Arsenic-induced oxidative stress and antioxidant defense in plants. Stresses 2022, 2, 179–209. [Google Scholar] [CrossRef]
- Chowrasia, S.; Nishad, J.; Mahato, R.; Kiran, K.; Rajkumari, N.; Panda, K.A.; Rawal, C.H.; Barman, M.; Mondal, K.T. Allantoin improves salinity tolerance in Arabidopsis and rice through synergid activation of abscisic acid and brassinosteroid biosynthesis. Plant Mol. Biol. 2023, 112, 143–160. [Google Scholar] [CrossRef]
- Mansoor, S.; Ali Wani, O.; Lone, J.K.; Manhas, S.; Kour, N.; Alam, P.; Ahmad, A.; Ahmad, P. Reactive oxygen species in plants: From source to sink. Antioxidants 2022, 11, 225. [Google Scholar] [CrossRef]
- Berwal, M.K.; Ram, C. Superoxide dismutase: A stable biochemical marker for abiotic stress tolerance in higher plants. In Abiotic and Biotic Stress in Plants; De Oliveira, A., Ed.; Intech Open: London, UK, 2018; pp. 1–10. [Google Scholar]
- Yang, C.; Dolatabadian, A.; Fernando, W.D. The wonderful world of intrinsic and intricate immunity responses in plants against pathogens. Can J. Plant Pathol. 2022, 44, 1–20. [Google Scholar] [CrossRef]
- Hunyadi, A. The mechanism(s) of action of antioxidants: From scavenging reactive oxygen/nitrogen species to redox signalling and the generation of bioactive secondary metabolites. Med. Res. Rev. 2019, 39, 2505–2533. [Google Scholar] [CrossRef]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; et al. Plant secondary metabolites as defense tools against herbivores for sustainable crop protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef]
- Watanabe, S.; Kounosu, Y.; Shimada, H.; Sakamoto, A. Arabidopsis xanthine dehydrogenase mutants defective in purine degradation show a compromised protective response to drought and oxidative stress. Plant Biotechnol. 2014, 14, 0117–0131. [Google Scholar] [CrossRef]
- Watanabe, S.; Matsumoto, M.; Hakomori, Y.; Takagi, H.; Shimada, H.; Sakamoto, A. The purine metabolite allantoin enhances abiotic stress tolerance through synergistic activation of abscisic acid metabolism. Plant Cell Environ. 2014, 37, 1022–1036. [Google Scholar] [CrossRef]
- Irani, S.; Todd, C.D. Ureide metabolism under abiotic stress in Arabidopsis thaliana. J. Plant Physiol. 2016, 199, 87–95. [Google Scholar] [CrossRef]
- Malik, V.M.; Lobo, J.M.; Stewart, C.; Irani, S.; Todd, C.D.; Gray, G.R. Growth irradiance affects ureide accumulation and tolerance to photoinhibition in Eutrema salsugineum (Thellungiella salsuginea). Photosynthetica 2016, 54, 93–100. [Google Scholar] [CrossRef]
- Kaur, H.; Chowrasia, S.; Gaur, V.S.; Mondal, T.K. Allantoin: Emerging role in plant abiotic stress tolerance. Plant Mol. Biol. Report. 2021, 39, 648–661. [Google Scholar] [CrossRef]
- Kaplan, F.; Kopka, J.; Haskell, D.W.; Zhao, W.; Schiller, K.C.; Gatzke, N.; Sung, D.Y.; Guy, C.L. Exploring the temperature-stress metabolome of Arabidopsis. Plant Physiol. 2004, 136, 4159–4168. [Google Scholar] [CrossRef] [PubMed]
- Savic, V.L.; Nikolic, V.D.; Arsic, I.A.; Stanojevic, L.P.; Najman, S.J.; Stojanovic, S.; Mladenovic-Ranisavljevic, I.I. Comparative study of the biological activity of allantoin and aqueous extract of the comfrey root. Phytother. Res. 2015, 29, 1117–1122. [Google Scholar] [CrossRef]
- Werner, A.K.; Romeis, T.; Witte, C.P. Ureide catabolism in Arabidopsis thaliana and Escherichia coli. Nat. Chem. Biol. 2010, 6, 19–21. [Google Scholar] [CrossRef]
- Brychkova, G.; Alikulov, Z.; Fluhr, R.; Sagi, M. A critical role for ureides in dark and senescence-induced purine remobilisation is unmasked in the Atxdh1 Arabidopsis mutant. Plant J. 2008, 54, 496–509. [Google Scholar] [CrossRef]
- Ashihara, H.; Stasolla, C.; Fujimura, T.; Crozier, A. Purine salvage in plants. Phytochemistry 2018, 147, 89–124. [Google Scholar] [CrossRef]
- Todd, C.D.; Polacco, J. AtAAH encodes a protein with allantoate amidohydrolase activity from Arabidopsis thaliana. Planta 2006, 223, 1108–1113. [Google Scholar] [CrossRef]
- Werner, A.K.; Sparkes, I.A.; Romeis, T.; Witte, C.P. Identification, biochemical characterization, and subcellular localization of allantoateamidohydrolases from Arabidopsis and soybean. Plant Physiol. 2008, 146, 418. [Google Scholar] [CrossRef]
- Fahad, S.; Khan, F.A.; Pandupuspitasari, N.; Hussain, S.; Khan, I.A.; Saeed, M.; Saud, S.; Hassan, S.; Adnan, M.; Arif, M.; et al. Suppressing photorespiration for the improvement in photosynthesis and crop yields: A review on the role of S-allantoin as a nitrogen source. J. Environ. Manag. 2019, 237, 644–651. [Google Scholar] [CrossRef]
- Pelissier, H.C.; Frerich, A.; Desimone, M.; Schumacher, K.; Tegeder, M. PvUPS1, an allantoin transporter in nodulated roots of French bean. Plant Physiol. 2004, 134, 664–675. [Google Scholar] [CrossRef]
- Tegeder, M.; Rentsch, D. Uptake and partitioning of amino acids and peptides. Mol. Plant 2010, 3, 997–1011. [Google Scholar] [CrossRef] [PubMed]
- Lescano, C.I.; Martini, C.; Gonzalez, C.A.; Desimone, M. Allantoin accumulation mediated by allantoinase downregulation and transport by ureide permease 5 confers salt stress tolerance to Arabidopsis plants. Plant Mol. Biol. 2016, 91, 581–595. [Google Scholar] [CrossRef] [PubMed]
- Desimone, M.; Catoni, E.; Ludewig, U.; Hilpert, M.; Schneider, A.; Kunze, R.; Tegeder, M.; Frommer, W.B.; Schumacher, K. A novel superfamily of transporters for allantoin and other oxo derivatives of nitrogen heterocyclic compounds in Arabidopsis. Plant Cell. 2002, 14, 847–856. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pelissier, H.C.; Tegeder, M. PvUPS1 plays a role in source-sink transport of allantoin in French bean (Phaseolus vulgaris). Funct. Plant Biol. 2007, 34, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Collier, R.; Tegeder, M. Soybean ureide transporters play a critical role in nodule development, function and nitrogen export. Plant J. 2012, 72, 355–367. [Google Scholar] [CrossRef]
- Redillas, M.C.F.R.; Bang, S.W.; Lee, D.K.; Kim, Y.S.; Jung, H.; Chung, P.J.; Suh, J.W.; Kim, J.K. Allantoin accumulation through over-expression of ureide permease1 improves rice growth under limited nitrogen conditions. Plant Biotech. J. 2019, 17, 1289–1301. [Google Scholar] [CrossRef]
- Soltabayeva, A.; Srivastava, S.; Kurmanbyeva, A.; Bekturova, A.; Fluhr, R.; Sagi, M. Early senescence in older leaves of low nitrate grown Atxdh1 uncovers a role for purine catabolism in N supply. Plant Physiol. 2018, 178, 1027–1044. [Google Scholar] [CrossRef]
- Nam, M.H.; Eunjung, B.; Taek, Y.K.; Yuran, K.; Eun, H.K.; Kyungwon, C.; Woong, J.P.; Beom-Gi, K.; In Sun, Y. Metabolite profiling of diverse rice germplasm and identification of conserved metabolic markers of rice roots in response to long-term mild salinity stress. Int. J. Mol. Sci. 2015, 16, 21959–21974. [Google Scholar] [CrossRef]
- Khadri, M.; Tejera, N.A.; Lluch, C. Alleviation of salt stress in common bean (Phaseolus vulgaris) by exogenous abscisic acid supply. J. Plant Growth Regul. 2006, 25, 110–119. [Google Scholar] [CrossRef]
- Schmidt, A.; Baumann, N.; Schwarzkopf, A.; Frommer, W.B.; Desimone, M. Comparative studies on Ureide Permeases in Arabidopsis thaliana and analysis of two alternative splice variants of AtUPS5. Planta 2006, 224, 1329–1340. [Google Scholar] [CrossRef]
- Irani, S.; Todd, C.D. Exogenous allantoin increases Arabidopsis seedlings tolerance to NaCl stress and regulates expression of oxidative stress response genes. J. Plant Physiol. 2018, 221, 43–50. [Google Scholar] [CrossRef]
- You, S.; Zhu, B.; Wang, F.; Han, H.; Sun, M.; Zhu, H.; Peng, R.; Yao, Q. A Vitis vinifera xanthine dehydrogenase gene, VvXDH, enhances salinity tolerance in transgenic Arabidopsis. Plant Biotechnol. Rep. 2017, 11, 147–160. [Google Scholar] [CrossRef]
- Nishad, J.; Panda, A.K.; Chowrasia, S.; Nirmala, C.; Mondal, T.K. Allantoin mediated regulation of miRNAs for short term salinity stress tolerance in Oryza sativa L. cv. IR-29. J. Plant Biochem. Biotech. 2022, 31, 953–960. [Google Scholar] [CrossRef]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine function in plants: Metabolism, regulation on development, and roles in abiotic stress responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef]
- Bueno, M.; Cordovilla, M.P. Polyamines in halophytes. Front. Plant Sci. 2019, 10, 439. [Google Scholar] [CrossRef] [PubMed]
- Tsaniklidis, G.; Pappi, P.; Tsafouros, A.; Charova, S.N.; Nikoloudakis, N.; Roussos, P.A.; Paschalidis, K.A.; Delis, C. Polyamine homeostasis in tomato biotic/abiotic stress cross-tolerance. Gene 2020, 727, 144230. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, D.; Wang, Z.; Zou, C.; Wang, B.; Zhang, H.; Gai, Z.; Zhang, P.; Wang, Y.; Li, C. Exogenous allantoin improves the salt tolerance of sugar beet by increasing putrescine metabolism and antioxidant activities. Plant Physiol. Biochem. 2020, 154, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Macovei, A.; Tuteja, N. microRNAs targeting DEAD-box helicases are involved in salinity stress response in rice (Oryza sativa L.). BMC Plant Biol. 2012, 12, 183. [Google Scholar] [CrossRef]
- Silvente, S.; Sobolev, A.P.; Lara, M. Metabolite adjustments in drought tolerant and sensitive soybean genotypes in response to water stress. PLoS ONE 2012, 7, 38554. [Google Scholar] [CrossRef] [PubMed]
- Itam, M.; Mega, R.; Tadano, S.; Abdelrahman, M.; Matsunaga, S.; Yamasaki, Y.; Akashi, K.; Tsujimoto, H. Metabolic and physiological responses to progressive drought stress in bread wheat. Sci. Rep. 2020, 10, 17189. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Bano, A.; Rahman, M.A.; Rathinasabapathi, B.; Babar, M.A. UPLC-HRMS-based untargeted metabolic profiling reveals changes in chickpea (Cicer arietinum) metabolome following long-term drought stress. Plant Cell Environ. 2019, 42, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Casartelli, A.; Melino, V.J.; Baumann, U.; Riboni, M.; Suchecki, R.; Jayasinghe, N.S.; Mendis, H.; Watanabe, M.; Erban, A.; Zuther, E.; et al. Opposite fates of the purine metabolite allantoin under water and nitrogen limitations in bread wheat. Plant Mol. Biol. 2019, 99, 477–497. [Google Scholar] [CrossRef]
- Pholo-tait, M.; Kgetse, T.; Tsheko, G.N.; Thedi, O.T.; Lethola, K.; Motlamme, E.O.; Ithuteng, M.I.; Ngwako, S. Genotypic variation in response to drought stress is associated with biochemical and transcriptional regulation of ureides metabolism in common bean (Phaseolus vulgaris L.). Acta Agric. Slov. 2022, 118, 1. [Google Scholar] [CrossRef]
- Alamillo, J.M.; Diaz-Leal, J.L.; Sanchez-Moran, M.V.; Pineda, M. Molecular analysis of ureide accumulation under drought stress in Phaseolus vulgaris L. Plant Cell Environ. 2010, 33, 1828–1837. [Google Scholar] [CrossRef]
- Watanabe, S.; Nakagawa, A.; Izumi, S.; Shimada, H.; Sakamoto, A. RNA interference-mediated suppression of xanthine dehydrogenase reveals the role of purine metabolism in drought tolerance in Arabidopsis. FEBS Lett. 2010, 584, 1181–1186. [Google Scholar] [CrossRef]
- Alam, R.; Rasheed, R.; Ashraf, M.A.; Hussain, I.; Ali, S. Allantoin alleviates chromium phytotoxic effects on wheat by regulating osmolyte accumulation, secondary metabolism, ROS homeostasis and nutrient acquisition. J. Hazard. Mater. 2023, 458, 131920. [Google Scholar] [CrossRef]
- Nourimand, M.; Todd, C.D. Allantoin increases cadmium tolerance in Arabidopsis via activation of antioxidant mechanisms. Plant Cell Physiol. 2016, 57, 2485–2496. [Google Scholar] [CrossRef]
- Nourimand, M.; Todd, C.D. Allantoin contributes to the stress response in cadmium-treated Arabidopsis roots. Plant Physiol. Biochem. 2017, 119, 103–109. [Google Scholar] [CrossRef]
- Iqbal, N.; Masood, A.; Nazar, R.; Syeed, S.; Khan, N.A. Photosynthesis, growth and antioxidant metabolism in mustard (Brassica juncea L.) cultivars differing in cadmium tolerance. Agric. Sci. China 2010, 9, 519–527. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015, 2015, 756120. [Google Scholar] [CrossRef]
- Dresler, S.; Hawrylak-Nowak, B.; Kovacik, J.; Pochwatka, M.; Hanaka, A.; Strzemski, M.; Sowa, I.; Wojciak-Kosior, M. Allantoin attenuates cadmium-induced toxicity in cucumber plants. Ecotoxicol. Environ. Saf. 2019, 170, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Nourimand, M.; Todd, C.D. There is a direct link between allantoin concentration and cadmium tolerance in Arabidopsis. Plant Physiol. Biochem. 2019, 135, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Dresler, S.; Hawrylak-Nowak, B.; Kovacik, J.; Wozniak, M.; Galazka, A.; Staniak, M.; Wojciak, M.; Sowa, I. Organic nitrogen modulates not only cadmium toxicity but also microbial activity in plants. J. Hazard. Mat. 2021, 402, 123887. [Google Scholar] [CrossRef]
- Dresler, S.; Rutkowska, E.; Bednarek, W.; Stanislawski, G.; Kubrak, T.; Bogucka-Kocka, A.; Wojcik, M. Selected secondary metabolites in Echium vulgare L. populations from nonmetalliferous and metalliferous areas. Phytochemistry 2017, 133, 4–14. [Google Scholar] [CrossRef]
- Dresler, S.; Bednarek, W.; Hawrylak-Nowak, B.; Wojcik, M. Morphometric and phytochemical profile of seeds of metallicolous and nonmetallicolous Echium vulgare populations. Biochem. Syst. Ecol. 2017, 70, 304–310. [Google Scholar] [CrossRef]
- Dresler, S.; Wojciak-Kosior, M.; Sowa, I.; Stanislawski, G.; Bany, I.; Wojcik, M. Effect of short-term Zn/Pb or long-term multi-metal stress on physiological and morphological parameters of metallicolous and nonmetallicolous Echium vulgare L. populations. Plant Physiol. Biochem. 2017, 115, 380–389. [Google Scholar] [CrossRef]
- Dresler, S.; Wojciak-Kosior, M.; Sowa, I.; Strzemski, M.; Sawicki, J.; Kovacik, J.; Blicharski, T. Effect of long-term strontium exposure on the content of phytoestrogens and allantoin in soybean. Int. J. Mol. Sci. 2018, 19, 3864. [Google Scholar] [CrossRef]
- Hanaka, A.; Dresler, S.; Wojciak-Kosior, M.; Strzemski, M.; Kovacik, J.; Latalski, M.; Zawislak, G.; Sowa, I. The impact of long-and short-term strontium treatment on metabolites and minerals in Glycine max. Molecules 2019, 24, 3825. [Google Scholar] [CrossRef]
- Nikiforova, V.J.; Daub, C.O.; Hesse, H.; Willmitzer, L.; Hoefgen, R. Integrative gene-metabolite network with implemented causality deciphers informational fluxes of sulphur stress response. J. Exp. Bot. 2005, 56, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.F.; Tahjib-Ul-Arif, M.; Sohag, A.A.M.; Latef, A.A.H.A.; Ragaey, M.M. Mechanistic insight of allantoin in protecting tomato plants against ultraviolet C stress. Plants 2021, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Soltabayeva, A.; Bekturova, A.; Kurmanbayeva, A.; Oshanova, D.; Nurbekova, Z.; Srivastava, S.; Standing, D.; Sagi, M. Ureides are accumulated similarly in response to UV-C irradiation and wounding in Arabidopsis leaves but are remobilized differently during recovery. J. Exp. Bot. 2022, 73, 1016–1032. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, M.; Dresler, S.; Tukiendorf, A. Physiological mechanisms of adaptation of Dianthus carthusianorum L. to growth on a Zn-Pb waste deposit-the case of chronic multi-metal and acute Zn stress. Plant Soil. 2015, 390, 237–250. [Google Scholar] [CrossRef]
- Izmailow, R.; Biskup, A. Reproduction of Echium vulgare L. (Boraginaceae) at contaminated sites. Acta Biol. Crac. Ser. Bot. 2003, 45, 69–75. [Google Scholar]
- Dresler, S.; Bednarek, W.; Wojcik, M. Effect of cadmium on selected physiological and morphological parameters in metallicolous and non-metallicolous populations of Echium vulgare L. Ecotoxicol. Environ. Saf. 2014, 104, 332–338. [Google Scholar] [CrossRef]
- Lal, N. Molecular mechanisms and genetic basis of heavy metal toxicity and tolerance in plants. In Plant Adaptation and Phytoremediation; Ashraf, M., Ozturk, M., Ahmad, M.S.A., Eds.; Springer: Dordrecht, The Netherlands; pp. 35–58.
- Brooks, C.; Hart, S.R.; Wendt, I. Realistic use of two-error regression treatments as applied to rubidium-strontium data. Rev. Geophys. 1972, 10, 551–577. [Google Scholar] [CrossRef]
- Kovacik, J.; Dresler, S.; Strzemski, M.; Sowa, I.; Babula, P.; Wojciak-Kosior, M. Nitrogen modulates strontium uptake and toxicity in Hypericum perforatum plants. J. Hazard. Mat. 2022, 425, 127894. [Google Scholar] [CrossRef]
- Dresler, S.; Kovacik, J.; Sowa, I.; Wojciak, M.; Strzemski, M.; Rysiak, A.; Babula, P.; Todd, C.D. Allantoin overaccumulation enhances production of metabolites under excess of metals but is not tightly regulated by nitric oxide. J. Hazard. Mat. 2022, 129, 138. [Google Scholar] [CrossRef]
- Irani, S.; Lobo, J.M.; Gray, G.R.; Todd, C.D. Allantoin accumulation in response to increased growth irradiance in Arabidopsis thaliana. Biol. Plant. 2018, 62, 181–187. [Google Scholar] [CrossRef]
- Castro, A.H.F.; Young, M.C.; Alvarenga, A.A.D.; Alves, J.D. Influence of photoperiod on the accumulation of allantoin in comfrey plants. Rev. Bras. Fisiol. Veg. 2001, 13, 49–54. [Google Scholar] [CrossRef]
- Brychkova, G.; Xia, Z.; Yang, G.; Yesbergenova, Z.; Zhang, Z.; Davydov, O.; Fluhr, R.; Sagi, M. Sulfite oxidase protects plants against sulfur dioxide toxicity. Plant J. 2007, 50, 696–709. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Hirashima, M.; Satoh, S.; Tanaka, A. The Arabidopsis-accelerated cell death gene ACD1 is involved in oxygenation of pheophorbide a: Inhibition of the pheophorbide a oxygenase activity does not lead to the “stay-green” phenotype in Arabidopsis. Plant Cell Physiol. 2003, 44, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Li, W.A.N.G.; Jiang, L.L.; Mika, N.; Shigeyuki, T.; Cheng, X.G. Excessive ammonia inhibited transcription of MsU2 gene and furthermore affected accumulation distribution of allantoin and amino acids in alfalfa Medicago sativa. J. Integr. Agric. 2015, 14, 1269–1282. [Google Scholar] [CrossRef]
- Coneva, V.; Simopoulos, C.; Casaretto, J.A.; El-Kereamy, A.; Guevara, D.R.; Cohn, J.; Zhu, T.; Guo, L.; Alexander, D.C.; Bi, Y.M.; et al. Metabolic and co-expression network-based analyses associated with nitrate response in rice. BMC Genom. 2014, 15, 1056. [Google Scholar] [CrossRef]
- Todd, C.D.; Tipton, P.A.; Blevins, D.G.; Piedras, P.; Pineda, M.; Polacco, J.C. Update on ureide degradation in legumes. J. Exp. Bot. 2006, 57, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Lescano, I.; Devegili, A.M.; Martini, C.; Tessi, T.M.; Gonzalez, C.A.; Desimone, M. Ureide metabolism in Arabidopsis thaliana is modulated by C:N balance. J. Plant Res. 2020, 133, 739–749. [Google Scholar] [CrossRef]
- Filippi, S.B.; Azevedo, R.A.; Sodek, L.; Mazzafera, P. Allantoin has a limited role as nitrogen source in cultured coffee cells. J. Plant Physiol. 2007, 164, 544–552. [Google Scholar] [CrossRef]
- Krapp, A.; Berthome, R.; Orsel, M.; Mercey-Boutet, S.; Yu, A.; Castaings, L.; Elftieh, S.; Major, H.; Renou, J.P.; Daniel-Vedele, F. Arabidopsis roots and shoots show distinct temporal adaptation patterns toward nitrogen starvation. Plant Physiol. 2011, 157, 1255–1282. [Google Scholar] [CrossRef]
- Lee, D.K.; Redillas, M.C.; Jung, H.; Choi, S.; Kim, Y.S.; Kim, J.K. A nitrogen molecular sensing system, comprised of the ALLANTOINASE and UREIDE PERMEASE 1 genes, can be used to monitor N status in rice. Front. Plant Sci. 2018, 9, 444. [Google Scholar] [CrossRef]
- Xin, W.; Zhang, L.; Gao, J.; Zhang, W.; Yi, J.; Zhen, X.; Bi, C.; He, D.; Liu, S.; Zhao, X. Adaptation mechanism of roots to low and high nitrogen revealed by proteomic analysis. Rice 2021, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Prosser, I.M.; Purves, J.V.; Saker, L.R.; Clarkson, D.T. Rapid disruption of nitrogen metabolism and nitrate transport in spinach plants deprived of sulphate. J. Exp. Bot. 2001, 52, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Katahira, R.; Ashihara, H. Profiles of pyrimidine biosynthesis, salvage and degradation in disks of potato (Solanum tuberosum L.) tubers. Planta 2002, 215, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Krantev, A.; Yordanova, R.; Janda, T.; Szalai, G.; Popova, L. Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J. Plant Physiol. 2008, 165, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Ishiga, Y.; Watanabe, S.; Konishi, T.; Egusa, M.; Akiyoshi, N.; Matsuura, T.; Mori, I.C.; Hirayama, T.; Kaminaka, H.; et al. Allantoin, a stress-related purine metabolite, can activate jasmonate signalling in a MYC2-regulated and abscisic acid-dependent manner. J. Exp. Bot. 2016, 67, 2519–2532. [Google Scholar] [CrossRef]
- Seiler, C.; Harshavardhan, V.T.; Rajesh, K.; Reddy, P.S.; Strickert, M.; Rolletschek, H.; Scholz, U.; Wobus, U.; Sreenivasulu, N. ABA biosynthesis and degradation contributing to ABA homeostasis during barley seed development under control and terminal drought-stress conditions. J. Exp. Bot. 2011, 62, 2615–2632. [Google Scholar] [CrossRef]
- Yang, X.F.; Li, L.L.; Xu, Y.; Kong, C.H. Kin recognition in rice (Oryza sativa) lines. N. Phytol. 2018, 220, 567–578. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, R.; Chandra, J.; Varghese, B.; Keshavkant, S. Allantoin: A Potential Compound for the Mitigation of Adverse Effects of Abiotic Stresses in Plants. Plants 2023, 12, 3059. https://doi.org/10.3390/plants12173059
Kaur R, Chandra J, Varghese B, Keshavkant S. Allantoin: A Potential Compound for the Mitigation of Adverse Effects of Abiotic Stresses in Plants. Plants. 2023; 12(17):3059. https://doi.org/10.3390/plants12173059
Chicago/Turabian StyleKaur, Rasleen, Jipsi Chandra, Boby Varghese, and S. Keshavkant. 2023. "Allantoin: A Potential Compound for the Mitigation of Adverse Effects of Abiotic Stresses in Plants" Plants 12, no. 17: 3059. https://doi.org/10.3390/plants12173059
APA StyleKaur, R., Chandra, J., Varghese, B., & Keshavkant, S. (2023). Allantoin: A Potential Compound for the Mitigation of Adverse Effects of Abiotic Stresses in Plants. Plants, 12(17), 3059. https://doi.org/10.3390/plants12173059






